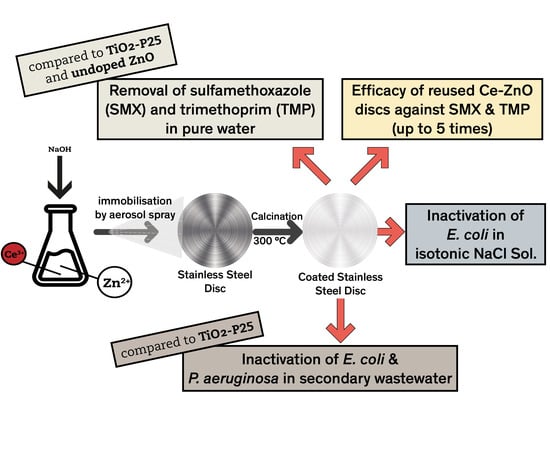
Immobilised Cerium-Doped Zinc Oxide as a Photocatalyst for the Degradation of Antibiotics and the Inactivation of Antibiotic-Resistant Bacteria
Abstract
1. Introduction
2. Results and Discussion
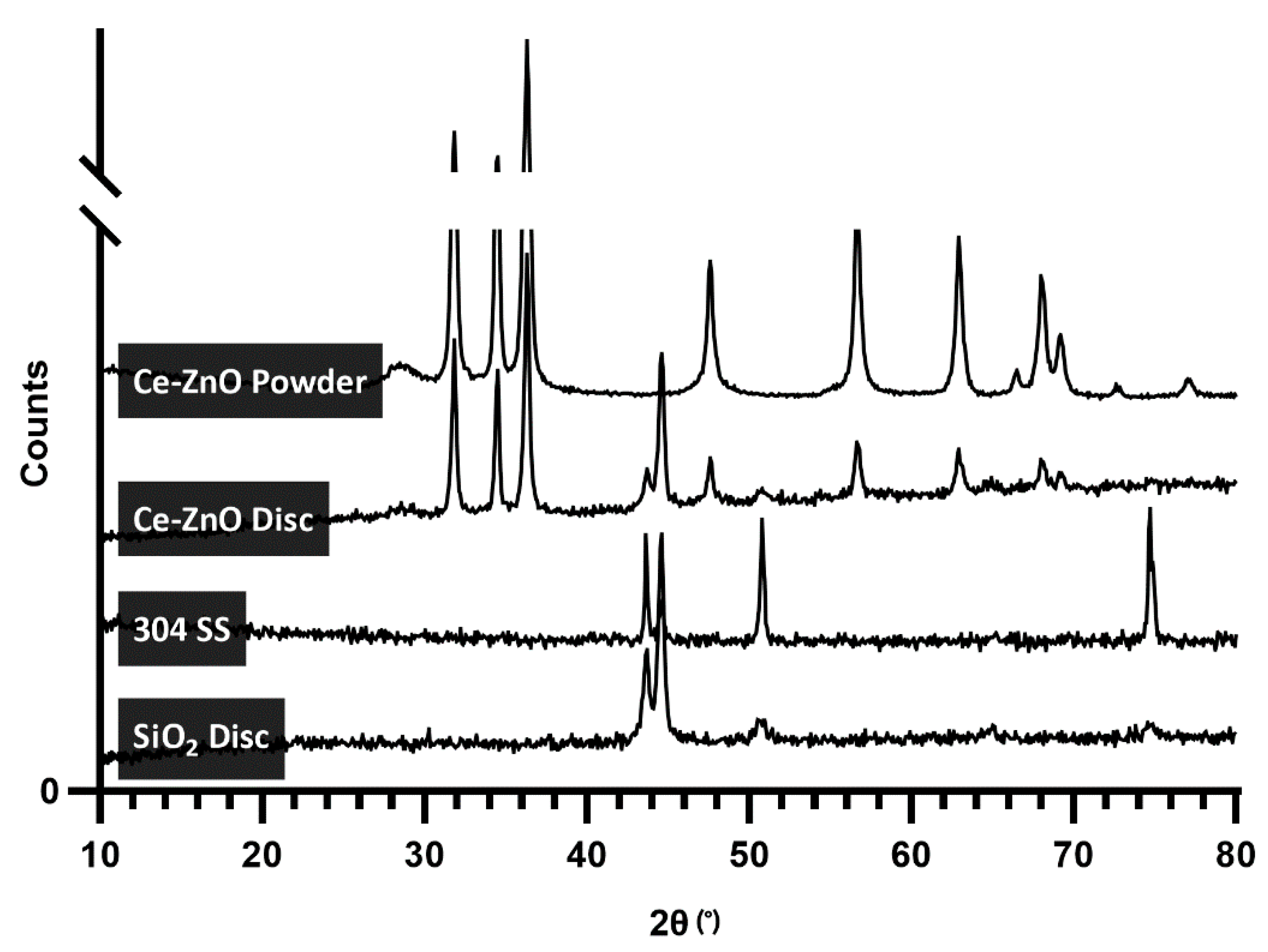
2.1. Photocatalyst Immobilisation and Characterisation
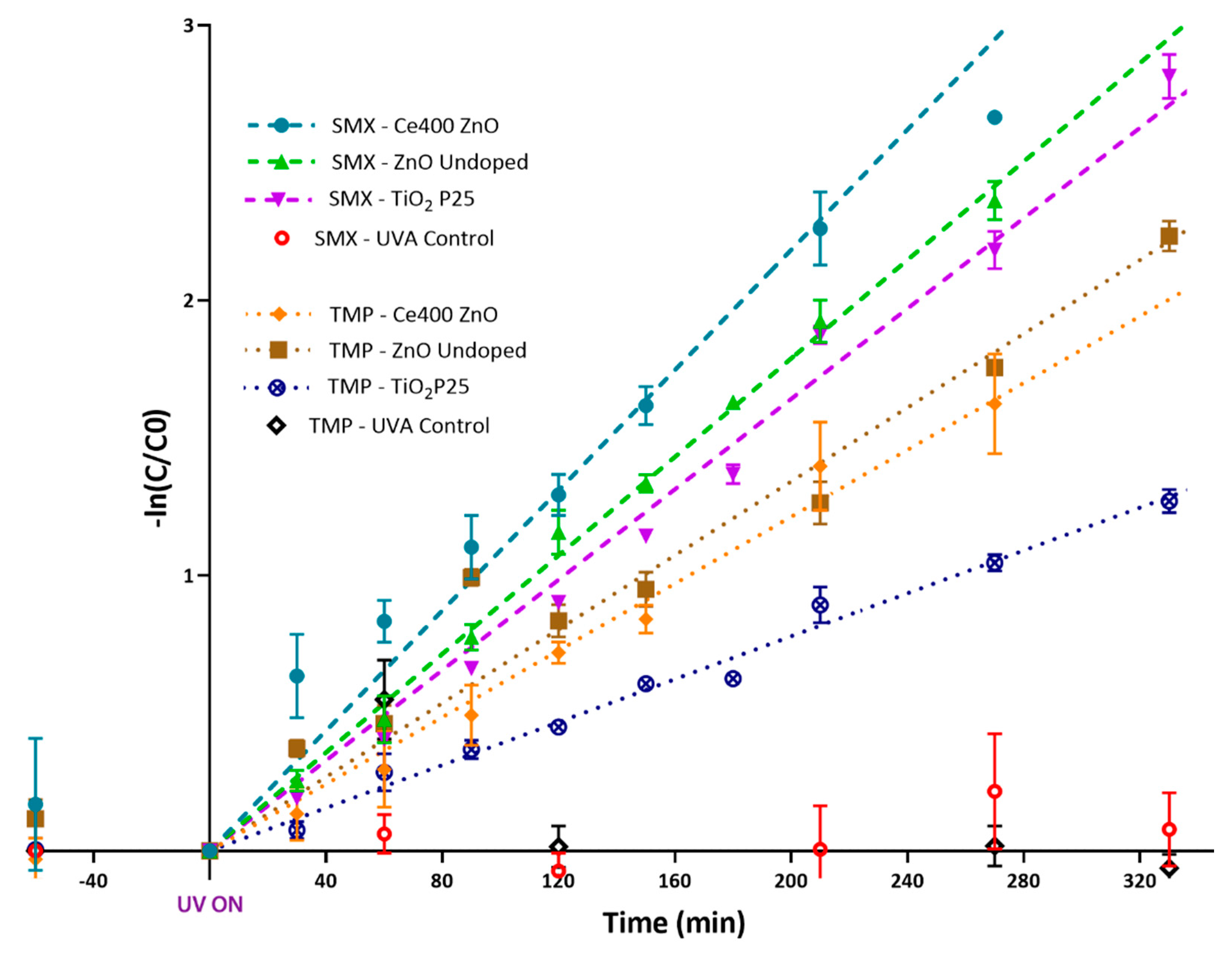
2.2. Photocatalytic Degradation of Antibiotics
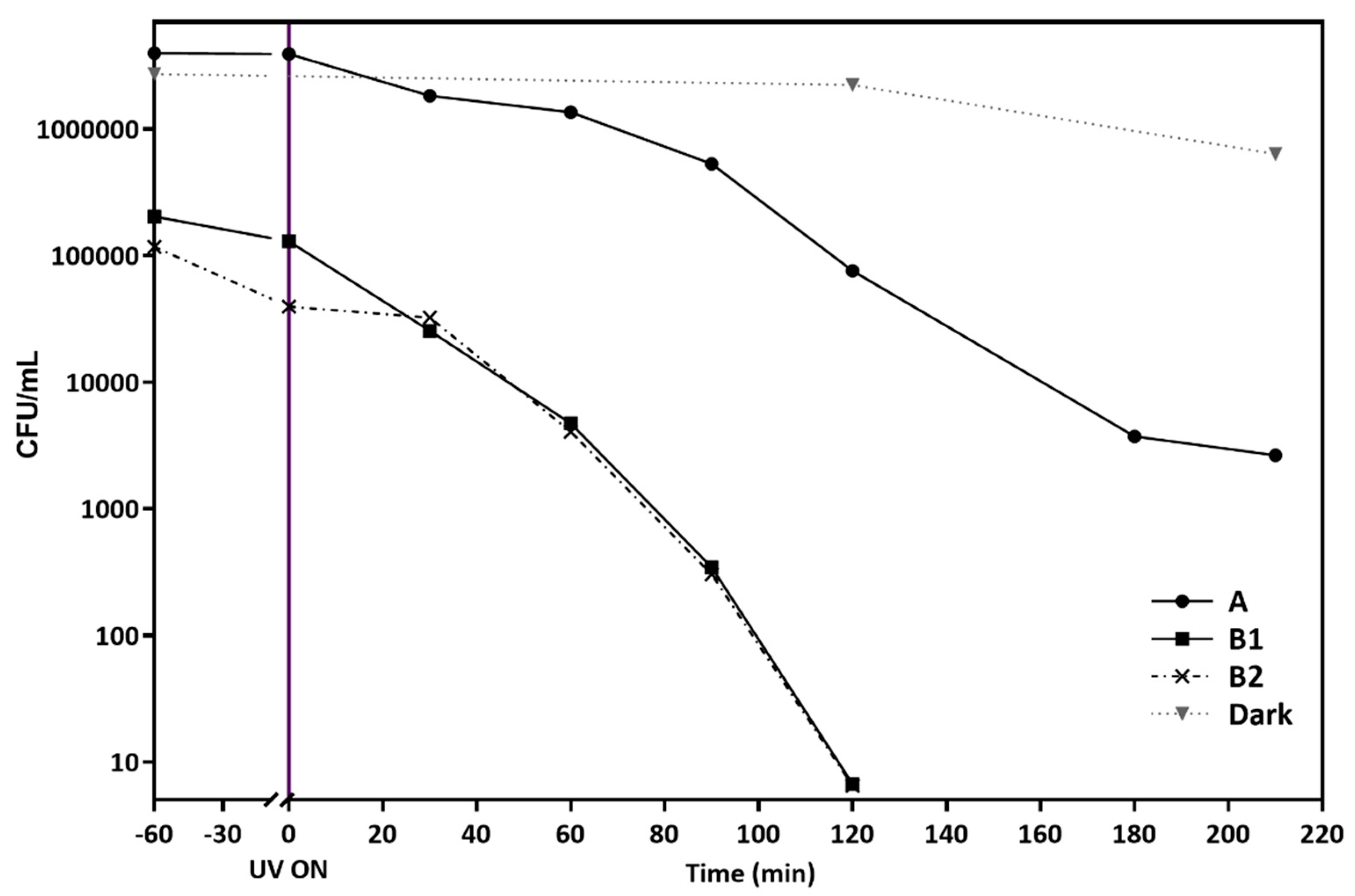
2.3. Photocatalytic Inactivation of E. coli in Isotonic Saline Solution
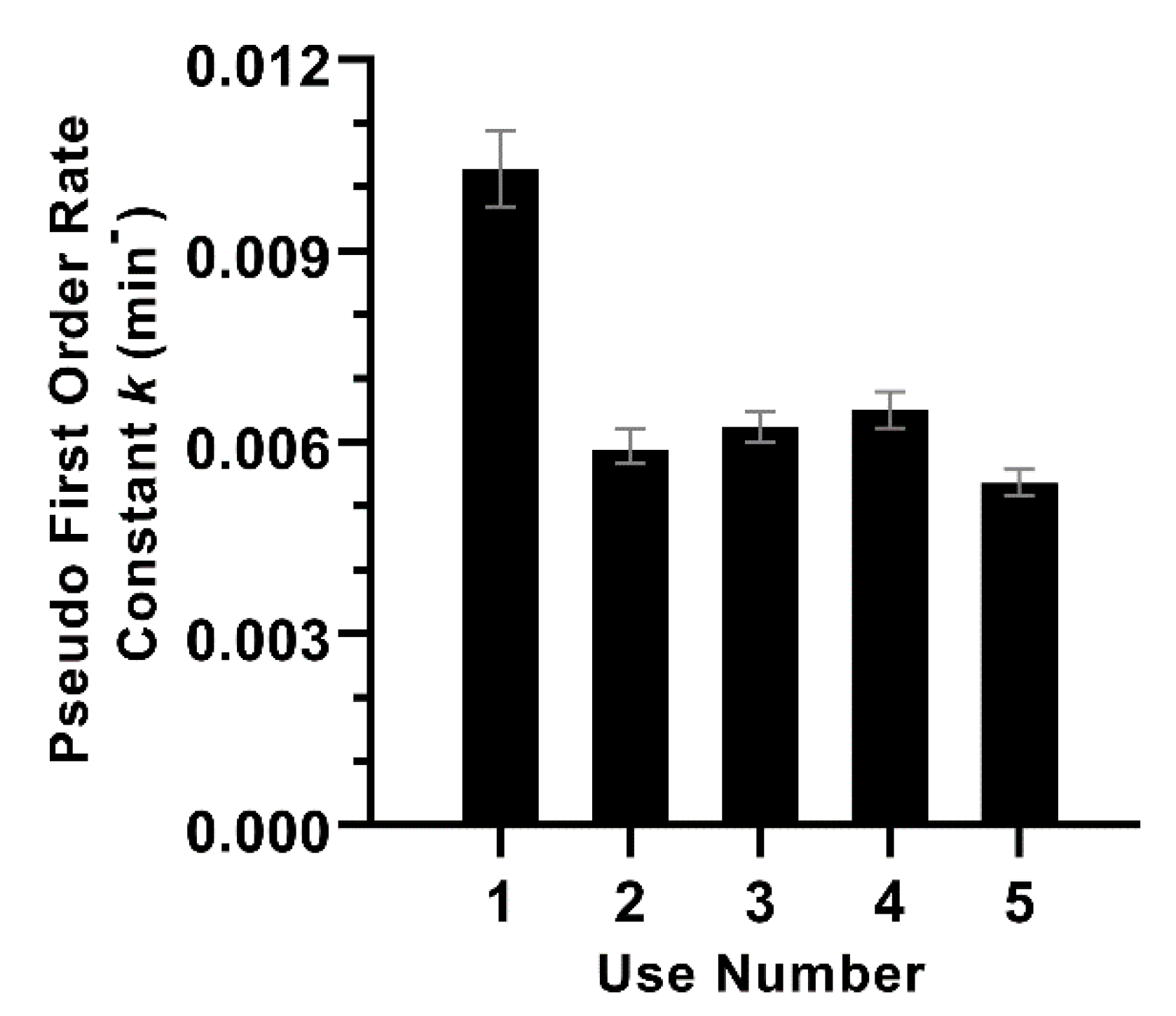
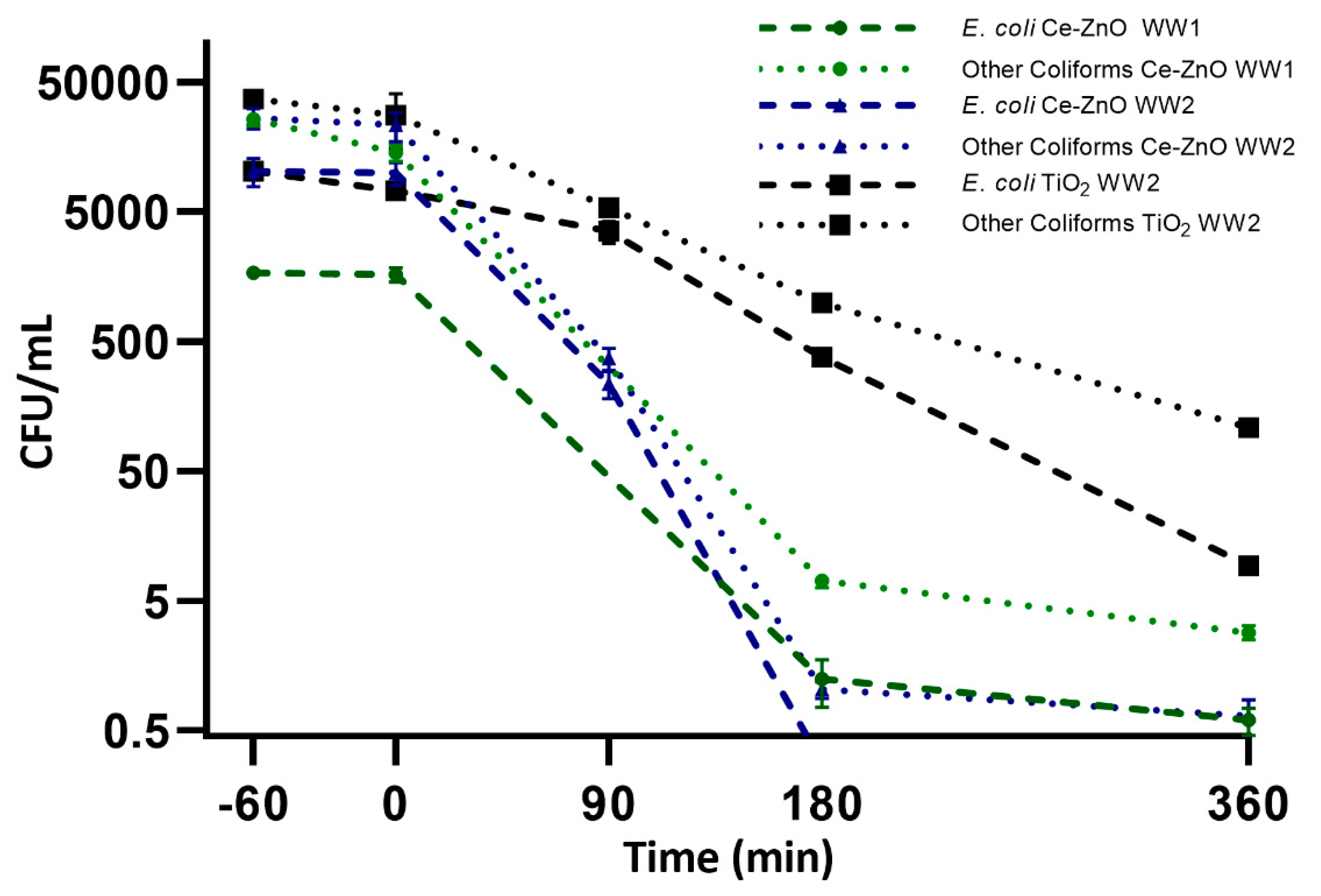
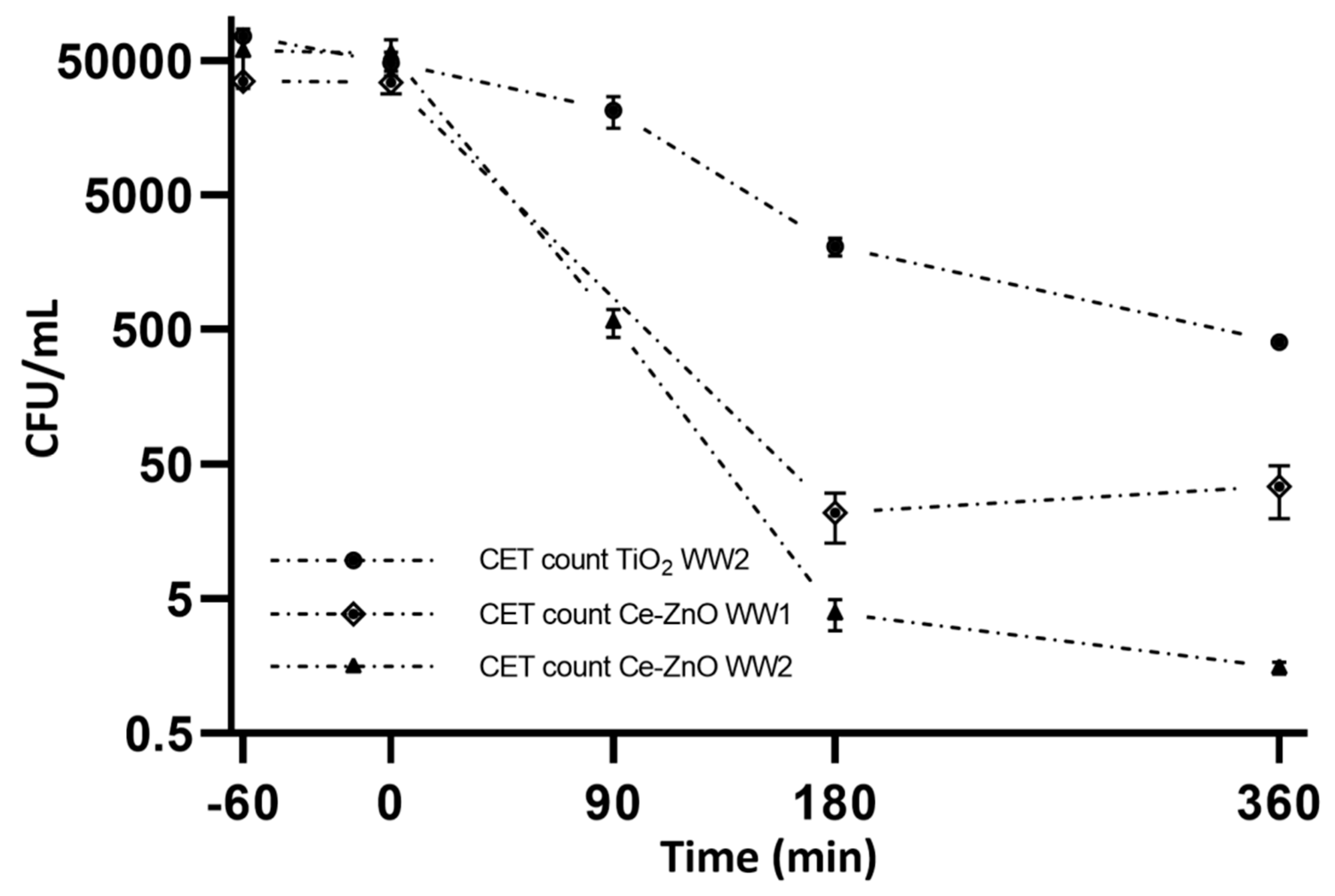
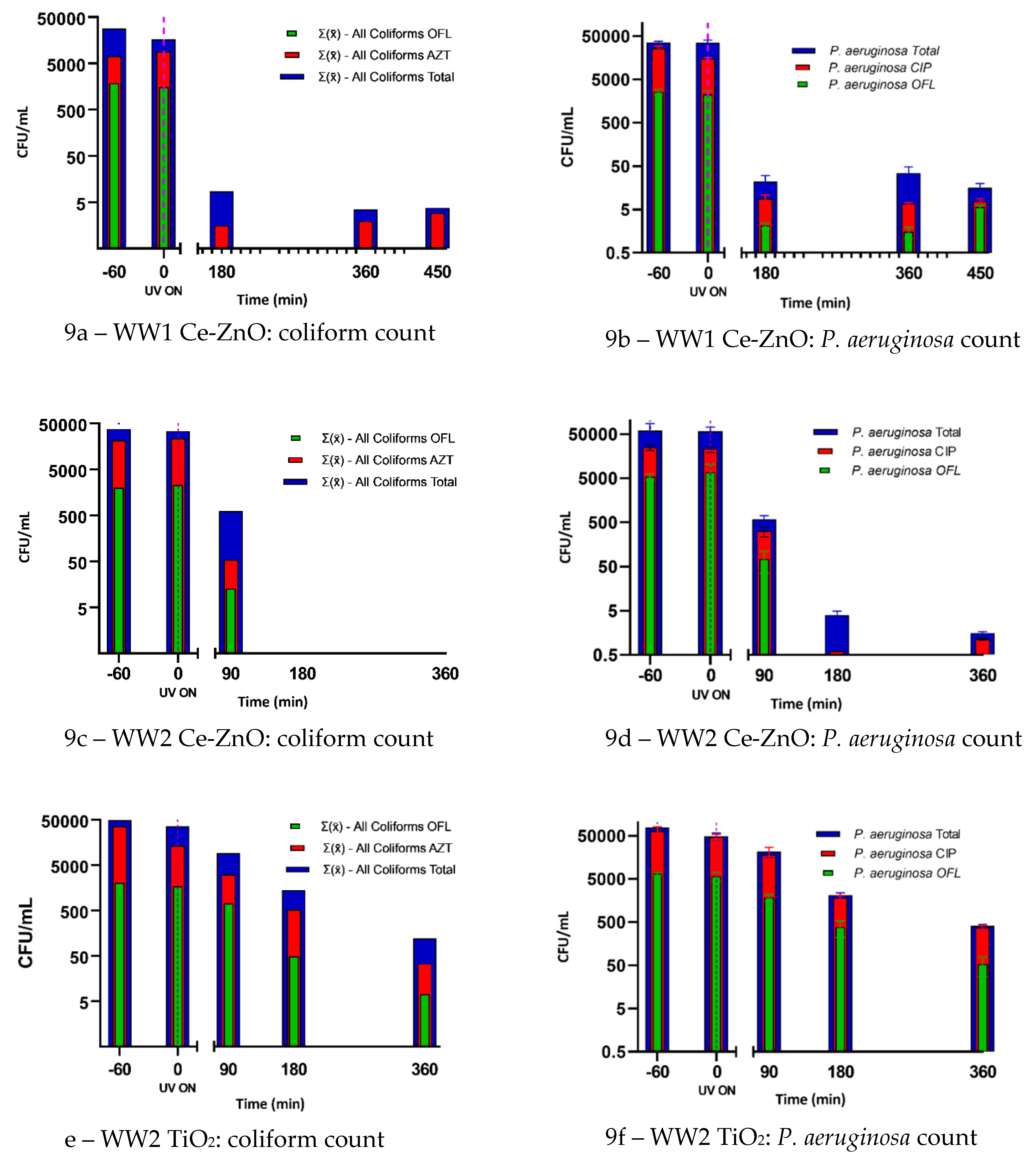
2.4. Photocatalytic Wastewater Disinfection
3. Materials and Methods
3.1. Wastewater Samples
3.2. Photocatalyst Synthesis, Immobilisation and Characterisation
3.3. Photocatalytic Experiments
3.4. Bacterial Count
3.5. Analytical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 14 February 2019).
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic-resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.J.; Andremont, A.; Bengtsson-Palme, J.; Brandt, K.K.; Husman, A.M.D.R.; Fagerstedt, P.; Fick, J.; Flach, C.-F.; Gaze, W.H.; Kuroda, M.; et al. Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ. Int. 2018, 117, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Manaia, C.M.; Rocha, J.; Scaccia, N.; Marano, R.; Radu, E.; Biancullo, F.; Cerqueira, F.; Fortunato, G.; Iakovides, I.C.; Zammit, I.; et al. Antibiotic resistance in wastewater treatment plants: Tackling the black box. Environ. Int. 2018, 115, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Antibiotic resistance in the environment: A link to the clinic? Curr. Opin. Microbiol. 2010, 13, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Bates, B.; Kundzewicz, Z.; Wu, S.; Palutikof, J. Climate Change and Water Technical Paper of the Intergovernmental Panel on Climate Change; IPCC Secretariat: Geneva, Switzerland, 2008; Volume 95, p. 96. Available online: https://digital.library.unt.edu/ark:/67531/metadc11958/m2/1/high_res_d/climate-change-water-en.pdf (accessed on 14 February 2019).
- BIO by Deloitte. Optimising Water Reuse in the EU—Final Report Prepared for the European Commission (DG ENV) Part 1. 07.0307/2013/658572/ENV.C1. 2015. Available online: http://ec.europa.eu/environment/water/blueprint/pdf/BIO_IA%20on%20water%20reuse_Final%20Part%20I.pdf (accessed on 14 February 2019).
- Rizzo, L.; Krätke, R.; Linders, J.; Scott, M.; Vighi, M.; De Voogt, P. Proposed EU minimum quality requirements for water reuse in agricultural irrigation and aquifer recharge: SCHEER scientific advice. Curr. Opin. Environ. Sci. Health 2018, 2, 7–11. [Google Scholar] [CrossRef]
- Murray, G.E.; Tobin, R.S.; Junkins, B.; Kushner, D.J. Effect of chlorination on antibiotic resistance profiles of sewage-related bacteria. Appl. Environ. Microbiol. 1984, 48, 73–77. [Google Scholar] [PubMed]
- Liu, S.-S.; Qu, H.-M.; Yang, D.; Hu, H.; Liu, W.-L.; Qiu, Z.-G.; Hou, A.-M.; Guo, J.; Li, J.-W.; Shen, Z.-Q.; et al. Chlorine disinfection increases both intracellular and extracellular antibiotic resistance genes in a full-scale wastewater treatment plant. Water Res. 2018, 136, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Su, C.; Zhou, J.; Xu, L.; Qian, Y.; Chen, H. Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Biochem. Eng. J. 2017, 317, 309–316. [Google Scholar] [CrossRef]
- Alexander, J.; Knopp, G.; Dötsch, A.; Wieland, A.; Schwartz, T. Ozone treatment of conditioned wastewater selects antibiotic resistance genes, opportunistic bacteria, and induce strong population shifts. Sci. Total Environ. 2016, 559, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Della Sala, A.; Fiorentino, A.; Puma, G.L. Disinfection of urban wastewater by solar driven and UV lamp–TiO2 photocatalysis: Effect on a multi drug resistant Escherichia coli strain. Water Res. 2014, 53, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D. Photocatalytic activity of a visible light active structured photocatalyst developed for municipal wastewater treatment. J. Clean. Prod. 2018, 175, 38–49. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mut. Res.-Rev. Mut. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Wildhaber, Y.S.; Mestankova, H.; Schärer, M.; Schirmer, K.; Salhi, E.; Von Gunten, U. Novel test procedure to evaluate the treatability of wastewater with ozone. Water Res. 2015, 75, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs. new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Van Grieken, R.; Marugan, J.; Sordo, C.; Martínez, P.; Pablos, C. Photocatalytic inactivation of bacteria in water using suspended and immobilized silver-TiO2. Appl. Catal. B-Environ. 2009, 93, 112–118. [Google Scholar] [CrossRef]
- Alrousan, D.M.; Dunlop, P.S.; McMurray, T.A.; Byrne, J.A. Photocatalytic inactivation of E. coli in surface water using immobilised nanoparticle TiO2 films. Water Res. 2009, 43, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Adán, C.; Magnet, A.; Fenoy, S.; Pablos, C.; Del Águila, C.; Marugán, J. Concomitant inactivation of Acanthamoeba spp. and Escherichia coli using suspended and immobilized TiO2. Water Res. 2018, 144, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Pablos, C.; Van Grieken, R.; Marugan, J.; Moreno, B.; Moreno-García, B. Photocatalytic inactivation of bacteria in a fixed-bed reactor: Mechanistic insights by epifluorescence microscopy. Catal. Today 2011, 161, 133–139. [Google Scholar] [CrossRef]
- Guz, L.; García, P.; Ponce, S.; Goyanes, S.; Marchi, M.C.; Candal, R.; Rodriguez, J.; Sanchez, L. Synthesis and Characterization of ZnO Nanorod Films on PET for Photocatalytic Disinfection of Water. J. Adv. Oxid. Tech. 2015, 18, 246–252. [Google Scholar]
- Yeber, M.C.; RodríguezJ; Freer, J.; Durán, N.; Mansilla, H.D. Photocatalytic degradation of cellulose bleaching effluent by supported TiO2 and ZnO. Chemosphere 2000, 41, 1193–1197. [Google Scholar] [CrossRef]
- Couto, S.R.; DomínguezA; Sanromán, A. Photocatalytic degradation of dyes in aqueous solution operating in a fluidised bed reactor. Chemosphere 2002, 46, 83–86. [Google Scholar] [CrossRef]
- Zammit, I.; Vaiano, V.; Iervolino, G.; Rizzo, L. Inactivation of an urban wastewater indigenous Escherichia coli strain by cerium-doped zinc oxide photocatalysis. RSC Adv. 2018, 8, 26124–26132. [Google Scholar] [CrossRef]
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: A review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Lassaletta, G.; Jiménez, V.; Justo, A.; González-Elipe, A.; Herrmann, J.-M.; Tahiri, H.; Ait-Ichou, Y. Preparation and characterization of TiO2 photocatalysts supported on various rigid supports (glass, quartz and stainless steel). Comparative studies of photocatalytic activity in water purification. Appl. Catal. B-Environ. 1995, 7, 49–63. [Google Scholar] [CrossRef]
- Cheary, R.W.; Ma-Sorrell, Y. Quantitative phase analysis by X-ray diffraction of martensite and austenite in strongly oriented orthodontic stainless steel wires. J. Mater. Sci. 2000, 35, 1105–1113. [Google Scholar] [CrossRef]
- Fathi, M.; Karimzadeh, F.; Dadfar, M.; Saatchi, A. Effect of TIG welding on corrosion behavior of 316L stainless steel. Mat. Lett. 2007, 61, 2343–2346. [Google Scholar]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photochemical Fate of Sulfa Drugs in the Aquatic Environment: Sulfa Drugs Containing Five-Membered Heterocyclic Groups. Environ. Sci. Technol. 2004, 38, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Dodd, M.C.; Buffle, M.-O.; Von Gunten, U. Oxidation of Antibacterial Molecules by Aqueous Ozone: Moiety-Specific Reaction Kinetics and Application to Ozone-Based Wastewater Treatment. Environ. Sci. Technol. 2006, 40, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Dionysiou, D.D.; Puma, G.L.; Ye, J.; Schneider, J.; Bahnemann, D. Photocatalysis: Applications; The Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Zhang, L.; Cheng, H.; Zong, R.; Zhu, Y. Photocorrosion Suppression of ZnO Nanoparticles via Hybridization with Graphite-like Carbon and Enhanced Photocatalytic Activity. J. Phys. Chem. C 2009, 113, 2368–2374. [Google Scholar] [CrossRef]
- Pekakis, P.A.; Xekoukoulotakis, N.P.; Mantzavinos, D. Treatment of textile dyehouse wastewater by TiO2 photocatalysis. Water Res. 2006, 40, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.N.; Sivasankar, B.; Sadasivam, V. Kinetic study on the photocatalytic degradation of salicylic acid using ZnO catalyst. J. Hazard. Mater. 2009, 166, 1357–1361. [Google Scholar] [PubMed]
- Fernandez-Ibanez, P.; Blanco, J.; Malato, S.; Nieves, F.L. Application of the colloidal stability of TiO2 particles for recovery and reuse in solar photocatalysis. Water Res. 2003, 37, 3180–3188. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dutta, B. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Tian, C.; Jiang, B.; Wu, A.; Zhang, Q.; Tian, G.; Fu, H. Facile synthesis of sheet-like ZnO assembly composed of small ZnO particles for highly efficient photocatalysis. J. Mater. Chem. A 2013, 1, 5700–5708. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Green and low cost tetracycline degradation processes by nanometric and immobilized TiO2 systems. Catal. Today 2017, 281, 38–44. [Google Scholar] [CrossRef]
- Pronina, N.; Klauson, D.; Moiseev, A.; Deubener, J.; Krichevskaya, M. Titanium dioxide sol–gel-coated expanded clay granules for use in photocatalytic fluidized-bed reactor. Appl. Catal. B-Envioron. 2015, 178, 117–123. [Google Scholar] [CrossRef]
- Akhavan, O. Lasting antibacterial activities of Ag–TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J. Colloid Interface Sci. 2009, 336, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Lalhriatpuia, C.; Tiwari, D.; Tiwari, A.; Lee, S.M. Immobilized Nanopillars-TiO2 in the efficient removal of micro-pollutants from aqueous solutions: Physico-chemical studies. Biochem. Eng. J. 2015, 281, 782–792. [Google Scholar]
- Van Doorslaer, X.; Demeestere, K.; Heynderickx, P.M.; Van Langenhove, H.; Dewulf, J. UV-A and UV-C induced photolytic and photocatalytic degradation of aqueous ciprofloxacin and moxifloxacin: Reaction kinetics and role of adsorption. Appl. Catal. B-Environ. 2011, 101, 540–547. [Google Scholar] [CrossRef]
- Ozer, L.Y.; Garlisi, C.; Oladipo, H.; Pagliaro, M.; Sharief, S.A.; Yusuf, A.; Almheiri, S.; Palmisano, G. Inorganic semiconductors-graphene composites in photo(electro)catalysis: Synthetic strategies, interaction mechanisms and applications. J. Photochem. Photobiol. C-Photochem. Rev. 2017, 33, 132–164. [Google Scholar] [CrossRef]
- Sarvari, M.H.; Sharghi, H. Reactions on a Solid Surface. A Simple, Economical and Efficient Friedel−Crafts Acylation Reaction over Zinc Oxide (ZnO) as a New Catalyst. J. Org. Chem. 2004, 69, 6953–6956. [Google Scholar] [CrossRef] [PubMed]
- Kiwi, J.; Nadtochenko, V. Evidence for the Mechanism of Photocatalytic Degradation of the Bacterial Wall Membrane at the TiO2 Interface by ATR-FTIR and Laser Kinetic Spectroscopy. Langmuir 2005, 21, 4631–4641. [Google Scholar] [CrossRef] [PubMed]
- Makwana, N.M.; Hazael, R.; McMillan, P.F.; Darr, J.A. Photocatalytic water disinfection by simple and low-cost monolithic and heterojunction ceramic wafers. Photochem. Photobiol. Sci. 2015, 14, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004, 38, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Grijpma, D.W.; Feijen, J.; Busscher, H.J.; Gottenbos, B.; Van Der Mei, H.C. Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J. Antimicrob. Chemother. 2001, 48, 7–13. [Google Scholar]
- Das, S.; Sinha, S.; Jayabalan, R.; Suar, M.; Mishra, A.; Tamhankar, A.J.; Tripathy, S.K.; Lundborg, C.S. Disinfection of Multidrug Resistant Escherichia coli by Solar-Photocatalysis using Fe-doped ZnO Nanoparticles. Sci. Rep. 2017, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, D.W.; Keefer, K.D. Structure of Soluble Silicates. MRS Proc. 1984, 32. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Micalizzi, G.R.; Bates, J.R.; Costanzo, S.D. Novel Method for Rapid Assessment of Antibiotic Resistance in Escherichia coli Isolates from Environmental Waters by Use of a Modified Chromogenic Agar. Appl. Environ. Microbiol. 2007, 73, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.V.; Vennes, J.W. Occurrence of multiple-antibiotic-resistant enteric bacteria in domestic sewage and oxidation lagoons. Appl. Environ. Microbiol. 1985, 50, 930–933. [Google Scholar] [PubMed]
- Carpa, R.; Oltean, A.; Popescu, O.; Lupan, I.; Kelemen, B.S. Release of Antibiotic-resistant bacteria by a Waste Treatment Plant from Romania. Microb. Environ. 2017, 32, 219–225. [Google Scholar]
- Kim, S.; Jensen, J.; Aga, D.; Weber, A. Fate of tetracycline resistant bacteria as a function of activated sludge process organic loading and growth rate. Water Sci. Technol. 2007, 55, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-J.; Hu, H.-Y.; Lu, S.-Q.; Li, Y.; Tang, F.; Lu, Y.; Wei, B. Monitoring and evaluation of antibiotic-resistant bacteria at a municipal wastewater treatment plant in China. Environ. Int. 2012, 42, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Enomoto, S. Nalidixic Acid Cetrimide Agar. Jpn. J. Microbiol. 1970, 14, 65–72. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Varela, A.R.; Ferro, G.; Vredenburg, J.; Yanık, M.; Vieira, L.; Rizzo, L.; Lameiras, C.; Manaia, C.M. Vancomycin resistant enterococci: From the hospital effluent to the urban wastewater treatment plant. Sci. Total Environ. 2013, 450, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Narciso-Da-Rocha, C.; Rocha, J.; Vaz-Moreira, I.; Lira, F.; Tamames, J.; Henriques, I.; Martinez, J.L.; Manaia, C.M. Bacterial lineages putatively associated with the dissemination of antibiotic resistance genes in a full-scale urban wastewater treatment plant. Environ. Int. 2018, 118, 179–188. [Google Scholar] [CrossRef] [PubMed]


| SMX | TMP | |||||||
|---|---|---|---|---|---|---|---|---|
| Ce400 ZnO | ZnO Undoped | TiO2 P25 | UVA Control | Ce400 ZnO | ZnO Undoped | TiO2P25 | UVA Control | |
| Std. Error | 2.63 × 10−4 | 8.80 × 10−5 | 9.90 × 10−5 | 1.52 × 10−4 | 1.44 × 10−4 | 1.79 × 10−4 | 5.50 × 10−5 | 3.32 × 10−4 |
| R2 | 0.943 | 0.993 | 0.988 | 0.083 | 0.956 | 0.941 | 0.983 | −0.248 |
| k (min−1) | 1.09 × 10−2 | 8.95 × 10−3 | 8.21 × 10−3 | 3.28 × 10−4 | 6.07 × 10−3 | 6.71 × 10−3 | 3.90 × 10−3 | 9.45 × 10−5 |
| t1/2 (min) | 63.6 | 77.4 | 84.4 | 2113.3 | 114.2 | 103.3 | 177.7 | 7334.9 |
| Initial (t = −60) | After 90 min Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Exp | OFL Resistant % | AZT Resistant % | CIP Resistant % | OFL Resistant % | AZT Resistant % | CIP Resistant % | |
| Ce ZnO | E. coli | 7.0–11.5 | 56.0–67.1 | N/A | 2.1–2.8 | 2.9–8.0 | N/A |
| Other Coliforms | 3.6–4.6 | 53.4–56.7 | N/A | 1.5–2.2 | 9.8–10.9 | N/A | |
| P. aeruginosa | 7.2–14.6 | N/A | 32.7-67.9 | 7.9–15.9 | N/A | 52.8–55.4 | |
| TiO2 | E. coli | 8.7-8.8 | 55.7–74.2 | N/A | 8.6–9.7 | 5.6–15.7 | N/A |
| Other Coliforms | 1.6–4.0 | 75.0–78.0 | N/A | 4.0–9.1 | 43.7–53.1 | N/A | |
| P. aeruginosa | 8.5–8.6 | N/A | 62.8–99.5 | 8.0–9.6 | N/A | 56.3–99.5 | |
| Monthly | Secondary Clarifier | |||||
|---|---|---|---|---|---|---|
| pH | Conductivity | BOD5 | COD | TSS | VSS | |
| (µS/Cm) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | ||
| Average | 7.2 | 707 | 31.3 | 90.3 | 33.5 | 31.4 |
| Min | 7.0 | 590 | 15.6 | 16.0 | 23.0 | 22.0 |
| Max | 7.5 | 859 | 95.3 | 204.0 | 76.0 | 76.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zammit, I.; Vaiano, V.; Ribeiro, A.R.; Silva, A.M.T.; Manaia, C.M.; Rizzo, L. Immobilised Cerium-Doped Zinc Oxide as a Photocatalyst for the Degradation of Antibiotics and the Inactivation of Antibiotic-Resistant Bacteria. Catalysts 2019, 9, 222. https://doi.org/10.3390/catal9030222
Zammit I, Vaiano V, Ribeiro AR, Silva AMT, Manaia CM, Rizzo L. Immobilised Cerium-Doped Zinc Oxide as a Photocatalyst for the Degradation of Antibiotics and the Inactivation of Antibiotic-Resistant Bacteria. Catalysts. 2019; 9(3):222. https://doi.org/10.3390/catal9030222
Chicago/Turabian StyleZammit, Ian, Vincenzo Vaiano, Ana R. Ribeiro, Adrián M. T. Silva, Célia M. Manaia, and Luigi Rizzo. 2019. "Immobilised Cerium-Doped Zinc Oxide as a Photocatalyst for the Degradation of Antibiotics and the Inactivation of Antibiotic-Resistant Bacteria" Catalysts 9, no. 3: 222. https://doi.org/10.3390/catal9030222
APA StyleZammit, I., Vaiano, V., Ribeiro, A. R., Silva, A. M. T., Manaia, C. M., & Rizzo, L. (2019). Immobilised Cerium-Doped Zinc Oxide as a Photocatalyst for the Degradation of Antibiotics and the Inactivation of Antibiotic-Resistant Bacteria. Catalysts, 9(3), 222. https://doi.org/10.3390/catal9030222