Effect of Preparation Method of Co-Ce Catalysts on CH4 Combustion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. Textural and Structural Characterization
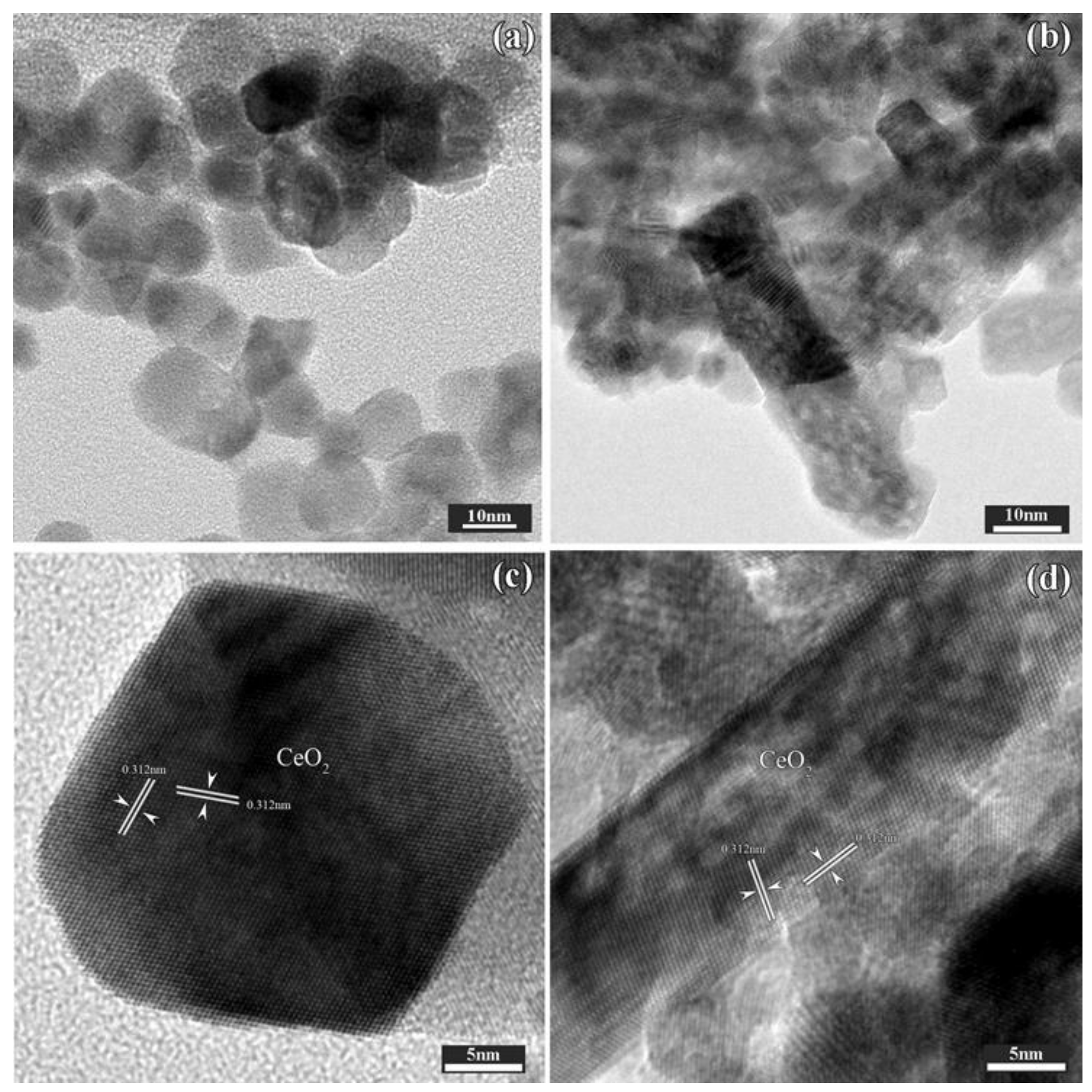
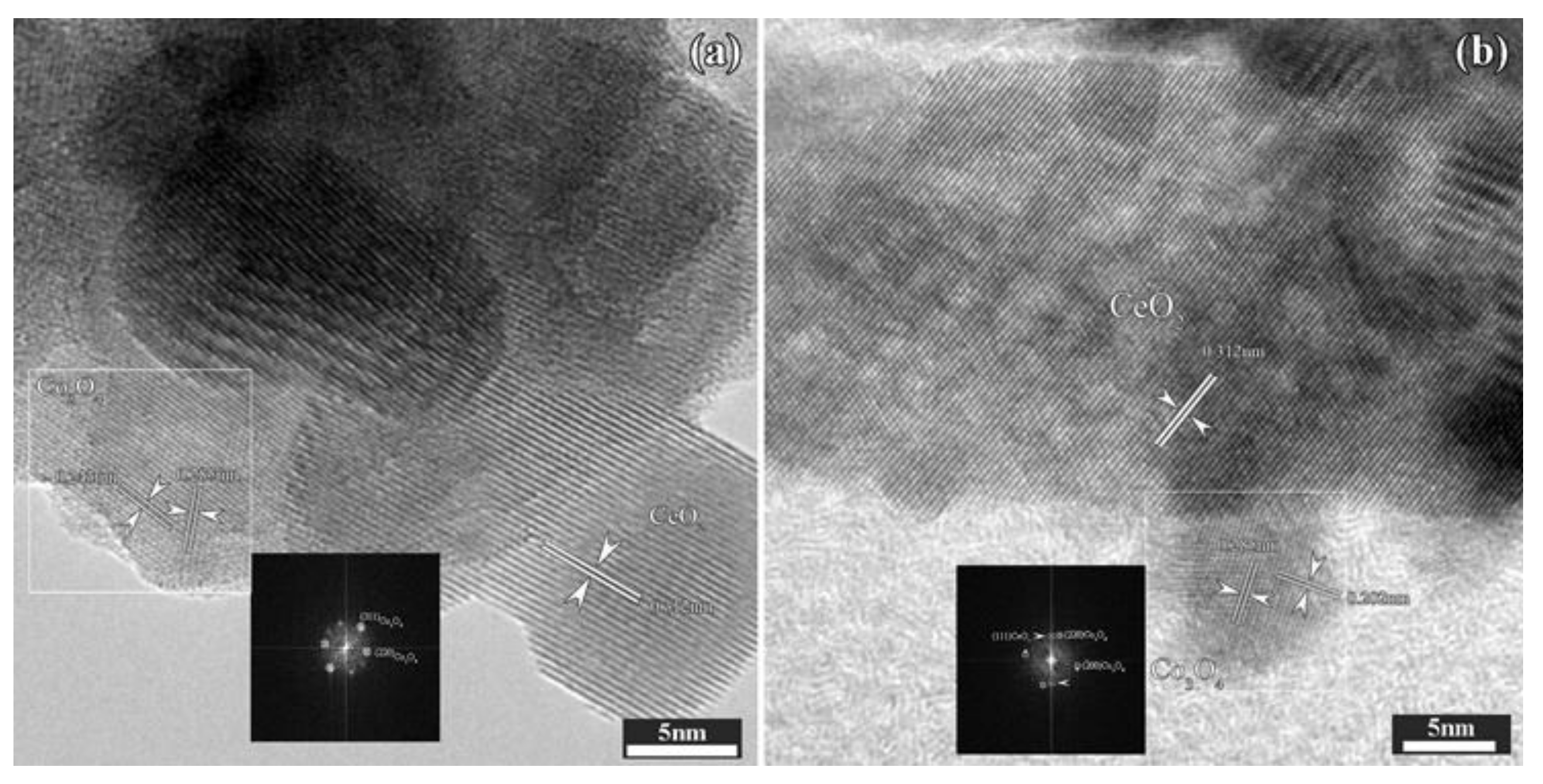
2.1.2. Morphological Characterization
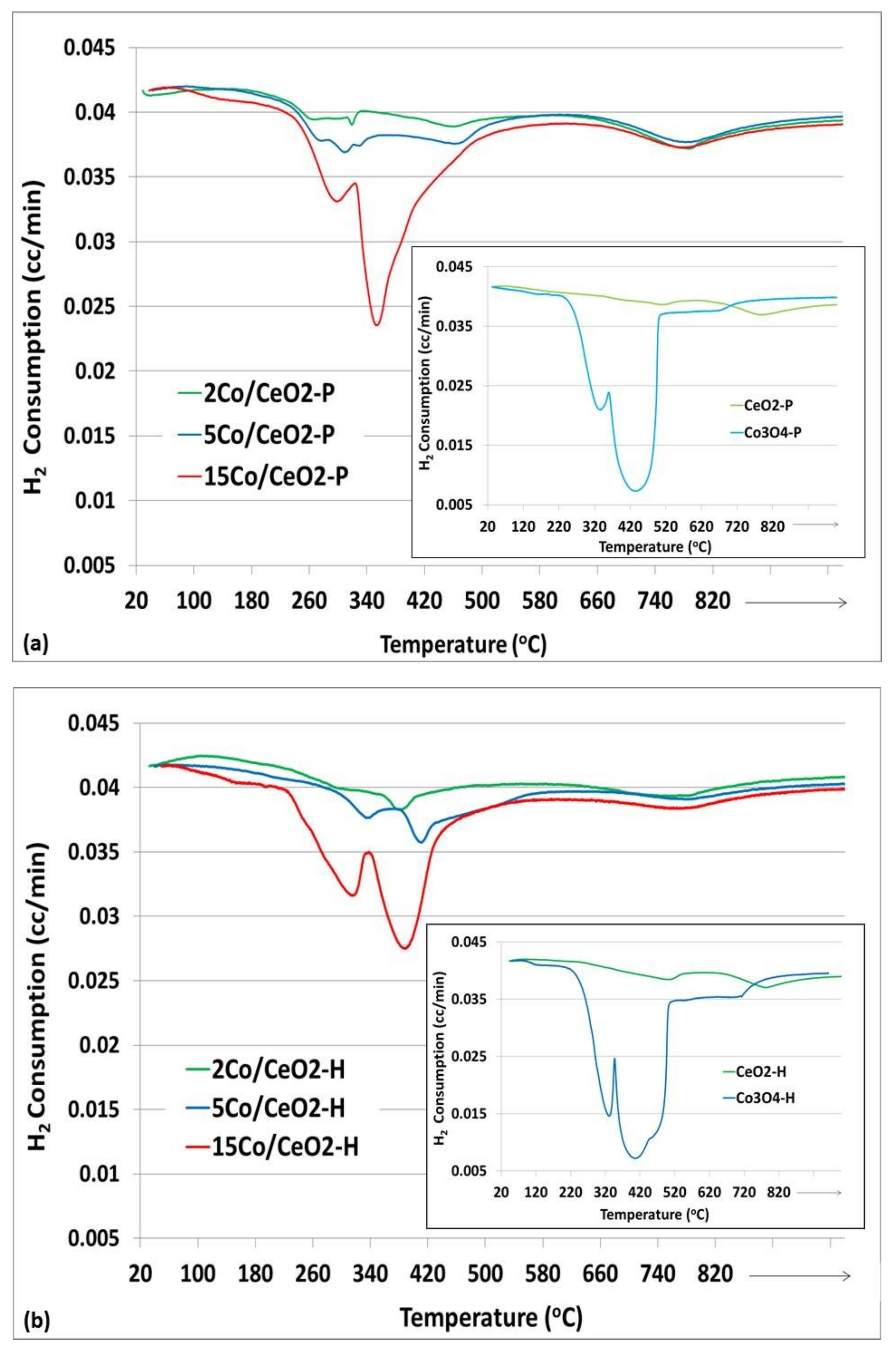
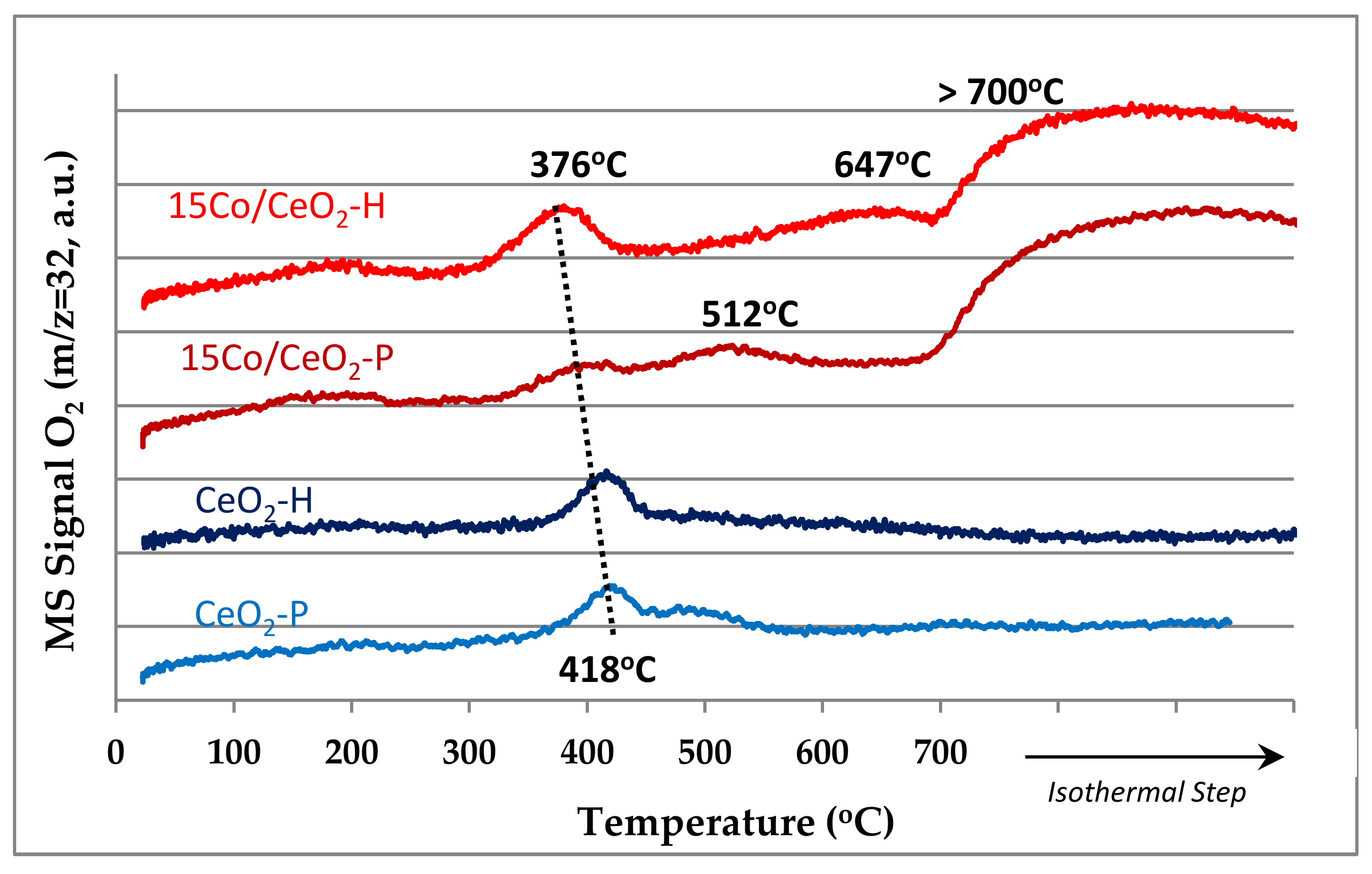
2.1.3. H2-TPR and O2-TPD Studies
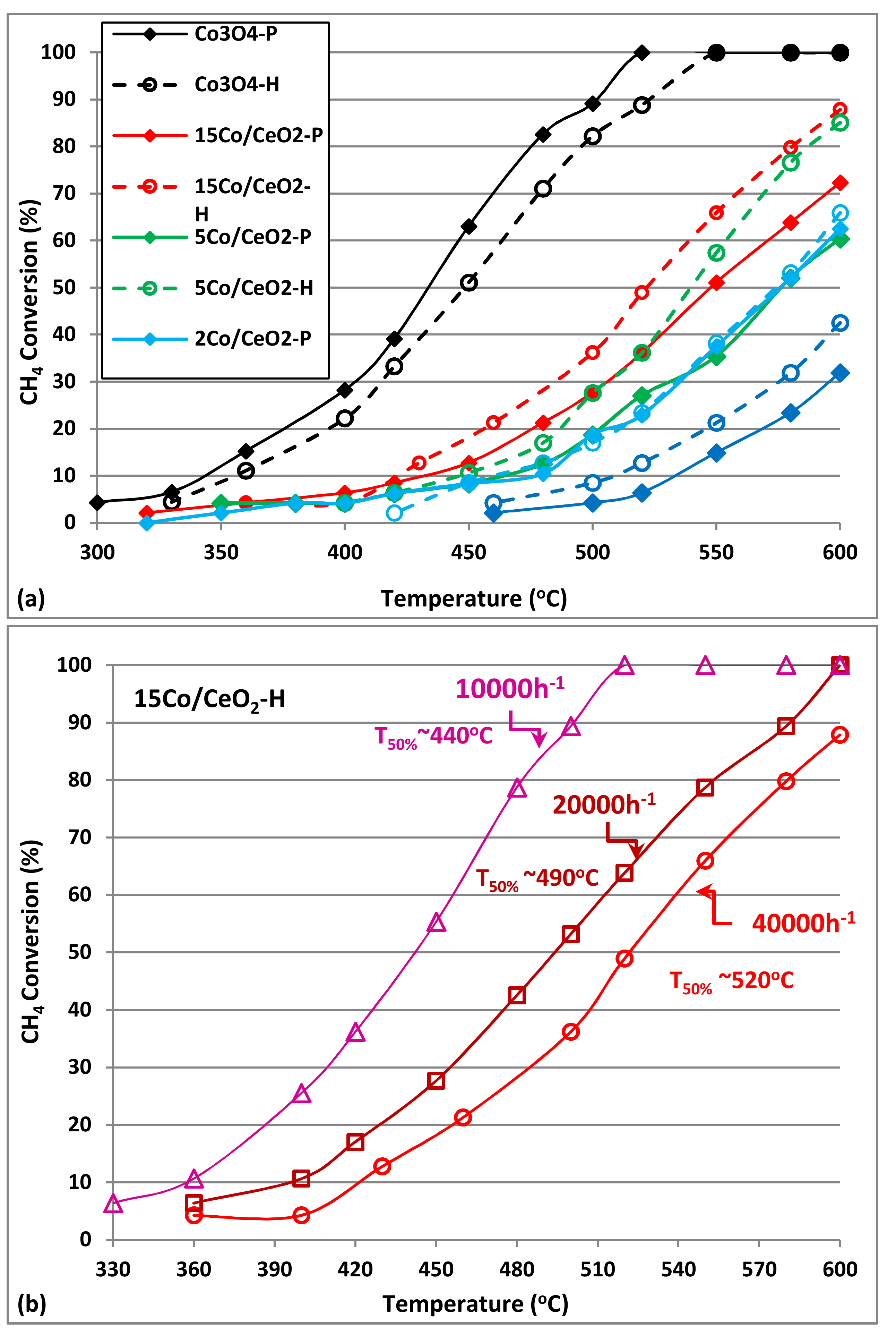
2.2. Catalytic Activity of Pure CeO2, Pure Co3O4 and Co3O4/CeO2 Oxides for Methane Oxidation
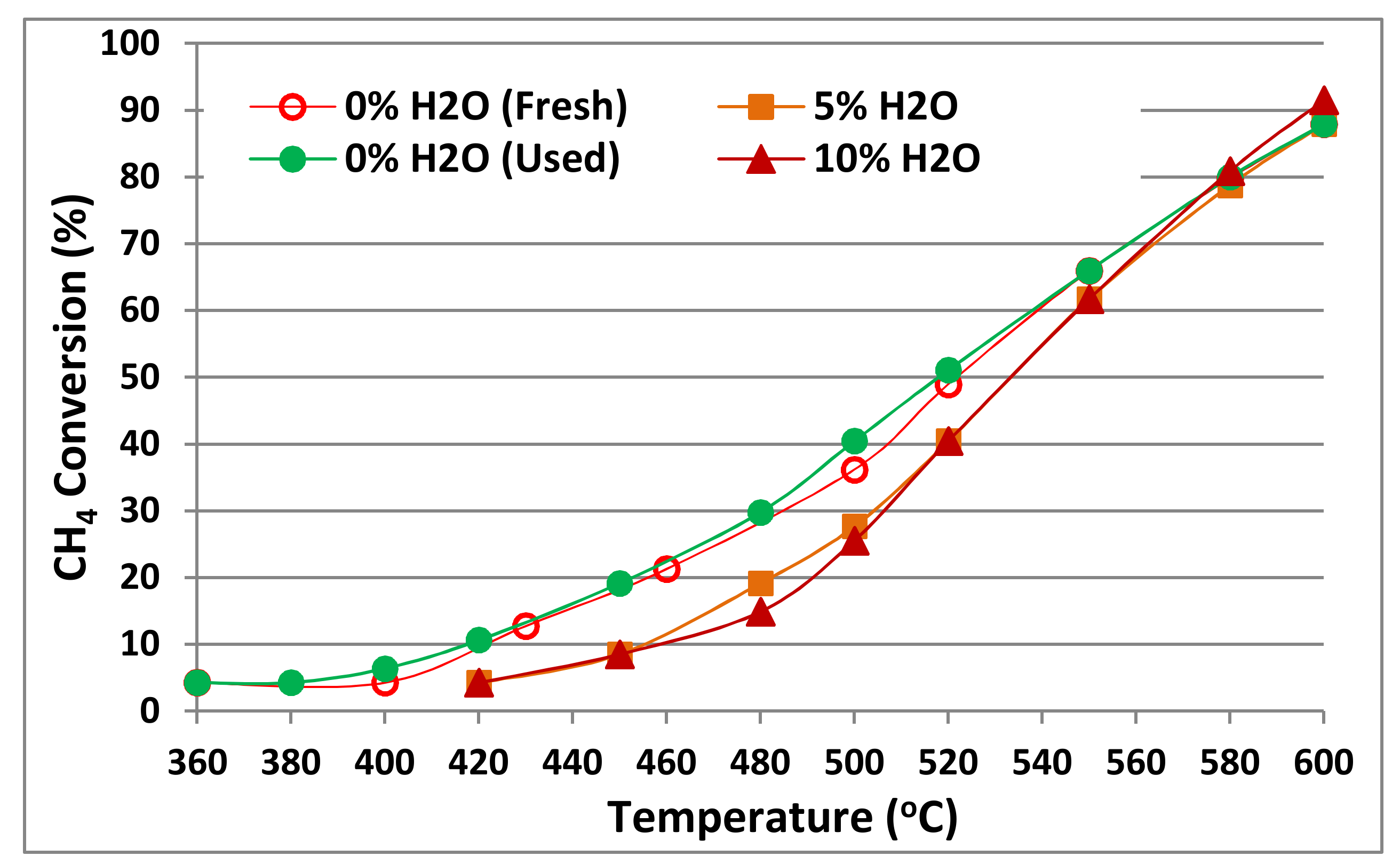
2.3. Catalyst Stability Tests
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, N.N.; Johnson, D.R.; McKain, D.L.; Wayne, W.S.; Li, H.; Rudek, J.; Mongold, R.A.; Sandoval, C.; Covington, A.N.; Hailer, J.T. Future methane emissions from the heavy-duty natural gas transportation sector for stasis, high, medium, and low scenarios in 2035. J. Air Waste Manag. 2017, 67, 1328–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrauto, R.J. Chemistry. Low-temperature oxidation of methane. Science 2012, 337, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Filip, M.; Todorova, S.; Shopska, M.; Ciobanu, M.; Papa, F.; Somacescu, S.; Munteanu, C.; Parvulescu, V. Effects of Ti loading on activity and redox behavior of metals in PtCeTi/KIT-6 catalysts for CH4 and CO oxidation. Catal. Today 2018, 306, 138–144. [Google Scholar] [CrossRef]
- Chen, J.; Arandiyan, H.; Gao, X.; Li, J. Recent Advances in Catalysts for Methane Combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Xie, S.; Wang, Z.; Dai, H. Catalytic removal of volatile organic compounds using ordered porous transition metal oxide and supported noble metal catalysts. Chin. J. Catal. 2016, 37, 1193–1205. [Google Scholar] [CrossRef]
- Dou, J.; Tang, Y.; Nie, L.; Andolina, C.M.; Zhang, X.; House, S.; Li, Y.; Yang, J.; Tao, F. Complete oxidation of methane on Co3O4/CeO2 nanocomposite: A synergic effect. Catal. Today 2018, 311, 48–55. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Q.; Li, Y. Selective Synthesis of Co3O4 Nanocrystal with Different Shape and Crystal Plane Effect on Catalytic Property for Methane Combustion. J. Am. Chem. Soc. 2008, 130, 16136–16137. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, S.; Lu, J.; Luo, M. Effect of structural properties of mesoporous Co3O4 catalysts on methane combustion. Chem. Res. Chin. Univ. 2016, 32, 808–811. [Google Scholar] [CrossRef]
- Teng, F.; Chen, M.D.; Li, G.Q.; Teng, Y.; Xu, T.G.; Hang, Y.C.; Yao, W.Q.; Santhanagopalan, S.; Meng, D.D.; Zhu, Y.F. High combustion activity of CH4 and catalluminescence properties of CO oxidation over porous Co3O4 nanorods. Appl. Catal. B 2011, 110, 133–140. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Xu, L.; Deng, Z.; Shi, W. Low temperature CO oxidation and CH4 combustion over Co3O4 nanosheets. Fuel 2017, 203, 419–429. [Google Scholar] [CrossRef]
- Choi, H.J.; Moon, J.; Shim, H.B.; Han, K.S.; Lee, E.G.; Jung, K.D. Preparation of Nanocrystalline CeO2 by the Precipitation Method and Its Improved Methane Oxidation Activity. J. Am. Ceram. Soc. 2006, 89, 343–345. [Google Scholar] [CrossRef]
- Nabih, N.; Schiller, R.; Lieberwirth, I.; Kockrick, E.; Frind, R.; Kaskel, S.; Weiss, C.K.; Landfester, K. Mesoporous CeO2 nanoparticles synthesized by an inverse miniemulsion technique and their catalytic properties in methane oxidation. Nanotechnology 2011, 22, 135606. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Lu, G.; Guo, Y.; Wang, Y.; Guo, Y. Effect of water vapor on the CO and CH4 catalytic oxidation over CeO2-MOx (M = Cu, Mn, Fe, Co, and Ni) mixed oxide. J. Rare Earths 2010, 28, 742. [Google Scholar] [CrossRef]
- Shi, L.M.; Chu, W.; Qu, F.F.; Hu, J.Y.; Li, M.M. Catalytic performance for methane combustion of supported Mn-Ce mixed oxides. J. Rare Earths 2008, 26, 836. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Xue, B. Catalytic combustion of methane over M (Ni, Co, Cu) supported on ceria–magnesia. Fuel Process. Technol. 2009, 90, 652. [Google Scholar] [CrossRef]
- Liotta, L.F.; Carlo, G.D.; Pantaleo, G.; Deganello, G. Co3O4/CeO2 and Co3O4/CeO2–ZrO2 composite catalysts for methane combustion: Correlation between morphology reduction properties and catalytic activity. Catal. Commun. 2005, 6, 329. [Google Scholar] [CrossRef]
- Honkanen, M.; Kärkkäinen, M.; Kolli, T.; Heikkinen, O.; Viitanen, V.; Zeng, L.; Jiang, H.; Kallinen, K.; Huuhtanen, M.; Keiski, R.L.; et al. Accelerated deactivation studies of the natural-gas oxidation catalyst—Verifying the role of sulfur and elevated temperature in catalyst aging. Appl. Catal. B Environ. 2016, 182, 439–448. [Google Scholar] [CrossRef]
- Colussi, S.; Arosio, F.; Montanari, T.; Busca, G.; Groppi, G.; Trovarelli, A. Study of sulfur poisoning on Pd/Al2O3 and Pd/CeO2/Al2O3 methane combustion catalysts. Catal. Today 2010, 155, 59–65. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Melchionna, M.; Duchoň, T.; Kús, P.; Chen, C.; Tsud, N.; Nasi, L.; Prince, K.C.; Veltruská, K.; et al. The effect of sulfur dioxide on the activity of hierarchical Pd-based catalysts in methane combustion. Appl. Catal. B Environ. 2017, 202, 72–83. [Google Scholar] [CrossRef]
- Wilburn, M.S.; Epling, W.S. Sulfur deactivation and regeneration of mono- and bimetallic Pd-Pt methane oxidation catalysts. Appl. Catal. B Environ. 2017, 206, 589–598. [Google Scholar] [CrossRef]
- Friberg, I.; Sadokhina, N.; Olsson, L. Complete methane oxidation over Ba modified Pd/Al2O3: The effect of water vapor. Appl. Catal. B Environ. 2018, 231, 242–250. [Google Scholar] [CrossRef]
- Toso, A.; Colussi, S.; Padigapaty, S.; de Leitenburg, C.; Trovarelli, A. High stability and activity of solution combustion synthesized Pd-based catalysts for methane combustion in presence of water. Appl. Catal. B Environ. 2018, 230, 237–245. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Chesalov, Y.A.; Ishchenko, A.V.; Kaichev, V.V.; Ismagilov, Z.R. Effect of Pt addition on sulfur dioxide and water vapor tolerance of Pd-Mn-hexaaluminate catalysts for high-temperature oxidation of methane. Appl. Catal. B Environ. 2017, 204, 89–106. [Google Scholar] [CrossRef]
- Liu, F.; Sang, Y.; Ma, H.; Li, Z.; Gao, Z. Nickel oxide as an effective catalyst for catalytic combustion of methane. J. Nat. Gas Sci. Eng. 2017, 41, 1–6. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Iliopoulou, E.F.; Carabineiro, S.A.C.; Konsolakis, M. Impact of the synthesis parameters on the solid state properties and the CO oxidation performance of ceria nanoparticles. RSC Adv. 2017, 7, 6160–6169. [Google Scholar] [CrossRef] [Green Version]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.C.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Marnellos, G.E.; Asad, M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Effect of cobalt loading on the solid state properties and ethyl acetate oxidation performance of cobalt-cerium mixed oxides. J. Colloid Interf. Sci. 2017, 496, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, S.; Zhang, Y.; Wang, Z.; Feng, J.; Song, S.; Zhang, H. CeO2 nanowires self-inserted into porous Co3O4 frameworks as high-performance “noble metal free” hetero-catalysts. Chem. Sci. 2016, 7, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Zhang, C.; He, H.; Teraoka, Y. Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl. Catal. B Environ. 2007, 75, 167–174. [Google Scholar] [CrossRef]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl. Catal. B Environ. 2013, 142–143, 677–683. [Google Scholar] [CrossRef]
- Lou, Y.; Ma, J.; Cao, X.; Wang, L.; Dai, Q.; Zhao, Z. Promoting Effects of In2O3 on Co3O4 for CO Oxidation: Tuning O2 Activation and CO Adsorption Strength Simultaneously. ACS Catal. 2014, 4, 4143–4152. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Song, J.; Huang, S.; Yang, N.; Zhang, J. Exploring the Effect of Co3O4 Nanocatalysts with Different Dimensional Architectures on Methane Combustion. ChemCatChem 2016, 8, 540. [Google Scholar] [CrossRef]
- Hu, L.H.; Peng, Q.; Li, Y.D. Low-temperature CH4 Catalytic Combustion over Pd Catalyst Supported on Co3O4 Nanocrystals with Well-Defined Crystal Planes. ChemCatChem 2011, 3, 868–874. [Google Scholar] [CrossRef]
- Li, Y.; Shen, W. Morphology-dependent nanocatalysts: Rod-shaped oxides. Chem. Soc. Rev. 2014, 43, 1543–74. [Google Scholar] [CrossRef] [PubMed]
- Manto, M.J.; Xie, P.; Wang, C. Catalytic Dephosphorylation Using Ceria Nanocrystals. ACS Catal. 2017, 7, 1931–1938. [Google Scholar] [CrossRef]


| Catalyst | Co (wt%) | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | Mean Particle Size (nm) 1 | |
|---|---|---|---|---|---|---|
| CeO2 | Co3O4 | |||||
| CeO2-P | - | 49.5 | 0.06 | 5.2 | 18.6 | - |
| CeO2-H | - | 110.9 | 0.55 | 19.9 | 11.8 | - |
| 2Co/CeO2-P | 2.1 | 43.3 | 0.06 | 5.8 | 16.3 | - |
| 2Co/CeO2-H | 2.3 | 100.3 | 0.49 | 19.5 | 8.1 | - |
| 5Co/CeO2-P | 5.1 | 39.6 | 0.05 | 5.3 | 20.5 | - |
| 5Co/CeO2-H | 5.1 | 92.1 | 0.23 | 9.5 | 9.4 | 17.8 |
| 15Co/CeO2-P | 14.6 | 38.8 | 0.09 | 9.3 | 16.2 | 76.6 |
| 15Co/CeO2-H | 15.4 | 78.9 | 0.14 | 7.3 | 6.4 | 16.0 |
| Co3O4-P | - | 15.5 | 0.14 | 47.6 | - | 42.2 |
| Co3O4-H | - | 12.8 | 0.04 | 11.4 | - | 33.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darda, S.; Pachatouridou, E.; Lappas, A.; Iliopoulou, E. Effect of Preparation Method of Co-Ce Catalysts on CH4 Combustion. Catalysts 2019, 9, 219. https://doi.org/10.3390/catal9030219
Darda S, Pachatouridou E, Lappas A, Iliopoulou E. Effect of Preparation Method of Co-Ce Catalysts on CH4 Combustion. Catalysts. 2019; 9(3):219. https://doi.org/10.3390/catal9030219
Chicago/Turabian StyleDarda, Sofia, Eleni Pachatouridou, Angelos Lappas, and Eleni Iliopoulou. 2019. "Effect of Preparation Method of Co-Ce Catalysts on CH4 Combustion" Catalysts 9, no. 3: 219. https://doi.org/10.3390/catal9030219
APA StyleDarda, S., Pachatouridou, E., Lappas, A., & Iliopoulou, E. (2019). Effect of Preparation Method of Co-Ce Catalysts on CH4 Combustion. Catalysts, 9(3), 219. https://doi.org/10.3390/catal9030219







