Silver-Mediated Methoxylation of Aryl C(sp2)–H Bonds Directing by DMEDA
Abstract
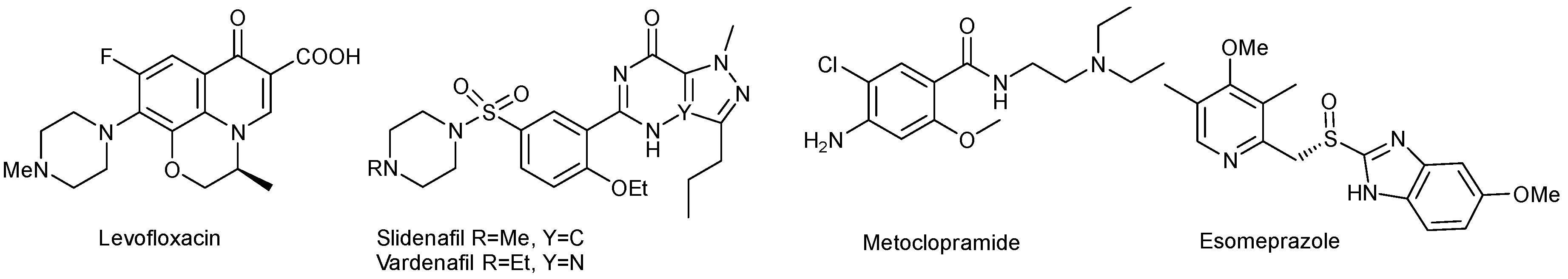
:1. Introduction
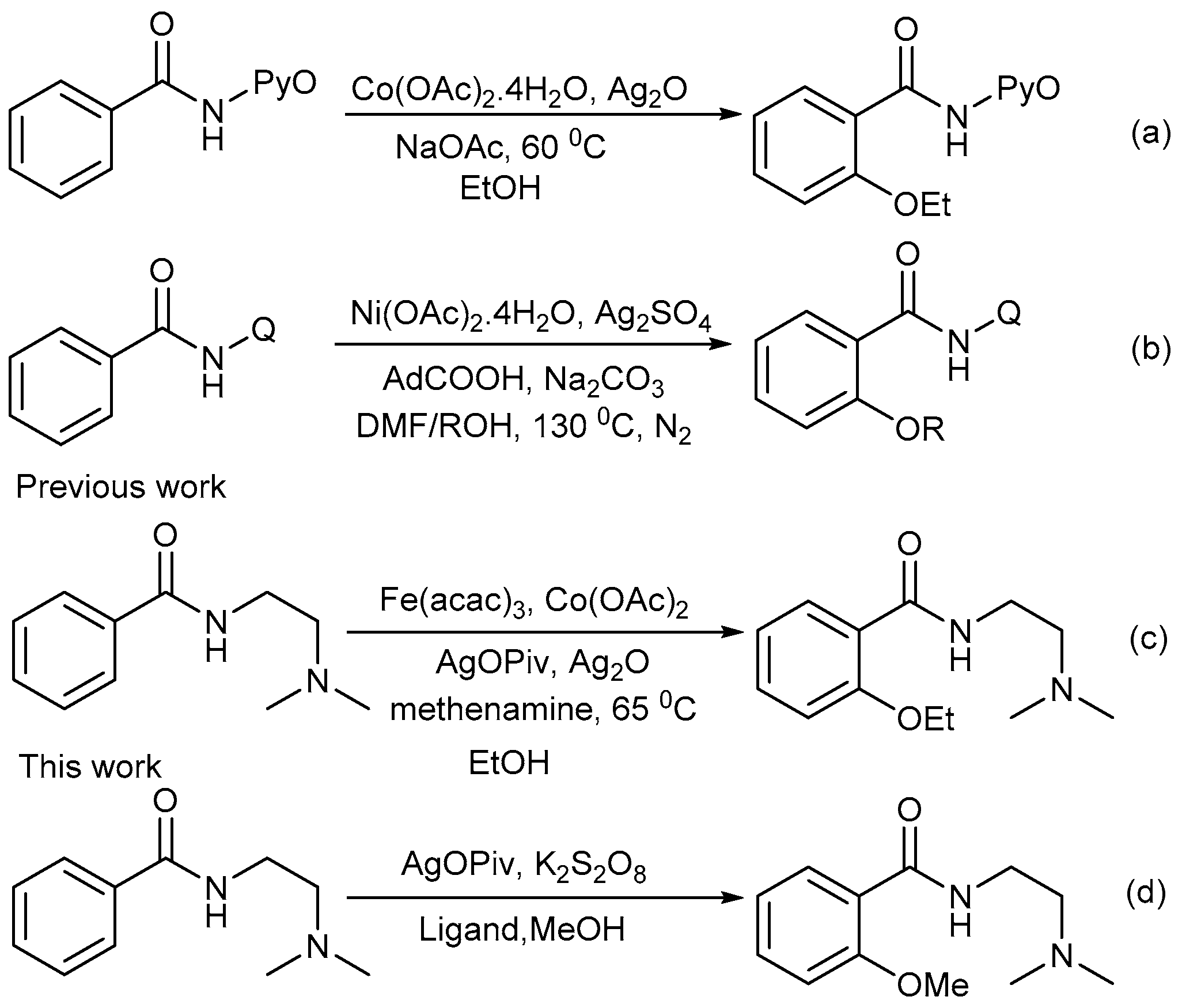
2. Results
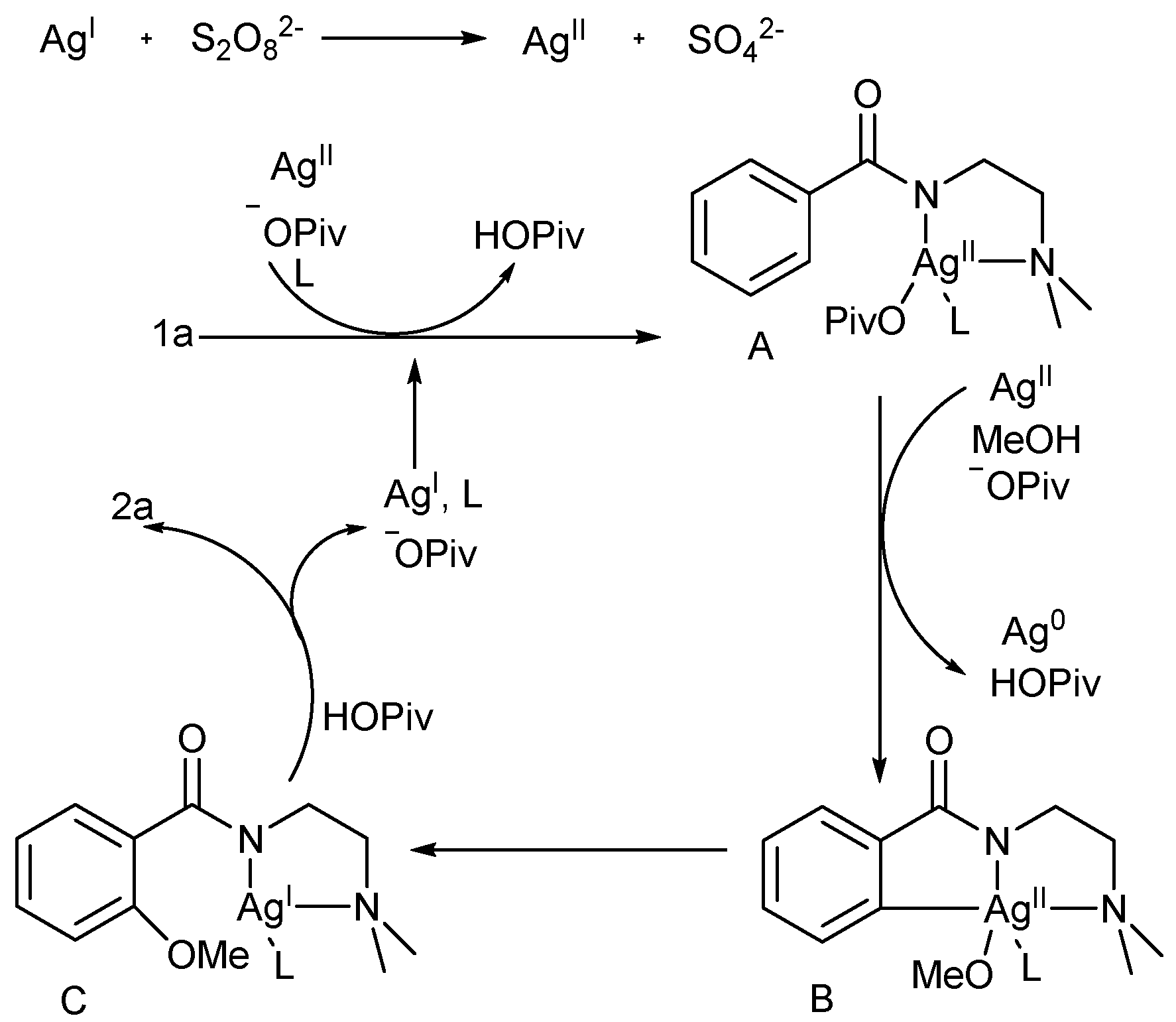
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. General Procedure for Silver-Mediated Methoxylation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maligres, P.E.; Li, J.; Krska, S.W.; Schreier, J.D.; Raheem, I.T. C–O cross-coupling of activated aryl and heteroaryl halides with aliphatic alcohols. Angew. Chem. Int. Ed. 2012, 51, 9071–9074. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Sunoj, R.B. Role of Explicit Solvents in Palladium(II)-Catalyzed Alkoxylation of Arenes: An Interesting Paradigm for Preferred Outer-Sphere Reductive Elimination over Inner-Sphere Pathway. Organometallics 2012, 31, 6466–6481. [Google Scholar] [CrossRef]
- Qiao, J.X.; Lam, P.Y.S. Copper-Promoted Carbon-Heteroatom Bond Cross-Coupling with Boronic Acids and Derivative. Synthesis 2011, 6, 829–856. [Google Scholar] [CrossRef]
- Monnier, F.; Taillefer, M. Catalytic C–C, C–N, and C–O Ullmann-Type Coupling Reactions. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Carbon–Heteroatom Bond Formation Catalysed by Organometallic Complexes. Nature 2008, 455, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.V.; Thomas, A.W. Modern Synthetic Methods for Copper-Mediated C(aryl)–O, C(aryl)–N, and C(aryl)–S Bond Formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, B.; Wang, C. Inert C–H Bond Transformations Enabled by Organometallic Manganese Catalysis. Acc. Chem. Res. 2018, 51, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhu, X.; Niu, J.-L.; Song, M.-P. High-Valent-Cobalt-Catalyzed C–H Functionalization Based on Concerted Metalation-Deprotonation and Single-Electron-Transfer Mechanisms. ChemCatChem 2016, 8, 1242–1263. [Google Scholar] [CrossRef]
- Su, B.; Cao, Z.-C.; Shi, Z.-J. Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations. Acc. Chem. Res. 2015, 48, 886–896. [Google Scholar] [CrossRef]
- Liu, C.; Liu, D.; Lei, A. Recent Advances of Transition-Metal Catalyzed Radical Oxidative Cross-Couplings. Acc. Chem. Res. 2014, 47, 3459–3470. [Google Scholar] [CrossRef]
- Qiu, F.-C.; Xie, Q.; Guan, B.-T. Palladium-Catalyzed ortho-Alkoxylation of Tertiary Benzamides: H2SO4 as an Effective Additive. Asian J. Org. Chem. 2018, 7, 107–110. [Google Scholar] [CrossRef]
- Li, S.; Zhu, W.; Gao, F.; Li, C.; Wang, J.; Liu, H. Palladium-Catalyzed Ortho-Alkoxylation of N-Benzoyl alpha-Amino Acid Derivatives at Room Temperature. J. Org. Chem. 2017, 82, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Yang, X.; Zong, Y.; Rao, Y. An Efficient Palladium-Catalyzed C–H Alkoxylation of Unactivated Methylene and Methyl Groups with Cyclic Hypervalent Iodine (I3+) Oxidants. Angew. Chem. Int. Ed. 2013, 52, 13606–13610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W.A.; Chen, G. Efficient Alkyl Ether Synthesis via Palladium-Catalyzed, Picolinamide-Directed Alkoxylation of Unactivated C(sp3)–H and C(sp2)–H Bonds at Remote Positions. J. Am. Chem. Soc. 2012, 134, 7313–7316. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Sunoj, R.B. Palladium(II)-Catalyzed Direct Alkoxylation of Arenes: Evidence for Solvent-Assisted Concerted Metalation Deprotonation. Org. Lett. 2011, 13, 4802–4805. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.B.; Duong, X.N.T.; Lu, H.D.; Cao, T.T.V.; Thanh, T. Auxiliary-Directed Etherification of sp2 C–H Bonds Under Heterogeneous Metal-Organic Framework Catalysis: Synthesis of Ethenzamide. RSC Adv. 2018, 8, 2829–2836. [Google Scholar] [CrossRef]
- Salvador, T.K.; Arnett, C.H.; Kundu, S.; Sapiezynski, N.G.; Bertke, J.A.; Boroujeni, M.R.; Warren, T.H. Copper Catalyzed sp3 C–H Etherification with Acyl Protected Phenols. J. Am. Chem. Soc. 2016, 138, 16580–16583. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, Z.; Hu, J.; Li, S.; Du, H. Copper-Catalyzed Intramolecular Alkoxylation of Purine Nucleosides: One-Step Synthesis of 5’-O,8-Cyclopurine Nucleosides. J. Org. Chem. 2015, 80, 9446–9453. [Google Scholar] [CrossRef]
- Yin, X.-S.; Li, Y.-C.; Yuan, J.; Gu, W.-J.; Shi, B.-F. Copper(II)-Catalyzed Methoxylation of Unactivated (hetero)Aryl C–H Bonds Using a Removable Bidentate Auxiliary. Org. Chem. Front. 2015, 2, 119–123. [Google Scholar] [CrossRef]
- Bhadra, S.; Dzik, W.I.; Goossen, L.J. Synthesis of Aryl Ethers from Benzoates through Carboxylate-Directed C–H-Activating Alkoxylation with Concomitant Protodecarboxylation. Angew. Chem. Int. Ed. 2013, 52, 2959–2962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-B.; Hao, X.-Q.; Zhang, S.-K.; Liu, Z.-J.; Zheng, X.-X.; Gong, J.-F.; Niu, J.-L.; Song, M.-P. Cobalt-Catalyzed C(sp2)–H Alkoxylation of Aromatic and Olefinic Carboxamides. Angew. Chem. Int. Ed. 2015, 54, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-N.; Du, C.; Zhu, X.; Wang, Z.-L.; Zhu, Y.; Chu, Z.-Y.; Niu, J.-L.; Song, M.-P. Cobalt-Catalyzed peri-Selective Alkoxylation of 1-Naphthylamine Derivatives. Beilstein J. Org. Chem. 2018, 14, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, N.; Sundararaju, B. Nickel-catalyzed C–H bond Alkoxylation of Amides with Alcohols. Asian J. Org. Chem. 2018, 7, 1368–1371. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, J.; Ding, C. Pharmaceutical-Oriented Iron-Catalyzed Ethoxylation of Aryl C(sp2)–H Bonds with Cobalt Co-Catalyst. Chemistryselect 2018, 3, 9803–9806. [Google Scholar] [CrossRef]
- Liu, S.-K.; Cui, X.-F.; Liu, X.-X.; Ma, H.-J.; Wei, D.-D.; Luo, X.-L.; Huang, G.-S. Cobalt(II)-Catalyzed Cross- Dehydrogenative Coupling Reaction of Benzamides with Alcohols to Build C–O Bond. Chemistryselect 2018, 3, 3989–3992. [Google Scholar] [CrossRef]
- Zhu, S.-Q.; Xu, X.-H.; Qing, F.-L. Silver-Mediated Oxidative C–H Difluoromethylation of Phenanthridines and 1,10-Phenanthrolines. Chem. Commun. 2017, 53, 11484–11487. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.-H.; Song, R.-J.; Hu, M.; Yang, Y.; Li, J.-H. Silver-Mediated Intermolecular 1,2-Alkylarylation of Styrenes with Carbonyl Alkyl Bromides and Indoles. Angew. Chem. Int. Ed. 2016, 55, 3187–3191. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Liao, P.; Zhang, L.; Tu, T.; Bi, X. Silver-Catalyzed Cross-Coupling of Isocyanides and Active Methylene Compounds by a Radical Process. Angew. Chem. Int. Ed. 2015, 54, 10618–10622. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Su, B.; Wang, Y.; Chen, K.; Jiang, X.; Zhang, Y.-F.; Zhang, X.-S.; Chen, G.; Cheng, Y.; Cao, Z.; et al. Silver-Catalysed Direct Amination of Unactivated C–H Bonds of Functionalized Molecules. Nature Commun. 2014, 5, 4707. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Guo, S.; Wang, L.; Tang, P. Silver-Catalyzed Oxidative Activation of Benzylic C–H Bonds for the Synthesis of Difluoromethylated Arenes. Angew. Chem. Int. Ed. 2014, 53, 5955–5958. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, F.; Jiang, X.; Zhou, Y.; Xu, X.; Tang, P. Silver-Promoted Oxidative Benzylic C–H Trifluoromethoxylation. Angew. Chem. Int. Ed. 2018, 57, 13266–13270. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, K.; Pan, J.; Qiu, X.; Luo, X.; Qin, Q.; Wei, J.; Wen, X.; Zhang, L.; Jiao, N. Silver-Catalyzed Remote Csp3-H Functionalization of Aliphatic Alcohols. Nature Commun. 2018, 9, 2625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhu, J.; Tong, C.; Ding, C. Pharmaceutical-Oriented Methoxylation of Aryl C(sp2)–H Bonds using Copper Catalysts. Synlett 2018, 29, 1451–1454. [Google Scholar]
- Girard, S.A.; Knauber, T.; Li, C.-J. The Cross-Dehydrogenative Coupling of Csp3–H Bonds: A Versatile Strategy for C–C Bond Formations. Angew. Chem. Int. Ed. 2014, 53, 74–100. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.S.; Dong, V.M. Catalytic Dehydrogenative Cross-Coupling: Forming Carbon-Carbon Bonds by Oxidizing Two Carbon-Hydrogen Bonds. Chem. Rev. 2011, 111, 1215–1292. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J. Cross-Dehydrogenative Coupling (CDC): Exploring C–C Bond Formations beyond Functional Group Transformations. Acc. Chem. Res. 2009, 42, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Vicente, R.; Kapdi, A.R. Transition-Metal-Catalyzed Direct Arylation of (Hetero)Arenes by C–H Bond Cleavage. Angew. Chem. Int. Ed. 2009, 48, 9792–9826. [Google Scholar] [CrossRef] [PubMed]
- Grochala, W.; Mazej, Z. Chemistry of Silver(II): A Cornucopia of Peculiarities. Philos. T. Roy. Soc. A 2015, 373, 12. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, A.; Jaron, T.; Mazej, Z.; Michalowski, T.; Szarek, P.; Grochala, W. Anomalous Chemical Shifts In X-ray Photoelectron Spectra of Sulfur-Containing Compounds of Silver (I) and (II). J. Electron Spectrosc. Relat. Phenom. 2015, 202, 38–45. [Google Scholar] [CrossRef]
- Seo, S.; Taylor, J.B.; Greaney, M.F. Protodecarboxylation of Benzoic Acids under Radical Conditions. Chem. Commun. 2012, 48, 8270–8272. [Google Scholar] [CrossRef]
- Anderson, J.M.; Kochi, J.K. Silver(I)-Catalyzed Oxidative Decarboxylation of Acids by Peroxydisulfate. Role of Silver(II). J. Am. Chem. Soc. 1970, 92, 1651–1659. [Google Scholar] [CrossRef]
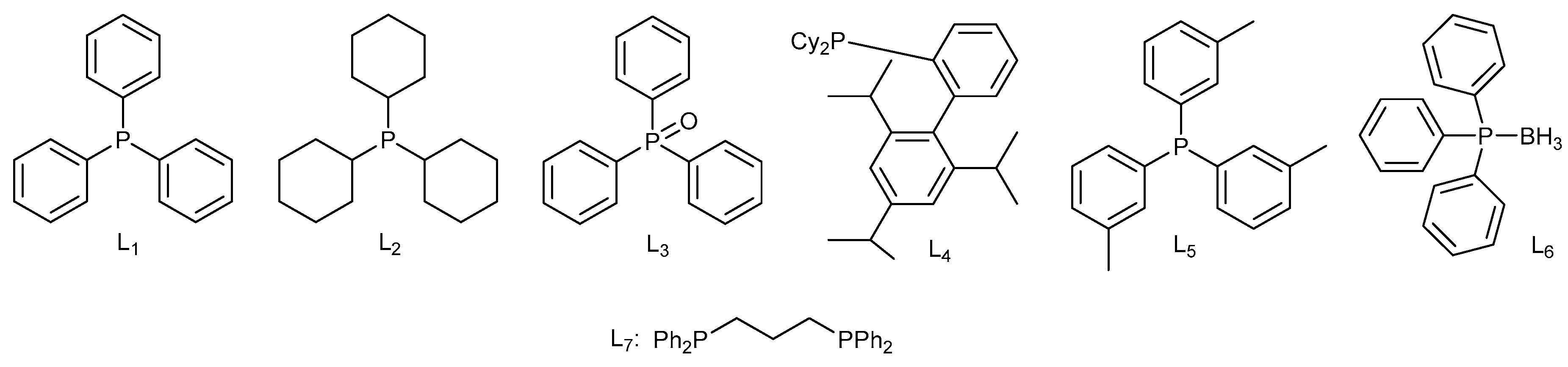
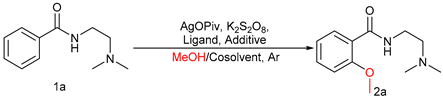 | ||||
|---|---|---|---|---|
| Entry | Ligand | Additive | Cosolvent | Yield [%] b |
| 1 | L1 | - | DMA | 63 |
| 2 | L2 | - | DMA | 54 |
| 3 | L3 | - | DMA | Trace |
| 4 | L4 | - | DMA | 17 |
| 5 | L5 | - | DMA | 51 |
| 6 | L6 | - | DMA | 9 |
| 7 | L7 | - | DMA | 36 |
| 8 | - | - | DMA | n.r |
| 9 | L1 | - | DMSO | 51 |
| 10 | L1 | - | DMF | 56 |
| 11 | L1 | - | Toluene | 4 |
| 12 | L1 | - | EA | 30 |
| 13 | L1 | Na2SO4 | DMA | 72 |
| 14 | L1 | MgSO4 | DMA | 78(71 c) |
| 15 | L1 | Na2CO3 | DMA | 30 |
| 16 | L1 | K2CO3 | DMA | n.r |
| 17 | L1 | Li2CO3 | DMA | 20 |
| 18 | L1 | Li3PO4 | DMA | 38 |
| 19 | L1 | NaOAc | DMA | 50 |
| 20 d | L1 | MgSO4 | DMA | 55 |
| 21 e | L1 | MgSO4 | DMA | n.r |
 | |||
|---|---|---|---|
| Entry | 1 | 2 | Yield b |
| 1 |  |  | 65 |
| 2 | 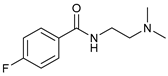 | 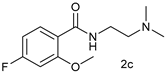 | 58 |
| 3 |  | 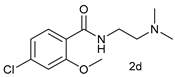 | 62 |
| 4 | 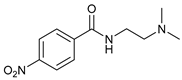 | 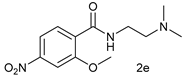 | 35 |
| 5 | 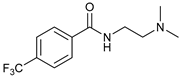 |  | 62 |
| 6 | 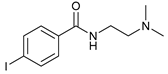 | 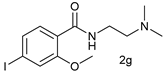 | 14 |
| 7 | 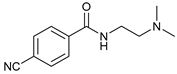 | 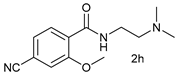 | 61 |
| 8 | 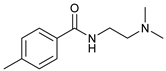 | 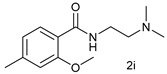 | 63 |
| 9 | 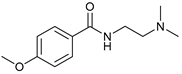 | 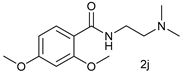 | 72 |
| 10 | 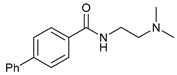 | 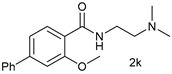 | 55 |
| 11 | 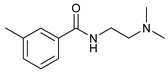 | 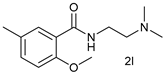 | 68 |
| 12 | 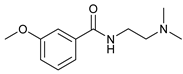 |  | 67 |
| 13 | 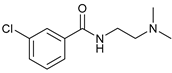 | 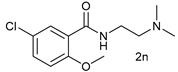 | 60 |
| 14 | 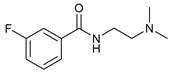 | 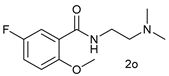 | 54 |
| 15 |  | 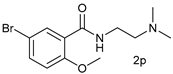 | 40 |
| 16 | 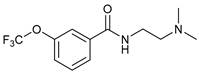 | 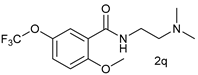 | 64 |
| 17 | 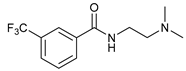 | 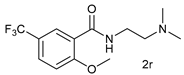 | 64 |
| 18 | 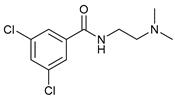 | 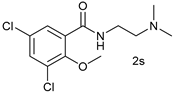 | 55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Zhu, J.; Xuan, L.; Ding, C. Silver-Mediated Methoxylation of Aryl C(sp2)–H Bonds Directing by DMEDA. Catalysts 2019, 9, 171. https://doi.org/10.3390/catal9020171
Zhang G, Zhu J, Xuan L, Ding C. Silver-Mediated Methoxylation of Aryl C(sp2)–H Bonds Directing by DMEDA. Catalysts. 2019; 9(2):171. https://doi.org/10.3390/catal9020171
Chicago/Turabian StyleZhang, Guofu, Jianfei Zhu, Lidi Xuan, and Chengrong Ding. 2019. "Silver-Mediated Methoxylation of Aryl C(sp2)–H Bonds Directing by DMEDA" Catalysts 9, no. 2: 171. https://doi.org/10.3390/catal9020171
APA StyleZhang, G., Zhu, J., Xuan, L., & Ding, C. (2019). Silver-Mediated Methoxylation of Aryl C(sp2)–H Bonds Directing by DMEDA. Catalysts, 9(2), 171. https://doi.org/10.3390/catal9020171





