New Oxidovanadium(IV) Complexes with 2,2′-bipyridine and 1,10-phenathroline Ligands: Synthesis, Structure and High Catalytic Activity in Oxidations of Alkanes and Alcohols with Peroxides
Abstract
:1. Introduction
2. Results and Discussion
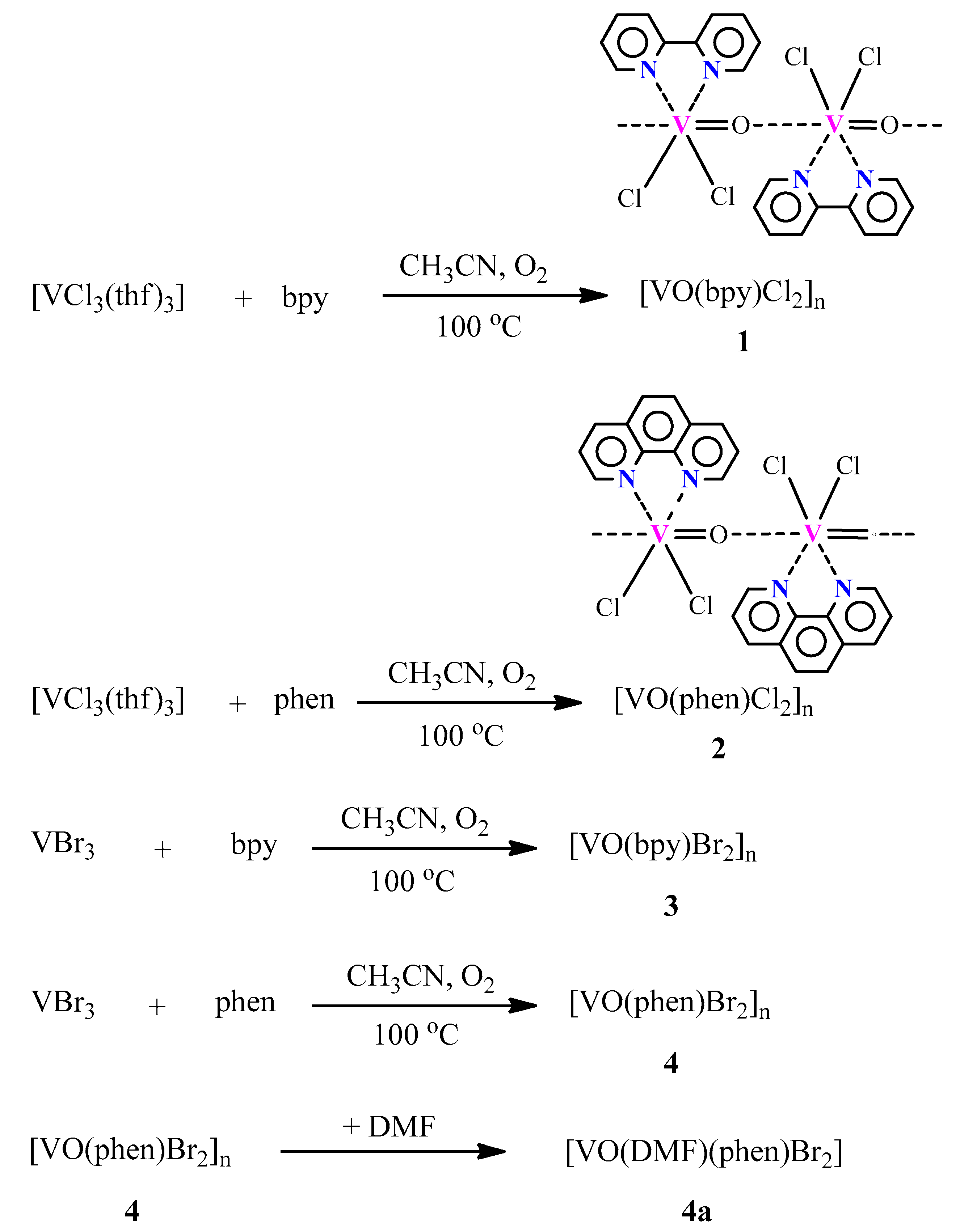
2.1. Synthesis and Characterization of Compounds 1–4a
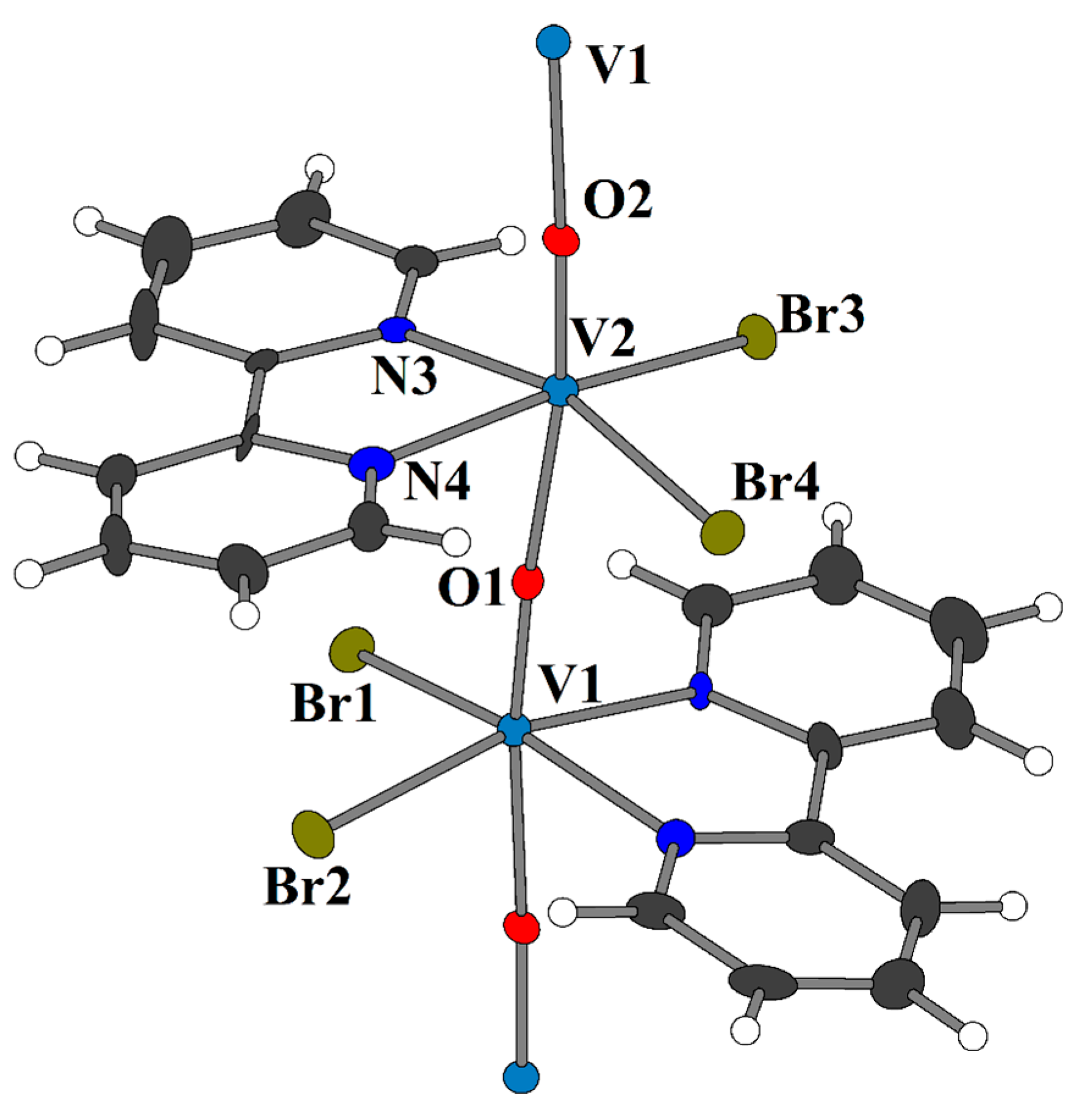
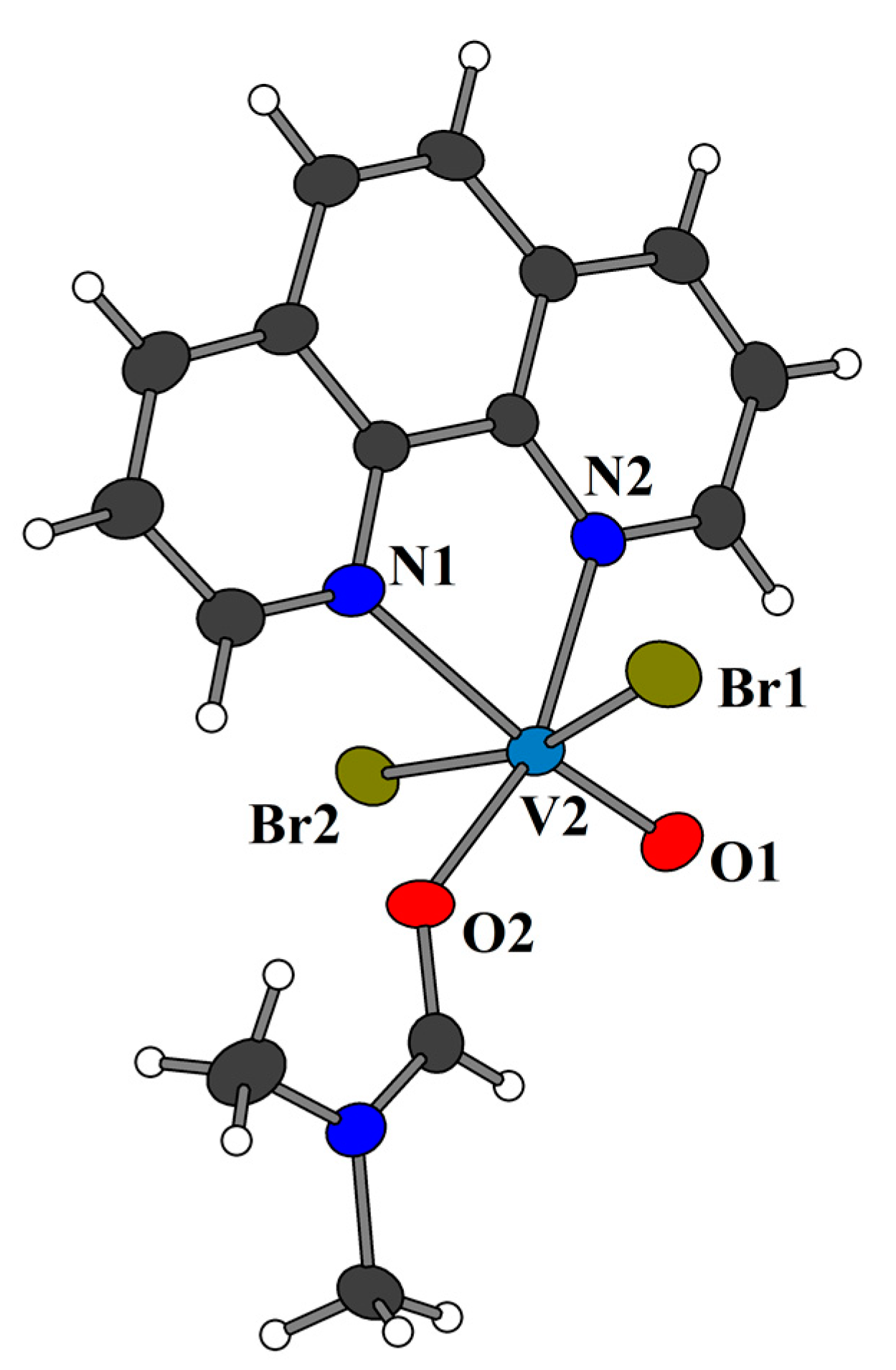
2.2. Crystal Structures of 3 and 4a
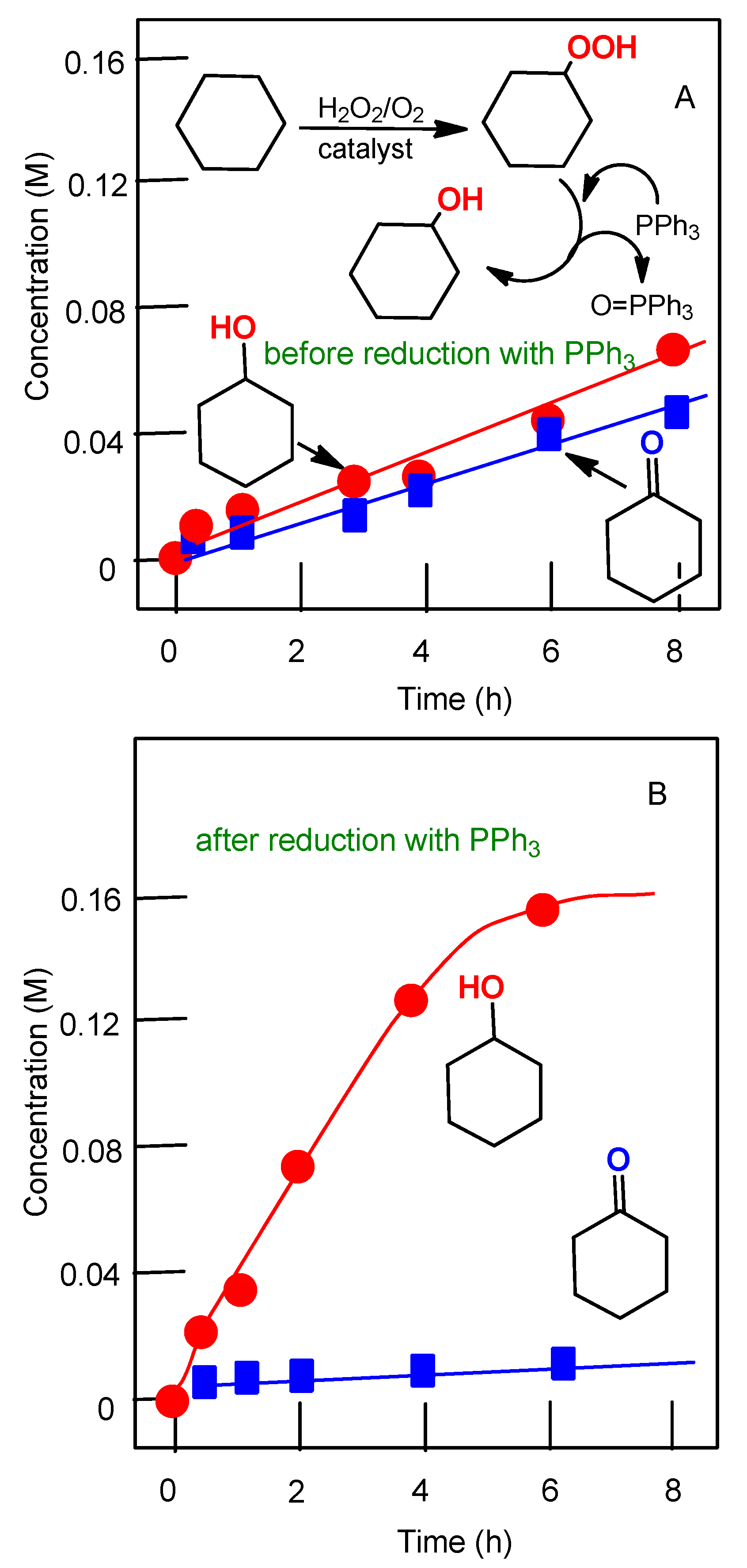
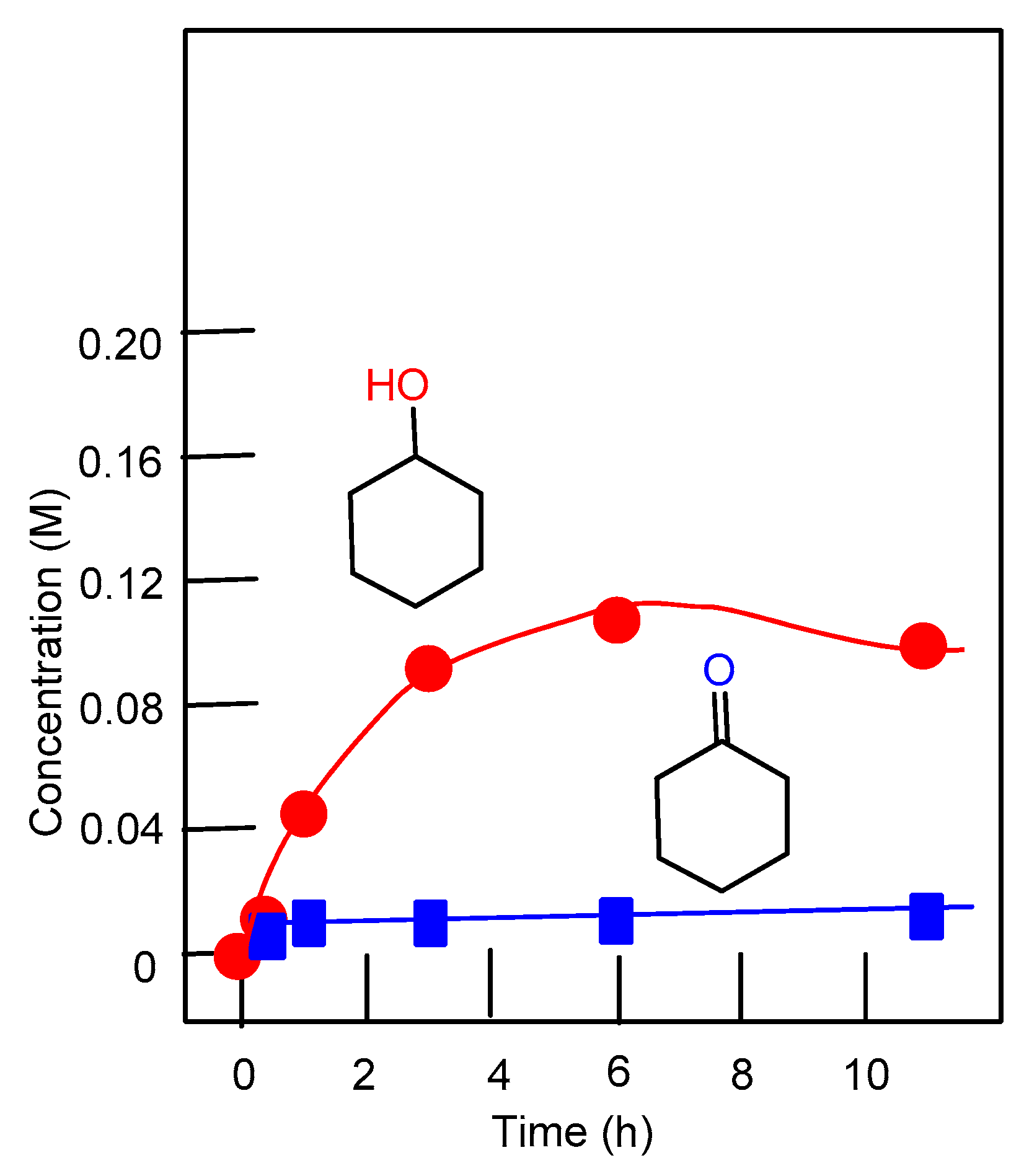
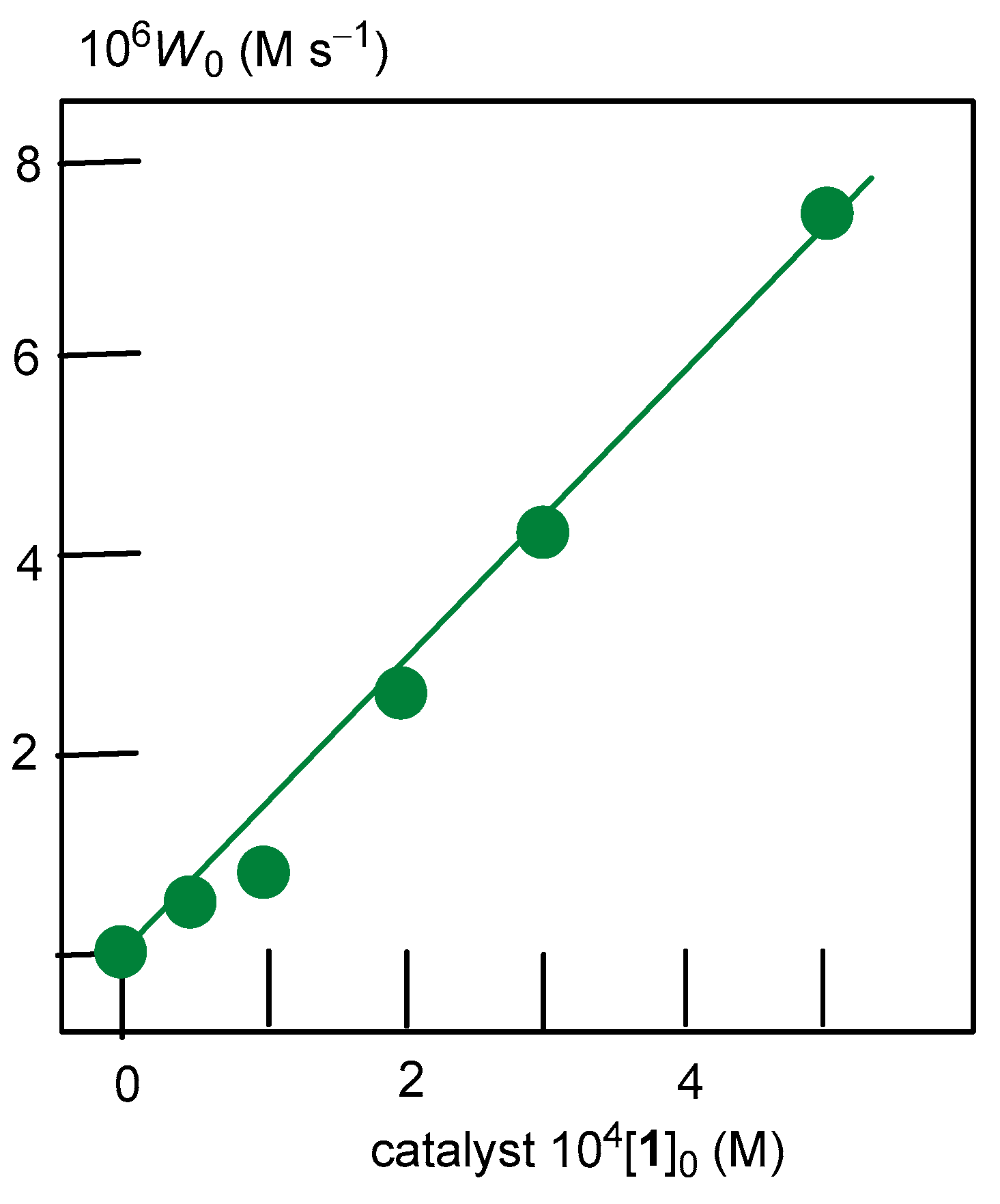
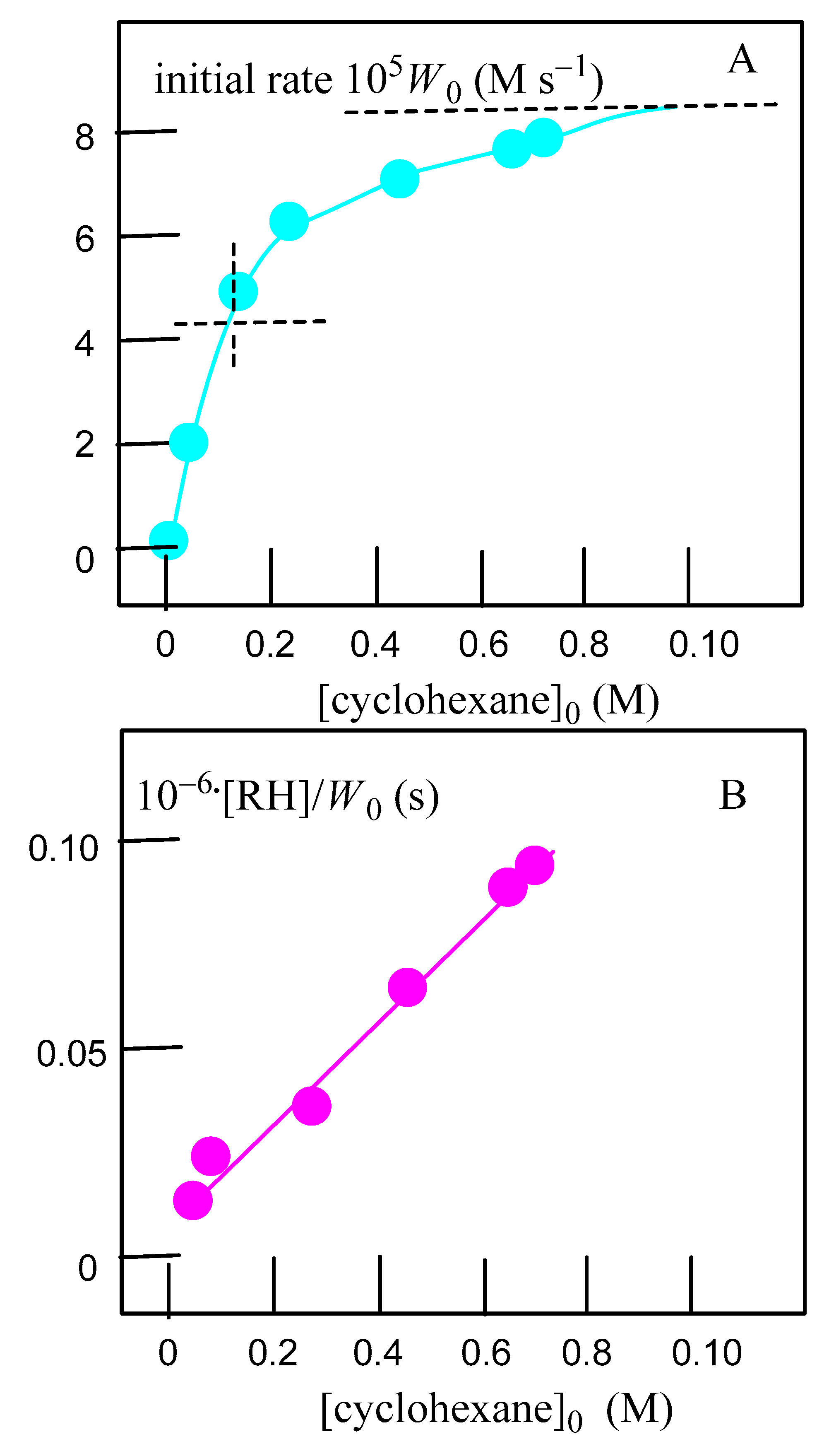
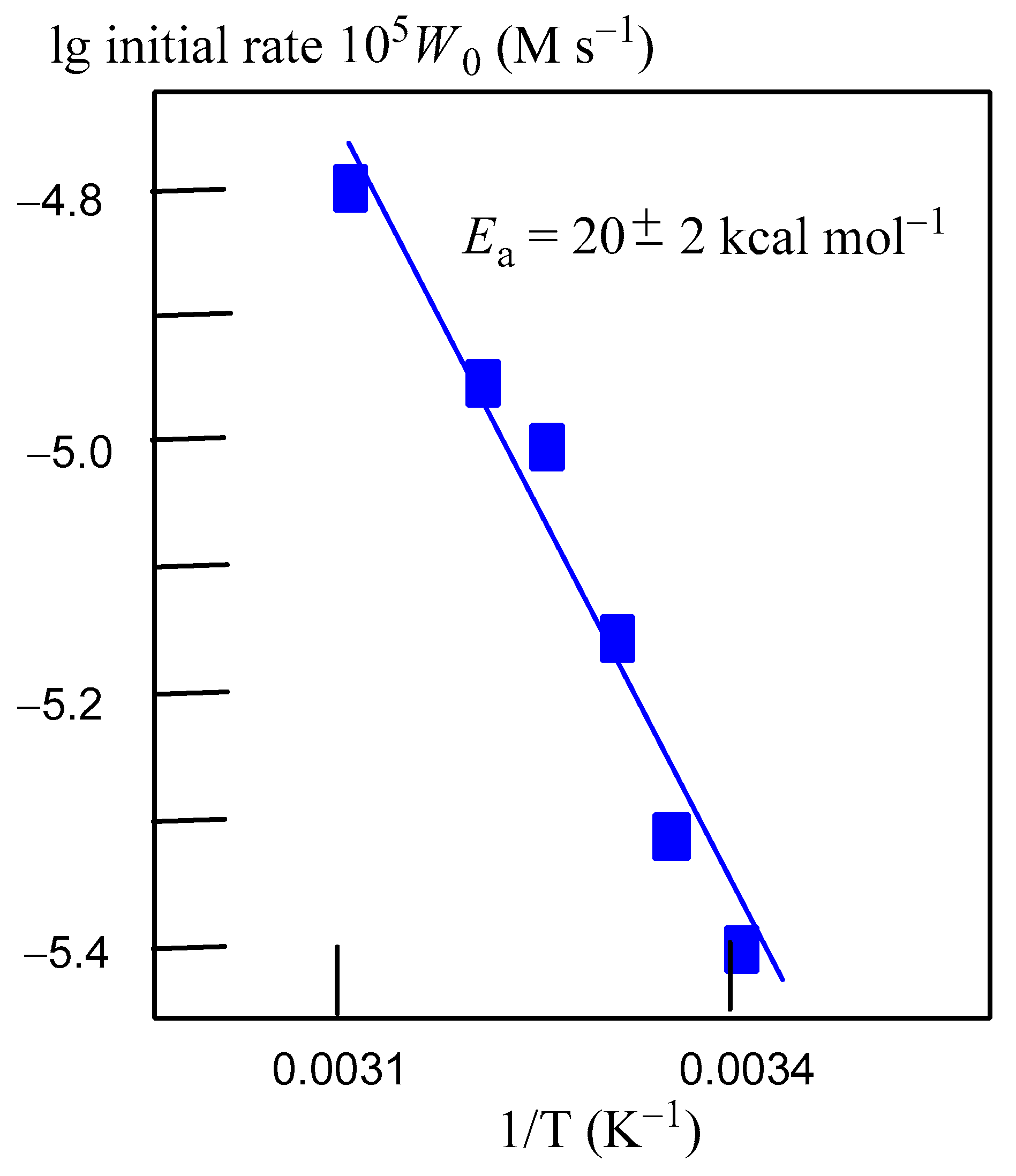
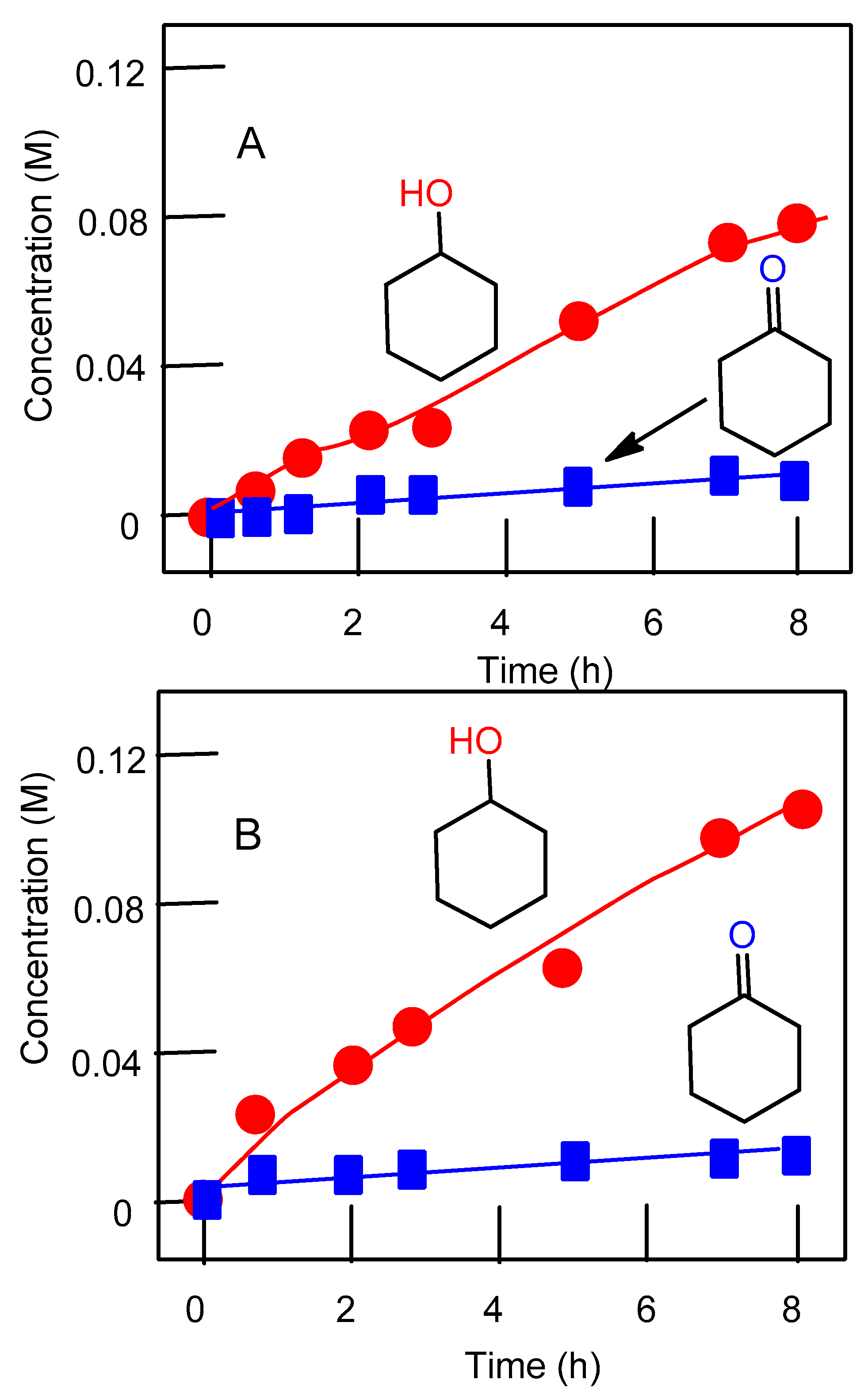
2.3. Oxidation of Alkanes with Peroxides Catalyzed by Complexes 1–4
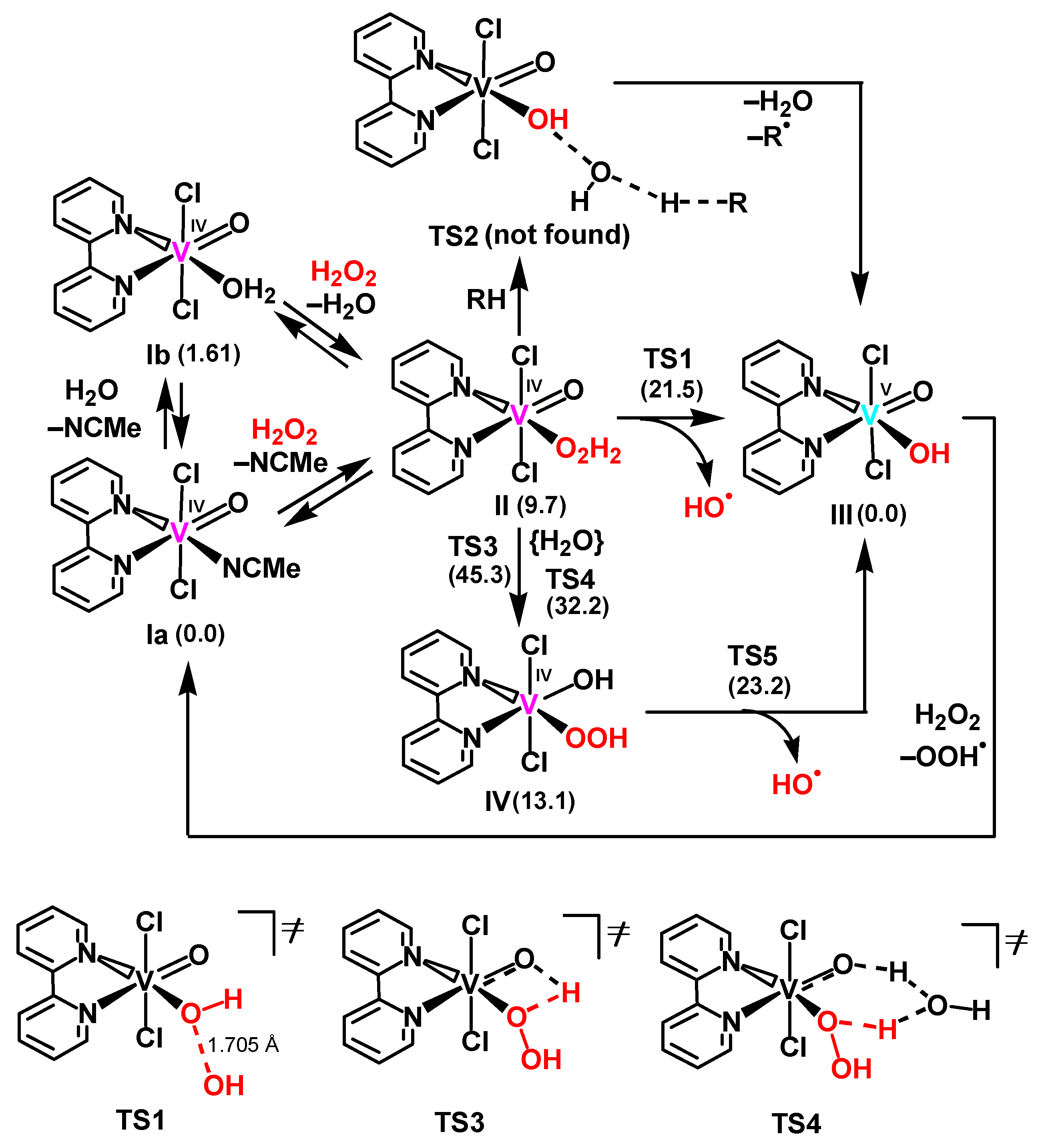
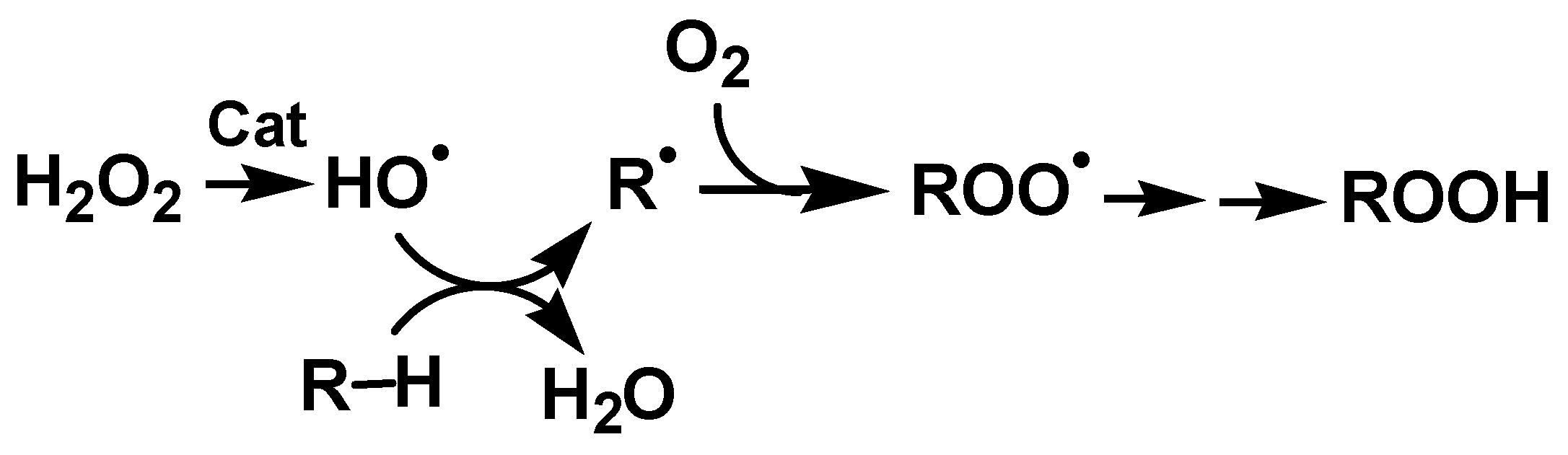
2.4. Theoretical Mechanistic Study
3. Experimental Section
3.1. Computational Details
Sg + [(–12.21 cal/mol•K) – 0.23(Sg – 12.21 cal/mol•K) + 5.87 cal/mol•K]
3.2. Materials
3.3. Physical Measurements
3.4. X-ray Crystallography
4. Conclusions
5. Syntheses of the Complexes
5.1. Synthesis of [VIVO(bpy)Cl2]n (1)
5.2. Synthesis of [VIVO(phen)Cl2]n (2)
5.3. Synthesis of [VIVO(bpy)Br2]n (3)
5.4. Synthesis of [VIVO(phen)Br2]n (4)
5.5. Oxidation of Alcohols and Hydrocarbons with Peroxides
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shilov, A.E.; Shul’pin, G.B. Activation of C–H Bonds by Metal Complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef] [PubMed]
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2002. [Google Scholar]
- Shul’pin, G.B. Oxidations of C–H Compounds Catalyzed by Metal Complexes. In Transition Metals for Organic Synthesis, 2nd ed.; Beller, M., Bolm, C., Eds.; Wiley-VCH: Weinheim, Germany; New York, NY, USA, 2004; Volume 2, pp. 215–242. [Google Scholar]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxidations. Mini-Rev. Org. Chem. 2009, 6, 95–104. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Selectivity enhancement in functionalization of C–H bonds: A review. Org. Biomol. Chem. 2010, 8, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Selectivity in C–H functionalizations. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Casella, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 6, Chapter 6.04; pp. 79–104. [Google Scholar]
- Wójtowicz-Młochowska, H. Synthetic utility of metal catalyzed hydrogen peroxide oxidation of C-H, C-C and C=C bonds in alkanes, arenes and alkenes: Recent advances. Arkivoc 2017, ii, 12–58. [Google Scholar]
- Shul’pin, G.B. Radical versus non-radical mechanisms. In Alkane Functionalization; Pombeiro, A.J.L., Ed.; Wiley, Inc.: Hoboken, NJ, USA, 2018; Chapter 3. [Google Scholar]
- Milan, M.; Salamone, M.; Costas, M.; Bietti, M. The Quest for Selectivity in Hydrogen Atom Transfer Based Aliphatic C–H Bond Oxygenation. Acc. Chem. Res. 2018, 51, 1984–1995. [Google Scholar] [CrossRef] [PubMed]
- Poon, J.-F.; Pratt, D.A. Recent Insights on Hydrogen Atom Transfer in the Inhibition of Hydrocarbon Autoxidation. Acc. Chem. Res. 2018, 51, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W. Manifestations of Noninnocent Ligand Behavior. Inorg. Chem. 2011, 50, 9752–9765. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W. The transition metal coordination chemistry of anion radicals. Coord. Chem. Rev. 1987, 76, 187–235. [Google Scholar] [CrossRef]
- Lyaskovskyy, V.; Bruin, B.D. Redox Non-Innocent Ligands: Versatile New Tools to Control Catalytic Reactions. ACS Catal. 2012, 2, 270–279. [Google Scholar] [CrossRef]
- Chirik, P.J.; Wieghardt, K. Radical Ligands Confer Nobility on Base-Metal Catalysts. Science 2010, 327, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Heyduk, A.F.; Zarkesh, R.A.; Nguyen, A.I. Designing catalysts for nitrene transfer using early transition metals and redox-active ligands. Inorg. Chem. 2011, 50, 9849–9863. [Google Scholar] [CrossRef] [PubMed]
- Praneeth, V.K.K.; Ringenberg, M.R.; Ward, T.R. Redox-Active Ligands in Catalysis. Angew. Chem. Int. Ed. 2012, 51, 10228–10234. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.D.; Brown, S.N. Nonclassical oxygen atom transfer as a synthetic strategy: Preparation of an oxorhenium(V) complex of the bis(3,5-di-tert-butyl-2-phenoxo)amide ligand. Inorg. Chem. 2013, 52, 7831–7833. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Vanadium complexes: Recent progress in oxidation catalysis. Coord. Chem. Rev. 2015, 301, 200–239. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Maurya, M.R. Vanadium complexes supported on organic polymers as sustainable systems for catalytic oxidations. Inorg. Chim. Acta 2017, 455, 415–428. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Organometallic Complexes as Catalysts in Oxidation of C–H Compounds. In Advances in Organometallic Chemistry and Catalysis; Pombeiro, A.J.L., Ed.; Wiley: Hoboken, NJ, USA, 2014; Chapter 1; pp. 3–14. [Google Scholar]
- Mandelli, D.; Shul’pina, L.S.; Kirillova, M.V.; Kirillov, A.M.; Carvalho, W.A.; Pombeiro, A.J.L.; Shul’pin, G.B. Oxidation of Glycerol with Hydrogen Peroxide catalyzed by Metal Complexes. In Advances in Organometallic Chemistry and Catalysis; Pombeiro, A.J.L., Ed.; Wiley: Hoboken, NJ, USA, 2014; Chapter 19; pp. 247–258. [Google Scholar]
- Shul’pin, G.B. Alkane-oxidizing systems based on metal complexes. Radical versus non-radical mechanisms. In Alkane Functionalization; Pombeiro, A.J.L., Ed.; Wiley: Hoboken, NJ, USA, 2018; Chapter 2. [Google Scholar]
- Butler, A. Mechanistic considerations of the vanadium haloperoxidases. Coord. Chem. Rev. 1999, 187, 17–35. [Google Scholar] [CrossRef]
- Wischang, D.; Brücher, O.; Hartung, J. Bromoperoxidases and functional enzyme mimics as catalysts for oxidative bromination—A sustainable synthetic approach. Coord. Chem. Rev. 2011, 255, 2204–2217. [Google Scholar]
- Fomenko, Y.S.; Gushchin, A.L.; Tkachev, A.V.; Vasilyev, E.S.; Abramov, P.A.; Nadolinny, V.A.; Syrokvashin, M.M.; Sokolov, M.N. First oxidovanadium complexes containing chiral derivatives of dihydrophenanthroline and diazafluorene. Polyhedron 2017, 135, 96–100. [Google Scholar] [CrossRef]
- King, A.E.; Nippe, M.; Atanasov, M.; Chantarojsiri, T.; Wray, C.A.; Bill, E.; Neese, F.; Long, J.R.; Chang, C.J. A Well-Defined Terminal Vanadium(III) Oxo Complex. Inorg. Chem. 2014, 53, 11388–11395. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.I.; Borer, L.L.; Olmstead, M.M. Vanadium(IV) and vanadium(V) complexes of salicyladimine ligands. Inorg. Chem. 2003, 42, 7410–7415. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.K.; Baker, R.T.; Gordon, J.C.; Scott, B.L.; Silks, L.A.P.; Thorn, D.L. Mechanism of Alcohol Oxidation by Dipicolinate Vanadium(V): Unexpected Role of Pyridine. J. Am. Chem. Soc. 2010, 132, 17804–17816. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.K.; Wu, R.; Silks, L.A.P. Mild and Selective Vanadium-Catalyzed Oxidation of Benzylic, Allylic, and Propargylic Alcohols Using Air. Org. Lett. 2011, 13, 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.K.; Wu, R.; Silks, L.A.P. C-C or C-O Bond Cleavage in a Phenolic Lignin Model xtc60 Compound: Selectivity Depends on Vanadium Catalyst. Angew. Chem. Int. Ed. 2012, 124, 3466–3469. [Google Scholar] [CrossRef]
- Zhang, G.; Scott, B.L.; Wu, R.; Silks, L.A.P.; Hanson, S.K. Aerobic Oxidation Reactions Catalyzed by Vanadium Complexes of Bis(Phenolate) Ligands. Inorg. Chem. 2012, 51, 7354–7361. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.K.; Baker, R.T.; Gordon, J.C.; Scott, B.L.; Thorn, D.L. Aerobic oxidation of lignin models using a base metal vanadium catalyst. Inorg. Chem. 2010, 49, 5611–5618. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, E.; Oyaizu, K. Oxovanadium (III–V) mononuclear complexes and their linear assemblies bearing tetradentate Schiff base ligands: Structure and reactivity as multielectron redox catalysts. Coord. Chem. Rev. 2003, 237, 213–228. [Google Scholar] [CrossRef]
- Chang, C.J.; Labinger, J.A.; Gray, H.B. Aerobic epoxidation of olefins catalyzed by electronegative vanadyl salen complexes. Inorg. Chem. 1997, 36, 5927–5930. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.E. Reinvestigation of the vanadium-oxygen stretch in the IR spectrum of bis [N-(4-chlorophenyl)salicylideneiminato] oxovanadium (IV). Inorg. Chem. 1991, 30, 1670–1671. [Google Scholar] [CrossRef]
- Matsuoka, N.; Kawamura, H.; Yoshioka, N. Magnetic property and crystal structure of bis [N-(4-chlorophenyl)salicylideneaminato] oxovanadium (IV). Chem. Phys. Lett. 2010, 488, 32–37. [Google Scholar] [CrossRef]
- Nakajima, K.; Kojima, M.; Azuma, S.; Kasahara, R.; Tsuchimoto, M.; Kubozono, Y.; Maeda, H.; Kashino, S.; Ohba, S.; Yoshikawa, Y.; et al. Interconversion between polymeric orange and monomeric green forms of a Schiff base-oxovanadium (IV) complex. Bull. Chem. Soc. Jpn. 1996, 69, 3207–3216. [Google Scholar] [CrossRef]
- Fairhurst, S.A.; Hughes, D.L.; Kleinkes, U.; Leigh, J.G.; Sanders, J.R.; Weisner, J. Non-planar co-ordination of the Schiff-base dianion N, N′-2, 2-dimethyltrimethylenebis [salicylideneiminate(2–)] to vanadium. J. Chem. Soc. Dalton Trans. 1995, 3, 321–326. [Google Scholar] [CrossRef]
- Tsuchimoto, M.; Hoshina, G.; Yoshioka, N.; Inoue, H.; Nakajima, K.; Kamishima, M.; Kojima, M.; Ohba, S. Mechanochemical reaction of polymeric oxovanadium (IV) complexes with Schiff base ligands derived from 5-nitrosalicylaldehyde and diamines. J. Solid State Chem. 2000, 153, 9–15. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Attanasio, D.; Suber, L. Efficient H2O2 oxidation of alkanes and arenes to alkyl peroxides and phenols catalyzed by the system vanadate–pyrazine-2-carboxylic acid. J. Catal. 1993, 142, 147–152. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Attanasio, D.; Suber, L. Oxidations by a H2O2–VO3––pyrazine-2-carboxylic acid reagent. 1. Oxidations of alkanes in CH3CN to produce alkyl peroxides. Russ. Chem. Bull. 1993, 42, 55–59. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Druzhinina, A.N.; Nizova, G.V. Oxidation with the H2O2–VO3––pyrazine-2-carboxylic acid reagent. 2. Oxidation of alcohols and aromatic hydrocarbons. Russ. Chem. Bull. 1993, 42, 1327–1329. [Google Scholar]
- Nizova, G.V.; Shul’pin, G.B. Oxidation by a H2O2–vanadium complex–2-pyrazinecarboxylic acid reagent. 3. Evidence for hydroxyl radical formation. Russ. Chem. Bull. 1994, 43, 1146–1148. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Süss-Fink, G. Oxidations by the reagent “H2O2–vanadium complex–pyrazine-2-carboxylic acid.” Part 4. Oxidation of alkanes, benzene and alcohols by an adduct of H2O2 with urea. J. Chem. Soc. Perkin Trans. 1995, 2, 1459–1463. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Drago, R.S.; Gonzalez, M. Oxidations by a “H2O2–vanadium complex–pyrazine-2-carboxylic acid” reagent. 5. Oxidation of lower alkanes with the formation of carbonyl compounds. Russ. Chem. Bull. 1996, 45, 2386–2388. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Guerreiro, M.C.; Schuchardt, U. Oxidations by the reagent O2–H2O2–vanadium complex–pyrazine-2-carboxylic acid. Part 7. Hydroperoxidation of higher alkanes. Tetrahedron 1996, 52, 13051–13062. [Google Scholar] [CrossRef]
- Guerreiro, M.C.; Schuchardt, U.; Shul’pin, G.B. Oxidation with the “O2–VO3––pyrazine-2-carboxylic acid” reagent. Part 6. Oxidation of n-heptane and cyclohexane. Direct determination of alkyl hydroperoxides by gas-liquid chromatography. Russ. Chem. Bull. 1997, 46, 749–754. [Google Scholar] [CrossRef]
- Nizova, G.V.; Süss-Fink, G.; Shul’pin, G.B. Oxidations by the reagent «O2–H2O2–vanadium complex–pyrazine-2-carboxylic acid»—8. Efficient oxygenation of methane and other lower alkanes in acetonitrile. Tetrahedron 1997, 53, 3603–3614. [Google Scholar] [CrossRef]
- Schuchardt, U.; Guerreiro, M.C.; Shul’pin, G.B. Oxidation with the ‘O2–H2O2–vanadium complex–pyrazine-2-carboxylic acid’ reagent. 9. Oxidation of cyclohexene and decalin. Russ. Chem. Bull. 1998, 47, 247–252. [Google Scholar] [CrossRef]
- Süss-Fink, G.; Nizova, G.V.; Stanislas, S.; Shul’pin, G.B. Oxidations by the reagent ‘O2–H2O2–vanadate anion–pyrazine-2-carboxylic acid’. Part 10. Oxygenation of methane in acetonitrile and water. J. Mol. Catal. A Chem. 1998, 130, 163–170. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Ishii, Y.; Sakaguchi, S.; Iwahama, T. Oxidations with the “O2–H2O2–vanadium complex–pyrazine-2-carboxylic acid” reagent. 11. Oxidation of styrene, phenylacetylene, and their derivatives with the formation of benzaldehyde and benzoic acid. Russ. Chem. Bull. 1999, 48, 887–890. [Google Scholar] [CrossRef]
- Süss-Fink, G.; Stanislas, S.; Shul’pin, G.B.; Nizova, G.V.; Stoeckli-Evans, H.; Neels, A.; Bobillier, C.; Claude, S. Oxidative functionalisation of alkanes: Synthesis, molecular structure and catalytic implications of anionic vanadium(V) oxo and peroxo complexes containing bidentate N,O ligands. J. Chem. Soc. Dalton Trans. 1999, 18, 3169–3175. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S.; Kitaygorodskiy, A.; Kulikova, V.S. Oxidations by the reagent "O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid" Part 12. Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin Trans. 2 2001, 8, 1351–1371. [Google Scholar] [CrossRef]
- De la Cruz, M.H.C.; Kozlov, Y.N.; Lachter, E.R.; Shul’pin, G.B. Oxidations by the reagent “O2–H2O2–vanadium derivative—Pyrazine-2-carboxylic acid”. Part 13. Kinetics and mechanism of the benzene hydroxylation. New J. Chem. 2003, 27, 634–638. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N. Kinetics and mechanism of alkane hydroperoxidation with tert-butyl hydroperoxide catalysed by a vanadate ion. Org. Biomol. Chem. 2003, 1, 2303–2306. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, L.G.; Kozlov, Y.N.; Süss-Fink, G.; Shul’pin, G.B. Oxidation of saturated hydrocarbons with peroxyacetic acid catalyzed by vanadium complexes. J. Mol. Catal. A Chem. 2004, 218, 171–177. [Google Scholar] [CrossRef]
- Kozlov, Y.N.; Nizova, G.V.; Shul’pin, G.B. Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 14. Competitive oxidation of alkanes and acetonitrile (solvent). J. Mol. Catal. A Chem 2005, 227, 247–253. [Google Scholar] [CrossRef]
- Jannini, M.J.D.M.; Shul’pina, L.S.; Schuchardt, U.; Shul’pin, G.B. Oxidation of alkanes with hydrogen peroxide catalyzed by the “vanadate-ion–pyrazine-2-carboxylic acid” system in the presence of pyridine (Part 15 of the series Oxidations by the reagent O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid). Petrol. Chem. 2005, 45, 413–418. [Google Scholar]
- Kozlov, Y.N.; Romakh, V.B.; Kitaygorodskiy, A.; Buglyó, P.; Süss-Fink, G.; Shul’pin, G.B. Oxidation of 2-Propanol and Cyclohexane by the Reagent “Hydrogen Peroxide-Vanadate Anion-Pyrazine-2-carboxylic Acid”: Kinetics and Mechanism. J. Phys. Chem. A 2007, 111, 7736–7752. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, M.V.; Kuznetsov, M.L.; Romakh, V.B.; Shul’pina, L.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L.; Shul’pin, G.B. Mechanism of oxidations with H2O2 catalyzed by vanadate anion or oxovanadium(V) triethanolaminate (vanadatrane) in combination with pyrazine-2-carboxylic acid (PCA): Kinetic and DFT studies. J. Catal. 2009, 267, 140–157. [Google Scholar] [CrossRef]
- Gusevskaya, E.V.; Menini, L.; Parreira, L.A.; Mesquita, R.A.; Kozlov, Y.N.; Shul’pin, G.B. Oxidation of isoeugenol to vanillin by the “H2O2–vanadate–pyrazine-2-carboxylic acid” reagent” <Part 17 of the series “Oxidations by the reagent ‘H2O2–vanadium derivative–pyrazine-2-carboxylic acid”>. J. Mol. Catal. A Chem. 2012, 363–364, 140–147. [Google Scholar] [CrossRef]
- Sutradhar, M.; Shvydkiy, N.V.; da Silva, M.F.C.G.; Kirillova, M.V.; Kozlov, Y.N.; Pombeiro, A.J.L.; Shul’pin, G.B. New binuclear oxovanadium(V) complex as a catalyst in combination with pyrazinecarboxylic acid (PCA) for efficient alkane oxygenation by H2O2. Dalton Trans. 2013, 42, 11791–11803. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, I.S.; Gushchin, A.L.; Shul’pina, L.S.; Ikonnikov, N.S.; Abramov, P.A.; Romashev, N.F.; Poryvaev, A.S.; Sheveleva, A.M.; Bogomyakov, A.S.; Shmelev, N.Y.; et al. New oxidovanadium (IV) complex with a BIAN ligand: Synthesis, structure, redox properties and catalytic activity. New J. Chem. 2018, 42, 16200–16210. [Google Scholar] [CrossRef]
- Triantafillou, G.D.; Tolis, E.I.; Terzis, A.; Deligiannakis, Y.; Raptopoulou, C.P.; Sigalas, M.P.; Kabanos, T.A. Monomeric Oxovanadium (IV) Compounds of the General Formula cis-[VIV(=O)(X)(LNN)2]+/0 {X= OH-, Cl-, SO42- and LNN= 2, 2’-Bipyridine (Bipy) or 4, 4 ‘-Disubstituted Bipy}. Inorg. Chem. 2004, 43, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Menati, S.; Rudbari, H.A.; Khorshidifard, M.; Jalilian, F. A new oxovanadium (IV) complex containing an O, N-bidentate Schiff base ligand: Synthesis at ambient temperature, characterization, crystal structure and catalytic performance in selective oxidation of sulfides to sulfones using H2O2 under solvent-free conditions. J. Mol. Struct. 2016, 1103, 94–102. [Google Scholar]
- Das, U.; Pattanayak, P.; Santra, M.K.; Chattopadhyay, S. Synthesis of new oxido-vanadium complexes: Catalytic properties and cytotoxicity. J. Chem. Res. 2018, 42, 57–62. [Google Scholar] [CrossRef]
- Kasahara, R.; Tsuchimoto, M.; Ohba, S.; Nakajima, K.; Ishida, H.; Kojima, M. Interconversion between polymeric and monomeric forms of oxovanadium (IV) complexes with tetradentate Schiff base ligands derived from (R, R)-2, 4-pentanediamine. Inorg. Chem. 1996, 35, 7661–7665. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Druzhinina, A.N. Hydroperoxidation of alkanes by atmospheric oxygen in the presence of hydroquinone or quinone catalyzed by copper(II) acetate under visible light irradiation. React. Kinet. Catal. Lett. 1992, 47, 207–211. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V. Formation of alkyl peroxides in oxidation of alkanes by H2O2 catalyzed by transition metal complexes. React. Kinet. Catal. Lett. 1992, 48, 333–338. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review . J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxidations. C. R. Chim. 2003, 6, 163–178. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Kudinov, A.R.; Mandelli, D. Extremely Efficient Alkane Oxidation by a New Catalytic Reagent H2O2/Os3(CO)12/Pyridine. Inorg. Chem. 2009, 48, 10480–10482. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Petrovskiy, P.V. Oxidation of alkanes and alcohols with hydrogen peroxide catalyzed by complex Os3(CO)10(-H)2. Appl. Organometal. Chem. 2010, 24, 464–472. [Google Scholar] [CrossRef]
- Knops-Gerrits, P.P.; Trujillo, C.A.; Zhan, B.Z.; Li, X.Y.; Rouxhet, P.; Jacobs, P.A. Oxidation catalysis with well-characterised vanadyl bis-bipyridine complexes encapsulated in NaY zeolite. Top. Catal. 1996, 3, 437–449. [Google Scholar] [CrossRef]
- Fornal, E.; Giannotti, C. Photocatalyzed oxidation of cyclohexane with heterogenized decatungstate. Photochem. Photobiol. A Chem. 2007, 188, 279–286. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Hydrocarbon Oxygenations with Peroxides Catalyzed by Metal Compounds. Mini-Rev. Org. Chem. 2009, 6, 95–104. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalyzed oxidation of C-H compounds with peroxides in unconventional solvents. Frontiers of Green Catalytic Selective Oxidations. Nat. Chem. 2019. Chapter 1. [Google Scholar]
- Shul’pin, G.B. C–H Functionalization: Thoroughly tuning ligands at a metal ion, a chemist can greatly enhance catalyst’s activity and selectivity. Dalton Trans. 2013, 42, 12794–12818. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. New Trends in Oxidative Functionalization of Carbon–Hydrogen Bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Olivo, G.; Lanzalunga, O.; Di Stefano, S. Non-Heme Imine-Based Iron Complexes as Catalysts for Oxidative Processes (Review). Adv. Synth. Catal. 2016, 358, 843–863. [Google Scholar] [CrossRef]
- Garcia-Bosch, I.; Siegel, M.A. Copper-Catalyzed Oxidation of Alkanes with H2O2 under a Fenton-like Regime. Angew. Chem. Int. Ed. 2016, 55, 12873–12876. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, A.L.; Kardasheva, Y.S.; Predeina, V.V.; Kluev, M.V.; Ramazanov, D.N.; Talanova, M.Y.; Karakhanov, E.A. Iron and copper complexes with nitrogen-containing ligands as catalysts for cyclohexane oxidation with hydrogen peroxide under mild reaction conditions. Petroleum Chem. 2012, 52, 318–326. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Chygorin, E.N.; Kokozay, V.N.; Bon, V.V.; Boča, R.; Kozlov, Y.N.; Shul’pina, L.S.; Jezierska, J.; Ozarowski, A.; Pombeiro, A.J.L.; et al. Heterometallic CoIII4FeIII2 Schiff Base Complex: Structure, Electron Paramagnetic Resonance, and Alkane Oxidation Catalytic Activity. Inorg. Chem. 2012, 51, 9110–9122. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Nesterov, D.S.; Shul’pina, L.S.; Pombeiro, A.J.L. A hydroperoxo-rebound mechanism of alkane oxidation with hydrogen peroxide catalyzed by binuclear manganese(IV) complex in the presence of an acid with involvement of atmospheric dioxygen” <Part 14 from the series “Oxidations by the system ‘hydrogen peroxide–[Mn2L2O3]2+ (L=1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid”>. Inorg. Chim. Acta 2017, 455, 666–676. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys. 1987, 86, 866–872. [Google Scholar] [CrossRef]
- Gryca, I.; CzerwiĔska, K.; Machura, B.; Chrobok, A.; Shul’pina, L.S.; Kuznetsov, M.L.; Nesterov, D.S.; Kozlov, Y.N.; Pombeiro, A.J.L.; Varyan, I.A.; et al. High Catalytic Activity of Vanadium Complexes in Alkane Oxidations with Hydrogen Peroxide: An Effect of 8-Hydroxyquinoline Derivatives as Noninnocent Ligands. Inorg. Chem. 2018, 57, 1824–1839. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher-order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Tomasi, J.; Persico, M. Molecular interactions in solution: An overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Wertz, G.H. Relationship between the gas-phase entropies of molecules and their entropies of solvation in water and 1-octanol. J. Am. Chem. Soc. 1980, 102, 5316–5322. [Google Scholar] [CrossRef]
- Cooper, J.; Ziegler, T. A density functional study of SN2 substitution at square-planar platinum(II) complexes. Inorg. Chem. 2002, 41, 6614–6622. [Google Scholar] [CrossRef] [PubMed]
- Brauer, G. Handbook of Preparative Inorganic Chemistry; Academic Press: New York, NY, USA, 1985; Volume 5. [Google Scholar]
- Sheldrick, G.M. SHELXT Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXT. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: A sample of the catalyst is not available from the authors. |


| 3 | 4a | |
| Crystal Data | ||
| Chemical formula | C10H8Br2N2OV | C15H15Br2N3O2V |
| Mr | 382.94 | 480.06 |
| Crystal system, space group | Monoclinic, P21 | Monoclinic, P21/n |
| Temperature (K) | 130 | 150 |
| a, b, c (Å) | 7.4626 (3), 17.5643 (7), 9.3510 (4) | 7.1041 (6), 14.0723 (9), 17.2455 (12) |
| β (°) | 92.037 (4) | 90.614 (3) |
| V (Å3) | 1224.91 (9) | 1724.0 (2) |
| µ(mm−1) | 7.31 | 5.22 |
| Crystal size (mm) | 0.25 × 0.06 × 0.06 | 0.10 × 0.06 × 0.05 |
| Data collection | ||
| Diffractometer | New Xcalibur, AtlasS2 | Bruker Apex Duo |
| Absorption correction | Multi-scan CrysAlis PRO 1.171.38.41 (Rigaku Oxford Diffraction, 2015) Empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. | Multi-scan SADABS (Bruker-AXS, 2004) |
| Tmin, Tmax | 0.739, 1000 | 0.545, 0.642 |
| Compound 3 | |||
| V1—O1 | 1.617 (5) | V2—Br3 | 2.4932 (16) |
| V1—O2 | 2.123 (5) | V2—Br4 | 2.4919 (16) |
| V1—Br1 | 2.4944 (16) | N1—V1 | 2.124 (7) |
| V1—Br2 | 2.5028 (16) | N2—V1 | 2.129 (8) |
| V2—O1 i | 2.125 (5) | N3—V2 | 2.124 (8) |
| V2—O2 | 1.617 (5) | N4—V2 | 2.136 (8) |
| V1—O1—V2 ii | 176.2 (4) | C10—N2—V1 | 125.3 (7) |
| V2—O2—V1 | 177.8 (4) | C11—N3—V2 | 125.2 (7) |
| C1—N1—V1 | 125.2 (7) | C15—N3—V2 | 115.4 (6) |
| C5—N1—V1 | 116.8 (6) | C16—N4—V2 | 115.5 (6) |
| C6—N2—V1 | 116.7 (6) | C20—N4—V2 | 124.5 (7) |
| Compound 4a | |||
| O1—V2 | 1.600 (3) | N2—V2 | 2.121 (3) |
| O2—V2 | 1.996 (3) | Br1—V2 | 2.5450 (8) |
| N1—V2 | 2.296 (3) | Br2—V2 | 2.5484 (7) |
| C14—O2—V2 | 129.3 (3) | C3—N2—V2 | 117.8 (3) |
| C1—N1—V2 | 129.6 (2) | C4—N2—V2 | 123.2 (3) |
| C2—N1—V2 | 112.6 (3) | ||
| n-Heptane | MeCH | 1,2-cis-DMCH | ||
|---|---|---|---|---|
| Entry | Catalyst | C(1):C(2):C(3):C(4) | 1°:2°:3° | trans/cis |
| 1 | 1 | 1.0:5.6:5.8:5.2 | 1.0:5.9:16.0 | 0.84 |
| 2 | 2 | 1.0:5.6:5.6:5.2 | 1.0:5.3:17.5 | 0.7 |
| 3 | 3 | 1.0:5.7:6.2:5.6 | 1.0:5.0:12.6 | 0.8 |
| 4 | 4 | 1.0:5.9:6.2:5.9 | 1.0:5.4:12.9 | 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fomenko, I.S.; Gushchin, A.L.; Abramov, P.A.; Sokolov, M.N.; Shul'pina, L.S.; Ikonnikov, N.S.; Kuznetsov, M.L.; Pombeiro, A.J.L.; Kozlov, Y.N.; Shul’pin, G.B. New Oxidovanadium(IV) Complexes with 2,2′-bipyridine and 1,10-phenathroline Ligands: Synthesis, Structure and High Catalytic Activity in Oxidations of Alkanes and Alcohols with Peroxides. Catalysts 2019, 9, 217. https://doi.org/10.3390/catal9030217
Fomenko IS, Gushchin AL, Abramov PA, Sokolov MN, Shul'pina LS, Ikonnikov NS, Kuznetsov ML, Pombeiro AJL, Kozlov YN, Shul’pin GB. New Oxidovanadium(IV) Complexes with 2,2′-bipyridine and 1,10-phenathroline Ligands: Synthesis, Structure and High Catalytic Activity in Oxidations of Alkanes and Alcohols with Peroxides. Catalysts. 2019; 9(3):217. https://doi.org/10.3390/catal9030217
Chicago/Turabian StyleFomenko, Iakov S., Artem L. Gushchin, Pavel A. Abramov, Maksim N. Sokolov, Lidia S. Shul'pina, Nikolay S. Ikonnikov, Maxim L. Kuznetsov, Armando J. L. Pombeiro, Yuriy N. Kozlov, and Georgiy B. Shul’pin. 2019. "New Oxidovanadium(IV) Complexes with 2,2′-bipyridine and 1,10-phenathroline Ligands: Synthesis, Structure and High Catalytic Activity in Oxidations of Alkanes and Alcohols with Peroxides" Catalysts 9, no. 3: 217. https://doi.org/10.3390/catal9030217
APA StyleFomenko, I. S., Gushchin, A. L., Abramov, P. A., Sokolov, M. N., Shul'pina, L. S., Ikonnikov, N. S., Kuznetsov, M. L., Pombeiro, A. J. L., Kozlov, Y. N., & Shul’pin, G. B. (2019). New Oxidovanadium(IV) Complexes with 2,2′-bipyridine and 1,10-phenathroline Ligands: Synthesis, Structure and High Catalytic Activity in Oxidations of Alkanes and Alcohols with Peroxides. Catalysts, 9(3), 217. https://doi.org/10.3390/catal9030217











