Nanosheet MFI Zeolites for Gas Phase Glycerol Dehydration to Acrolein
Abstract
1. Introduction
2. Results and Discussion
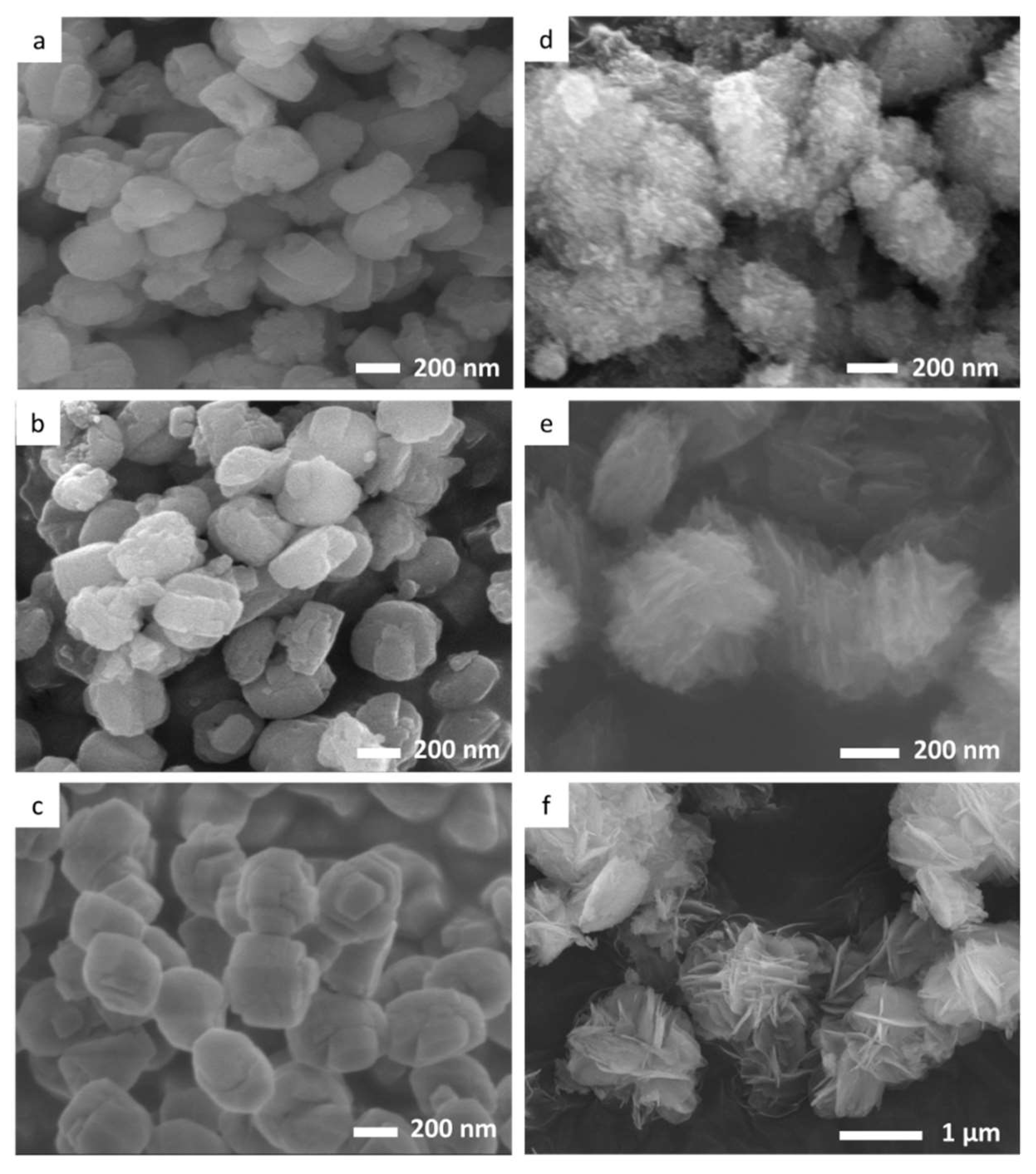
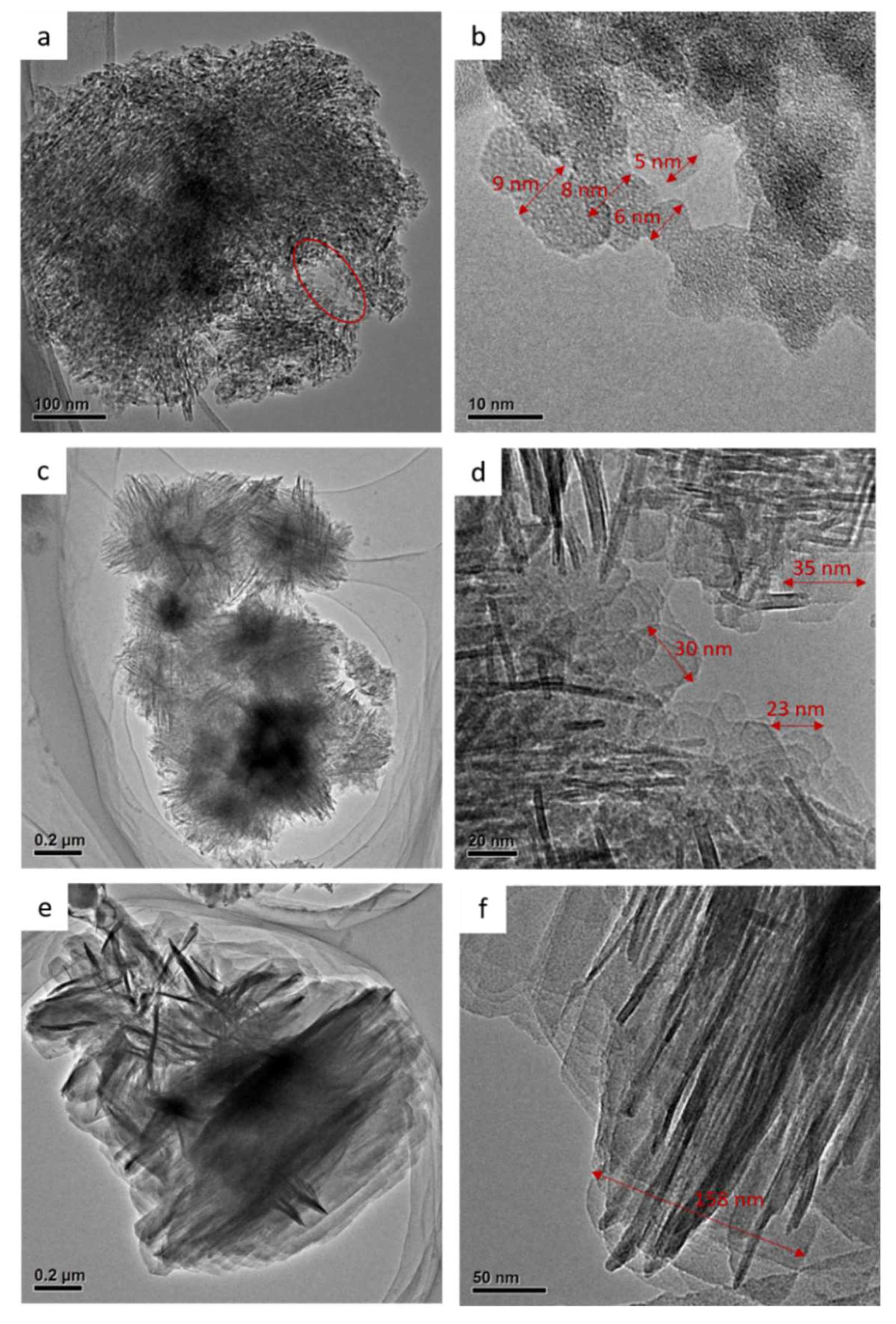
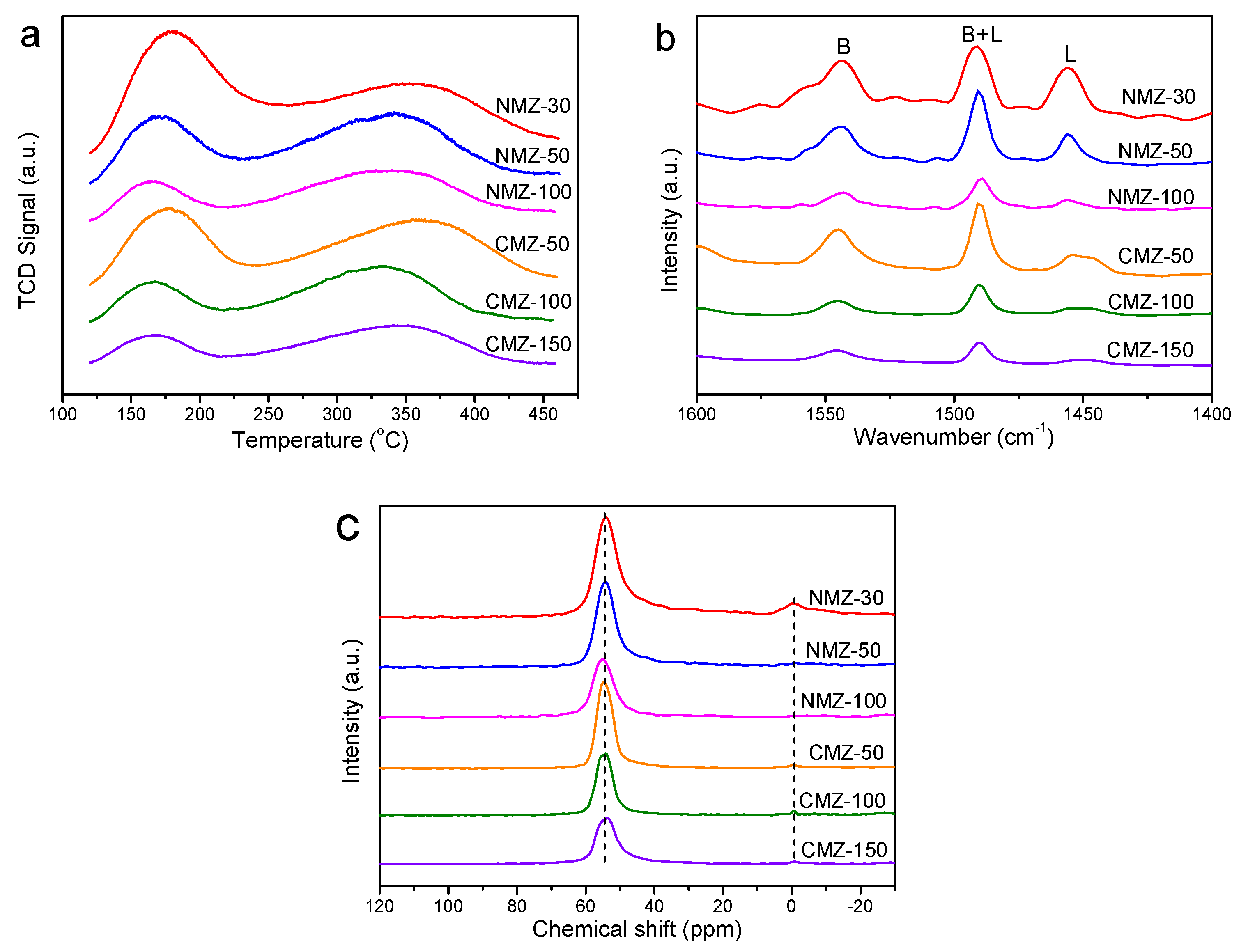
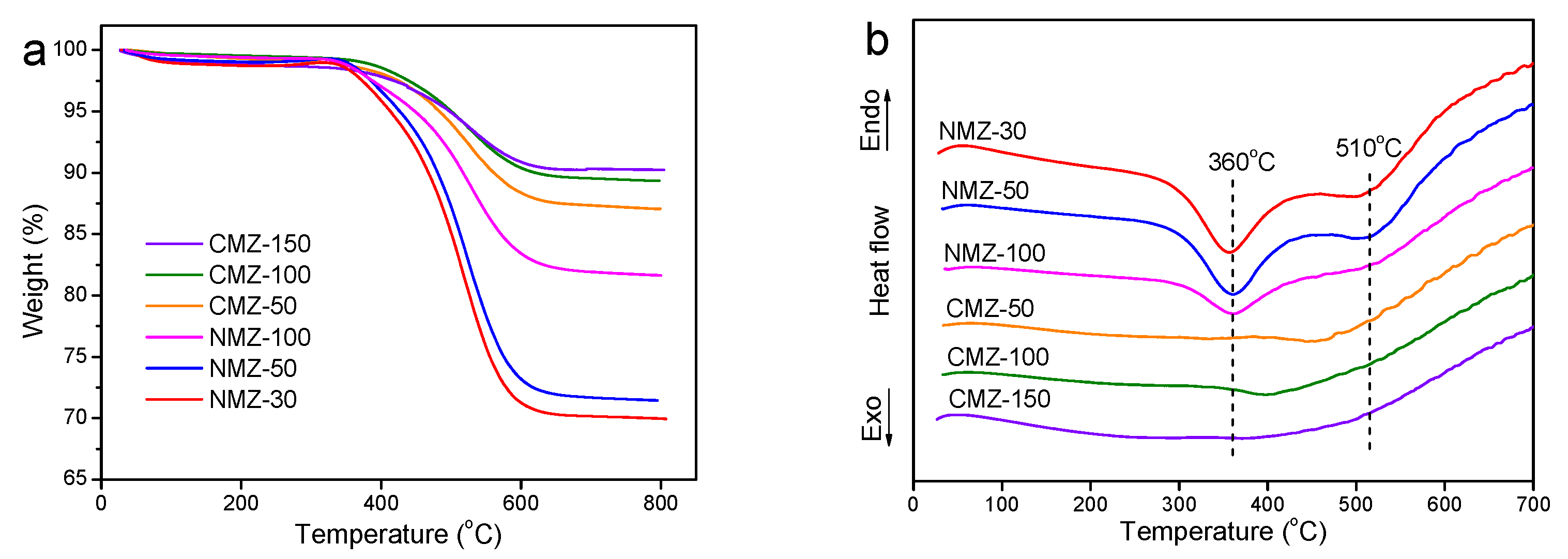
2.1. Characterization Results
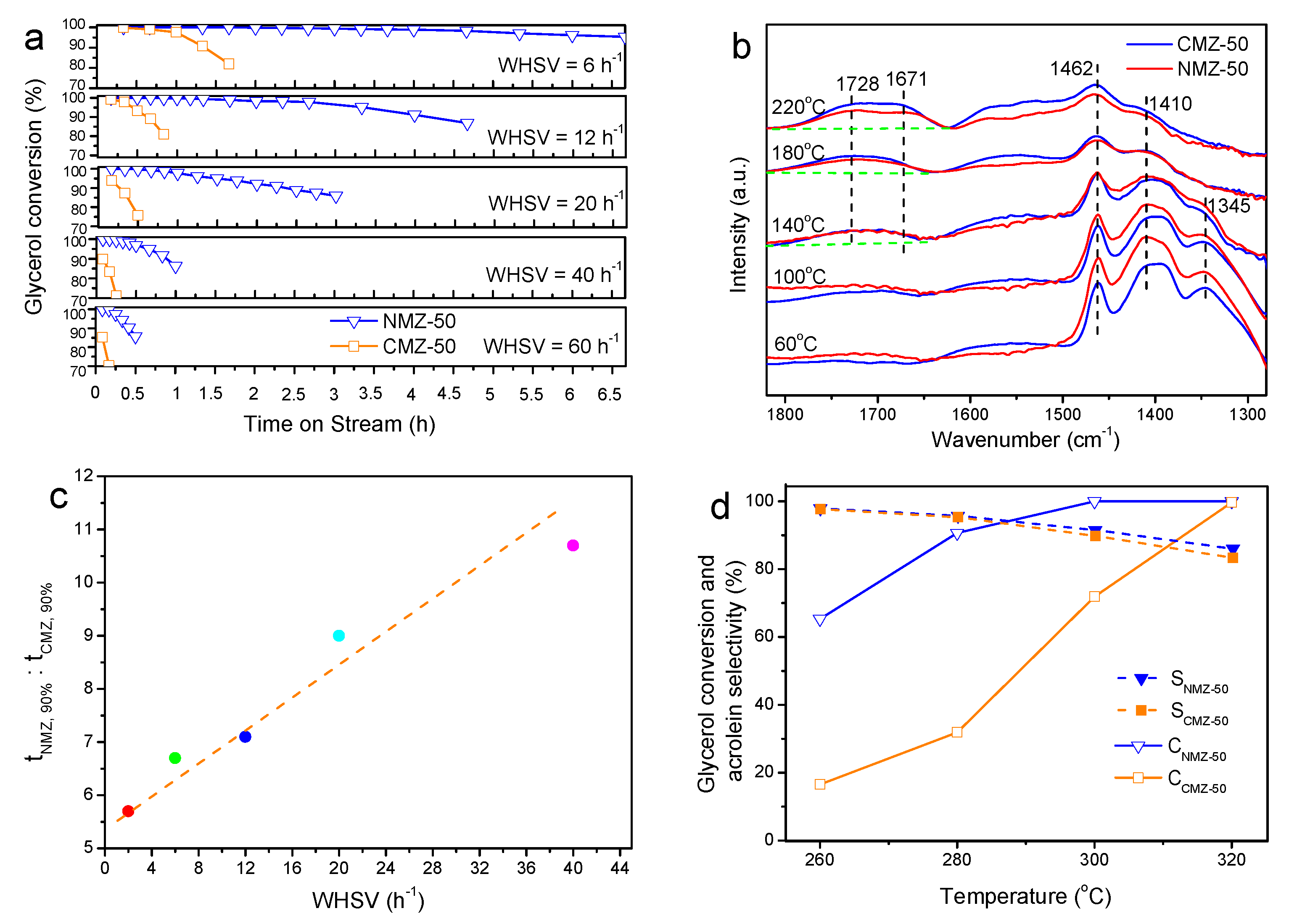
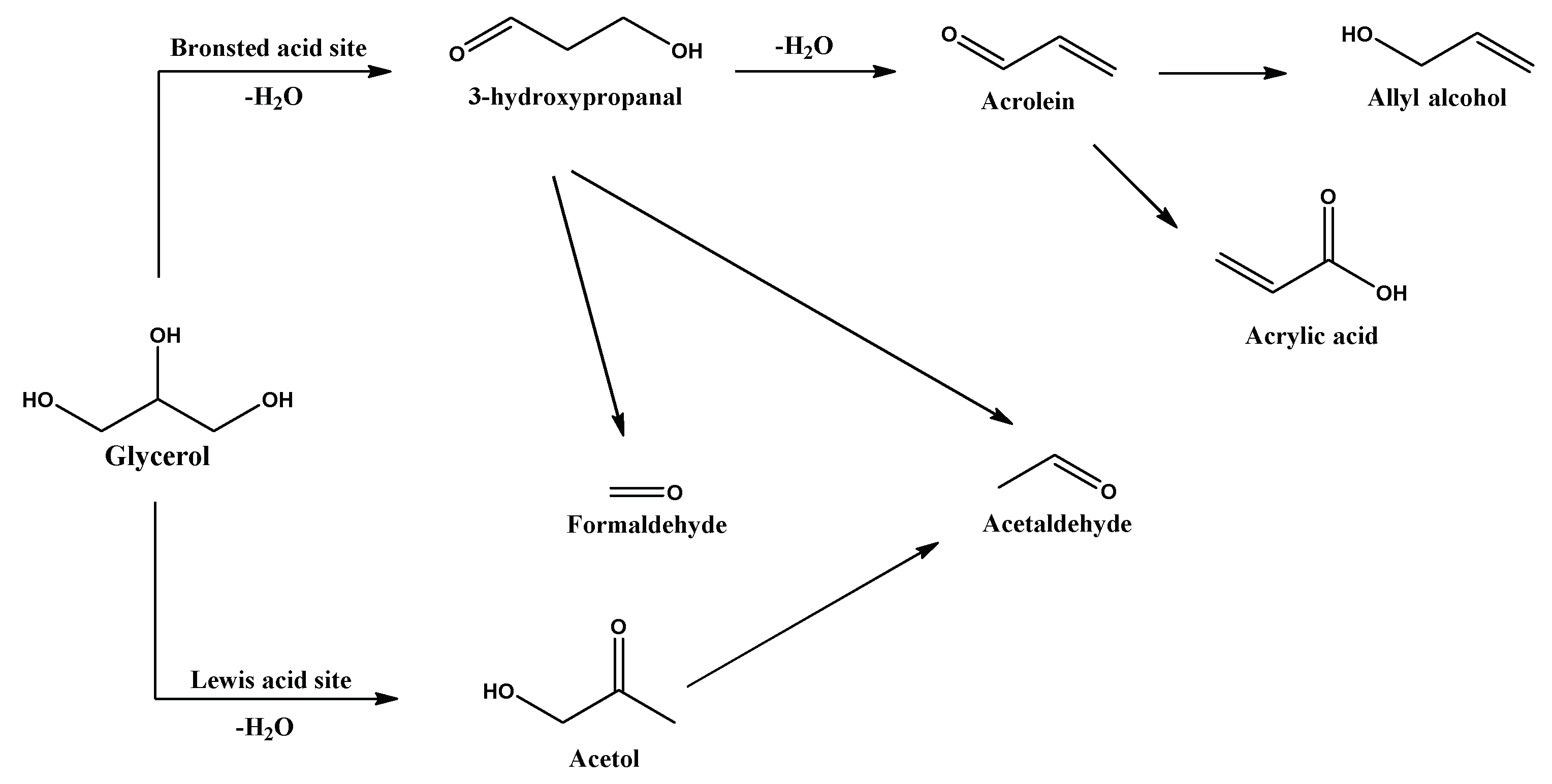
2.2. Effects of Nanosheet Structure on the Catalytic Performance for Glycerol Dehydration
2.3. Effects of Si/Al Ratios
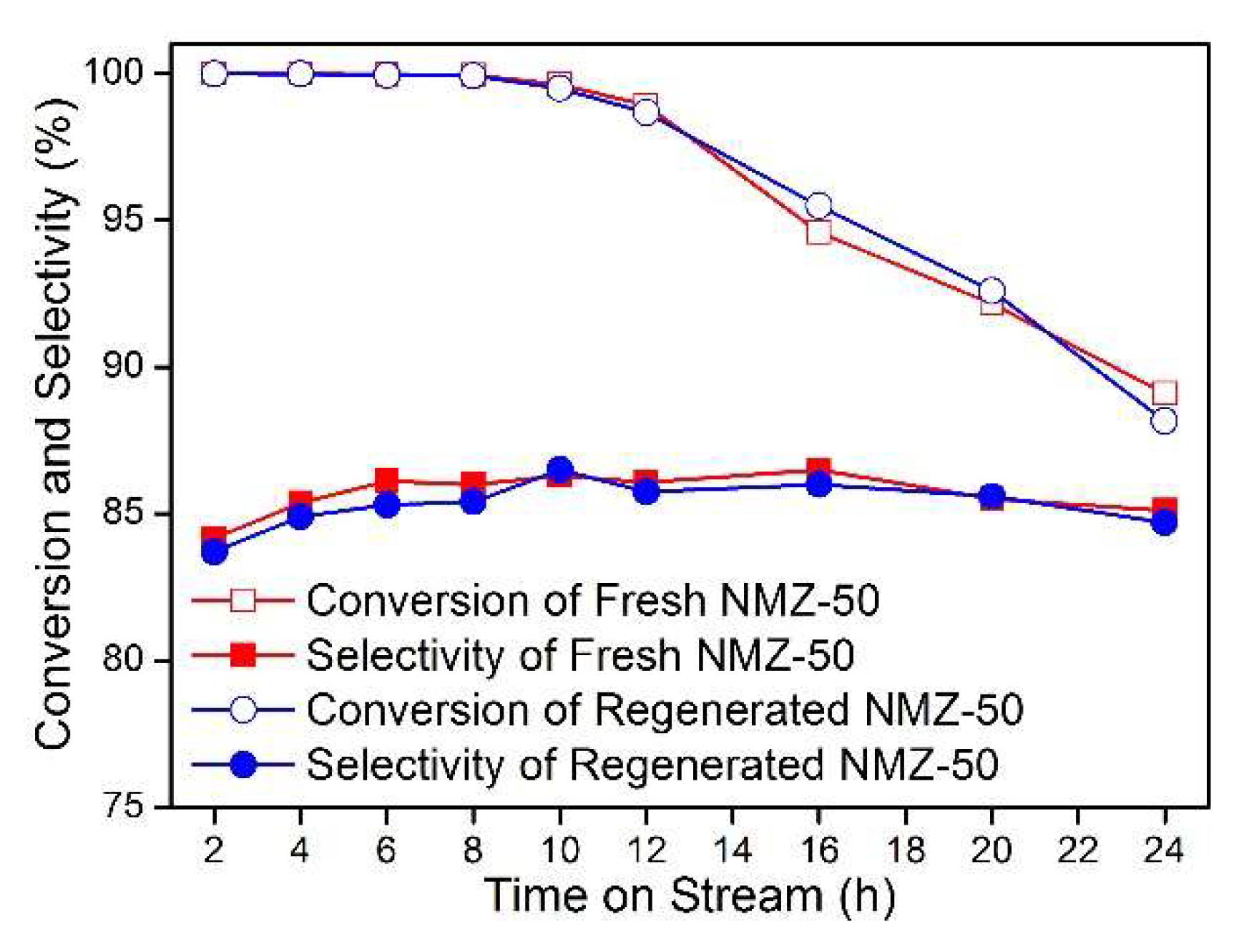
2.4. Catalyst Reutilization
3. Experimental
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B: Environ. 2016, 193, 75–92. [Google Scholar] [CrossRef]
- Cespi, D.; Passarini, F.; Mastragostino, G.; Vassura, I.; Larocca, S.; Iaconi, A.; Chieregato, A.; Dubois, J.L.; Cavani, F. Glycerol as feedstock in the synthesis of chemicals: A life cycle analysis for acrolein production. Green Chem. 2015, 17, 343–355. [Google Scholar] [CrossRef]
- Chai, S.H.; Wang, H.P.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: gas-phase dehydration of glycerol over 12-tungstophosphoric acid supported on ZrO2 and SiO2. Green Chem. 2008, 10, 1087–1093. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.P.; Contreras, J.L.; Salmones, J.; Tapia, C.; Zeifert, B.; Navarrete, J.; Vazquez, T.; Garcia, D.C. Catalytic Dehydration of Glycerol to Acrolein over a Catalyst of Pd/LaY Zeolite and Comparison with the Chemical Equilibrium. Catalysts 2017, 7, 73. [Google Scholar] [CrossRef]
- Carrico, C.S.; Cruz, F.T.; Santos, M.B.; Oliveira, D.S.; Pastore, H.O.; Andrade, H.M.C.; Mascarenhas, A.J.S. MWW-type catalysts for gas phase glycerol dehydration to acrolein. J. Catal. 2016, 334, 34–41. [Google Scholar] [CrossRef]
- Huang, L.; Qin, F.; Huang, Z.; Zhuang, Y.; Ma, J.; Xu, H.; Shen, W. Hierarchical ZSM-5 Zeolite Synthesized by an Ultrasound-Assisted Method as a Long-Life Catalyst for Dehydration of Glycerol to Acrolein. Ind. Eng. Chem. Res. 2016, 55, 7318–7327. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Dumeignil, F. Recent developments in the field of catalytic dehydration of glycerol to acrolein. ACS Catal. 2013, 3, 1819–1834. [Google Scholar] [CrossRef]
- Zhou, C.H.; Beltramini, J.N.; Fan, Y.X.; Lu, G. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Ulgen, A.; Hoelderich, W.F. Conversion of glycerol to acrolein in the presence of WO3/TiO2 catalysts. Appl. Catal. A: Gen. 2011, 400, 34–38. [Google Scholar] [CrossRef]
- Martinuzzi, I.; Azizi, Y.; Zahraa, O.; Leclerc, J.P. Deactivation study of a heteropolyacid catalyst for glycerol dehydration to form acrolein. Chem. Eng. Sci. 2015, 134, 663–670. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol as a potential renewable raw material for acrylic acid production. Green Chem. 2017, 19, 3186–3213. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Capron, M.; Dumeignil, F. Towards the sustainable production of acrolein by glycerol dehydration. ChemSusChem 2009, 2, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ye, X.P.; Bozell, J.J. A comparative review of petroleum-based and bio-based acrolein production. ChemSusChem 2012, 5, 1162–1180. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Ren, S.; Ye, X.P. Glycerol dehydration to acrolein catalyzed by ZSM-5 zeolite in supercritical carbon dioxide medium. ChemSusChem 2016, 9, 3268–3271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Jiang, Y.; Hunger, M.; Huang, J. Cooperativity of Brønsted and Lewis acid sites on zeolite for glycerol dehydration. ACS Catal. 2014, 4, 1144–1147. [Google Scholar] [CrossRef]
- García-Sancho, C.; Cecilia, J.A.; Moreno-Ruiz, A.; Mérida-Robles, J.M.; Santamaría-onzález, J.; Moreno-Tost, R.; Maireles-Torres, P. Influence of the niobium supported species on the catalytic dehydration of glycerol to acrolein. Appl. Catal. B: Environ. 2015, 179, 139–149. [Google Scholar]
- Liu, R.; Wang, T.; Jin, Y. Catalytic dehydration of glycerol to acrolein over HPW supported on Cs+ modified SBA-15. Catal. Today 2014, 233, 127–132. [Google Scholar] [CrossRef]
- Chai, S.H.; Wang, H.P.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: Investigation of solid acid-base catalysts for gas-phase dehydration of glycerol. Green Chem. 2007, 9, 1130–1136. [Google Scholar] [CrossRef]
- Jia, C.J.; Liu, Y.; Schmidt, W.; Lu, A.H.; Schüth, F. Small-sized HZSM-5 zeolite as highly active catalyst for gas phase dehydration of glycerol to acrolein. J. Catal. 2010, 269, 71–79. [Google Scholar] [CrossRef]
- Possato, L.G.; Diniz, R.N.; Garetto, T.; Pulcinelli, S.H.; Santilli, C.V.; Martins, L. A comparative study of glycerol dehydration catalyzed by micro/mesoporous MFI zeolites. J. Catal. 2013, 300, 102–112. [Google Scholar] [CrossRef]
- Ding, J.F.; Ma, T.L.; Shao, R.; Xu, W.; Wang, P.F.; Song, X.Y.; Guan, R.F.; Yeung, K.L.; Han, W. Gas phase dehydration of glycerol to acrolein on an amino siloxane-functionalized MCM-41 supported Wells-Dawson type H6P2W18O62 catalyst. New J. Chem. 2018, 42, 14271–14280. [Google Scholar] [CrossRef]
- Atia, H.; Armbruster, U.; Martin, A. Dehydration of glycerol in gas phase using heteropolyacid catalysts as active compounds. J. Catal. 2008, 258, 71–82. [Google Scholar] [CrossRef]
- Chai, S.H.; Wang, H.P.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: Gas-phase dehydration of glycerol over Nb2O5 catalyst. J. Catal. 2007, 250, 342–349. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S. Thermo-kinetic and diffusion studies of glycerol dehydration to acrolein using HSiW-gamma-Al2O3 supported ZrO2 solid acid catalyst. Renew. Energy. 2017, 114, 794–804. [Google Scholar] [CrossRef]
- Ma, T.; Ding, J.; Shao, R.; Xu, W.; Yun, Z. Dehydration of glycerol to acrolein over Wells-Dawson and Keggin type phosphotungstic acids supported on MCM-41 catalysts. Chem. Eng. J. 2017, 316, 797–806. [Google Scholar] [CrossRef]
- Kim, Y.T.; Jung, K.D.; Park, E.D. A comparative study for gas-phase dehydration of glycerol over H-zeolites. Appl. Catal. A: Gen. 2011, 393, 275–287. [Google Scholar] [CrossRef]
- Hemelsoet, K.; Van der Mynsbrugge, J.; De Wispelaere, K.; Waroquier, W.; Van Speybroeck, V. Unraveling the reaction mechanisms governing methanol-to-olefins catalysis by theory and experiment. ChemPhysChem 2013, 14, 1526–1545. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Jung, K.D.; Park, E.D. Gas-phase dehydration of glycerol over ZSM-5 catalysts. Micro. Meso. Mater. 2010, 131, 28–36. [Google Scholar] [CrossRef]
- Kongpatpanich, K.; Nanok, T.; Boekfa, B.; Probst, M.; Limtrakul, J. Structures and reaction mechanisms of glycerol dehydration over H-ZSM-5 zeolite, a density functional theory study. Phys. Chem. Chem. Phys. 2011, 13, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lv, Y.; Qu, Y.; Zhang, G.; Xi, Y.; Phillips, D.L.; Liu, C. A combined experimental and computational study of the catalytic dehydration of glycerol on microporous zeolites: An investigation of the reaction mechanism and acrolein selectivity. Phys. Chem. Chem. Phys. 2013, 15, 20120–20133. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Huber, G.W.; Sauvanaud, L.; O’Connor, P. Biomass to chemicals: Catalytic conversion of glycerol/water mixtures into acrolein, reaction network. J. Catal. 2008, 257, 163–171. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Z.; Huang, L.; Zhang, H.; Song, K.; Wang, L.; Shi, Z.; Ma, J.; Zhuang, J.; Shen, W.; Zhang, Y.; Xu, H.; Tang, Y. Dehydration of glycerol to acrolein over hierarchical ZSM-5 zeolites: Effects of mesoporosity and acidity. ACS Catal. 2015, 5, 2548–2558. [Google Scholar] [CrossRef]
- Suprun, W.; Lutecki, M.; Haber, T.; Papp, H. Acidic catalysts for the dehydration of glycerol: Activity and deactivation. J. Mol. Catal. A: Chem. 2009, 309, 71–78. [Google Scholar] [CrossRef]
- Chen, L.H.; Li, X.Y.; Rooke, J.C.; Zhang, Y.H.; Yang, X.Y.; Tang, Y.; Xiao, F.S.; Su, B.L. Hierarchically structured zeolites: Synthesis, mass transport properties and applications. J. Mater. Chem. 2012, 22, 17381–17403. [Google Scholar] [CrossRef]
- Chal, R.; Gérardin, C.; Bulut, M.; Donk, S.V. Overview and Industrial Assessment of synthesis strategies towards zeolites with mesopores. ChemCatChem 2011, 3, 67–81. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.C.; Jo, C.; Kim, T.W.; Kim, C.U.; Jeong, S.Y.; Chae, H.J. Structural and physicochemical effects of MFI zeolite nanosheets for the selective synthesis of propylene from methanol. Micro. Meso. Mater. 2016, 222, 1–8. [Google Scholar] [CrossRef]
- Hu, S.; Shan, J.; Zhang, Q.; Wang, Y.; Liu, Y.; Gong, Y.; Wu, Z.; Dou, T. Selective formation of propylene from methanol over high-silica nanosheets of MFI zeolite. Appl. Catal. A: Gen. 2012, 445, 215–220. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Park, W.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Pillared MFI zeolite nanosheets of a single-unit-cell thickness. J. Am. Chem. Soc. 2010, 132, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Brus, J.; Kobera, L.; Schoefberger, W.; Urbanova, M.; Klein, P.; Sazama, P.; Tabor, E.; Sklenak, S.; Fishchuk, A.V.; Dedecek, J. Structure of framework aluminum Lewis sites and perturbed aluminum atoms in zeolites as determined by Al-27{H-1} REDOR (3Q) MAS NMR Spectroscopy and DFT/Molecular Mechanics. Angew. Chem. Int. Ed. 2015, 54, 541–545. [Google Scholar]
- Bauerl, F.; Karge, H.G. Characterization of coke on zeolites. In Book Molecular Sieves; Springer: Berlin, Germany, 2007; Volume 5, pp. 249–364. [Google Scholar]
- Foo, G.S.; Wei, D.; Sholl, D.S.; Sievers, C. Role of Lewis and Brønsted acid sites in the dehydration of glycerol over niobia. ACS Catal. 2014, 4, 3180–3192. [Google Scholar] [CrossRef]
- Oliveira, A.S.D.; Vasconcelos, S.J.S.; Sousa, J.R.; Sousa, F.F.; Filho, J.M.; Oliveira, A.C. Catalytic conversion of glycerol to acrolein over modified molecular sieves: Activity and deactivation studies. Chem. Eng. J. 2011, 168, 765–774. [Google Scholar] [CrossRef]
- Copeland, J.R.; Foo, G.S.; Harrison, L.A.; Sievers, C. In situ ATR-IR study on aqueous phase reforming reactions of glycerol over a Pt/gamma-Al2O3 catalyst. Catal. Today. 2013, 205, 49–59. [Google Scholar] [CrossRef]
- Yi, X.D.; Zhang, X.B.; Weng, W.Z.; Wan, H.L. Studies on the reaction pathways for the selective oxidation of propane to acrolein over MoPO/SiO2 catalyst by IR spectroscopy. J. Mol. Catal. A: Chem. 2007, 277, 202–209. [Google Scholar] [CrossRef]
- Ravenelle, R.M.; Copeland, J.R.; Pelt, A.H.V.; Crittenden, J.C.; Sievers, C. Stability of Pt/gamma-Al2O3 Catalysts in Model Biomass Solutions. Top Catal. 2012, 55, 162–174. [Google Scholar] [CrossRef]
- Schmidt, F.; Hoffmann, C.; Giordanino, F.; Bordiga, S.; Simon, P.; Carrillo-Cabrera, W.; Kaskel, S. Coke location in microporous and hierarchical ZSM-5 and the impact on the MTH reaction. J. Catal. 2013, 307, 238–245. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, C.; Xue, Y.; Wu, J.; Wang, J.; Fan, W. Graphene oxide: An efficient acid catalyst for alcoholysis and esterification reactions. ChemCatChem 2014, 6, 3080–3083. [Google Scholar] [CrossRef]
- Madeira, F.F.; Ben Tayeb, K.; Pinard, L.; Vezin, H.; Maury, S.; Cadran, N. Ethanol transformation into hydrocarbons on ZSM-5 zeolites: Influence of Si/Al ratio on catalytic performances and deactivation rate. Study of the radical species role. Appl. Catal A: Gen. 2012, 443, 171–180. [Google Scholar] [CrossRef]



| Sample | Si/Ala | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | ||||
|---|---|---|---|---|---|---|---|
| Total | Microb | Externalc | Total | Mesod | |||
| NMZ-30 | 28 | 543 | 271 | 272 | 0.67 | 0.63 | |
| NMZ-50 | 46 | 563 | 267 | 296 | 0.71 | 0.66 | |
| NMZ-100 | 94 | 432 | 241 | 191 | 0.53 | 0.45 | |
| CMZ-50 | 48 | 355 | 262 | 93 | 0.21 | 0.10 | |
| CMZ-100 | 96 | 363 | 275 | 88 | 0.22 | 0.12 | |
| CMZ-150 | 143 | 370 | 279 | 91 | 0.21 | 0.11 | |
| Sample | TOS (h) | Conversion (%) | Product Selectivity (%) | ||
|---|---|---|---|---|---|
| Acrolein | Acetol | Othersb | |||
| NMZ-30 | 4 | 99.7 | 81.8 | 7.9 | 10.3 |
| 8 | 98.3 | 82.8 | 8.4 | 8.8 | |
| NMZ-50 | 4 | 99.8 | 85.4 | 4.9 | 9.7 |
| 8 | 99.7 | 85.9 | 5.3 | 8.8 | |
| NMZ-100 | 4 | 99.2 | 86.8 | 4.7 | 8.5 |
| 8 | 97.1 | 86.3 | 5.2 | 8.5 | |
| CMZ-50 | 4 | 91.3 | 84.3 | 5.1 | 10.6 |
| 8 | <80.0 | - | - | - | |
| CMZ-100 | 4 | 95.4 | 84.7 | 4.8 | 10.5 |
| 8 | 89.9 | 85.1 | 5.0 | 9.9 | |
| CMZ-150 | 4 | 93.5 | 86.5 | 4.7 | 8.8 |
| 8 | 87.3 | 85.8 | 4.4 | 9.8 | |
| Sample | TOSa (h) | Coke Contentb (%) | Coke Deposition Ratec (gcoke gcat−1 h−1) | Surface Aread (m2 g−1)/Percentage Remained (%) | ||
|---|---|---|---|---|---|---|
| Total | Micro | External | ||||
| NMZ-50 | 36 | 27.5 | 1.05 × 10−2 | 296/52.6 | 99/37.1 | 197/66.6 |
| CMZ-50 | 7 | 11.7 | 1.89 × 10−2 | 66/18.5 | 42/16.0 | 24/25.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, J.; Li, Z.; Zhu, S.; Liu, H.; Li, J.; Wang, J.; Fan, W. Nanosheet MFI Zeolites for Gas Phase Glycerol Dehydration to Acrolein. Catalysts 2019, 9, 121. https://doi.org/10.3390/catal9020121
Shan J, Li Z, Zhu S, Liu H, Li J, Wang J, Fan W. Nanosheet MFI Zeolites for Gas Phase Glycerol Dehydration to Acrolein. Catalysts. 2019; 9(2):121. https://doi.org/10.3390/catal9020121
Chicago/Turabian StyleShan, Jianfeng, Zhikai Li, Shanhui Zhu, Huan Liu, Junfen Li, Jianguo Wang, and Weibin Fan. 2019. "Nanosheet MFI Zeolites for Gas Phase Glycerol Dehydration to Acrolein" Catalysts 9, no. 2: 121. https://doi.org/10.3390/catal9020121
APA StyleShan, J., Li, Z., Zhu, S., Liu, H., Li, J., Wang, J., & Fan, W. (2019). Nanosheet MFI Zeolites for Gas Phase Glycerol Dehydration to Acrolein. Catalysts, 9(2), 121. https://doi.org/10.3390/catal9020121






