Application of Heterogeneous Catalytic Ozonation for Refractory Organics in Wastewater
Abstract
:1. Introduction
2. Advanced Oxidation Processes (AOPs)
3. Heterogeneous Catalytic Ozonation
- Metal oxides
- Metal or metal oxides on supports
- Carbon materials
- Minerals modified with metals
4. Metal Oxides
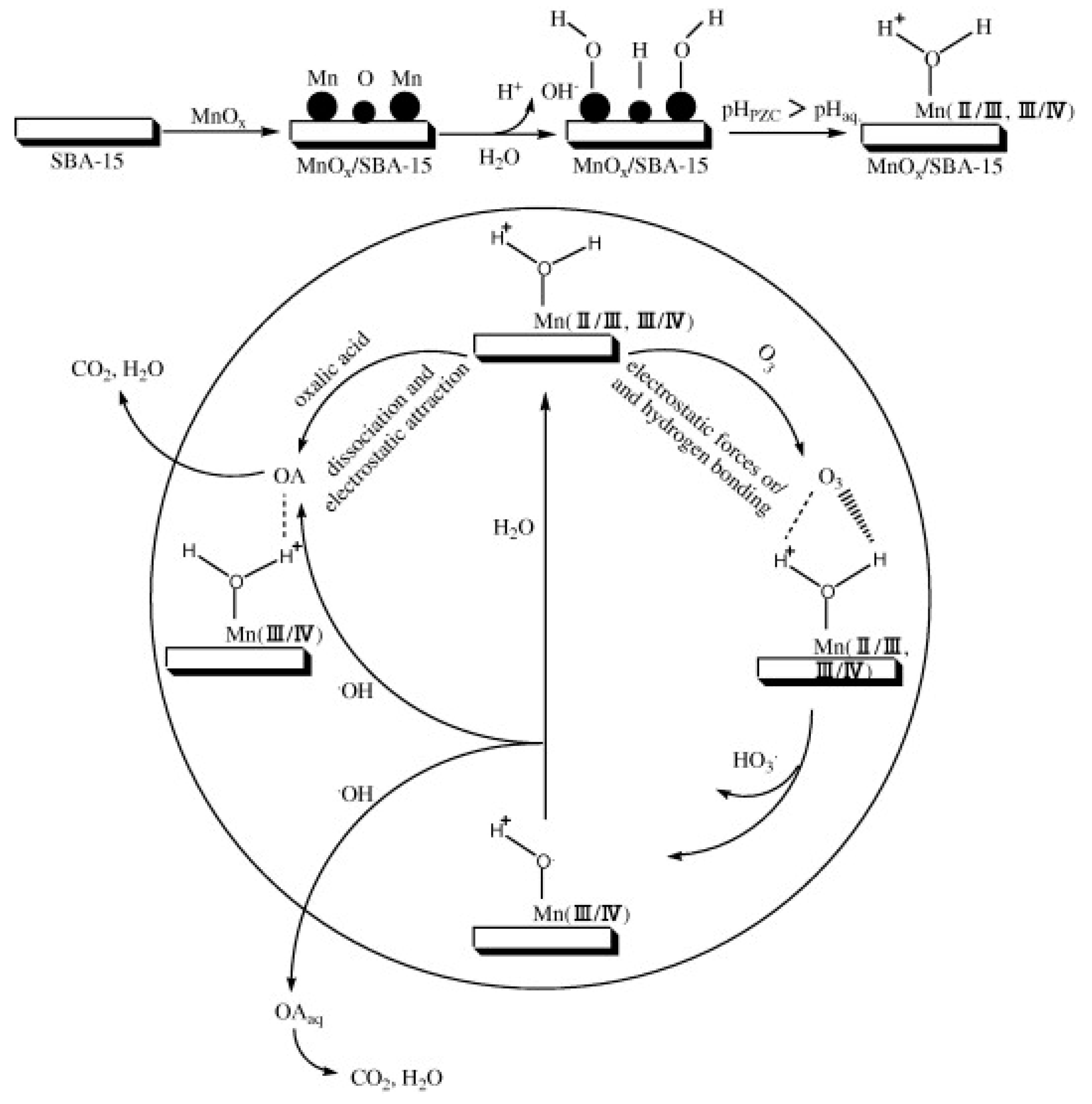
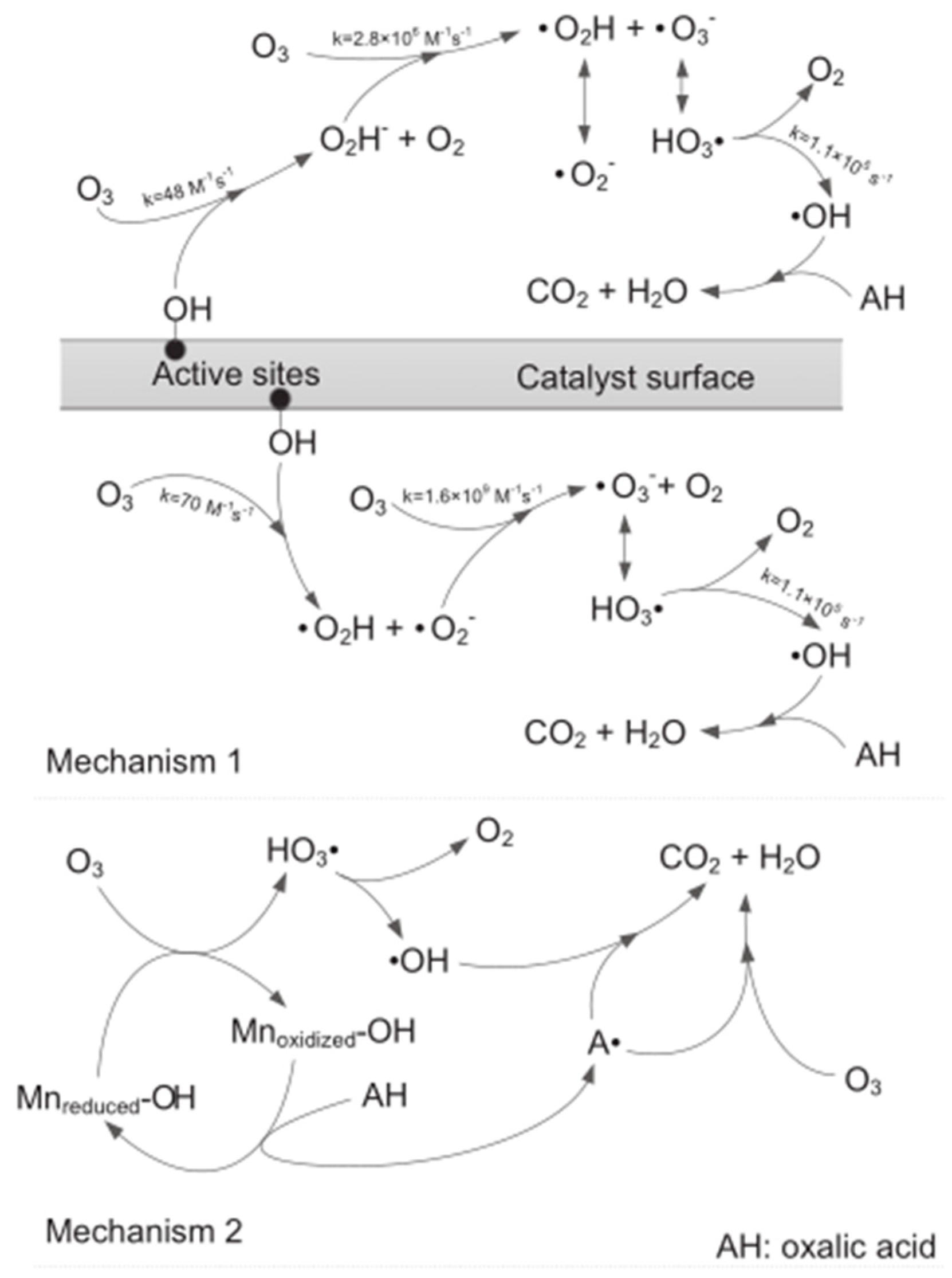
4.1. Manganese Oxide
4.2. Titanium Dioxide
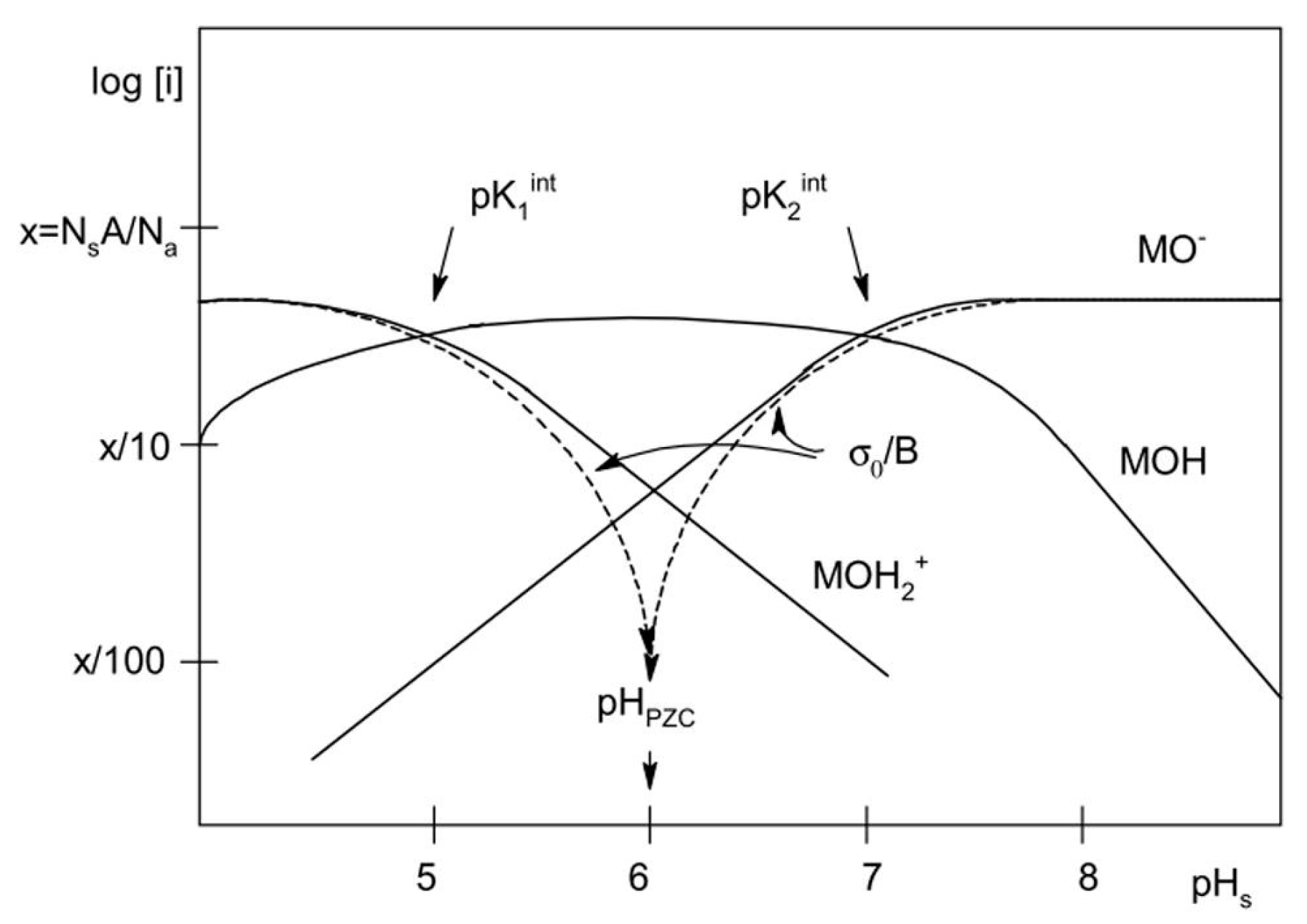
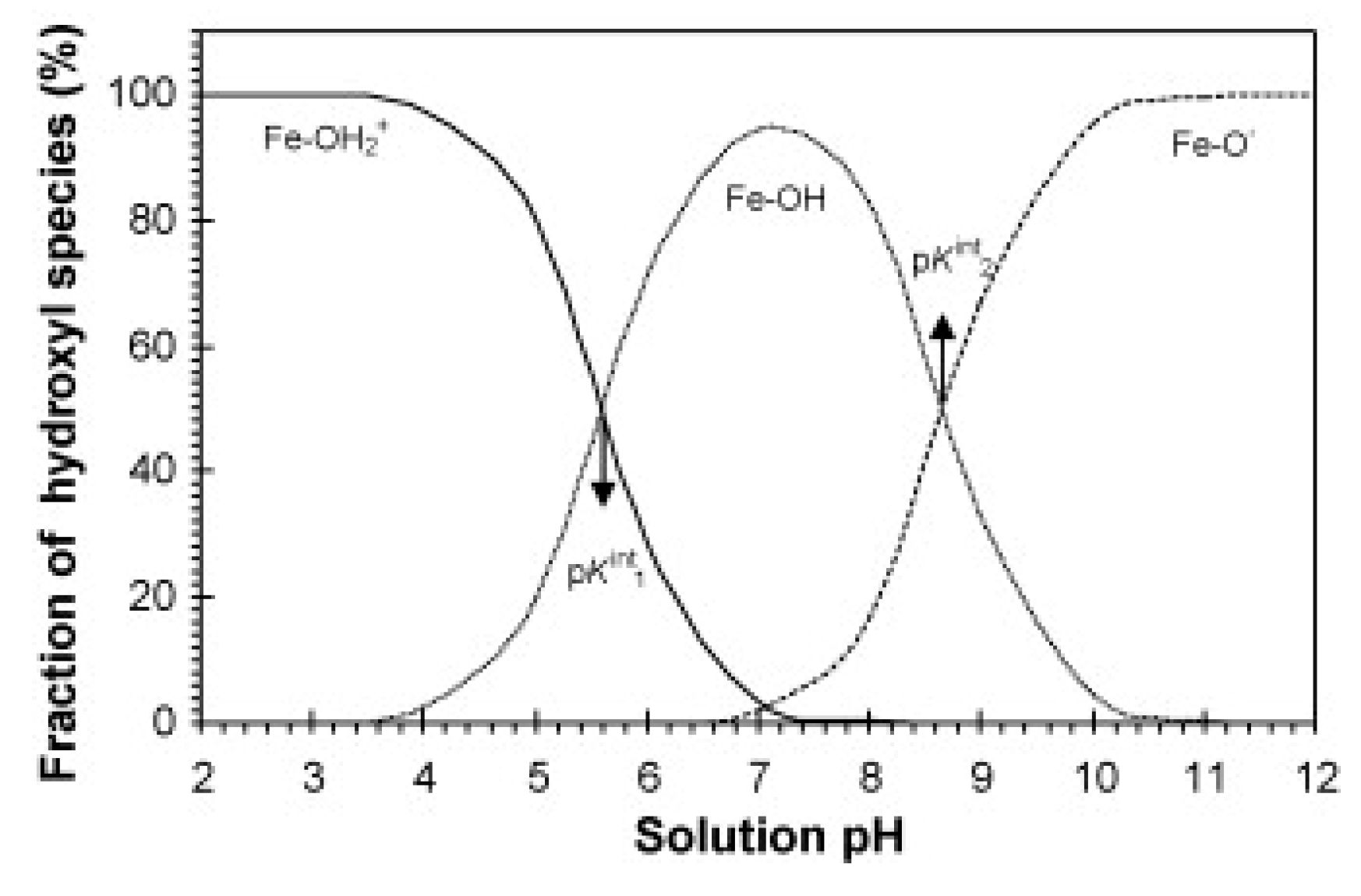
4.3. Iron Oxides
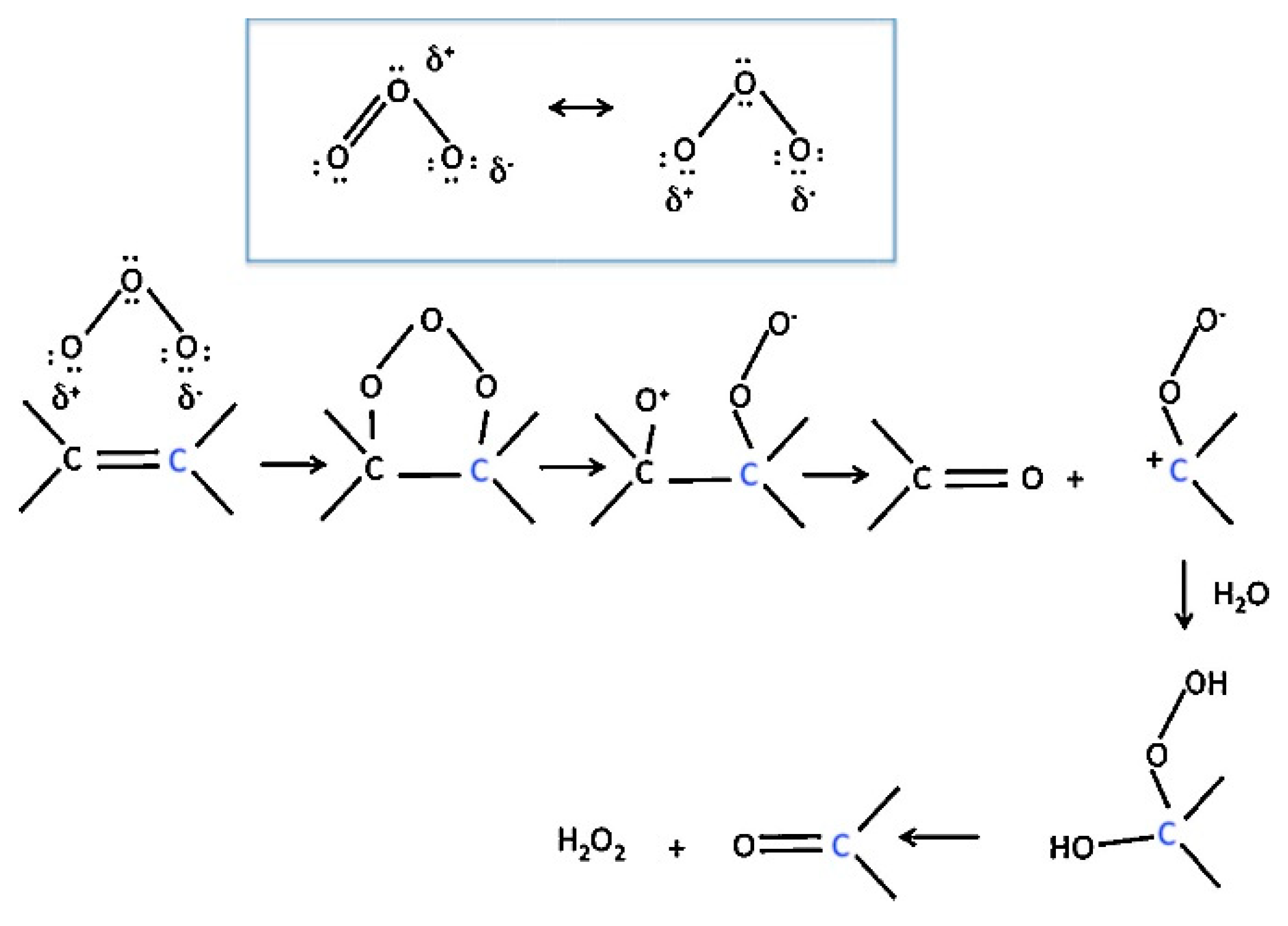
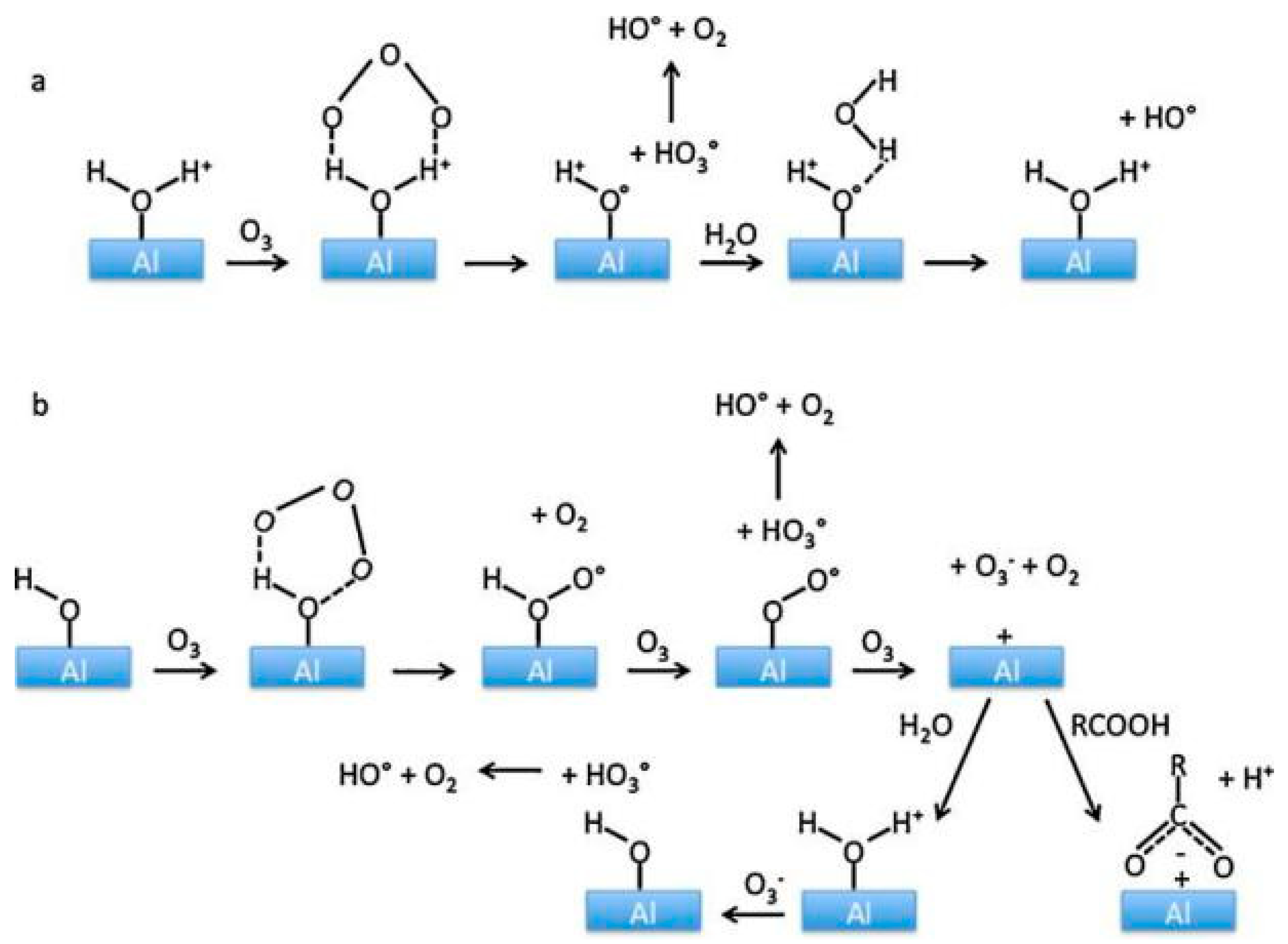
4.4. Aluminum Oxides
4.5. Magnesium Oxide
- Direct oxidation with O3 molecules on MgO surface:
- Radical type catalytic oxidation on MgO surface:
- Direct oxidation with O3 molecules in the bulk solution:
- Radical type catalytic oxidation in the bulk solution:
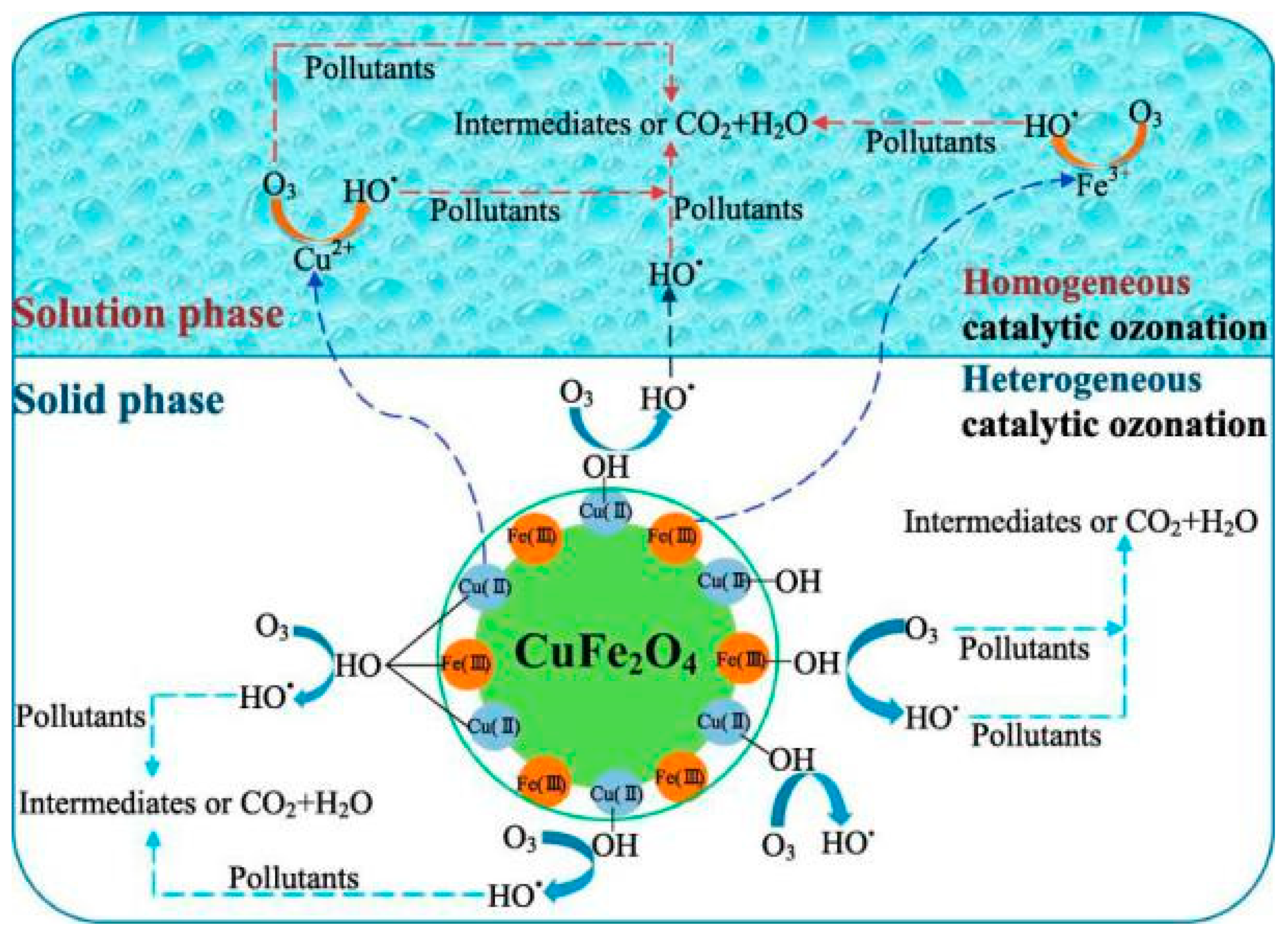
4.6. Bimetallic Oxides
5. Metal or Metal Oxides on Supports
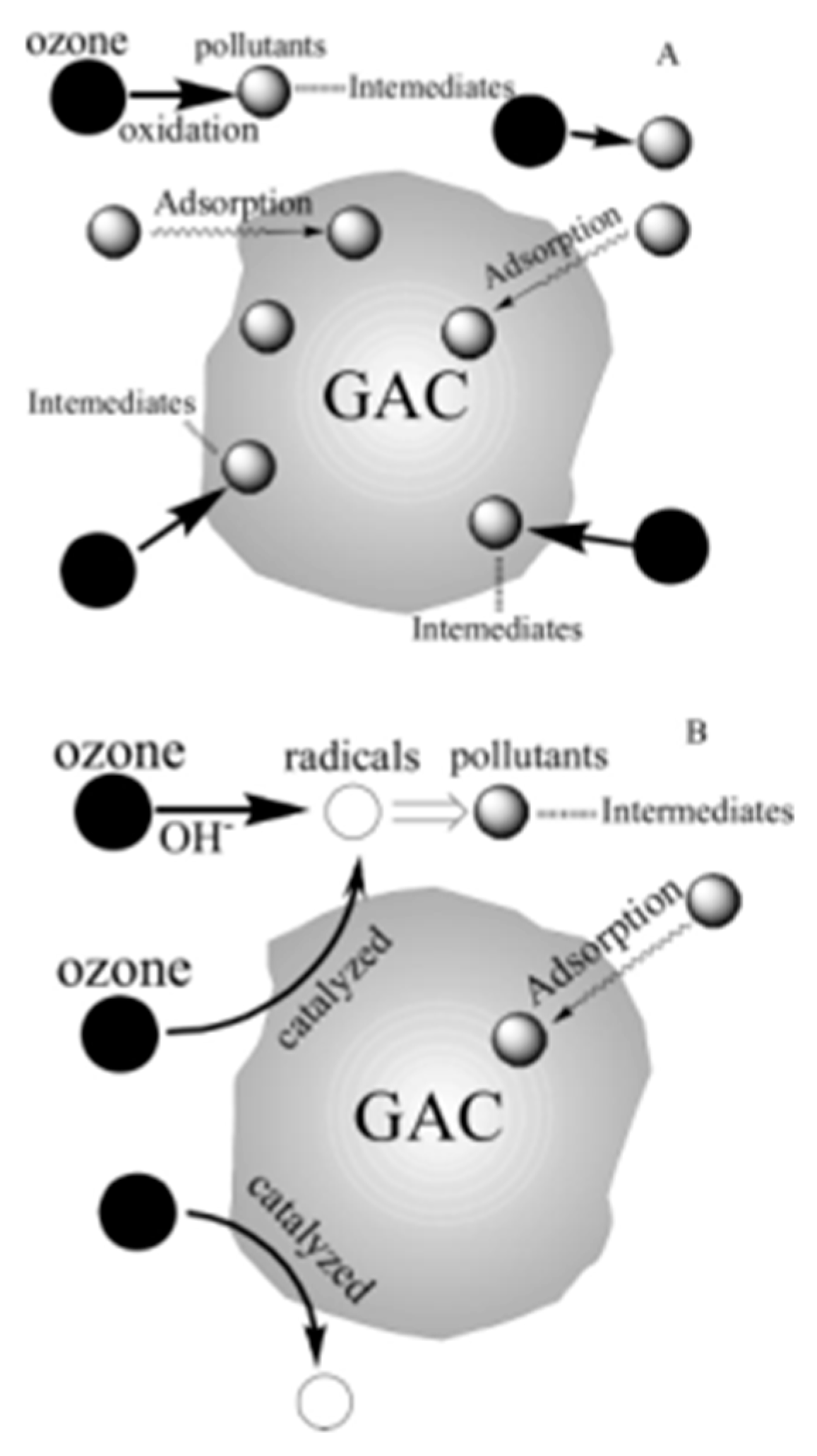
6. Carbon Materials
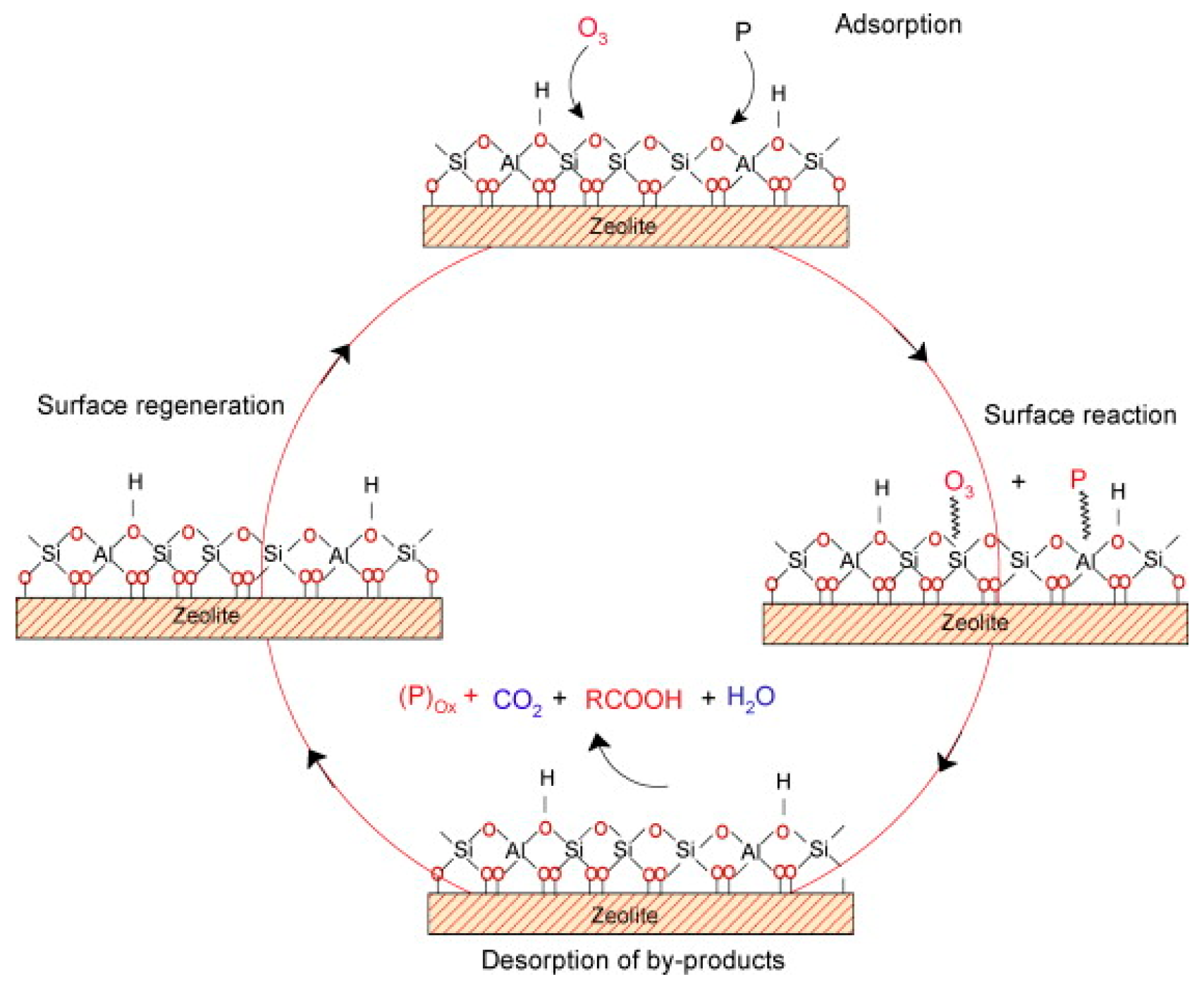
7. Minerals Modified with Metals
8. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akar, S.T.; Uysal, R. Untreated clay with high adsorption capacity for effective removal of C.I. Acid Red 88 from aqueous solutions: Batch and dynamic flow mode studies. Chem. Eng. J. 2010, 162, 591–598. [Google Scholar] [CrossRef]
- Martínez, F.; Melero, J.A.; Botas, J.Á.; Pariente, M.I.; Molina, R. Treatment of Phenolic Effluents by Catalytic Wet Hydrogen Peroxide Oxidation over Fe2O3/SBA-15 Extruded Catalyst in a Fixed-Bed Reactor. Ind. Eng. Chem. Res. 2007, 46, 4396–4405. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation—A Review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Shokri, A.; Mahanpoor, K. Degradation of ortho-toluidine from aqueous solution by the TiO2/O3 process. Int. J. Ind. Chem. 2017, 8, 101–108. [Google Scholar] [CrossRef]
- Wang, J.L.; Lou, Y.Y.; Xu, C.; Song, S.; Liu, W.P. Magnetic lanthanide oxide catalysts: An application and comparison in the heterogeneous catalytic ozonation of diethyl phthalate in aqueous solution. Sep. Purif. Technol. 2016, 159, 57–67. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W.; Chapin, D.H. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Linden, K.G.; Mohseni, M. 2.8-Advanced Oxidation Processes: Applications in Drinking Water Treatment. Compr. Water Q. Purif. 2014, 2, 148–172. [Google Scholar]
- Ameta, S.C.; Ameta, R. Introduction. In Advanced Oxidation Processes for Waste Water Treatment; Ameta, S.C., Ed.; Udaipur: Rajasthan, India, 2018; pp. 1–12. ISBN 978-0-12-810499-6. [Google Scholar]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Kim, K.H.; Ihm, S.K. Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: A review. J. Hazard. Mater. 2011, 186, 16–34. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent-A review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Boczkaj, G.; Fernandes, A.; Makoś, P. Study of Different Advanced Oxidation Processes for Wastewater Treatment from Petroleum Bitumen Production at Basic pH. Ind. Eng. Chem. Res. 2017, 56, 8806–8814. [Google Scholar] [CrossRef]
- Fernandes, A.; Makoś, P.; Khan, J.A.; Boczkaj, G. Pilot scale degradation study of 16 selected volatile organic compounds by hydroxyl and sulfate radical based advanced oxidation processes. J. Clean. Prod. 2019, 208, 54–64. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Highly effective degradation of selected groups of organic compounds by cavitation based AOPs under basic pH conditions. Ultrason. Sonochem. 2018, 45, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Effective method of treatment of industrial effluents under basic pH conditions using acoustic cavitation-A comprehensive comparison with hydrodynamic cavitation processes. Chem. Eng. Process.-Process 2018, 128, 103–113. [Google Scholar] [CrossRef]
- Boczkaj, G.; Gągol, M.; Klein, M.; Przyjazny, A. Effective method of treatment of effluents from production of bitumens under basic pH conditions using hydrodynamic cavitation aided by external oxidants. Ultrason. Sonochem. 2018, 40, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Vittenet, J.; Aboussaoud, W.; Mendret, J.; Pic, J.S.; Debellefontaine, H.; Lesage, N.; Faucher, K.; Manero, M.H.; Thibault-Starzyk, F.; Leclerc, H. Catalytic ozonation with γ-Al2O3 to enhance the degradation of refractory organics in water. Appl. Catal. A Gen. 2014, 504, 519–532. [Google Scholar] [CrossRef]
- Maddila, S.; Dasireddy, V.D.B.C.; Jonnalagadda, S.B. Dechlorination of tetrachloro-o-benzoquinone by ozonation catalyzed by cesium loaded metal oxides. Appl. Catal. B Environ. 2013, 138–139, 149–160. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Monteiro, D.C.M.; Órfão, J.J.M.; Pereira, M.F.R. Cerium, manganese and cobalt oxides as catalysts for the ozonation of selected organic compounds. Chemosphere 2009, 74, 818–824. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Q.; Zhang, A.L.; Song, S.; Liu, Z.W.; Chen, J.M.; Xu, X.H.; Liu, W.P. γ-Al2O3 Modified with Praseodymium: An Application in the Heterogeneous Catalytic Ozonation of Succinic Acid in Aqueous Solution. Ind. Eng. Chem. Res. 2010, 49, 12345–12351. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Catalytic ozonation of sulfonated aromatic compounds in the presence of activated carbon. Appl. Catal. B Environ. 2008, 83, 150–159. [Google Scholar] [CrossRef]
- Wu, J.; Ma, L.M.; Chen, Y.L.; Cheng, Y.Q.; Liu, Y.; Zha, X.S. Catalytic ozonation of organic pollutants from bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst: Removal and pathways. Water Res. 2016, 92, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Bai, Z.Y. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2016, 312, 79–98. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Oyama, S.T. Ozone Decomposition over Manganese Oxide Supported on ZrO2 and TiO2: A Kinetic Study Using in Situ Laser Raman Spectroscopy. J. Catal. 2001, 199, 282–290. [Google Scholar] [CrossRef]
- Hu, C.; Xing, S.T.; Qu, J.H.; He, H. Catalytic Ozonation of Herbicide 2,4-D over Cobalt Oxide Supported on Mesoporous Zirconia. J. Phys. Chem. C 2008, 112, 5978–5983. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Kasprzyk-Hordern, B. Catalytic ozonation of chlorinated VOCs on ZSM-5 zeolites and alumina: Formation of chlorides. Appl. Catal. B Environ. 2017, 200, 274–282. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Mechanisms of catalytic ozonation on alumina and zeolites in water: Formation of hydroxyl radicals. Appl. Catal. B Environ. 2012, 123–124, 94–106. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, R.P.; Zhao, X.; Q, J.H. Effect of manganese ion on the mineralization of 2,4-dichlorophenol by ozone. Chemosphere 2008, 72, 1006–1012. [Google Scholar] [CrossRef]
- Nawrocki, J.; Rigney, M.; Mccormick, A.; Carr, P.W. Chemistry of zirconia and its use in chromatography. J. Chromatogr. A 1993, 657, 229–282. [Google Scholar] [CrossRef]
- Yang, L.; Hu, C.; Nie, Y.L.; Q, J.H. Catalytic ozonation of selected pharmaceuticals over mesoporous alumina-supported manganese oxide. Environ. Sci. Technol. 2009, 43, 2525–2529. [Google Scholar] [CrossRef]
- Ma, J.; Sui, M.H.; Chen, Z.L.; Wang, L.N. Degradation of Refractory Organic Pollutants by Catalytic Ozonation-Activated Carbon and Mn-Loaded Activated Carbon as Catalysts. Ozone Sci. Eng. 2004, 26, 3–10. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xie, Y.B.; Sun, H.Q.; Xiao, J.D.; Cao, H.B.; Wang, S.B. 2D/2D nano-hybrids of γ-MnO2 on reduced graphene oxide for catalytic ozonation and coupling peroxymonosulfate activation. J. Hazard. Mater. 2016, 301, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lu, Y.T.; Lu, C.; Zhu, M.S.; Zhai, C.Y.; Du, Y.K.; Yang, P. Efficient catalytic ozonation of bisphenol-A over reduced graphene oxide modified sea urchin-like α-MnO2 architectures. J. Hazard. Mater. 2015, 294, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.Q.; Li, L.S.; Yan, H.H.; Hong, X.T.; Hui, K.S.; Pan, Z.Q. Influence of the surface hydroxyl groups of MnOx/SBA-15 on heterogeneous catalytic ozonation of oxalic acid. Chem. Eng. J. 2014, 242, 348–356. [Google Scholar] [CrossRef]
- Rosal, R.; Gonzalo, M.S.; Rodríguez, A.; García-Calvo, E. Catalytic ozonation of fenofibric acid over alumina-supported manganese oxide. J. Hazard. Mater. 2010, 183, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wen, W.J.; Kong, L.J.; Tian, S.H.; Ding, F.C.; Xiong, Y. Magnetically Separable and Durable MnFe2O4 for Efficient Catalytic Ozonation of Organic Pollutants. Ind. Eng. Chem. Res. 2014, 53, 6297–6306. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.X.; Hong, H. Transition metal doped cryptomelane-type manganese oxide catalysts for ozone decomposition. Appl. Catal. B Environ. 2017, 201, 503–510. [Google Scholar] [CrossRef]
- Xu, H.M.; Yan, N.Q.; Qu, Z.; Liu, W.; Mei, J.; Huang, W.J.; Zhao, S.J. Gaseous Heterogeneous Catalytic Reactions over Mn-based Oxides for Environmental Applications-A Critical Review. Environ. Sci. Technol. 2017, 51, 8879–8892. [Google Scholar] [CrossRef]
- Tong, S.P.; Liu, W.P.; Leng, W.H.; Zhang, Q.Q. Characteristics of MnO2 catalytic ozonation of sulfosalicylic acid and propionic acid in water. Chemosphere 2003, 50, 1359–1364. [Google Scholar] [CrossRef]
- Luo, K.; Zhao, S.X.; Wang, Y.F.; Zhao, S.J.; Zhang, X.H. Synthesis of petal-like δ- MnO2 and its catalytic ozonation performance. New J. Chem. 2018, 42, 6770–6777. [Google Scholar] [CrossRef]
- Nawaz, F.; Xie, Y.B.; Cao, H.B.; Xiao, J.D.; Wang, Y.Q.; Zhang, X.H.; Li, M.J.; Duan, F. Catalytic ozonation of 4-nitrophenol over an mesoporous α-MnO2 with resistance to leaching. Catal. Today 2015, 258, 595–601. [Google Scholar] [CrossRef]
- Nawaz, F.; Xie, Y.; Xiao, J.D.; Cao, H.B.; Ghazi, Z.A.; Guo, Z.; Chen, Y. The influence of the substituent to phenol oxidation rate and reactive species in cubic MnO2 catalytic ozonation. Catal. Sci. Technol. 2016, 6, 7875–7884. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.Q.; Ang, H.M.; Tadé, M.O.; Wang, S.B. Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B Environ. 2013, 142–143, 729–735. [Google Scholar] [CrossRef]
- Andreozzi, R.; Insola, A.; Caprio, V.; Marotta, R.; Tufano, V. The use of manganese dioxide as a heterogeneous catalyst for oxalic acid ozonation in aqueous solution. Appl. Catal. A Gen. 1996, 138, 75–81. [Google Scholar] [CrossRef]
- Nawaz, F.; Cao, H.B.; Xie, Y.B.; Xiao, J.D.; Chen, Y.; Ghazi, Z.A. Selection of active phase of MnO2 for catalytic ozonation of 4-nitrophenol. Chemosphere 2017, 168, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.H.; Teng, F.; Bulgan, G.; Zong, R.L.; Zhu, Y.F. Effect of Phase Structure of MnO2 Nanorod Catalyst on the Activity for CO Oxidation. J. Phys. Chem. C 2008, 112, 5307–5315. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.Q.; Ang, H.M.; Tadé, M.O.; Wang, S.B. Different crystallographic one-dimensional MnO2 nanomaterials and their superior performance in catalytic phenol degradation. Environ. Sci. Technol. 2013, 47, 5882–5887. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.M.; Yang, H.X.; He, K.; Song, S.Q.; Zhang, A.M. β-MnO2 nanowires: A novel ozonation catalyst for water treatment. Appl. Catal. B Environ. 2009, 85, 155–161. [Google Scholar] [CrossRef]
- Kapteijn, F.; Vanlangeveld, A.D.V.; Moulijn, J.A.; Andreini, A.; Vuurman, M.A.; Turek, A.M.; Jehng, J.M.; Wachs, I.E. Alumina-Supported Manganese Oxide Catalysts: I. Characterization: Effect of Precursor and Loading. J. Catal. 1994, 150, 94–104. [Google Scholar] [CrossRef]
- Yin, M.C.; Li, Z.S.; Kou, J.H.; Zou, Z.G. Mechanism Investigation of Visible Light-Induced Degradation in a Heterogeneous TiO2/Eosin Y/Rhodamine B System. Environ. Sci. Technol. 2009, 43, 8361–8366. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.M.; Jiang, P.P.; Wang, G.L.; Zhang, J.J.; Li, K.; Feng, C.Y. An α-MnO2 nanotube used as a novel catalyst in ozonation: Performance and the mechanism. New J. Chem. 2014, 38, 1743–1750. [Google Scholar] [CrossRef]
- Tan, X.Q.; Wan, Y.F.; Huang, Y.J.; He, C.; Zhang, Z.L.; He, Z.Y.; Hu, L.L.; Zeng, J.W.; Shu, D. Three-dimensional MnO2 porous hollow microspheres for enhanced activity as ozonation catalysts in degradation of bisphenol A. J. Hazard. Mater. 2017, 321, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Li, Y. Understanding the Reaction Mechanism of Photocatalytic Reduction of CO2 with H2O on TiO2-Based Photocatalysts: A Review. Aerosol Air Q. Res. 2014, 14, 453–469. [Google Scholar] [CrossRef]
- Liu, L.M.; Li, S.C.; Cheng, H.Z.; Diebold, U.; Selloni, A. Growth and organization of an organic molecular monolayer on TiO2: Catechol on anatase (101). J. Am. Chem. Soc. 2011, 133, 7816–7823. [Google Scholar] [CrossRef] [PubMed]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009, 2, 745–758. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Gonzalo, M.S.; García-Calvo, E. Catalytic ozonation of naproxen and carbamazepine on titanium dioxide. Appl. Catal. B Environ. 2008, 84, 48–57. [Google Scholar] [CrossRef]
- Yang, Y.X.; Ma, J.; Qin, Q.D.; Zhai, X.D. Degradation of nitrobenzene by nano-TiO2 catalyzed ozonation. J. Mol. Catal. A Chem. 2007, 267, 41–48. [Google Scholar] [CrossRef]
- Farbod, M.; Khademalrasool, M. Synthesis of TiO2 nanoparticles by a combined sol–gel ball milling method and investigation of nanoparticle size effect on their photocatalytic activities. Powder Technol. 2011, 214, 344–348. [Google Scholar] [CrossRef]
- Saliby, I.E.; Okour, Y.; Shon, H.K.; Kandasamy, J.; Lee, W.E.; Kim, J.H. TiO2 nanoparticles and nanofibres from TiCl4 flocculated sludge: Characterisation and photocatalytic activity. J. Ind. Eng. Chem. 2012, 18, 1033–1038. [Google Scholar] [CrossRef]
- Rosal, R.; Gonzalo, M.S.; Rodríguez, A.; García-Calvo, E. Ozonation of clofibric acid catalyzed by titanium dioxide. J. Hazard. Mater. 2009, 169, 411–418. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.H.; He, Z.Q.; Zhang, A.L.; Chen, J.M. Impacts of morphology and crystallite phases of titanium oxide on the catalytic ozonation of phenol. Environ. Sci. Technol. 2010, 44, 3913–3918. [Google Scholar] [CrossRef] [PubMed]
- Trancik, J.E.; Barton, S.C.; Hone, J. Transparent and catalytic carbon nanotube films. Nano Lett. 2008, 8, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, J.M.; Abbatt, J.P.D. Diffuse reflectance FTIR study of the interaction of alumina surfaces with ozone and water vapor. J. Phys. Chem. A 2005, 109, 9028–9034. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Lurot, F.; Schrotter, J.C. Catalytic ozonation of refractory organic model compounds in aqueous solution by aluminum oxide. Appl. Catal. B Environ. 2004, 47, 15–25. [Google Scholar] [CrossRef]
- Hernández-Alonso, M.D.; Coronado, J.M.; Soria, J.; Conesa, J.C.; Loddo, V.; Addamo, M.; Augugliaro, V. EPR and kinetic investigation of free cyanide oxidation by photocatalysis and ozonation. Res. Chem. Intermed. 2007, 33, 205–224. [Google Scholar] [CrossRef]
- Anandan, S.; Wu, J.J. Effective Degradation of Fipronil Using Combined Catalytic Ozonation Processes. Ozone Sci. Eng. 2015, 37, 186–190. [Google Scholar] [CrossRef]
- Ye, M.M.; Chen, Z.L.; Zhang, T.Q.; Shao, W.Y. Effect of calcination temperature on the catalytic activity of nanosized TiO2 for ozonation of trace 4-chloronitrobenzene. Water Sci. Technol. 2012, 66, 479–486. [Google Scholar] [CrossRef]
- Shahamat, Y.D.; Farzadkia, M.; Nasseri, S.; Mahvi, A.H.; Gholami, M.; Esrafili, A. Magnetic heterogeneous catalytic ozonation: A new removal method for phenol in industrial wastewater. J. Environ. Health Sci. Eng. 2014, 12, 50. [Google Scholar] [CrossRef]
- Lan, B.Y.; Huang, R.H.; Li, L.S.; Yan, H.H.; Liao, G.Z.; Wang, X.; Zhang, Q.Y. Catalytic ozonation of p-chlorobenzoic acid in aqueous solution using Fe-MCM-41 as catalyst. Chem. Eng. J. 2013, 219, 346–354. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, J. Catalytic ozonation of trace nitrobenzene in water with synthetic goethite. J. Mol. Catal. A Chem. 2008, 279, 82–89. [Google Scholar] [CrossRef]
- Zhuang, H.F.; Han, H.J.; Hou, B.L.; Jia, S.Y.; Zhao, Q. Heterogeneous catalytic ozonation of biologically pretreated Lurgi coal gasification wastewater using sewage sludge based activated carbon supported manganese and ferric oxides as catalysts. Bioresour. Technol. 2014, 166, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Chen, W.Y.; Ma, L.M.; Wang, H.W.; Fan, J.H. Industrial wastewater advanced treatment via catalytic ozonation with an Fe-based catalyst. Chemosphere 2017, 195, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Oputu, O.; Chowdhury, M.; Nyamayaro, K.; Fatoki, O.; Fester, V. Catalytic activities of ultra-small β-FeOOH nanorods in ozonation of 4-chlorophenol. J. Environ. Sci. 2015, 35, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, Z.; Younesi, H.; Zinatizadeh, A.A. Preparation, characterization and photocatalytic application of TiO2/Fe-ZSM-5 nanocomposite for the treatment of petroleum refinery wastewater: Optimization of process parameters by response surface methodology. Chemosphere 2016, 159, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Hadavifar, M.; Younesi, H.; Zinatizadeh, A.A.; Mahdad, F.; Li, Q.; Ghasemi, Z. Application of integrated ozone and granular activated carbon for decolorization and chemical oxygen demand reduction of vinasse from alcohol distilleries. J. Environ. Manag. 2016, 170, 28–36. [Google Scholar] [CrossRef]
- Wang, C.; Li, A.M.; Shuang, C.D. The effect on ozone catalytic performance of prepared-FeOOH by different precursors. J. Environ. Manag. 2018, 228, 158–164. [Google Scholar] [CrossRef]
- Stumm, W. Introduction. In Chemistry of the Solid-Water Interface: Processes at the Mineral-Water and Particle-Water Interface in Natural Systems; WILEY-VCH Verlag GmbH & Co.: Zurich, Switzerland, 1992; pp. 13–41. ISBN 978-0-471-57672-3. [Google Scholar]
- Sui, M.H.; Sheng, L.; Lu, K.X.; Tian, F. FeOOH catalytic ozonation of oxalic acid and the effect of phosphate binding on its catalytic activity. Appl. Catal. B Environ. 2010, 96, 94–100. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.Z.; Ma, J. Novel relationship between hydroxyl radical initiation and surface group of ceramic honeycomb supported metals for the catalytic ozonation of nitrobenzene in aqueous solution. Environ. Sci. Technol. 2009, 43, 4157–4163. [Google Scholar] [CrossRef]
- Buhler, R.E.; Staehelin, J.; Hoigne, J. Ozone Decompositlon in Water Studied by Pulse Radiolysis. 1. HO2/O2− and HO3/O3− as Intermediates. J. Phys. Chem. 1984, 88, 2560–2564. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, L.; Sun, Z.Z.; Liu, H.L. Mechanism of heterogeneous catalytic ozonation of nitrobenzene in aqueous solution with modified ceramic honeycomb. Appl. Catal. B Environ. 2009, 89, 326–334. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.J.; Ma, J.; Tian, H.; Qiang, Z.M. Surface hydroxyl groups of synthetic α-FeOOH in promoting ·OH generation from aqueous ozone: Property and activity relationship. Appl. Catal. B Environ. 2008, 82, 131–137. [Google Scholar] [CrossRef]
- Zhu, S.M.; Dong, B.Z.; Yu, Y.H.; Bu, L.J.; Deng, J.; Zhou, S.Q. Heterogeneous catalysis of ozone using ordered mesoporous Fe3O4 for degradation of atrazine. Chem. Eng. J. 2017, 328, 527–535. [Google Scholar] [CrossRef]
- Xiong, Z.K.; Lai, B.; Yuan, Y.; Cao, J.Y.; Yang, P.; Zhou, Y.X. Degradation of p-nitrophenol (PNP) in aqueous solution by a micro-size Fe0/O3 process (mFe0/O3): Optimization, kinetic, performance and mechanism. Chem. Eng. J. 2016, 302, 137–145. [Google Scholar] [CrossRef]
- Yin, R.L.; Guo, W.Q.; Zhou, X.J.; Zheng, H.S.; Du, J.S.; Wu, Q.L.; Chang, J.S.; Ren, N.Q. Enhanced sulfamethoxazole ozonation based on magnetic Fe3O4 nanoparticles by noble-metal-free catalysis: Catalytic performance and degradation mechanism. RSC Adv. 2016, 6, 19265–19270. [Google Scholar] [CrossRef]
- Aghaeinejad-Meybodi, A.; Ebadi, A.; Shafiei, S.; Khataee, A.; Kiadehi, A.D. Degradation of Fluoxetine using catalytic ozonation in aqueous media in the presence of nano-γ-alumina catalyst: Experimental, modeling and optimization study. Sep. Purif. Technol. 2019, 211, 551–563. [Google Scholar] [CrossRef]
- Keykavoos, R.; Mankidy, R.; Ma, H.; Jones, P.; Soltan, J. Mineralization of bisphenol A by catalytic ozonation over alumina. Sep. Purif. Technol. 2013, 107, 310–317. [Google Scholar] [CrossRef]
- Qi, F.; Xu, B.; Chen, Z.; Feng, L.; Zhang, L.; Sun, D. Catalytic ozonation of 2-isopropyl-3-methoxypyrazine in water by γ-AlOOH and γ-Al2O3: Comparison of removal efficiency and mechanism. Chem. Eng. J. 2013, 219, 527–536. [Google Scholar] [CrossRef]
- Kiadehi, A.D.; Ebadi, A.; Aghaeinejad-Meybodi, A. Removal of methyl tert-butyl ether (MTBE) from aqueous medium in the presence of nano-perfluorooctyl alumina (PFOAL): Experimental study of adsorption and catalytic ozonation processes. Sep. Purif. Technol. 2017, 182, 238–246. [Google Scholar] [CrossRef]
- Qi, F.; Xu, B.B.; Chen, Z.L.; Ma, J. Catalytic ozonation for degradation of 2, 4, 6-trichloroanisole in drinking water in the presence of gamma-AlOOH. Water Environ. Res. A Res. Publ. Water Environ. Fed. 2009, 81, 592–597. [Google Scholar]
- Moussavi, G.; Khavanin, A.; Alizadeh, R. The integration of ozonation catalyzed with MgO nanocrystals and the biodegradation for the removal of phenol from saline wastewater. Appl. Catal. B Environ. 2010, 97, 160–167. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.H.; Lu, J.; Xiong, Y. Catalytic performance of MgO with different exposed crystal facets towards the ozonation of 4-chlorophenol. Appl. Catal. A Gen. 2015, 506, 118–125. [Google Scholar] [CrossRef]
- Moussavi, G.; Yazdanbakhsh, A.; Heidarizad, M. The removal of formaldehyde from concentrated synthetic wastewater using O3/MgO/H2O2 process integrated with the biological treatment. J. Hazard. Mater. 2009, 171, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Khosravi, R.; Omran, N.R. Development of an efficient catalyst from magnetite ore: Characterization and catalytic potential in the ozonation of water toxic contaminants. Appl. Catal. A Gen. 2012, 445-446, 42–49. [Google Scholar] [CrossRef]
- Richards, R.; Mulukutla, R.S.; Mishakov, I.; Chesnokov, V.; Volodin, A.; Zaikovski, V.; Sun, N.J.; Klabunde, K.J. Nanocrystalline ultra high surface area magnesium oxide as a selective base catalyst. Scr. Mater. 2001, 44, 1663–1666. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bedilo, A.F.; Richards, R.M.; Chesnokov, V.V.; Volodin, A.M.; Zaikovskii, V.I.; Buyanov, R.A.; Klabunde, K.J. Nanocrystalline MgO as a dehydrohalogenation catalyst. J. Catal. 2002, 206, 40–48. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Moussavi, G.; Khavanin, A.; Alizadeh, R. The investigation of catalytic ozonation and integrated catalytic ozonation/biological processes for the removal of phenol from saline wastewaters. J. Hazard. Mater. 2009, 171, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Gulková, K.; Šolcová, O.; Zdražil, M. Preparation of MgO catalytic support in shaped mesoporous high surface area form. Microporous Mesoporous Mater. 2004, 76, 137–149. [Google Scholar] [CrossRef]
- Moussavi, G.; Mahmoudi, M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard. Mater. 2009, 168, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ma, W.C.; Han, H.J.; Han, Y.X.; Ma, W.W. Catalytic ozonation of quinoline using Nano-MgO: Efficacy, pathways, mechanisms and its application to real biologically pretreated coal gasification wastewater. Chem. Eng. J. 2017, 327, 91–99. [Google Scholar] [CrossRef]
- Mashayekh-Salehi, A.; Moussavi, G.; Yaghmaeian, K. Preparation, characterization and catalytic activity of a novel mesoporous nanocrystalline MgO nanoparticle for ozonation of acetaminophen as an emerging water contaminant. Chem. Eng. J. 2017, 310, 157–169. [Google Scholar] [CrossRef]
- Joseph, Y.; Ranke, W.; Weiss, W. Water on FeO (111) and Fe3O4 (111): Adsorption behavior on different surface terminations. J. Phys. Chem. 2000, 104, 3224–3236. [Google Scholar] [CrossRef]
- Ma, J.; Graham, N.J.D. Degradation of atrazine by manganese-catalysed ozonation: Influence of humic substances. Water Res. 1999, 33, 785–793. [Google Scholar] [CrossRef]
- Moussavi, G.; Mahmoudi, M. Degradation and biodegradability improvement of the reactive red 198 azo dye using catalytic ozonation with MgO nanocrystals. Chem. Eng. J. 2009, 152, 1–7. [Google Scholar] [CrossRef]
- Mohammadi, L.L.; Bazrafshan, E.; Noroozifar, M.; Ansari-Moghaddam, A.; Barahuie, F.; Balarak, D. Removing 2,4-Dichlorophenol from aqueous environments by heterogeneous catalytic ozonation process using synthetized MgO nanoparticles. Water Sci. Technol. 2017, 76, 3054–3068. [Google Scholar] [CrossRef] [PubMed]
- Kermani, M.; Asl, F.B.; Farzadkia, M.; Esrafili, A.; Arian, S.S.; Khazaei, M.; Shahamat, Y.D.; Zeynalzadeh, D. Heterogeneous catalytic ozonation by Nano-MgO is better than sole ozonation for metronidazole degradation, toxicity reduction, and biodegradability improvement. Desalin. Water Treat. 2015, 57, 16435–16444. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.D.; Kong, L.J.; Tu, Y.T.; Lu, J.; Xiong, Y. Efficient degradation of nitrobenzene by an integrated heterogeneous catalytic ozonation and membrane separation system with active MgO(111) catalyst. Desalin. Water Treat. 2014, 56, 2168–2180. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, H.B.; Croué, J.P. Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: Efficiency, stability, and mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef]
- Xu, Y.; Ai, J.; Zhang, H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.H.; Chen, C.C. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and Beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.Y.; Zheng, Y.Y.; Dacquin, J.P.; Royer, S.; Zhang, H. Mechanism and kinetics of catalytic ozonation for elimination of organic compounds with spinel-type CuAl2O4 and its precursor. Sci. Total Environ. 2019, 651, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, D.L.; Peng, S.H.; Feng, Y.; Liu, Z.G. Enhanced mineralization of dimethyl phthalate by heterogeneous ozonation over nanostructured Cu-Fe-O surfaces: Synergistic effect and radical chain reactions. Environ. Pollut. 2019, 209, 588–597. [Google Scholar] [CrossRef]
- Liu, P.X.; Ren, Y.M.; Ma, W.J.; Ma, J.; Du, Y.C. Degradation of shale gas produced water by Magnetic porous MFe2O4 (M = Cu, Ni, Co and Zn) heterogeneous catalyzed ozone. Chem. Eng. J. 2018, 345, 98–106. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Wei, C.H.; Wu, K.Y.; Zhou, H.T.; Hu, Y.; Preis, S. Mechanistic evaluation of ferrite AFe2O4 (A = Co, Ni, Cu, and Zn) catalytic performance in oxalic acid ozonation. Appl. Catal. A Gen. 2017, 547, 60–68. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, F.Z.; Zhang, Y.H.; Pan, Z.C.; Lai, B. Catalytic ozonation of N,N-dimethylacetamide (DMAC) in aqueous solution using nanoscaled magnetic CuFe2O4. Chem. Eng. J. 2018, 193, 368–377. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Yang, Q.; Wang, J.L. Catalytic ozonation of sulfamethazine using Ce0.1Fe0.9OOH as catalyst: Mineralization and catalytic mechanisms. Chem. Eng. J. 2016, 300, 169–176. [Google Scholar] [CrossRef]
- Dai, Q.Z.; Zhang, Z.; Zhan, T.T.; Hu, Z.T.; Chen, J.M. Catalytic Ozonation for the Degradation of 5-Sulfosalicylic Acid with Spinel-Type ZnAl2O4 Prepared by Hydrothermal, Sol-Gel, and Coprecipitation Methods: A Comparison Study. ACS Omega 2018, 3, 6506–6512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dong, Y.M.; Jiang, P.P.; Wang, G.L.; Zhang, J.J.; Zhang, C. ZnAl2O4 as a novel high-surface-area ozonation catalyst: One-step green synthesis, catalytic performance and mechanism. Chem. Eng. J. 2015, 260, 623–630. [Google Scholar] [CrossRef]
- Lu, J.; Wei, X.D.; Yu, C.; Tian, S.H.; Xiong, Y. Role of Mg in Mesoporous MgFe2O4 for Efficient Catalytic Ozonation of Acid Orange II. J. Chem. Technol. Biotechnol. 2015, 91, 985–993. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xie, Y.B.; Sun, H.Q.; Xiao, J.D.; Cao, H.B.; Wang, S.B. Hierarchically shape-controlled mixed-valence calcium manganites for catalytic ozonation of aqueous phenolic compounds. Catal. Sci. Technol. 2015, 6, 2918–2929. [Google Scholar] [CrossRef]
- Xing, S.T.; Lu, X.Y.; Ren, L.M.; Ma, Z.C. Characterization and reactivity of Mn-Ce-O composites for catalytic ozonation of antipyrine. RSC Adv. 2015, 5, 60279–60285. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.M.; Wang, G.L.; Jiang, P.P.; Zhang, J.J.; Wu, L.N.; Li, K. Novel magnetically separable nanomaterials for heterogeneous catalytic ozonation of phenol pollutant: NiFe2O4 and their performances. Chem. Eng. J. 2013, 219, 295–302. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhou, Z.M.; Jing, G.H.; Fang, J.H. Catalytic ozonation of Acid Red B in aqueous solution over a Fe-Cu-O catalyst. Sep. Purif. Technol. 2013, 115, 129–135. [Google Scholar] [CrossRef]
- Wu, J.J.; Muruganandham, M.; Chang, L.T.; Lee, G.J.; Batalova, V.N.; Mokrousov, G.M. Catalytic Ozonation of Oxalic Acid Using SrTiO3 Catalyst. Ozone Sci. Eng. 2011, 33, 74–79. [Google Scholar] [CrossRef]
- Bing, J.S.; Li, L.S.; Lan, B.Y.; Liao, G.Z.; Zeng, J.Y.; Zhang, Q.Y.; Li, X.K. Synthesis of cerium-doped MCM-41 for ozonation of p -chlorobenzoic acid in aqueous solution. Appl. Catal. B Environ. 2012, 115–116, 16–24. [Google Scholar] [CrossRef]
- Huang, R.H.; Yan, H.H.; Li, L.S.; Deng, D.Y.; Shu, Y.H.; Zhang, Q.Y. Catalytic activity of Fe/SBA-15 for ozonation of dimethyl phthalate in aqueous solution. Appl. Catal. B Environ. 2011, 106, 264–271. [Google Scholar] [CrossRef]
- Sun, Q.Q.; Wang, Y.; Li, L.S.; Bing, J.S.; Wang, Y.X.; Yan, H.H. Mechanism for enhanced degradation of clofibric acid in aqueous by catalytic ozonation over MnOx/SBA-15. J. Hazard. Mater. 2015, 286, 276–284. [Google Scholar] [CrossRef]
- Huang, Y.X.; Sun, Y.; Xu, Z.H.; Luo, M.Y.; Zhu, C.L.; Li, L. Removal of aqueous oxalic acid by heterogeneous catalytic ozonation with MnOx/sewage sludge-derived activated carbon as catalysts. Sci. Total Environ. 2017, 575, 50–57. [Google Scholar] [CrossRef]
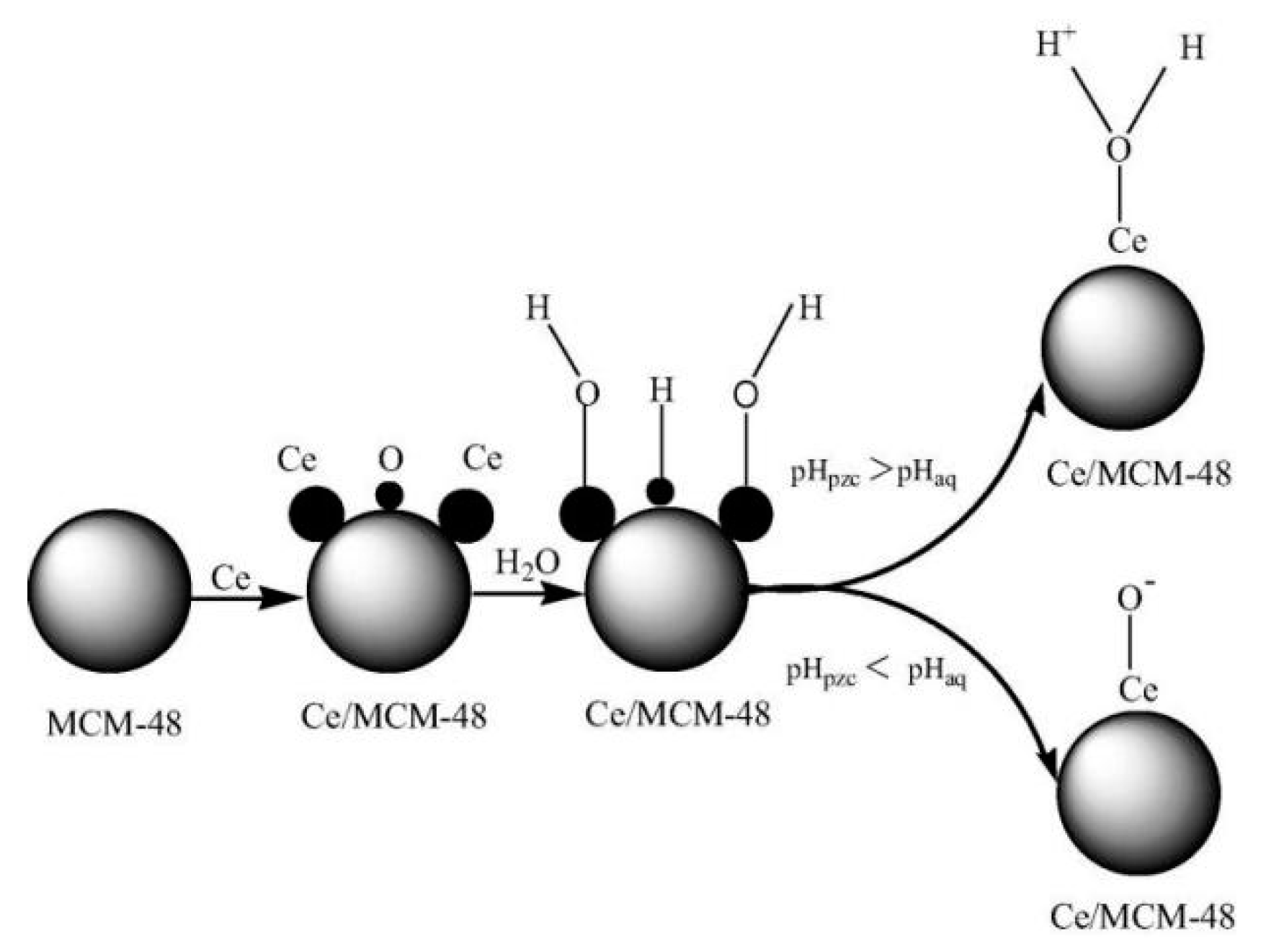
- Li, S.Y.; Tang, Y.M.; Chen, W.R.; Hu, Z.; Li, X.K.; Li, L.S. Heterogeneous catalytic ozonation of clofibric acid using Ce/MCM-48: Preparation, reaction mechanism, comparison with Ce/MCM-41. J. Colloid Interface Sci. 2017, 504, 238–246. [Google Scholar] [CrossRef]
- Roshani, B.; Mcmaster, I.; Rezaei, E.; Soltan, J. Catalytic ozonation of benzotriazole over alumina supported transition metal oxide catalysts in water. Sep. Purif. Technol. 2014, 135, 158–164. [Google Scholar] [CrossRef]
- Bing, J.S.; Hu, C.; Nie, Y.L.; Yang, M.; Qu, J.H. Mechanism of catalytic ozonation in Fe2O3/Al2O3@SBA-15 aqueous suspension for destruction of ibuprofen. Environ. Sci. Technol. 2015, 49, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Lou, Y.Y.; Zhuang, X.W.; Song, S.; Liu, W.P.; Xu, C. Magnetic Pr6O11/SiO2 @Fe3O4 particles as the heterogeneous catalyst for the catalytic ozonation of acetochlor: Performance and aquatic toxicity. Sep. Purif. Technol. 2018, 197, 63–69. [Google Scholar] [CrossRef]
- Wang, Z.M.; Ma, H.; Zhang, C.; Feng, J.; Pu, S.Y.; Ren, Y.M.; Wang, Y. Enhanced catalytic ozonation treatment of dibutyl phthalate enabled by porous magnetic Ag-doped ferrospinel MnFe2O4 materials: Performance and mechanism. Chem. Eng. J. 2018, 354, 42–52. [Google Scholar] [CrossRef]
- Xiong, Z.K.; Cao, J.Y.; Lai, B.; Yang, P. Comparative study on degradation of p-nitrophenol in aqueous solution by mFe0/Cu/O3 and mFe0/O3 processes. J. Ind. Eng. Chem. 2018, 59, 196–207. [Google Scholar] [CrossRef]
- Peng, J.L.; Lai, L.D.; Jiang, X.; Jiang, W.J.; Lai, B. Catalytic ozonation of succinic acid in aqueous solution using the catalyst of Ni/Al2O3 prepared by electroless plating-calcination method. Sep. Purif. Technol. 2018, 195, 138–148. [Google Scholar] [CrossRef]
- Li, X.K.; Chen, W.R.; Li, L.S. Catalytic Ozonation of Oxalic Acid in the Presence of Fe2O3-Loaded Activated Carbon. Ozone Sci. Eng. 2018, 40, 448–456. [Google Scholar] [CrossRef]
- Chen, W.R.; Li, X.K.; Pan, Z.Q.; Ma, S.S.; Li, L.S. Synthesis of MnOx/SBA-15 for Norfloxacin degradation by catalytic ozonation. Sep. Purif. Technol. 2017, 173, 99–104. [Google Scholar] [CrossRef]
- Shen, T.D.; Wang, Q.W.; Tong, S.P. Solid Base MgO/Ceramic Honeycomb Catalytic Ozonation of Acetic Acid in Water. Ind. Eng. Chem. Res. 2017, 56, 10965–10971. [Google Scholar] [CrossRef]
- Huang, Y.J.; Xu, W.J.; Hu, L.L.; Zeng, J.W.; He, C.; Tan, X.Q.; He, Z.Y.; Zhang, Q.; Shu, D. Combined adsorption and catalytic ozonation for removal of endocrine disrupting compounds over MWCNTs/Fe3O4 composites. Catal. Today 2017, 297, 143–150. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Yang, Q.; Wang, J.L. Catalytic ozonation of dimethyl phthalate using Fe3O4/multi-wall carbon nanotubes. Environ. Technol. 2016, 38, 2048–2057. [Google Scholar] [CrossRef]
- Liu, Y.F.; Li, G.X.; Zhang, Z.L.; Gao, X.; Niu, J.R.; Zhao, J.N.; Li, Z.X. Catalytic Ozonation of Bisphenol A in Aqueous Medium by Mn-Fe/Al2O3 Catalyst. J. Adv. Oxid. Technol. 2016, 19, 358–365. [Google Scholar] [CrossRef]
- Lu, X.H.; Zhang, Q.Y.; Yang, W.Q.; Li, X.K.; Zeng, L.X.; Li, L.S. Catalytic ozonation of 2,4-dichlorophenoxyacetic acid over novel Fe-Ni/AC. RSC Adv. 2015, 5, 10537–10545. [Google Scholar] [CrossRef]
- Bibiana, B.C.; Jafar, S.; Gustavo, A.P. Mineralization of Ibuprofen and Humic Acid through Catalytic Ozonation. Ozone Sci. Eng. 2015, 38, 203–210. [Google Scholar]
- Li, Z.X.; Zhao, J.N.; Zhong, W.Z.; Duan, E.; Li, G.X.; Liu, Y.F.; Gao, X. Efficiency and Kinetics of Catalytic Ozonation of Acid Red B over Cu-Mn/γ-Al2O3 Catalysts. Ozone Sci. Eng. 2014, 37, 287–293. [Google Scholar] [CrossRef]
- Qi, L.I.; Yao, J.; You, H.; Zhang, R.; Feng, C.H.; Agtmaal, S.V. Oxidation products and degradation pathways of 4-chlorophenol by catalytic ozonation with MnOx/γ-Al2O3/TiO2 as catalyst in aqueous solution. J. Environ. Sci. Health Part A 2014, 49, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.L.; Poznyak, T.; Valenzuela, M.A.; Tiznado, H.; Chairez, I. Surface interactions and mechanistic studies of 2,4-dichlorophenoxyacetic acid degradation by catalytic ozonation in presence of Ni/TiO2. Chem. Eng. J. 2013, 222, 426–434. [Google Scholar] [CrossRef]
- Alvárez, P.M.; García-Araya, J.F.; Beltrán, F.J.; Giráldez, I.; Jaramillo, J.; Gómez-Serrano, V. The influence of various factors on aqueous ozone decomposition by granular activated carbons and the development of a mechanistic approach. Carbon 2006, 44, 3102–3112. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rivas, J.; Álvarez, P.; Montero-de-Espinosa, R. Kinetics of Heterogeneous Catalytic Ozone Decomposition in Water on an Activated Carbon. Ozone Sci. Eng. 2002, 24, 227–237. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Ozone Decomposition in Water Catalyzed by Activated Carbon: Influence of Chemical and Textural Properties. Ind. Eng. Chem. Res. 2006, 45, 2715–2721. [Google Scholar] [CrossRef]
- Jans, U.; Hoigné, J. Activated Carbon and Carbon Black Catalyzed Transformation of Aqueous Ozone into OH-Radicals. Ozone Sci. Eng. 1998, 20, 67–90. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; Gunten, U.V.; Rivera-Utrilla, J. Efficiency of activated carbon to transform ozone into OH radicals: Influence of operational parameters. Water Res. 2005, 39, 3189–3198. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.G.; Órfão, J.J.M.; Pereira, M.F.R. Catalytic ozonation of sulphamethoxazole in the presence of carbon materials: Catalytic performance and reaction pathways. J. Hazard. Mater. 2012, 239–240, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.G.; Órfão, J.J.M.; Pereira, M.F.R. Ozonation of sulfamethoxazole promoted by MWCNT. Catal. Commun. 2013, 35, 82–87. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Influence of the surface chemistry of multi-walled carbon nanotubes on their activity as ozonation catalysts. Carbon 2010, 48, 4369–4381. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Ma, J.; Cui, Y.H.; Zhang, B.P. Effect of ozonation pretreatment on the surface properties and catalytic activity of multi-walled carbon nanotube. Appl. Catal. B Environ. 2009, 92, 301–306. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Activated carbon catalytic ozonation of oxamic and oxalic acids. Appl. Catal. B Environ. 2008, 79, 237–243. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Pocostales, J.P.; Alvarez, P.M.; Jaramillo, J. Mechanism and kinetic considerations of TOC removal from the powdered activated carbon ozonation of diclofenac aqueous solutions. J. Hazard. Mater. 2009, 169, 532–538. [Google Scholar] [CrossRef]
- Restivo, J.; Órfão, J.J.M.; Pereira, M.F.R.; Vanhaecke, E.; Rönning, M.; Iouranova, T.; Kiwi-Minsker, L.; Armenise, S.; Garcia-Bordejé, E. Catalytic ozonation of oxalic acid using carbon nanofibres on macrostructured supports. Water Sci. Technol. 2012, 65, 1854–1862. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, X.W.; Lei, L.C. Degradation of Aqueous p-Nitrophenol by Ozonation Integrated with Activated Carbon. Ind. Eng. Chem. Res. 2008, 47, 6809–6815. [Google Scholar] [CrossRef]
- Leili, M.; Moussavi, G.; Naddafi, K. Degradation and mineralization of furfural in aqueous solutions using heterogeneous catalytic ozonation. Desalin. Water Treat. 2013, 51, 6789–6797. [Google Scholar] [CrossRef]
- Guzman-Perez, C.A.; Soltan, J.; Robertson, J. Kinetics of catalytic ozonation of atrazine in the presence of activated carbon. Sep. Purif. Technol. 2011, 79, 8–14. [Google Scholar] [CrossRef]
- Wang, Y.X.; Cao, H.B.; Chen, L.I.; Chen, C.M.; Duan, X.G.; Xie, Y.B.; Song, W.Y.; Sun, H.Q.; Wang, S.B. Tailored synthesis of active reduced graphene oxides from waste graphite: Structural defects and pollutant-dependent reactive radicals in aqueous organics decontamination. Appl. Catal. B Environ. 2018, 229, 71–80. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xie, Y.B.; Sun, H.Q.; Xiao, J.D.; Cao, H.B.; Wang, S.B. Efficient Catalytic Ozonation over Reduced Graphene Oxide for p-Hydroxylbenzoic Acid (PHBA) Destruction: Active Site and Mechanism. ACS Appl. Mater. Interfaces 2016, 8, 9710–9720. [Google Scholar] [CrossRef] [PubMed]
- Tizaoui, C.; Mohammad-Salim, H.; Suhartono, J. Multi-walled carbon nanotubes for heterogeneous nanocatalytic ozonation. Ozone Sci. Eng. 2015, 37, 269–278. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Gonçalves, A.G.; Delgado, J.J.; Órfão, J.J.M.; Pereira, M.F.R. Modification of carbon nanotubes by ball-milling to be used as ozonation catalysts. Catal. Today 2015, 249, 199–203. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Ma, J.; Cui, Y.H.; Zhao, L.; Zhang, B.P. Factors affecting the catalytic activity of multi-walled carbon nanotube for ozonation of oxalic acid. Sep. Purif. Technol. 2011, 78, 147–153. [Google Scholar] [CrossRef]
- Chen, C.M.; Yan, X.; Yoza, B.A.; Zhou, T.T.; Li, Y.; Zhan, Y.L.; Wang, Q.H.; Li, Q.X. Efficiencies and mechanisms of ZSM5 zeolites loaded with cerium, iron, or manganese oxides for catalytic ozonation of nitrobenzene in water. Sci. Total Environ. 2018, 612, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Catalytic ozonation for the removal of organic contaminants in water on ZSM-5 zeolites. Appl. Catal. B Environ. 2014, 154–155, 110–122. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Mechanisms of catalytic ozonation: An investigation into superoxide ion radical and hydrogen peroxide formation during catalytic ozonation on alumina and zeolites in water. Appl. Catal. B Environ. 2013, 129, 437–449. [Google Scholar] [CrossRef]
- Valdés, H.; Tardón, R.F.; Zaror, C.A. Role of surface hydroxyl groups of acid-treated natural zeolite on the heterogeneous catalytic ozonation of methylene blue contaminated waters. Chem. Eng. J. 2012, 211–212, 388–395. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J.M.; Chen, Z.L.; Yu, L. Pumice-catalyzed ozonation degradation of p-chloronitrobenzene in aqueous solution. Adv. Mater. Res. 2012, 117–118, 414–419. [Google Scholar] [CrossRef]
- Gao, G.Y.; Shen, J.M.; Chu, W.; Chen, Z.L.; Yuan, L. Mechanism of enhanced diclofenac mineralization by catalytic ozonation over iron silicate-loaded pumice. Sep. Purif. Technol. 2017, 173, 55–62. [Google Scholar] [CrossRef]
- Xu, B.B.; Qi, F.; Zhang, J.Z.; Li, H.N.; Sun, D.Z.; Robert, D.; Chen, Z.L. Cobalt modified red mud catalytic ozonation for the degradation of bezafibrate in water: Catalyst surface properties characterization and reaction mechanism. Chem. Eng. J. 2016, 284, 942–952. [Google Scholar] [CrossRef]
- Li, H.N.; Xu, B.B.; Qi, F.; Sun, D.Z.; Chen, Z.L. Degradation of bezafibrate in wastewater by catalytic ozonation with cobalt doped red mud: Efficiency, intermediates and toxicity. Appl. Catal. B Environ. 2014, 152–153, 342–351. [Google Scholar] [CrossRef]
- Xu, B.B.; Qi, F.; Sun, D.Z.; Chen, Z.L.; Robert, D. Cerium doped red mud catalytic ozonation for bezafibrate degradation in wastewater: Efficiency, intermediates, and toxicity. Chemosphere 2016, 146, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Xia, Y.J.; Li, Q.W.; Qi, F.; Xu, B.B.; Chen, Z.L. Synchronously Degradation Benzotriazole and Elimination Bromate by Perovskite Oxides Catalytic Ozonation: Performance and Reaction Mechanism. Sep. Purif. Technol. 2018, 197, 261–270. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.D.; Ma, J.; Lu, X.H.; Qi, J.Y.; Song, S. Strong promoted catalytic ozonation of atrazine at low temperature using tourmaline as catalyst: Influencing factors, reaction mechanisms and pathways. Chem. Eng. J. 2018, 354, 113–125. [Google Scholar] [CrossRef]
- Peng, J.L.; Yan, J.F.; Chen, Q.X.; Jiang, X.; Yao, G.; Lai, B. Natural mackinawite catalytic ozonation for N, N-dimethylacetamide (DMAC) degradation in aqueous solution: Kinetic, performance, biotoxicity and mechanism. Chemosphere 2018, 210, 831–842. [Google Scholar] [CrossRef]
- Wang, Y.D.; Ma, W.F.; Yoza, B.A.; Xu, Y.Y.; Li, Q.X.; Chen, C.M.; Wang, Q.H.; Gao, Y.; Guo, S.H.; Zhan, Y. L Investigation of Catalytic Ozonation of Recalcitrant Organic Chemicals in Aqueous Solution over Various ZSM-5 Zeolites. Catalysts 2018, 8, 128. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Waheed, S.; Joya, K.S.; Kazmi, M. Catalytic ozonation of paracetamol on zeolite A: Non-radical mechanism. Catal. Commun. 2018, 112, 15–20. [Google Scholar] [CrossRef]
- Khataee, A.; Rad, T.S.; Fathinia, M. The role of clinoptilolite nanosheets in catalytic ozonation process: Insights into the degradation mechanism, kinetics and the toxicity. J. Taiwan Inst. Chem. Eng. 2017, 77, 205–215. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J.M.; Chen, Z.L.; Guan, X.H. Role of Fe/pumice composition and structure in promoting ozonation reactions. Appl. Catal. B Environ. 2016, 180, 707–714. [Google Scholar] [CrossRef]
- Qi, F.; Li, H.H.; Xu, B.B.; Sun, D.Z. Heating activated red mud catalytic ozonation for degradation nitrobenzene from aqueous solution: Performance and influence of preparation factors. J. Nanosci. Nanotechnol. 2014, 14, 6984–6990. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Xu, B.B.; Chen, Z.L.; Ma, J.; Sun, D.Z.; Zhang, L.Q.; Wu, F.C. Ozonation catalyzed by the raw bauxite for the degradation of 2,4,6-trichloroanisole in drinking water. J. Hazard. Mater. 2009, 168, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.M.; Yang, H.X.; He, K.; Wu, X.; Zhang, A.M. Catalytic activity and stability of Y zeolite for phenol degradation in the presence of ozone. Appl. Catal. B Environ. 2008, 82, 163–168. [Google Scholar] [CrossRef]


| Advantages |
|
| Disadvantages |
|
| Oxidizing Agent | Electrochemical Oxidation Potential (EOP) (V) | EOP Relative to Chlorine |
|---|---|---|
| Fluorine | 3.06 | 2.25 |
| Hydroxyl radical | 2.80 | 2.05 |
| Oxygen atomic | 2.42 | 1.78 |
| TiO2+hv 1 | 2.35 | 1.72 |
| Ozone | 2.08 | 1.52 |
| Persulfate | 2.01 | 1.48 |
| Perbromate | 1.85 | 1.35 |
| Hydrogen peroxide | 1.78 | 1.30 |
| Perhydroxyl radical | 1.70 | 1.25 |
| Hypochlorite | 1.49 | 1.10 |
| Bromate | 1.48 | 1.09 |
| Chlorine | 1.36 | 1.00 |
| Dichromate | 1.33 | 0.98 |
| Chlorine dioxide | 1.27 | 0.93 |
| Permanganate | 1.24 | 0.91 |
| Oxygen (molecular) | 1.23 | 0.90 |
| Perchlorate | 1.20 | 0.89 |
| Bromine | 1.09 | 0.80 |
| Iodine 2 | 0.54 | 0.39 |
| Catalyst | Pollutants | Operation Conditions | Comments | References | |
|---|---|---|---|---|---|
| δ-MnO2 | Bisphenol A (BPA) | [Cat] = 0.1 g/L | [O3] = 4 mg/L | The strong interaction among the catalyst surface, ozone and organic molecules rather than hydroxyl radicals were responsible for the degradation of bisphenol A. | Luo et al. (2018) [41] |
| [Pull]0 = 50 mg/L | [%] = 68.2% | ||||
| [Time] = 20 min | |||||
| α-MnO2 | 4-Nitrophenol (4-NP) | [Cat] = 0.1 g/L | [O3] = 5.0 mg/min | Crystal phase was a vital factor determining the catalytic activity of MnO2. | Nawaz et al. (2017) [46] |
| [Pull]0 = 50 mg/L | pH = 7.0 | ||||
| [Time] = 30 min | [%] = 100% | ||||
| MnO2 | BPA | [Cat] = 0.05 g/L | [O3] = 2 mg/L | Two three-dimensional (3D) MnO2 were synthesized and showed the excellent adsorption capacity and catalytic activity. Catalytic ozonation of BPA was dominated by •O2− and •OH. | Tan et al. (2017) [53] |
| [Pull]0 = 50 mg/L | [%] = 90% | ||||
| [Time] = 30 min | |||||
| α-MnO2 | Phenol | [Cat] = 1.0 g/L | [O3] = 0.80 mg/min | The surface hydroxyl groups acted as the active sites in producing active oxygen species, and Lewis acid sites as the reactive centers for catalytic ozonation. | Zhao et al. (2014) [52] |
| [Pull]0 = 300 mg/L | pH = 6.4 | ||||
| [Time] = 60 min | |||||
| MnO2 | Phenol | [Cat] = 0.2 g/L | [O3] = 2.5 mg/min | MnO2 had higher active than Mn2O3 and Mn3O4 due to its higher electron transfer ability and higher amount of oxygen defects or surface hydroxyl groups. | Nawaz et al. (2016) [43] |
| [Pull]0 = 23 mg/L | pH = 7.0 | ||||
| [Time] = 60 min | [%] = 100% | ||||
| Catalyst | Pollutants | Operation Conditions | Comments | References | |
|---|---|---|---|---|---|
| TiO2 | Naproxen and carbamazepine | [Cat] = 1.0 g/L | [O3] = 40 mg/L | The adsorption of organics could play a vital role in the reaction. The improvement of mineralization would not merely depend on the production of hydroxyl radicals from ozone. | Rosal et al. (2008) [58] |
| [Pull]0 = 15 mg/L | pH = 5.0 | ||||
| [Time] = 20 min Naproxen: [%] = 62% Carbamazepine: [%] = 73% | |||||
| Nano-TiO2 | Mitrobenzene | [Cat] = 0.1 g/L | [O3] = 0.367 mg/L | The increase of dose of catalyst did not affect the degradation of nitrobenzene. TiO2-catalyzed ozonation followed a radical-type mechanism. | Yang et al. (2007) [59] |
| [Pull]0 = 0.06 mg/L | pH = 10.0 | ||||
| [Time] = 20 min | [%] > 60% | ||||
| TiO2 | Ortho-toluidine (OT) | [Cat] = 1.2 g/L | [O3] = 16.6 mg/L | The degradation of OT was higher at neutral pH than at alkaline or acidic ones. Degradation of OT followed pseudo-first order kinetics. OT oxidation was occurred through hydroxyl radical mechanism. | Shokri et al. (2017) [5] |
| [Pull]0 = 50 mg/L | pH = 7.0 | ||||
| [Time] = 60 min | [%] = 96% | ||||
| TiO2 | 4-chloronitrobenzene (4-CNB) | [Cat] = 0.2 mg/L | [O3] = 0.35 mg/L | The catalyst exhibited the best catalytic activity for removing 4-CNB, which was 9 times higher than ozone alone. Degradation of TiO2 followed a radical-type mechanism. | Ye et al. (2012) [67] |
| [Pull]0 = 0.1 mg/L | pH = 5.3 | ||||
| [Time] = 20 min | [%] = 64% | ||||
| Catalyst | Pollutants | Operation Conditions | Comments | References | |
|---|---|---|---|---|---|
| β-FeOOH | 4-chlorophenol (4-CP) | [Cat] = 0.1 g/L | [O3] = 0.6 mg/min | The catalytic ability of the β-FeOOH during ozonation process was found to be shown at lower pH. | Oputu et al. (2015) [74] |
| [Pull]0 = 2 × 10−3 mol/L | pH = 3.5 | ||||
| [Time] = 40 min | [%] = 99% | ||||
| FeOOH | Oxalic acid | [Cat] = 2.0 g/L | [O3] = 0.45 mg/min | FeOOH could effectively improve the generation of hydroxyl radicals (·OH). Hydroxyl groups in different situations such as neutral state and positive charge state could act as the active sites for the decomposition of ozone and the generation of hydroxyl radicals. | Sui et al. (2010) [79] |
| [Pull]0 = 1 × 10−5 mol/L | pH = 7.0 | ||||
| [Time] = 30 min | [%] = 54% | ||||
| α-FeOOH | Nitrobenzene | [Cat] = 0.2 g/L | [O3] = 1.2 mg/L | The neutral surface hydroxyl species of α-FeOOH had high activity to catalyze ·OH generation from aqueous ozone due to the surface OH-ozone interaction. | Zhang al. (2008) [83] |
| [Pull]0 = 2.96 × 10−6 mol/L | pH = 7.3 | ||||
| [Time] = 15 min | |||||
| Fe3O4 | Atrazine (ATZ) | [Cat] = 0.2 g/L | [O3] = 4.8 mg/L | The redox cycles of Fe2+/Fe3+ benefited the generation of ·OH. Mesoporous Fe3O4 presented low iron leaching, good stability and easy to separate. | Zhu et al. (2017) [84] |
| [Pull]0 = 5.0 × 10−6 mol/L | pH = 9.5 | ||||
| [Time] = 2 min | [%] = 95% | ||||
| Micro-size Fe0 (mFe0) | P-nitrophenol (PNP) | [Cat] = 40 g/L | [O3] = 7.6 mg/L | High degradation of PNP in aqueous solution was due to the synergetic effect between O3 and Fe0. | Xiong et al. (2016) [85] |
| [Pull]0 = 3.6 × 10−3 mol/L | pH = 5.3 | ||||
| [Time] = 60 min | [%] = 89.5% | ||||
| Catalyst | Pollutants | Operation Conditions | Comments | References | |
|---|---|---|---|---|---|
| Alumina | Bisphenol A (BPA) | [Cat] = 1.0 g/L | [O3] = 4.5 mg/L | The increase of alumina catalyst dose from 0.5 g/L to 4 g/L did not exhibit a big effect on the TOC removal. | Keykavoos et al. (2013) [87] |
| [Pull]0 = 10 mg/L | pH = 5.0 | ||||
| [Time] = 60 min | [%] = 90% | ||||
| γ-Al2O3 | 2,4-dimethylphenol (2,4-DMP) | [Cat] = 2.0 g/L | [O3] = 2 × 10−6 mg/L | It was found that the reaction followed hydroxyl radical mechanism. No adsorption of 2,4-DMP occurred on γ-Al2O3. | Vittenet et al. (2015) [18] |
| [Pull]0 = 50 mg/L | pH = 4.5 | ||||
| [Time] = 22 min | [%] = 100% | ||||
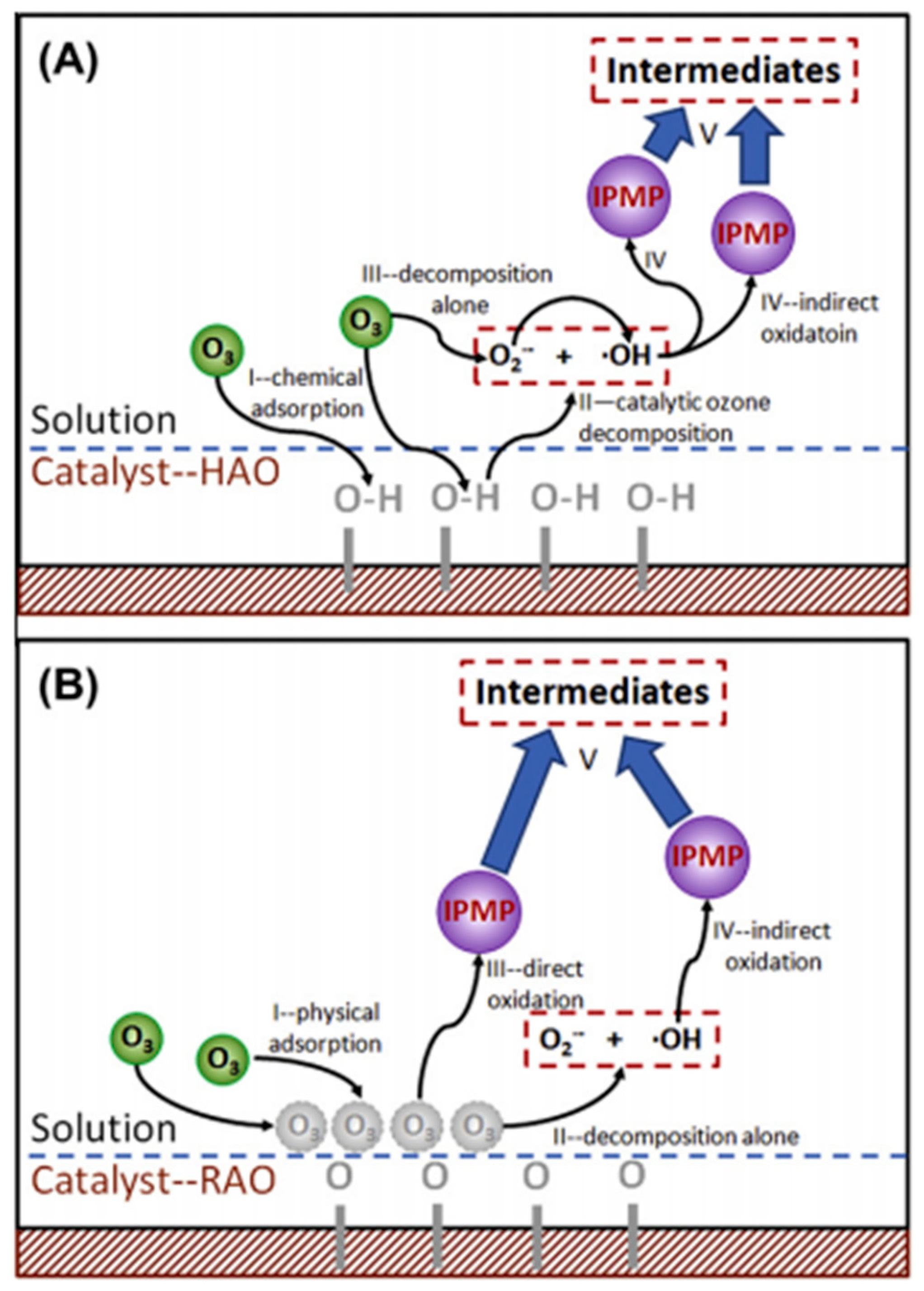
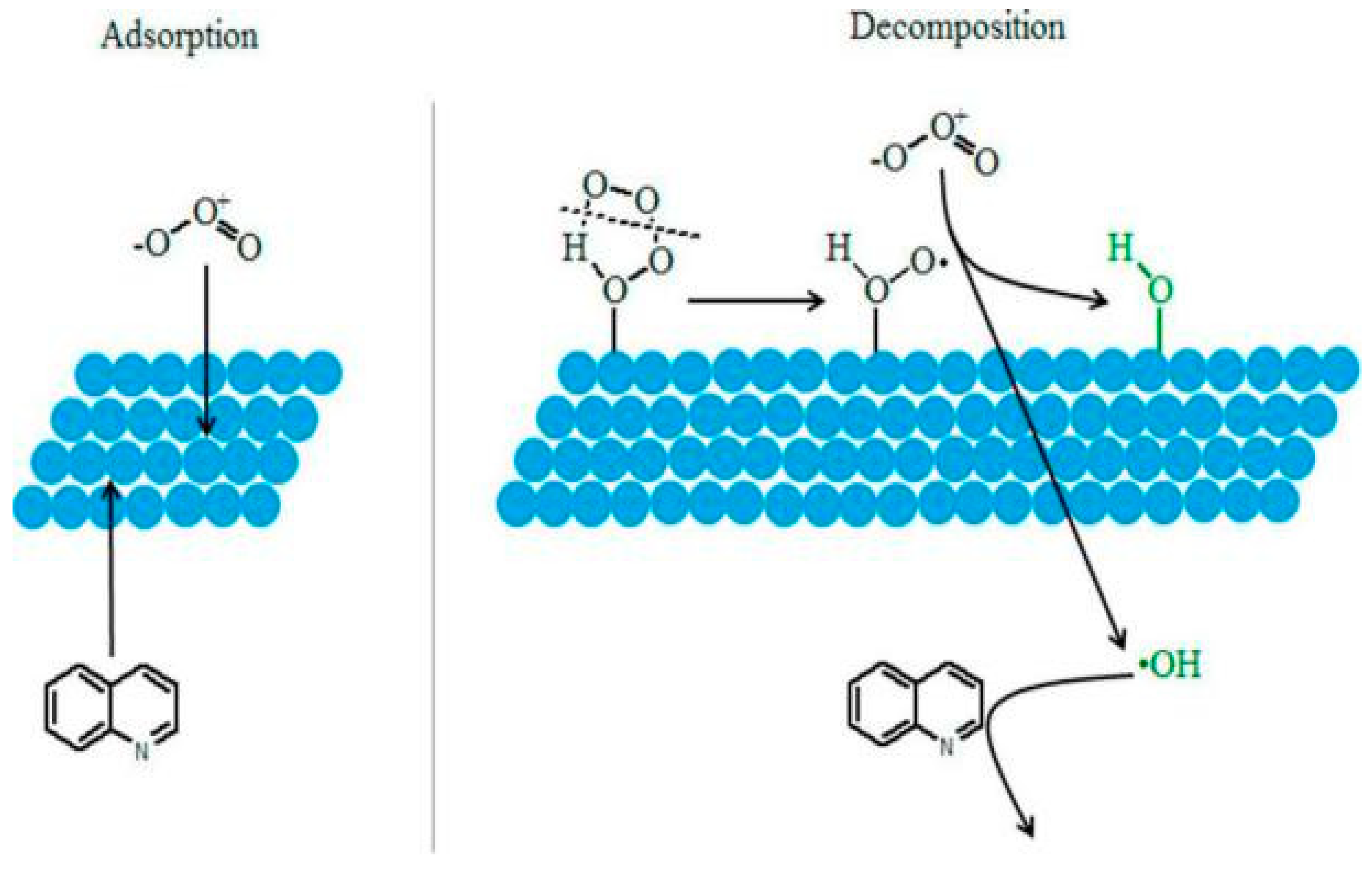
| γ-AlOOH (HAO) and γ-Al2O3 (RAO) | 2-isopropyl-3-meth-oxypyrazine (IPMP) | [Cat] = 0.5 g/L | [O3] = 0.5 mg/L | Both HAO and RAO showed the good stability with low aluminum ions leaching. Surface hydroxyl groups were important reaction sites for HAO but not for RAO. | Qi et al. (2013) [88] |
| [Pull]0 = 0.04 mg/L | pH = 7.05 | ||||
| [Time] = 10 min HAO: [%] = 94.2% RAO: [%] = 90.0% | |||||
| Nano-perfluorooctyl alumina (PFOAL) | Tert-butyl ether (MTBE) | [Cat] = 5.0 g/L | pH = 6.7 | Degradation using PFOAL catalyst was about two times higher than single ozonation. The adsorption of MTBE on PFOAL followed pseudo-second-order kinetics. | Kiadehi et al. (2017) [90] |
| [Pull]0 = 1.0 g/L | [%] = 98.18% | ||||
| [Time] = 90 min | |||||
| γ-AlOOH | 2,4, 6-trichloroanisole (TCA) | [Cat] = 0.2 g/L | [O3] = 0.5 mg/L | The significant decrease in the TCA degradation rate due to the transformation of the crystal phase and the reduction of surface hydroxyl groups. | Qi et al. (2009) [91] |
| [Pull]0 = 0.025 mg/L | pH = 6.0 | ||||
| [Time] = 10 min | [%] = 80% | ||||
| Catalyst | Pollutants | Operation Conditions | Comments | References | |
|---|---|---|---|---|---|
| MgO | 4-chlorophenol | [Cat] = 1.0 g/L | [O3] =2.5 mg/min | The pseudo first-order reaction constant of 4-chlorophenol removal in catalytic ozonation using MgO (111), MgO (110), MgO (200) catalyst mixed with ozone were 4.8, 2.5, 3.1 times higher than that of ozonation alone, respectively. | Chen et al. (2015) [93] |
| [Pull]0 = 100 mg/L | pH = 6.2 | ||||
| [Time] = 30 min | [%] = 99.3% | ||||
| Nano-MgO | Quinoline | [Cat] = 0.2 g/L | [O3] =2.0 mg/L | Nano-MgO could accelerate ozone decomposition and behave via a hydroxyl radical mechanism. The catalyst remained stable catalytic ability. | Zhu et al. (2017) [102] |
| [Pull]0 = 20 mg/L | pH = 6.8 | ||||
| [Time] = 15 min | [%] = 90.7% | ||||
| MgO | Acetaminophen (ACT) | [Cat] = 2.0 g/L | [O3] = 1.8 mg/min | Kinetics of ACT oxidation showed that the rate of degradation and mineralization ACT was 18.8 times and 7.8 times in the MgO/O3 process compared to the ozonation alone. Degradation of ACT was governed by hydroxyl radical mechanism. | Mashayekh-Salehi et al. (2017) [103] |
| [Pull]0 = 50 mg/L | pH = 5.4 | ||||
| [Time] = 15 min | [%] = 100% | ||||
| MgO | 2,4-Dichlorophenol (2,4-DCP) | [Cat] = 0.3 mg/L | pH > 7.0 | Effect of operational parameters like solution pH, ozonation time, dose of MgO and initial 2,4-DCP concentration. | Mohammadi et al. (2017) [107] |
| [Pull]0 = 50 mg/L | [%] = 99.99% | ||||
| [Time] = 50 min | |||||
| Nano-MgO | Metronidazole (MNZ) | [Cat] = 0.25 g/L | [O3] = 8.3 mg/min | The introduction of MgO nanocrystals contributed to the increase of MNZ removal and the decrease of required time compared to the conventional ozonation. | Kermani et al. (2015) [108] |
| [Pull]0 = 40 mg/L | pH = 10.0 | ||||
| [Time] = 20 min | [%] = 93.5% | ||||
| MgO(111) | Nitrobenzene | [Cat] = 1.0 g/L | [O3] = 5.0 mg/L | Catalytic activities of three catalyst followed MgO (111) > CP-MgO > MnOx for nitrobenzene mineralizaton. MgO (111) had amount of surface O2− Lewis basic sites. | Chen et al. (2014) [109] |
| [Pull]0 = 50 mg/L | pH = 12.0 | ||||
| [Time] = 30 min | [%] = 95.7% | ||||
| Catalyst | Pollutants | Operation Conditions | Comments | References | |
|---|---|---|---|---|---|
| Ce0.1Fe0.9OOH | Sulfamethazine (SMT) | [Cat] = 0.4 g/L | [O3] = 20 mg/L | Ce0.1Fe0.9OOH was prepared by isomorphous substitution method. The catalyst enhanced the mineralization efficiency of SMT depending on the dosage of ozone and catalyst. | Bai et al. (2016) [118] |
| [Pull]0 = 10 mg/L | pH = 7.0 | ||||
| [Time] = 10 min | [%] = 100% | ||||
| ZnAl2O4 | 5-Sulfosalicylic acid (SSal) | [Cat] = 0.2 g/L | [O3] = 5.0 mg/min | The ZnAl2O4 catalyst was prepared by hydrothermal, sol-gel, and coprecipitation methods were compared. Catalyst prepared by hydrothermal method showed better catalytic activity in ozonation. Degradation of SSal followed radical mechanism. | Dai et al. (2018) [119] |
| [Pull]0 = 500 mg/L | pH = 7.0 | ||||
| [Time] = 60 min | [%] = 64.8% | ||||
| ZnAl2O4 | Phenol | [Cat] = 1.0 g/L | [O3] = 0.75 mg/min | After using 4 times of catalyst, the removal rate of phenol slightly decreased by 5.7%. Hydroxyl radicals reacted with phenol in bulk solution. ZnAl2O4 was applied in a wide pH range from 3.3 to 9.3. | Zhao et al. (2016) [120] |
| [Pull]0 = 300 mg/L | pH = 6.4 | ||||
| [Time] = 60 min | [%] = 73.4% | ||||
| MgFe2O4 | Acid Orange II (AOII) | [Cat] = 0.1 g/L | [O3] = 5.0 mg/L | MgFe2O4 had the most catalytic activity among MgO, Fe2O3 and MgO+Fe2O3 and possessed a reaction rate constant at least 2.3 times compared to that of NiFe2O4, MnFe2O4 and CuFe2O4. | Lu et al. (2015) [121] |
| [Pull]0 = 50 mg/L | pH = 6.5 | ||||
| [Time] = 40 min | [%] = 94.1% | ||||
| CaMn3O6 and CaMn4O8 | 4-nitrophenol | [Cat] = 0.1 g/L | [O3] = 50 mg/L | The superoxide radicals and singlet oxygen other than hydroxyl radicals were responsible for the degradation and mineralization of 4-nitrophenol. The CaMn3O6 and CaMn4O8 exhibited much higher catalytic activities and stabilities than manganese oxides. | Wang et al. (2015) [122] |
| [Pull]0 = 50 mg/L | pH = 5.7 | ||||
| CaMn3O6: [Time] = 45 min | |||||
| CaMn4O8: [Time] = 30 min | [%] = 100% | ||||
| Mn-Ce-O | Antipyrine | [Cat] = 0.1 g/L | [O3] = 20 mg/L | Catalytic ozonaion was governed by hydroxyl radical mechanism. A strengthen of the contribution of surface reactions with a decrease of pH. | Xing et al. (2015) [123] |
| [Pull]0 = 40 mg/L | pH = 6.5 | ||||
| [Time] = 2 min | [%] = 100% | ||||
| NiFe2O4 | Phenol | [Cat] = 1.0 g/L | [O3] = 0.75 mg/min | The NiFe2O4-H and NiFe2O4-C were prepared by hydrothermal and calcined treatments, respectively. Presence of NiFe2O4-H promoted the degradation of phenol, but NiFe2O4-C was noneffective. Lewis acid sites were behaved as reactive centers for catalytic ozonation. | Zhao et al. (2013) [124] |
| [Pull]0 = 300 mg/L | pH = 6.5 | ||||
| [Time] = 60 min | [%] = 97.6% | ||||
| Fe-Cu-O | Acid Red B (ARB) | [Cat] = 1.0 g/L | [O3] = 30 mg/min | The Fe-Cu-O indicated good stability after four successive recycles. Degradation of ARB followed pseudo-first-order rate equation. | Liu et al. (2013) [125] |
| [Pull]0 = 100 mg/L | pH = 6.8 | ||||
| [Time] = 10 min | [%] = 98% | ||||
| SrTiO3 | OA | [Cat] = 1.25 g/L | [O3] = 18.4 mg/L | Catalyst indicated good stability and efficiency after four successive cycles. | Wu et al. (2011) [126] |
| [Pull]0 = 100 mg/L | pH = 3.0 | ||||
| [Time] = 60 min | [%] = 45.8% | ||||
| Catalyst | Pollutants | Operation Conditions | Comments | References | |
|---|---|---|---|---|---|
| MnOx/sewage sludge-derived activated carbon (MnOx/SAC) | Oxalic acid | [Cat] = 100 mg/L | [O3] = 5.0 mg/L | The reaction mechanism involved both surface reactions and reactions in the bulk water, but dominantly surface reactions. | Huang et al. (2017) [130] |
| [Pull]0 = 80 mg/L | pH = 3.5 | ||||
| [Time] = 60 min | [%] = 92.2% | ||||
| Ce/MCM-48 and Ce/MCM-41 | Clofibric acid | [Cat] = 0.4 mg/L | [O3] = 1.7 mg/min | Addition of phosphate and sodium hydrogen sulfite into the reaction indicated hydroxyl radical mechanism for catalytic ozonation. | Li et al. (2017) [131] |
| [Pull]0 = 10 mg/L | pH = 4 | ||||
| [Time] = 120 min Ce/MCM-48: [%] = 64% Ce/MCM-41: [%] =54% | |||||
| Fe2O3/Al2O3@SBA-15 | Ibuprofen | [Cat] = 1.25 g/L | [O3] = 30 mg/L | The Fe2O3/Al2O3@SBA-15 had high catalytic activity for Ibuprofen due to the surface oxygen atom, ·OHads and O2•−. | Bing et al. (2015) [133] |
| [Pull]0 = 10 mg/L | pH = 7.0 | ||||
| [Time] = 60 min | [%] = 90% | ||||
| Pr6O11/SiO2 @Fe3O4 | Acetochlor | [Cat] = 0.5 g/L | [O3] = 60 mL/min | Pr6O11/SiO2 @Fe3O4 was proved to be stable and recyclable. The catalytic ozonation process followed an ·OH reaction mechanism. | Wang et al. (2018) [134] |
| [Pull]0 = 20 mg/L | [%] = 37.3% | ||||
| [Time] = 120 min | |||||
| Ag/MnFe2O4 | Di-n-butyl phthalate (DBP) | [Cat] = 10 mg/L | [O3] = 0.68 mg/min | Ag/MnFe2O4 had highly porous structure with good magnetic property. Catalytic ozonation accelerated ozonation of DBP compared to the ozone-alone and undoped MnFe2O4 systems due to the increase of density of surface hydroxyl groups and electron transfer and cycle. | Wang et al. (2018) [135] |
| [Pull]0 = 0.5 mg/L | pH = 7.3 | ||||
| [Time] = 60 min | [%] = 75.3% | ||||
| mFe/Cu | P-Nitrophenol (PNP) | [Cat] = 20 g/L | [O3] = 5.42 mg/L | The reaction mechanism of the mFe/Cu/O3 included catalytic ozonation, Fenton-like and/or peroxone reaction, adsorption and coagulation. | Xiong et al. (2018) [136] |
| [Pull]0 = 500 mg/L | pH = 5.4 | ||||
| [Time] = 30 min | [%] = 93.6% | ||||
| Ni/Al2O3 | Succinic acid (SA) | [Cat] = 10 g/L | [O3] = 300 mL/min | The preparation parameters and operational parameters had an effect on catalytic ozonation. Catalytic ozonation occurred via hydroxyl radical mechanism. | Peng et al. (2018) [137] |
| [Pull]0 = 200 mg/L | pH = 8.0 | ||||
| [Time] = 60 min | [%] = 100% | ||||
| Fe2O3/AC | OA | [Cat] = 0.71 g/L | [O3] = 0.8 mg/min | Acidic condition benefited OA removal in the Fe2O3/AC/O3 process. A hydroxyl radical mechanism was proven in catalytic ozonation. | Li et al. (2018) [138] |
| [Pull]0 = 30 mg/L | pH = 3.3 | ||||
| [Time] = 60 min | [%] = 89.2% | ||||
| MnOx/SBA-15 | Norfloxacin | [Cat] = 0.1 g/L | [O3] = 1.7 mg/min | Toxicological tests showed that a high detoxification was achieved after 30 min. | Chen et al. (2017) [139] |
| [Pull]0 = 10 mg/L | pH = 5.0 | ||||
| [Time] = 60 min | [%] = 54% | ||||
| MgO/ceramic honeycomb (MgO/CH) | Acetic acid | [Cat] = 20 g/L | [O3] = 45.5 mg/min | MgO/CH had a good reusability property by recycling test. Catalytic ozonation was governed by hydroxyl radical mechanism. | Shen et al. (2017) [140] |
| [Pull]0 = 100 mg/L | [%] = 81.6% | ||||
| [Time] = 30 min | |||||
| MWCNTs/Fe3O4 | Bisphenol A (BPA) | [Cat] = 0.5 g/L | [O3] = 3.0 mg/L | MWCNTs/Fe3O4 had excellent catalytic activity, simple separation and good stability. | Huang et al. (2017) [141] |
| [Pull]0 = 50 mg/L | pH = 7.0 | ||||
| [Time] = 40 min | [%] = 90% | ||||
| Fe3O4/multi-wall carbon nanotubes | Dimethyl phthalate (DMP) | [Cat] = 0.3 mg/L | [O3] = 4.8 mg/min | The acidic sites of catalyst benefited ozone decomposition. Fe3O4 crystal structure was stable after five runs. | Bai et al. (2016) [142] |
| [Pull]0 = 20 mg/L | pH = 6.8 | ||||
| [Time] = 30 min | [%] = 96% | ||||
| Mn-Fe/Al2O3 | BPA | [Cat] = 5.0 g/L | [O3] = 3.2 mg/min | Hydroxyl radicals played a vital role in catalytic ozonation. Mn-Fe/Al2O3 exhibited good reusability and stability. | Liu et al. (2016) [143] |
| [Pull]0 = 50 mg/L | pH = 7 | ||||
| [Time] = 30 min | [%] = 84.1% | ||||
| Fe-Ni/AC | 2,4-dichlorophenoxyacetic acid (2,4-D) | [Cat] = 0.5 g/L | [O3] = 0.8 mg/min | The degradation rate constant of 2,4-D with Fe-Ni/AC/O3 was 1.6 times higher than that with AC/O3 and 1.9 times than that with ozonation alone. Degradation of 2,4-D followed the pseudo first order reaction model. | Lu et al. |
| [Pull]0 = 10 mg/L | pH = 4.2 | (2015) [144] | |||
| [Time] = 60 min | [%] = 72% | ||||
| MnO2-CuO/γ-Al2O3 | Ibuprofen and Humic Acid | [Cat] = 1.0 g/L | [O3] = 6.4 g/min | The noncatalytic mineralization increased by 10% due to the presence of humic acid. Adsorption played a major role in catalytic ozonation process. | Bibiana et al. (2015) [145] |
| Ibuprofen: [Pull]0 = 5 mg/L Humic Acid: [Pull]0 = 15 mg/L | |||||
| [Time] = 60 min | pH = 5.6 | ||||
| Ibuprofen: [%] = 55% Humic Acid: [%] = 75% | |||||
| Cu-Mn/γ-Al2O3 | Acid Red B | [Cat] = 4.0 g/L | [O3] = 4.26 mg/min | Cu-Mn/γ-Al2O3 catalytic ozonation of Acid Red B followed the pseudo-first-order kinetics reaction model. The reaction followed hydroxyl radical mechanism. | Li et al. (2014) [146] |
| [Pull]0 = 250 mg/L | pH = 8.5 | ||||
| [Time] = 20 min | [%] = 99.35% | ||||
| MnOx/γ-Al2O3/TiO2(MAT) | 4-chlorophenol (4-CP) | [Cat] = 2.0 g/L | [O3] = 2.0 mg/L | 4-CP was oxidized primarily by hydroxyl radical mechanism. | Qi et al. (2014) [147] |
| [Pull]0 = 100 mg/L | pH = 6.6 | ||||
| [Time] = 100 min | [%] = 94.1% | ||||
| Ni/TiO2 | 2,4-D | [Cat] = 0.1 g/L | [O3] = 25 mg/L | Ni/TiO2 had high catalytic activity in catalytic ozonation for the mineralization of 2,4-D due to the synergic effect between ·OH and O3. | Rodríguez et al. (2013) [148] |
| [Pull]0 = 80 mg/L | pH = 3.1 | ||||
| [Time] = 20 min | [%] = 97% | ||||
| Catalyst | Pollutants | Operation Conditions | Comments | References | |
|---|---|---|---|---|---|
| MWCNTs | Sulfamethoxazole (SMX) | [Cat] = 0.14 g/L | [O3] = 50 mg/L | The MWCNTs with various surface chemical properties were synthesized by oxidative and thermal treatments. MWCNTs significantly promoted the mineralization degree compared to ozonation alone. | Gonçalves et al. (2013) [155] |
| [Pull]0 = 50 mg/L | pH = 4.8 | ||||
| [Time] = 30 min | [%] = 100% | ||||
| Reduced graphene oxide (rGO) | P-Hydroxylbenzoic Acid (PHBA) | [Cat] = 0.2 g/L | [O3] = 20 mg/L | The reactive oxygen species (ROS) including superoxide radical (·O2−) and singlet oxygen (1O2) were responsible for PHBA degradation in catalytic ozonation process. The electron-rich carbonyl groups were acted as the active sites for the catalytic reaction. | Wang et al. (2016) [165] |
| [Pull]0 = 5 mg/L | pH = 3.5 | ||||
| [Time] = 60 min | [%] = 95% | ||||
| Carbon nanotubes (CNTs) | Methyl orange (MO) | [Cat] = 10 mg/L | [O3] = 2 mg/L | The degradation of MO increased with pH from 2 to 3, while a reverse trend with the pH increased from 3 to 9. MO oxidation in solution occurred via molecular ozone. | Tizaoui et al. (2015) [166] |
| [Pull]0 = 20 mg/L | pH = 3.0 | ||||
| [Time] = 2 min | [%] = 61% | ||||
| Multi-walled carbon nanotubes (MWCNT) | Oxalic acid | [Cat] = 0.14 mg/L | pH = 3.0 | The ball-milled MWCNT exhibited better results for the degradation of oxalic acid compared to the unmilled MWCNT. | Soares et al. (2015) [167] |
| [Pull]0 = 90 mg/L | |||||
| MWCNT | Oxalic acid | [Cat] = 0.1 g/L | [O3] = 20 mg/min | Catalyst dosage and the reaction temperature showed positive effects on the removing of oxalic acid in catalytic ozonation. With the increase of initial pH from 1.0–3.0, the oxalic acid removal increases, in contrast, decreasing with further increasing of pH from 3.0 to 6.1. | Liu et al. (2011) [168] |
| [Pull]0 = 90 mg/L | pH = 3.0 | ||||
| [Time] = 40 min | [%] = 79.4% | ||||
| Catalyst | Pollutants | Operation Conditions | Comments | References | |
|---|---|---|---|---|---|
| Iron silicate- loaded pumice (FSO/PMC) | Diclofenac (DCF) | [Cat] = 0.8 g/L | [O3] = 5.52 mg/L | The DCF mineralization was enhanced in FSO/PMC catalytic ozonation process due to the improvement of mass transfer of aqueous ozone, increase of the solubility of aqueous ozone, and acceleration of the generation of ·OH radicals. | Gao et al. (2017) [173] |
| [Pull]0 = 29.6 mg/L | pH = 7.0 | ||||
| [Time] = 60 min | [%] = 73.3% | ||||
| Cobalt-loaded red mud (RM) | Bezafibrate (BZF) | [Cat] = 50 mg/L | [O3] = 0.5 mg/L | Surface cobalt loading contributed to the change of the structure, surface chemical properties and catalytic activity of Co/RM. Degradation of BZF followed hydroxyl radical mechanism. | Xu et al. (2016) [175] |
| [Pull]0 = 10 mg/L | pH = 6.5 | ||||
| [Time] = 30 min | |||||
| LaCoO3 | Benzotriazole (BZA) | [Cat] = 0.5 mg/L | [O3] = 2.0 mg/L | The surface hydroxyl groups of LaCoO3 accelerated the decomposition of ozone to generate more radicals. | Zhang et al. (2018) [178] |
| [Pull]0 = 10 mg/L | pH = 6.4 | ||||
| [Time] = 15 min | [%] = 100% | ||||
| Tourmaline | Atrazine (ATZ) | [Cat] = 1.0 g/L | [O3] = 3.0 mg/L | Catalytic ozonation using tourmaline resulted in higher ATZ removal efficiency compared to single ozonation. | Wang et al. (2018) [179] |
| [Pull]0 = 1.1 mg/L | pH = 7.0 | ||||
| [Time] = 10 min | [%] = 98% | ||||
| Natural mackinawite (NM) | N,N-dimethylacetamide (DMAC) | [Cat] = 3.5 g/L | [O3] = 0.3 L/min | Degradation of DMAC was governed by hydroxyl radical mechanism. | Peng et al. (2018) [180] |
| [Time] = 20 min | pH = 6.8 | ||||
| [%] = 95.4% | |||||
| ZSM-5 Zeolites | Nitrobenzene | [Cat] = 1.0 g/L | [O3] = 5 mg/min | NaZSM-5 catalytic ozonation of nitrobenzene by adsorption and direct ozonation for the first use and direct ozonation and •OH mediated oxidation for after eight recycles. The more Si-O bonds on zeolite surfaces contributed to the higher catalytic activity of NaZSM-5. | Wang et al. (2018) [181] |
| [Pull]0 = 100 mg/L | pH = 7.2 | ||||
| [Time] = 50 min | [%] = 74% | ||||
| Zeolite4A (Z4A) | Paracetamol | [Cat] = 11 g/L | [O3] = 0.9 mg/min | The Z4A did not promote the decomposition of ozone to produce superoxide ion radical and hydroxyl radicals. It was found that the catalytic ozonation reaction followed a non-radical mechanism. | Ikhlaq et al. (2018) [182] |
| [Pull]0 = 50 mg/L | pH = 7.12 | ||||
| [Time] = 60 min | [%] = 90.68% | ||||
| Clinoptilolite | Nalidixic acid (NA) | [Cat] = 6 g/L | [O3] = 0.25 mg/min | The hydroxyl and superoxide radicals, adsorption and catalyst active sites played a vital role in catalytic ozonation of NA. | Khataee et al. (2017) [183] |
| [Pull]0 = 20 mg/L | pH = 7.0 | ||||
| [Time] = 60 min | [%] = 73.8% | ||||
| Fe/pumice | P-chloronitrobenzene (p-CNB) | [Cat] = 0.5 g/L | [O3] = 0.9 mg/L | The uncharged surfaces hydroxyl groups were responsible for catalytic activity of the Fe/pumice. Degradation of p-CNB followed hydroxyl radical mechanism | Yuan et al. (2016) [184] |
| [Pull]0 = 0.1 mg/L | pH = 6.0 | ||||
| [Time] = 15 min | [%] = 90.8% | ||||
| Red mud (RM) | Nitrobenzene | [Cat] = 0.5 g/L | [O3] = 1.0 mg/L | Hydroxyl radical played a role in nitrobenzene degradation. | Qi et al. (2014) [185] |
| [Pull]0 = 1.0 mg/L | pH = 7.0 | ||||
| [Time] = 40 min | |||||
| Raw bauxite | 2,4,6-trichloroanisole (TCA) | [Cat] = 0.2 g/L | [O3] = 0.5 mg/L | Presence of the raw bauxite in ozonation improved the degradation of TCA. Producing •OH in the catalytic ozonation process due to the introduction of the raw bauxite. | Qi et al. (2009) [186] |
| [Pull]0 = 1 × 10−4 mg/L | pH = 6.0 | ||||
| [Time] = 10 min | [%] = 95.2% | ||||
| Y zeolite | Phenol | [Cat] = 4.2 g/L | [O3] = 0.3 mg/min | Y zeolite enhanced the decomposition of ozone and the production of hydroxyl. Y zeolite showed excellent repetitive-use performance even after 10 runs of experiments. | Dong et al. (2008) [187] |
| [Pull]0 = 100 mg/L | [%] = 50.9% | ||||
| [Time] = 45 min | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhang, H.; Wang, F.; Xiong, X.; Tian, K.; Sun, Y.; Yu, T. Application of Heterogeneous Catalytic Ozonation for Refractory Organics in Wastewater. Catalysts 2019, 9, 241. https://doi.org/10.3390/catal9030241
Wang B, Zhang H, Wang F, Xiong X, Tian K, Sun Y, Yu T. Application of Heterogeneous Catalytic Ozonation for Refractory Organics in Wastewater. Catalysts. 2019; 9(3):241. https://doi.org/10.3390/catal9030241
Chicago/Turabian StyleWang, Bing, Huan Zhang, Feifei Wang, Xingaoyuan Xiong, Kun Tian, Yubo Sun, and Tingting Yu. 2019. "Application of Heterogeneous Catalytic Ozonation for Refractory Organics in Wastewater" Catalysts 9, no. 3: 241. https://doi.org/10.3390/catal9030241
APA StyleWang, B., Zhang, H., Wang, F., Xiong, X., Tian, K., Sun, Y., & Yu, T. (2019). Application of Heterogeneous Catalytic Ozonation for Refractory Organics in Wastewater. Catalysts, 9(3), 241. https://doi.org/10.3390/catal9030241




