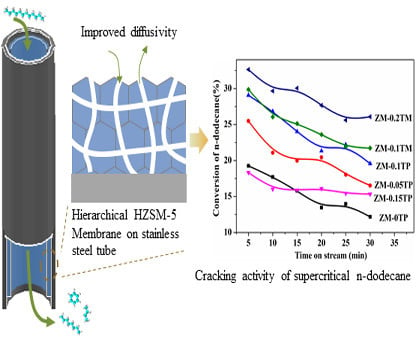
Fabrication of the Hierarchical HZSM-5 Membrane with Tunable Mesoporosity for Catalytic Cracking of n-Dodecane
Abstract
:1. Introduction
2. Results and Discussion
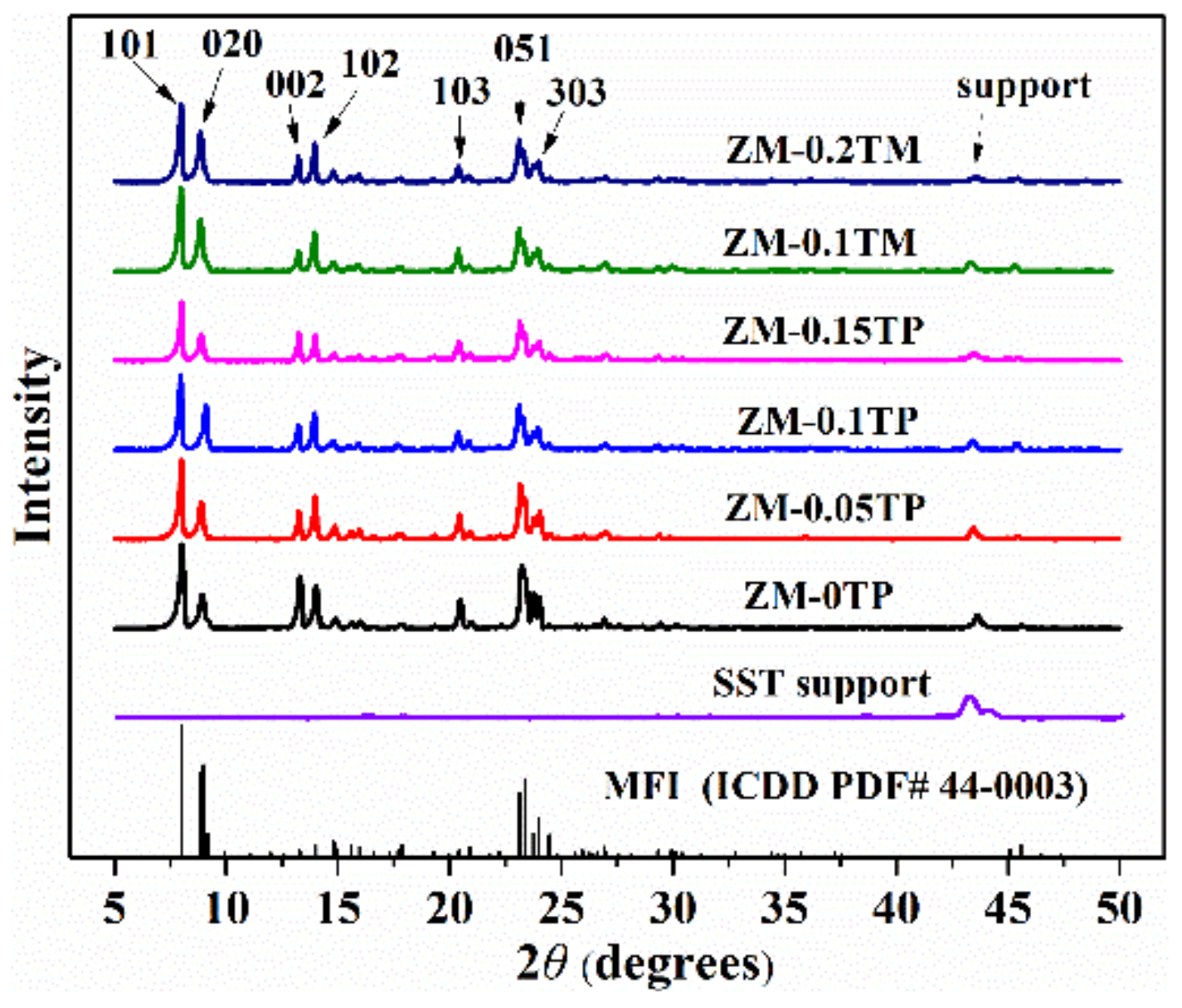
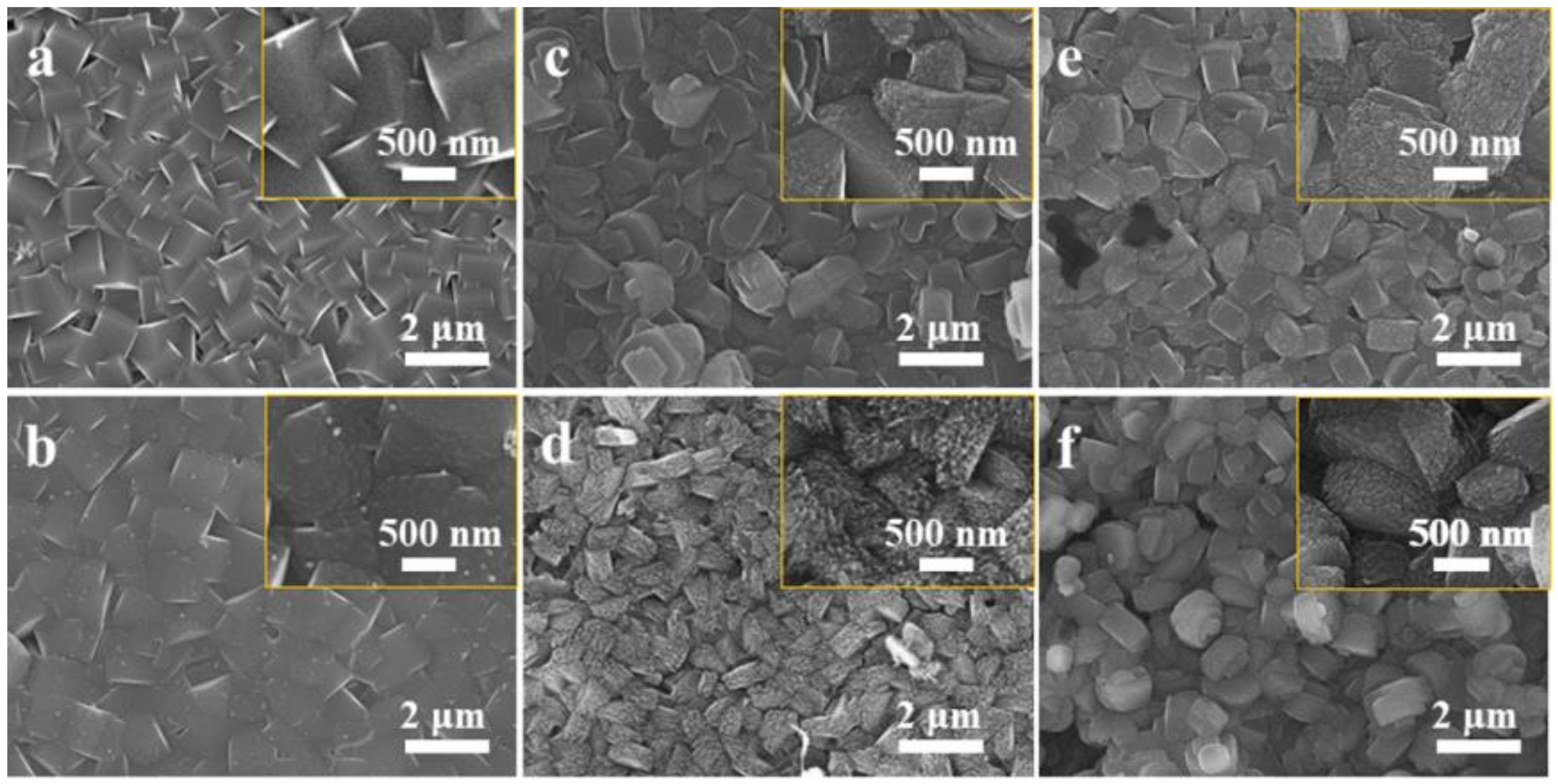
2.1. Structures Characterization
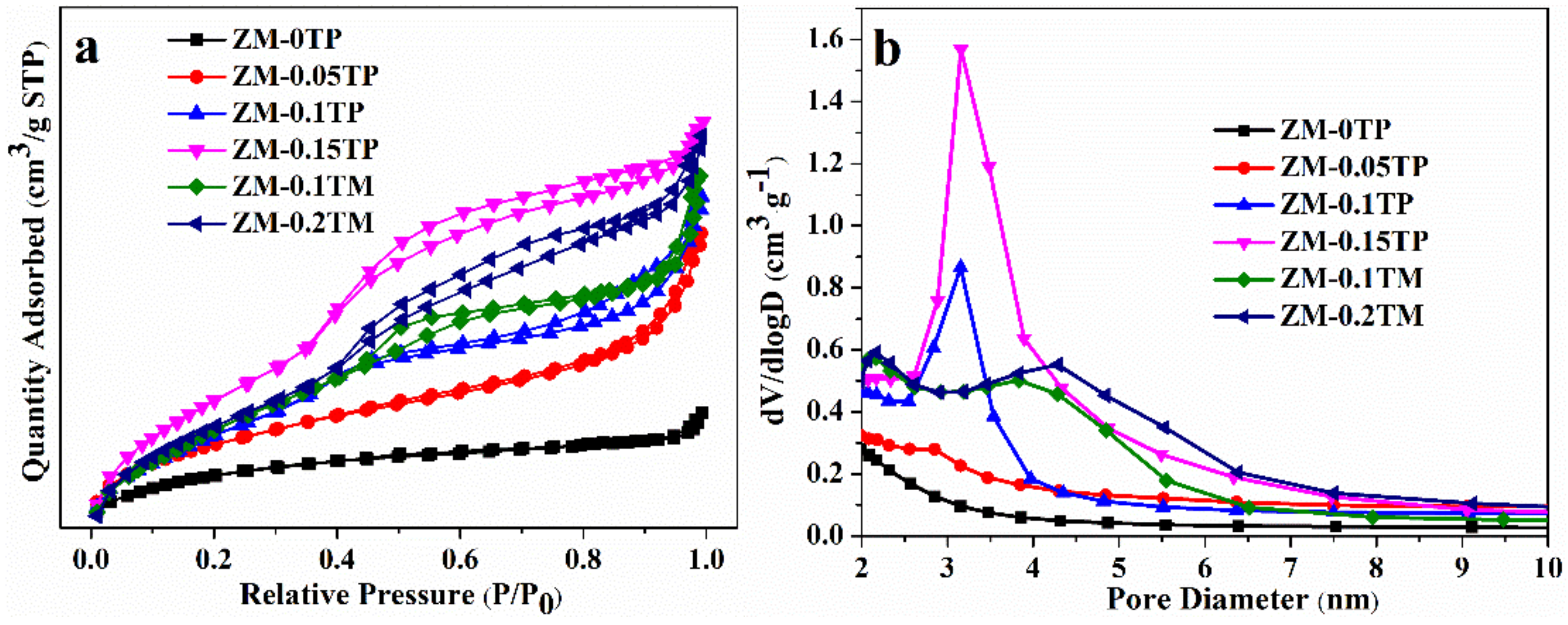
2.2. Gas Permeation Properties
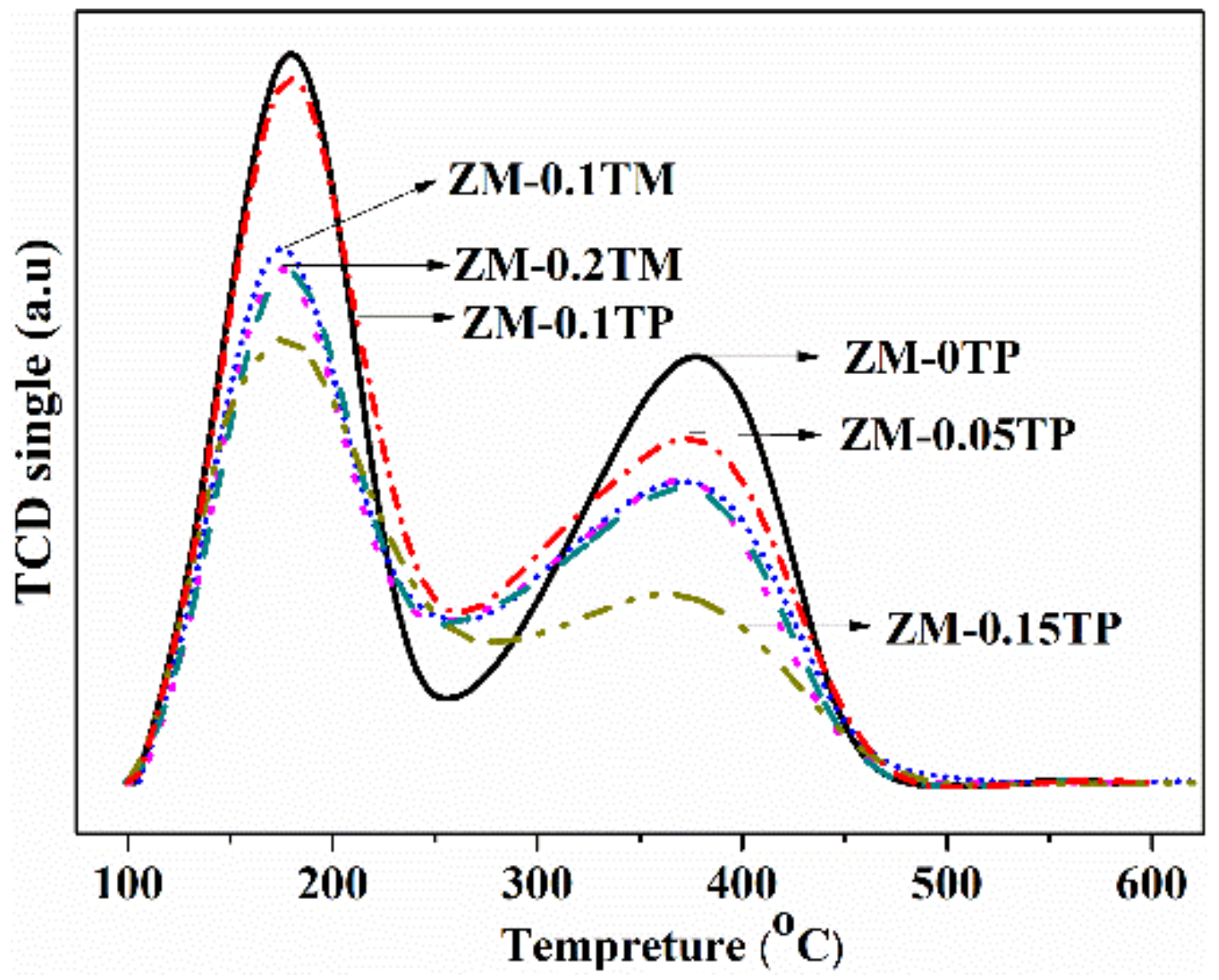
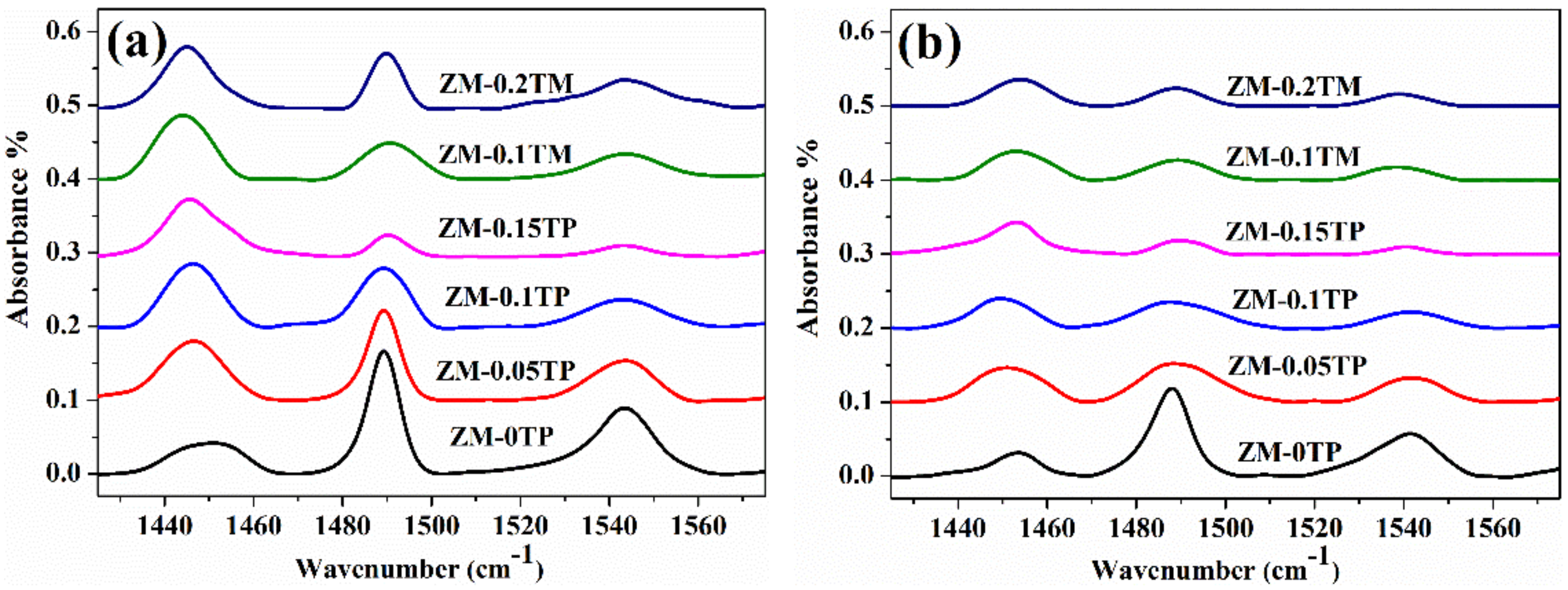
2.3. Compositions and Acid Properties
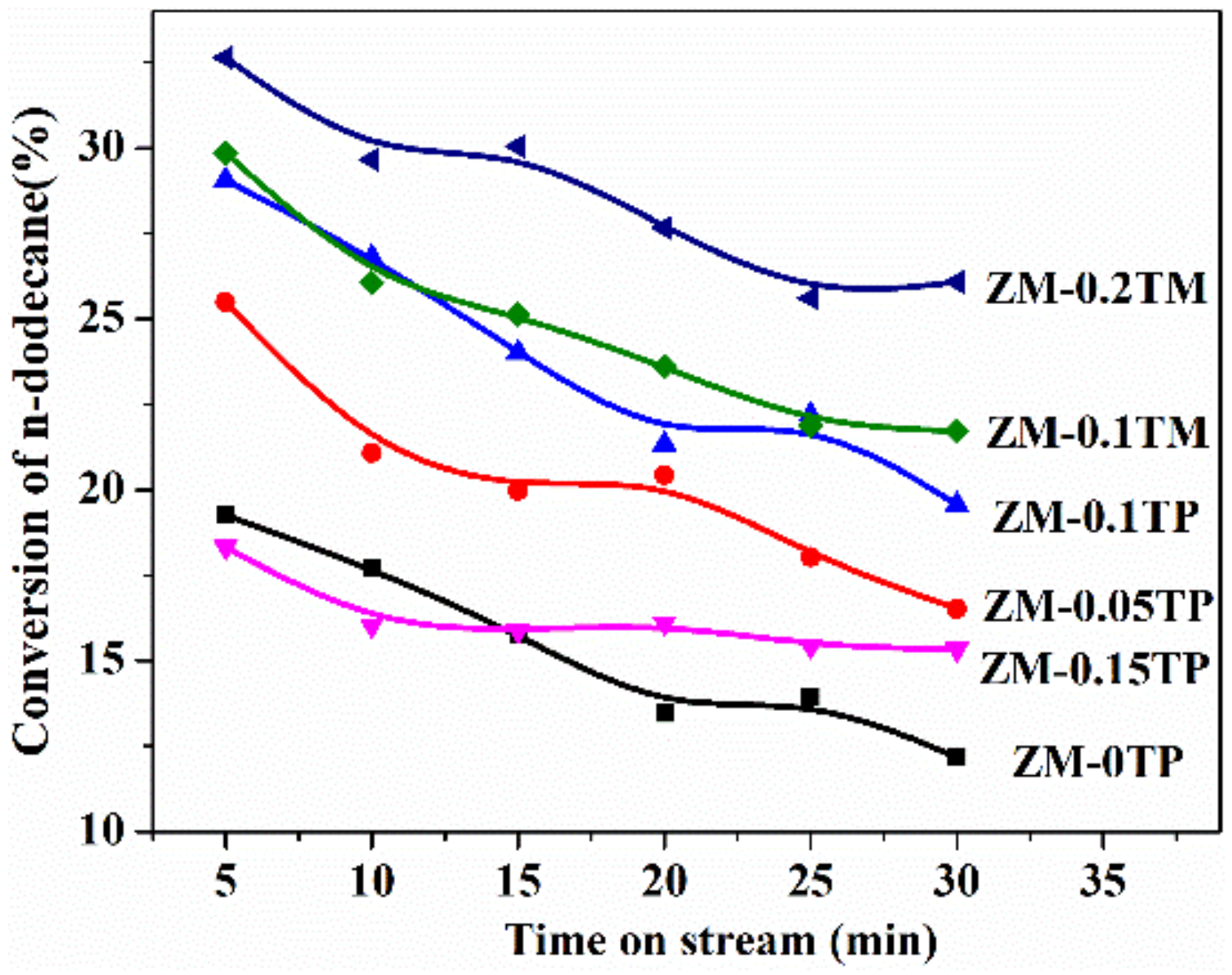
2.4. Catalytic Cracking Performances
2.4.1. Catalytic Cracking Activities
2.4.2. Product Distribution
3. Experiment
3.1. Materials
3.2. Preparation of the Seed Layers
3.3. Preparation of the Zeolite Membranes
3.4. Characterization
3.5. N2 Gas Permeation Test
3.6. Catalytic Cracking of n-dodecane
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, H.; Spadaccini, L.J.; Sobel, D.R. Fuel-cooled thermal management for advanced aeroengines. J. Eng. Gas Turb. Power 2004, 126, 284–293. [Google Scholar] [CrossRef]
- Xian, X.; Liu, G.; Zhang, X.; Wang, L.; Mi, Z. Catalytic cracking of n-dodecane over HZSM-5 zeolite under supercritical conditions: Experiments and kinetics. Chem. Eng. Sci. 2010, 65, 5588–5604. [Google Scholar] [CrossRef]
- Luo, J.; Gorte, R.J. High pressure cracking of n-hexane over H-ZSM-5. Catal. Lett. 2013, 143, 313–316. [Google Scholar] [CrossRef]
- Kim, J.; Hyeon, D.H.; Park, S.H.; Chun, B.; Jeong, B.H.; Han, J.; Kim, S.H. Catalytic endothermic reactions of exo-tetrahydrodicyclopentadiene with zeolites and improvement of heat of reactions. Catal. Today 2014, 232, 63–68. [Google Scholar] [CrossRef]
- Yue, L.; Li, G.; He, G.; Guo, Y.; Xu, L.; Fang, W. Impacts of hydrogen to carbon ratio (H/C) on fundamental properties and supercritical cracking performance of hydrocarbon fuels. Chem. Eng. J. 2016, 283, 1216–1223. [Google Scholar] [CrossRef]
- Wu, H.; Li, G. ZSM-5 crystals grown on the wall of a long tubular reactor as a structured catalyst for cracking of endothermic fuels. Appl. Catal. A Gen. 2012, 423, 108–113. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.; Zhang, X.; Zhao, D.; Wang, L. Effects of aluminum sources on synthesis and catalytic performance of b-oriented ZSM-5 coatings. Micropor. Mesopor. Mat. 2017, 244, 164–170. [Google Scholar] [CrossRef]
- Tian, Y.; Qiu, Y.; Hou, X.; Wang, L.; Liu, G. Catalytic cracking of JP-10 over HZSM-5 nanosheets. Energ. Fuel. 2017, 31, 11987–11994. [Google Scholar] [CrossRef]
- Vu, X.H.; Armbruster, U.; Martin, A. Micro/mesoporous zeolitic composites: Recent developments in synthesis and catalytic applications. Catalysts 2016, 6, 183. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Yang, W.; Tang, Y.; Xie, Z. Recent advances of pore system construction in zeolite-catalyzed chemical industry processes. Chem. Soc. Rev. 2015, 44, 8877–8903. [Google Scholar] [CrossRef]
- Caro, J. Hierarchy in inorganic membranes. Chem. Soc. Rev. 2016, 45, 3468–3478. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, L.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B. Hierarchically porous materials: Synthesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qu, S.; Wang, L.; Zhang, X.; Liu, G. Preparation and performance of hierarchical HZSM-5 coatings on stainless-steeled microchannels for catalytic cracking of hydrocarbons. Catal. Today 2013, 216, 64–70. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, B.; Yang, H.; Yan, W. Synthesis of isomorphous MFI nanosheet zeolites for supercritical catalytic cracking of n-dodecane. Appl. Catal. A Gen. 2017, 533, 90–98. [Google Scholar] [CrossRef]
- Diao, Z.; Wang, L.; Zhang, X.; Liu, G. Catalytic cracking of supercritical n-dodecane over meso-HZSM-5@Al-MCM-41 zeolites. Chem. Eng. Sci. 2015, 135, 452–460. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, G.; Meng, F.; Qu, S.; Wang, L.; Zhang, X. Catalytic cracking of supercritical n-dodecane over wall-coated HZSM-5 zeolites with micro- and nanocrystal sizes. Energ. Fuel. 2011, 26, 1220–1229. [Google Scholar] [CrossRef]
- Richter, H.; Voß, H.; Voigt, I.; Diefenbacher, A.; Schuch, G.; Steinbach, F.; Caro, J. High-flux ZSM-5 membranes with an additional non-zeolite pore system by alcohol addition to the synthesis batch and their evaluation in the 1-butene/i-butene separation. Sep. Purif. Technol. 2010, 72, 388–394. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, D.; Pu, S.; Liu, G. Remarkable catalytic activities of hydrothermally synthesized bilayered HZSM-5 coatings with additional nonzeolite pores. Ind. Eng. Chem. Res. 2015, 54, 5246–5253. [Google Scholar] [CrossRef]
- Liu, X.; Ge, P.; Zhang, Y.; Zhang, B.; Yao, Z.; Wang, Z.; Li, X.; Hu, M. Highly oriented thin membrane fabrication with hierarchically porous zeolite seed. Cryst. Growth Des. 2018, 18, 4544–4554. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Hao, J.; Liu, G.; Ma, X.; Hu, S. Synthesis of HZSM-5 coatings on the inner surface of stainless steel tubes and their catalytic performance in n-dodecane cracking. Appl. Catal. A Gen. 2013, 462, 271–277. [Google Scholar] [CrossRef]
- Tarach, K.; Góra-Marek, K.; Tekla, J.; Brylewska, K.; Datka, J.; Mlekodaj, K.; Makowski, W.; Igualada López, M.C.; Martínez Triguero, J.; Rey, F. Catalytic cracking performance of alkaline-treated zeolite Beta in the terms of acid sites properties and their accessibility. J. Catal. 2014, 312, 46–57. [Google Scholar] [CrossRef]
- Srivastava, R. Synthesis and applications of ordered and disordered mesoporous zeolites: Present and future prospective. Catal. Today 2017, 309, 172–188. [Google Scholar] [CrossRef]
- Choi, M.; Cho, H.S.; Srivastava, R.; Venkatesan, C.; Choi, D.; Ryoo, R. Amphiphilic organosilane-directed synthesis of crystalline zeolite with tunable mesoporosity. Nat. Mater. 2006, 5, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xu, X.; Yao, X.; Liu, H.; Gu, X. Fabrication of novel hierarchical ZSM-5 zeolite membranes with tunable mesopores for ultrafiltration. J. Memb. Sci. 2018, 549, 446–455. [Google Scholar] [CrossRef]
- Feng, Y.; Meng, Y.; Li, F.; Lv, Z.; Xue, J. Synthesis of mesoporous LTA zeolites with large BET areas. J. Porous Mater 2013, 20, 465–471. [Google Scholar] [CrossRef]
- Carvalho, K.; Urquieta-Gonzalez, E. Microporous–mesoporous ZSM-12 zeolites: Synthesis by using a soft template and textural, acid and catalytic properties. Catal. Today 2015, 243, 92–102. [Google Scholar] [CrossRef]
- Wang, L.; Diao, Z.; Tian, Y.; Xiong, Z.; Liu, G. Catalytic cracking of endothermic hydrocarbon fuels over ordered meso-HZSM-5 zeolites with Al-MCM-41 shells. Energ. Fuel. 2016, 30, 6977–6983. [Google Scholar] [CrossRef]
- Ishihara, A.; Hashimoto, T.; Nasu, H. Large mesopore generation in an amorphous silica-alumina by controlling the pore size with the gel skeletal reinforcement and its application to catalytic cracking. Catalysts 2012, 2, 368–385. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, H.; Yan, W. Strategies to enhance the catalytic performance of ZSM-5 zeolite in hydrocarbon cracking: A review. Catalysts 2017, 7, 367. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M. Nanosized and hierarchical zeolites: A short review. Chinese J. Catal. 2016, 37, 447–467. [Google Scholar] [CrossRef]
- Cho, K.; Cho, H.S.; De Ménorval, L.; Ryoo, R. Generation of mesoporosity in LTA zeolites by organosilane surfactant for rapid molecular transport in catalytic application. Chem. Mater. 2009, 21, 5664–5673. [Google Scholar] [CrossRef]
- Han, Y.; Pitukmanorom, P.; Zhao, L.; Ying, J. Generalized synthesis of mesoporous shells on zeolite crystals. Small 2011, 7, 326–332. [Google Scholar] [CrossRef]
- Ji, M.; Liu, G.; Chen, C.; Wang, L.; Zhang, X.; Hu, S.; Ma, X. Catalytic performances of b-oriented bi-layered HZSM-5 coatings for cracking of hydrocarbon fuels. Appl. Catal. A Gen. 2014, 482, 8–15. [Google Scholar] [CrossRef]
- Serrano, D.P.; García, R.A.; Vicente, G.; Linares, M.; Procházková, D.; Čejka, J. Acidic and catalytic properties of hierarchical zeolites and hybrid ordered mesoporous materials assembled from MFI protozeolitic units. J. Catal. 2011, 279, 366–380. [Google Scholar] [CrossRef]
- Na, J.; Liu, G.; Zhou, T.; Ding, G.; Hu, S.; Wang, L. Synthesis and catalytic performance of ZSM-5/MCM-41 zeolites with varying mesopore size by surfactant-directed recrystallization. Catal. Lett. 2013, 143, 267–275. [Google Scholar] [CrossRef]
- Qu, S.; Liu, G.; Meng, F.; Wang, L.; Zhang, X. Catalytic cracking of supercritical n-dodecane over wall-coated HZSM-5 with different Si/Al ratios. Energ. Fuel. 2011, 25, 2808–2814. [Google Scholar] [CrossRef]
- Koekkoek, A.; Tempelman, C.; Degirmenci, V.; Guo, M.; Feng, Z.; Li, C.; Hensen, E. Hierarchical zeolites prepared by organosilane templating: A study of the synthesis mechanism and catalytic activity. Catal. Today 2011, 168, 96–111. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Martı́nez-Triguero, J.; Pergher, S. Delaminated zeolites: Combining the benefits of zeolites and mesoporous materials for catalytic uses. J. Catal. 1999, 186, 57–63. [Google Scholar] [CrossRef]
- Ishihara, A.; Inui, K.; Hashimoto, T.; Nasu, H. Preparation of hierarchical β and Y zeolite-containing mesoporous silica–aluminas and their properties for catalytic cracking of n-dodecane. J. Catal. 2012, 295, 81–90. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, Z.; Kumakiri, I.; Tanaka, K.; Chen, X.; Kita, H. Preparation and characterization of high water perm-selectivity ZSM-5 membrane without organic template. J. Memb. Sci. 2012, 415, 57–65. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lippens, B.C.; De Boer, J.H. Studies on pore systems in catalysts: V. The t method. J. Catal. 1965, 4, 319–323. [Google Scholar] [CrossRef]
- Groen, J.C.; Jansen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Optimal aluminum-assisted mesoporosity development in MFI zeolites by desilication. J. Phys. Chem. B 2004, 108, 13062–13065. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]

| Membranes | Rca (%) | δ b (μm) | Loading amounts (mg·cm−2) | Density c (g·cm−3) | N2 permeance mol·Pa−1·s−1·m−2 | rdd (%) |
|---|---|---|---|---|---|---|
| ZM-0TP | 100 | 5.08 ± 0.25 | 1.40 ± 0.07 | 2.76 | 2.7×10−6 | 36.79 |
| ZM-0.05TP | 87 | 5.27 ± 0.24 | 1.37 ± 0.06 | 2.60 | 4.1×10−5 | 35.17 |
| ZM-0.1TP | 71 | 5.43 ± 0.31 | 1.35 ± 0.09 | 2.49 | 1.6×10−4 | 32.72 |
| ZM-0.15TP | 61 | 6.14 ± 0.27 | 1.31 ± 0.05 | 2.13 | 5.4×10−3 | 16.35 |
| ZM-0.1TM | 68 | 5.59 ± 0.32 | 1.41 ± 0.06 | 2.52 | 2.2×10−4 | 27.26 |
| ZM-0.2TM | 68 | 5.72 ± 0.24 | 1.34 ± 0.10 | 2.34 | 8.5×10−4 | 20.10 |
| Samples | SBET (m2·g−1) | Sexter (m2·g−1) | Vt (cm3·g−1) | Vmicro (cm3·g−1) | Si/Al a | Acid density b (μmol NH3·g−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | BS | LW | LS | T | TW | TS | ||||||
| ZM-0TP | 432 | 146 | 0.231 | 0.133 | 71 | 86 | 82 | 46 | 37 | 251 | 132 | 119 |
| ZM-0.05TP | 491 | 218 | 0.353 | 0.126 | 86 | 47 | 43 | 78 | 66 | 234 | 125 | 109 |
| ZM-0.1TP | 515 | 316 | 0.384 | 0.091 | 90 | 40 | 39 | 69 | 63 | 211 | 109 | 102 |
| ZM-0.15TP | 586 | 425 | 0.453 | 0.071 | 107 | 15 | 10 | 88 | 35 | 148 | 103 | 45 |
| ZM-0.1TM | 528 | 344 | 0.390 | 0.083 | 92 | 38 | 36 | 72 | 66 | 212 | 110 | 102 |
| ZM-0.2TM | 533 | 349 | 0.433 | 0.085 | 93 | 41 | 37 | 67 | 62 | 207 | 108 | 99 |
| Products | ZM-0TP | ZM-0.05TP | ZM-0.1TP | ZM-0.15TP | ZM-0.1TM | ZM-0.2TM |
|---|---|---|---|---|---|---|
| methane | 4.03 | 3.58 | 3.34 | 2.37 | 2.97 | 2.45 |
| ethylene | 4.79 | 5.42 | 5.77 | 4.89 | 6.43 | 6.31 |
| ethane | 4.66 | 4.37 | 5.04 | 3.58 | 5.87 | 5.62 |
| propane | 5.42 | 6.01 | 7.45 | 4.92 | 7.04 | 7.33 |
| propylene | 9.11 | 10.45 | 13.26 | 11.07 | 12.76 | 13.7 |
| iso-butane | 0.59 | 0.46 | 0.39 | 0.24 | 0.37 | 0.31 |
| n-butane | 3.35 | 2.39 | 2.01 | 1.43 | 2.10 | 2.05 |
| trans-2-butene | 1.38 | 1.17 | 0.92 | 0.87 | 1.08 | 1.74 |
| iso-butene | 1.46 | 1.41 | 1.57 | 1.35 | 1.69 | 1.66 |
| 1-butene | 4.07 | 2.98 | 2.51 | 2.85 | 2.56 | 3.29 |
| cis-2-butene | 1.21 | 0.85 | 1.39 | 0.58 | 0.74 | 0.85 |
| pentene | 6.72 | 7.37 | 6.24 | 5.49 | 6.38 | 6.62 |
| n-pentane | 5.95 | 6.19 | 5.76 | 3.32 | 5.85 | 4.54 |
| hexene | 4.74 | 5.83 | 5.85 | 6.31 | 5.26 | 5.93 |
| n-hexane | 2.57 | 5.09 | 4.13 | 3.25 | 3.87 | 3.71 |
| benzene | 0.24 | 0.21 | 0.18 | 0.07 | 0.13 | 0.09 |
| heptene | 4.58 | 5.56 | 5.57 | 7.02 | 6.09 | 5.84 |
| n-heptane | 4.03 | 2.83 | 3.11 | 3.59 | 2.78 | 2.47 |
| toluene | 0.41 | 0.35 | 0.30 | 0.17 | 0.32 | 0.21 |
| octene | 5.37 | 3.15 | 6.85 | 7.45 | 6.34 | 6.67 |
| n-octane | 3.41 | 2.06 | 1.36 | 2.73 | 1.38 | 1.52 |
| nonene | 5.27 | 3.97 | 4.88 | 7.16 | 5.97 | 5.06 |
| n-nonane | 4.16 | 3.68 | 2.56 | 3.23 | 2.04 | 2.24 |
| decene | 4.79 | 5.97 | 3.92 | 6.86 | 4.16 | 4.49 |
| n-decane | 2.64 | 2.33 | 1.19 | 1.75 | 1.06 | 0.85 |
| undecene | 1.80 | 1.78 | 1.54 | 3.64 | 1.73 | 1.86 |
| n-undecane | 2.94 | 3.89 | 2.44 | 2.87 | 2.62 | 2.07 |
| dodecene | 0.31 | 0.65 | 0.47 | 0.94 | 0.41 | 0.52 |
| C3=/C3 | 1.68 | 1.74 | 1.78 | 2.25 | 1.81 | 1.87 |
| i-C4=/i-C4 | 2.47 | 3.07 | 4.03 | 5.63 | 4.57 | 5.35 |
| Average conversion (%) | 15.39 | 20.26 | 23.84 | 16.18 | 24.71 | 28.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, Z.; Cheng, L.; Hou, X.; Rong, D.; Lu, Y.; Yue, W.; Sun, D. Fabrication of the Hierarchical HZSM-5 Membrane with Tunable Mesoporosity for Catalytic Cracking of n-Dodecane. Catalysts 2019, 9, 155. https://doi.org/10.3390/catal9020155
Diao Z, Cheng L, Hou X, Rong D, Lu Y, Yue W, Sun D. Fabrication of the Hierarchical HZSM-5 Membrane with Tunable Mesoporosity for Catalytic Cracking of n-Dodecane. Catalysts. 2019; 9(2):155. https://doi.org/10.3390/catal9020155
Chicago/Turabian StyleDiao, Zhenheng, Lushi Cheng, Xu Hou, Di Rong, Yanli Lu, Wenda Yue, and De Sun. 2019. "Fabrication of the Hierarchical HZSM-5 Membrane with Tunable Mesoporosity for Catalytic Cracking of n-Dodecane" Catalysts 9, no. 2: 155. https://doi.org/10.3390/catal9020155
APA StyleDiao, Z., Cheng, L., Hou, X., Rong, D., Lu, Y., Yue, W., & Sun, D. (2019). Fabrication of the Hierarchical HZSM-5 Membrane with Tunable Mesoporosity for Catalytic Cracking of n-Dodecane. Catalysts, 9(2), 155. https://doi.org/10.3390/catal9020155