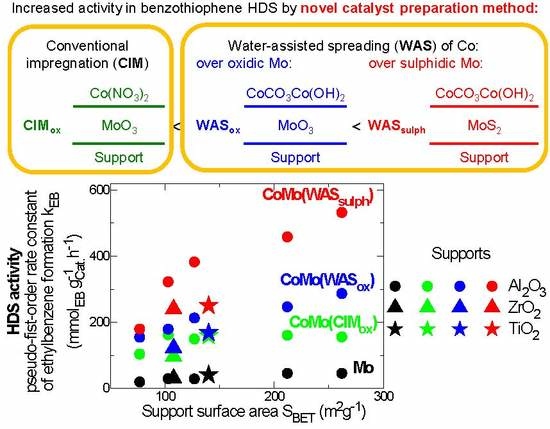
Deposition of Co onto Supported MoS2 by Water-Assisted Spreading: Increased Activity Promotion in Hydrodesulphurization of 1-Benzothiophene
Abstract
1. Introduction
2. Results and Discussion
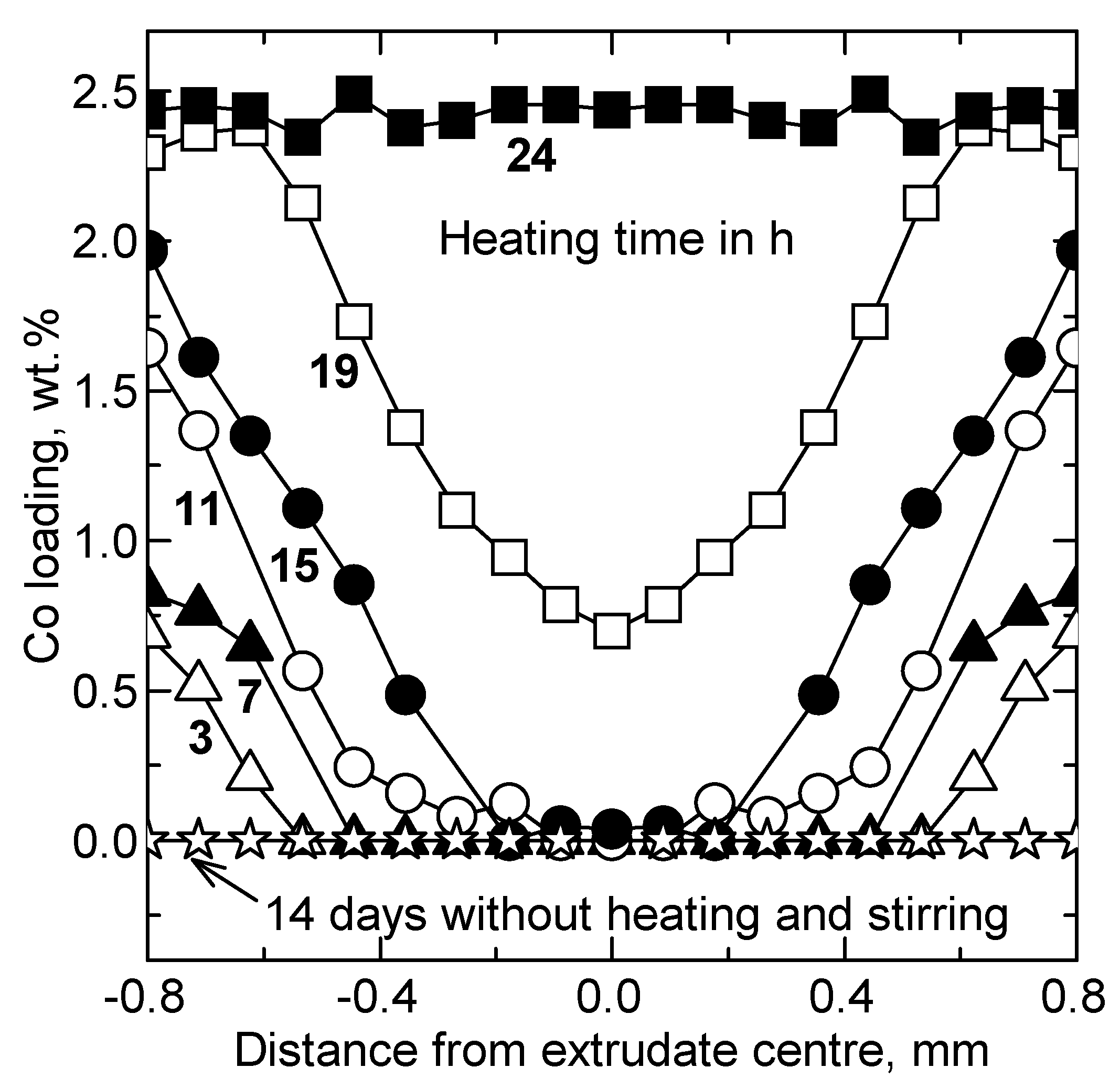
2.1. Concept of Co Deposition onto Sulphidic Mo Catalysts
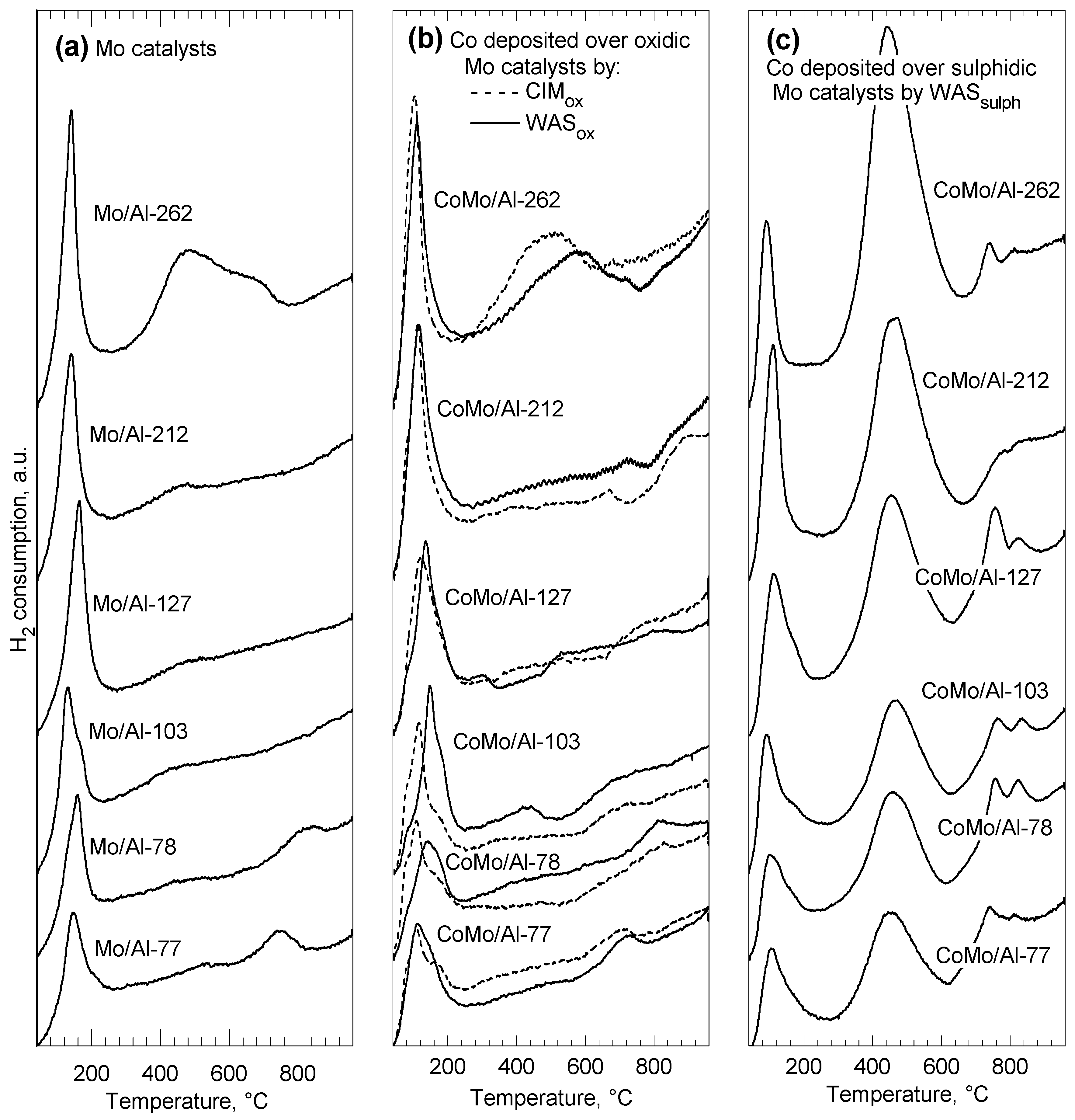
2.2. TPR and XPS of Sulphided Catalysts
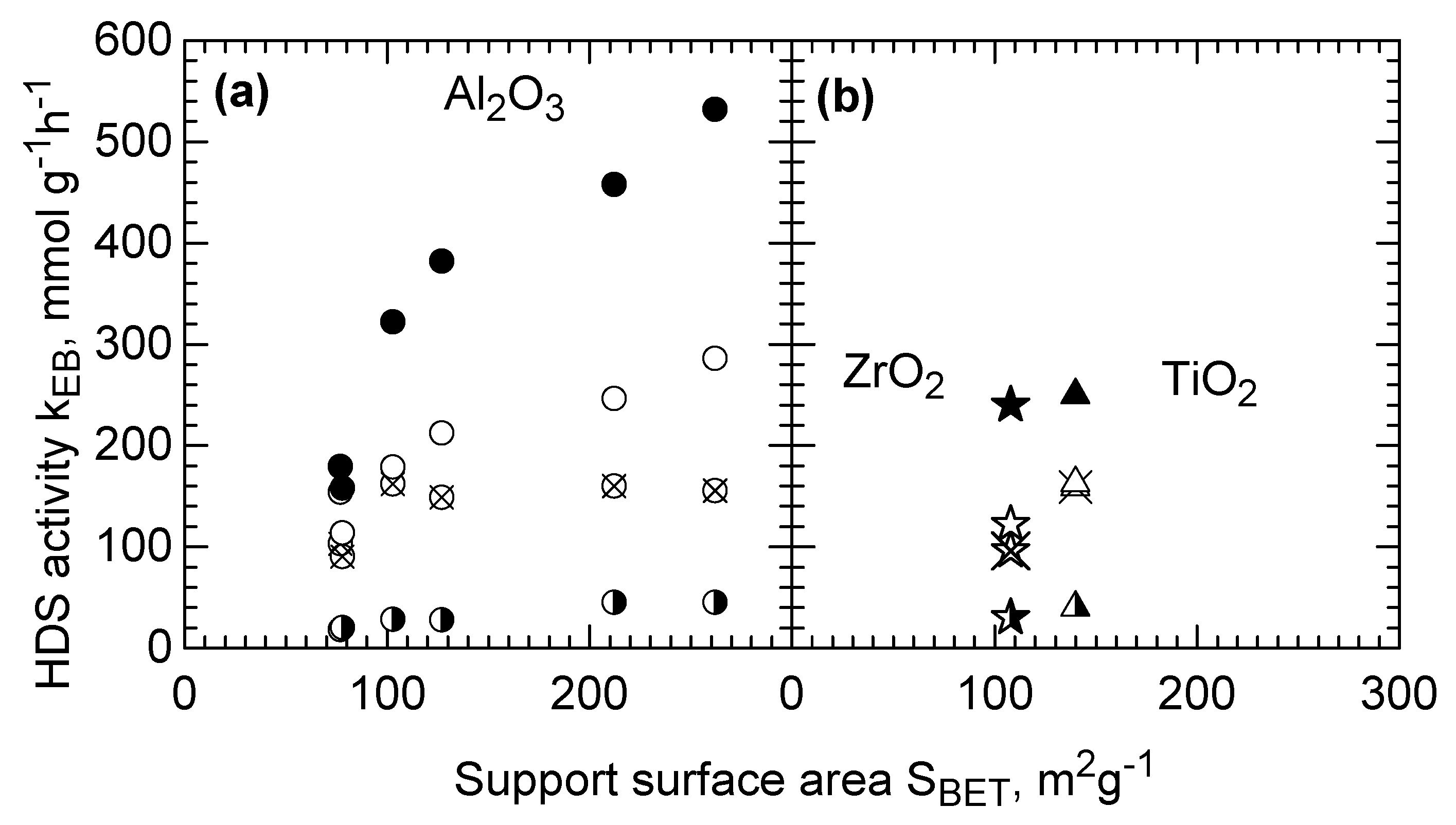
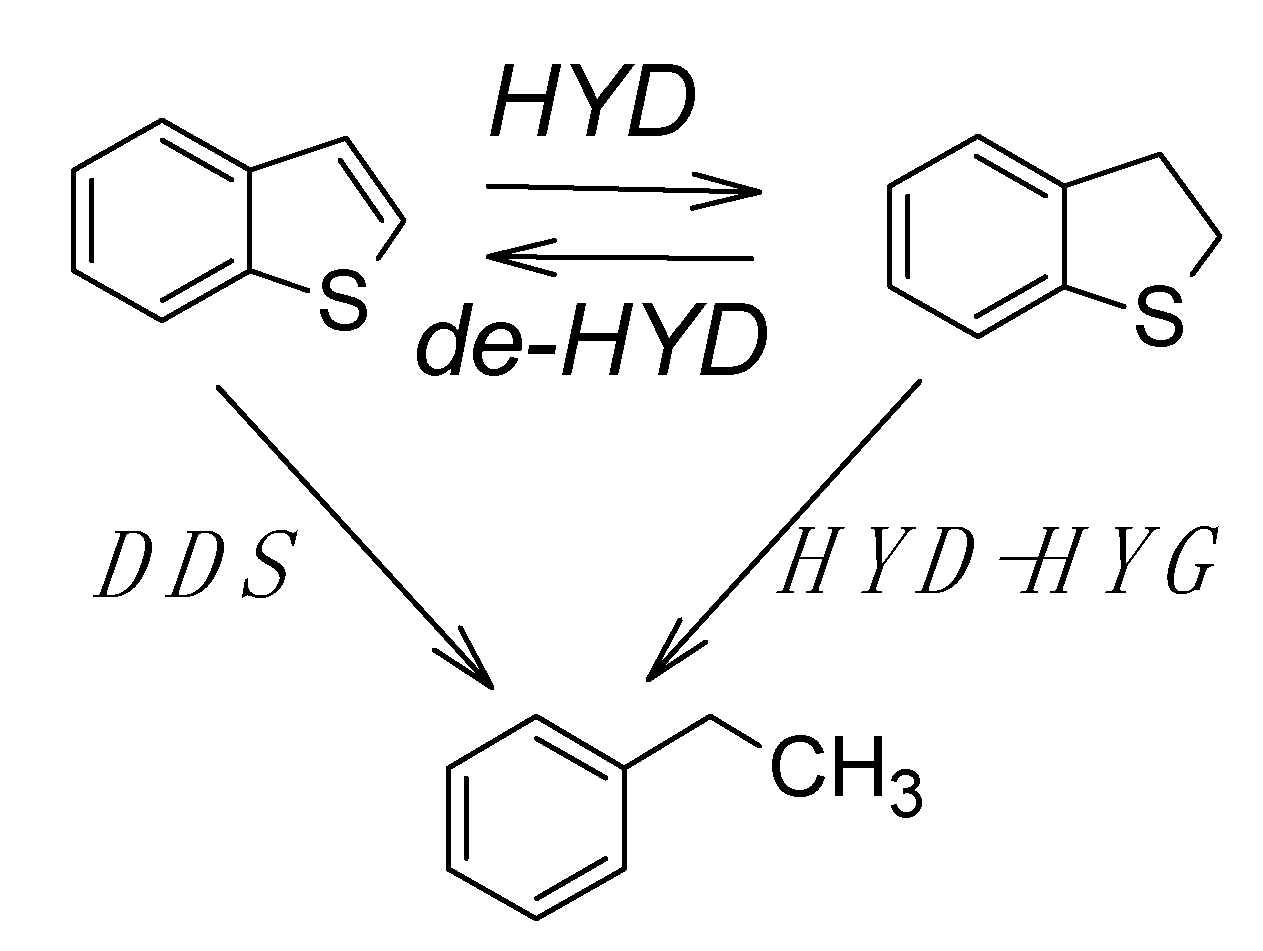
2.3. HDS Activity
3. Materials and Methods
3.1. Catalysts Preparation
3.1.1. Mo Deposition
3.1.2. Co Deposition
3.1.3. WASsulph over Extrudates
3.2. Reference Catalyst
3.3. Characterization
3.4. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toulhoat, H.; Raybaud, P. Catalysis by Transition Metal Sulphides; Editions Technip: Paris, France, 2013; pp. 1–787. [Google Scholar]
- Lélias, M.A.; Kooyman, P.J.; Mariey, L.; Oliviero, L.; Travert, A.; Van Gestel, J.; Van Veen, J.A.R.; Maugé, F. Effect of NTA addition on the structure and activity of the active phase of cobalt-molybdenum sulfide hydrotreating catalysts. J. Catal. 2009, 267, 14–23. [Google Scholar] [CrossRef]
- Escobar, J.; Barrera, M.C.; De los Reyes, J.A.; Toledo, J.A.; Santes, V.; Colín, J.A. Effect of chelating ligands on Ni–Mo impregnation over wide-pore ZrO2–TiO2. J. Mol. Catal. A 2008, 287, 33–40. [Google Scholar] [CrossRef]
- Bremmer, G.M.; Van Haandel, L.; Hensen, E.J.M.; Frenken, J.W.M.; Kooyman, P.J. The effect of oxidation and resulfidation on (Ni/Co)MoS2 hydrodesulfurisation catalysts. Appl. Catal. B Environ. 2019, 243, 145–150. [Google Scholar] [CrossRef]
- Bezverzkyy, I.; Afanasiev, P.; Lacroix, M. Promotion of highly loaded MoS2/Al2O3 hydrodesulfurization catalysts prepared in aqueous solution. J. Catal. 2005, 230, 133–139. [Google Scholar] [CrossRef]
- Ji, Y.; Afanasiev, P.; Vrinat, M.; Li, W.; Li, C. Promoting effects in hydrogenation and hydrodesulfurization reactions on the zirconia and titania supported catalysts. Appl. Catal. A Gen. 2004, 257, 157–164. [Google Scholar] [CrossRef]
- Afanasiev, P. Mixed TiO2–ZrO2 support for hydrotreating, obtained by co-precipitation from Zr basic carbonate and Ti oxosulfate. Catal. Commun. 2008, 9, 734–739. [Google Scholar] [CrossRef]
- Farag, H.; Whitehurst, D.D.; Mochida, I. Synthesis of Active Hydrodesulfurization Carbon-Supported Co-Mo Catalysts. Relationships between Preparation Methods and Activity/Selectivity. Ind. Eng. Chem. Res. 1998, 37, 3533–3539. [Google Scholar] [CrossRef]
- Gulková, D.; Zdražil, M. Increased promotion by deposition of Co acetylacetonate over sulphided Mo/TiO2 hydrodesulphurization catalyst. React. Kinet. Catal. Lett. 2007, 91, 379–384. [Google Scholar] [CrossRef]
- Kaluža, L.; Gulková, D.; Šolcová, O.; Žilková, N.; Čejka, J. Hydrotreating catalysts supported on organized mesoporous alumina: Optimization of Mo deposition and promotional effects of Co and Ni. Appl. Catal. A Gen. 2008, 351, 93–101. [Google Scholar] [CrossRef]
- Maugé, F.; Vallet, A.; Bachelier, J.; Duchet, J.C.; Lavalley, J.C. Preparation, Characterization, and Activity of Sulfided Catalysts Promoted by Co(CO)3NO Thermodecomposition. J. Catal. 1996, 162, 88–95. [Google Scholar]
- Okamoto, Y.; Ishihara, S.; Kawano, M.; Satoh, M.; Kubota, T. Preparation of Co–Mo/Al2O3 model sulfide catalysts for hydrodesulfurization and their application to the study of the effects of catalyst preparation. J. Catal. 2003, 217, 12–22. [Google Scholar] [CrossRef]
- Kaluža, L.; Vít, Z.; Zdražil, M. Preparation and properties of filled monolayer of MoO3 deposited on Al2O3 supports by solvent-assisted spreading. Appl. Catal. A Gen. 2005, 282, 247–253. [Google Scholar] [CrossRef]
- Kaluža, L.; Zdražil, M. Deposition of CoO onto MoO3/Al2O3 hydrodesulfurization catalysts by solvent assisted spreading. Top. Catal. 2007, 45, 191–194. [Google Scholar] [CrossRef]
- Leyrer, J.; Mey, D.; Knözinger, H. Spreading Behavior of Molybdenum Trioxide on Alumina and Silica: A Raman Microscopy Study. J. Catal. 1990, 124, 349–356. [Google Scholar] [CrossRef]
- Kisfaludi, G.; Leyrer, J.; Knözinger, H.; Prins, R. An EXAFS Study of the Spreading of MoO3 on the Surface of γ-Al2O3. J. Catal. 1991, 130, 192–201. [Google Scholar] [CrossRef]
- Günther, S.; Marsi, M.; Kolmakov, A.; Kiskinova, M.; Noeske, M.; Taglauer, E.; Mestl, G.; Schubert, U.A.; Knözinger, H. Photoelectron Spectromicroscopic Study of the Spreading Behavior of MoO3 on Titania and Alumina Model Supports. J. Phys. Chem. B 1997, 101, 10004–10011. [Google Scholar] [CrossRef]
- Kaluža, L.; Gulková, D.; Vít, Z.; Zdražil, M. Effect of support type on the magnitude of synergism and promotion in CoMo sulphide hydrodesulphurisation catalyst. Appl. Catal. A Gen. 2007, 324, 30–35. [Google Scholar] [CrossRef]
- Das, K.K. Electrokinetics of mineral particles. In Interfacial Electrokinetics and Electrophoresis; Delgado, A.V., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 799–824. [Google Scholar]
- Lewis, R.; Edwards, H.G.M. Handbook of Raman Spectroscopy; Marcel Dekker Inc.: New York, NY, USA, 2001; pp. 1–1049. [Google Scholar]
- Golasa, K.; Grzeszczyk, M.; Korona, K.P.; Bozek, R.; Binder, J.; Szczytko, J.; Wysmolek, A.; Babinski, A. Optical Properties of Molybdenum Disulfide (MoS2). Acta Phys. Pol. A 2013, 124, 849–851. [Google Scholar] [CrossRef]
- Mestl, G.; Srinivasan, T.K.K. Raman Spectroscopy of Monolayer-Type Catalysts: Supported Molybdenum Oxides. Cat. Rev. Sci. Eng. 1998, 40, 451–570. [Google Scholar] [CrossRef]
- Debecker, D.P.; Stoyanova, M.; Rodemerck, U.; Gaigneaux, E.M. Preparation of MoO3/SiO2-Al2O3 metathesis catalysts via wet impregnation with different Mo precursors. J. Mol. Catal. A 2011, 340, 65–76. [Google Scholar] [CrossRef]
- Zingg, D.S.; Makovsky, L.E.; Tischer, R.E.; Brown, F.R.; Hercules, D.M. A Surface Spectroscopic Study of Molybdenum-Alumina Catalysts Using X-ray Photoelectron, Ion-Scattering, and Raman Spectroscopies. J. Phys. Chem. 1980, 84, 2898–2906. [Google Scholar] [CrossRef]
- Dufresne, P.; Payen, P.; Grimblot, J.; Bonnelle, J.P. Study of Ni-Mo-γ-Al2O3 Catalysts by X-ray Photoelectron and Raman Spectroscopy. Comparison with Co-Mo-γ-Al2O3 Catalysts. J. Phys. Chem. 1981, 85, 2344–2351. [Google Scholar] [CrossRef]
- Afanasiev, P. Calculation of MoS2 slabs morphology descriptors from transmission electron microscopy data revisited. Case study of the influence of citric acid and treatment conditions on the properties of MoS2/Al2O3. Appl. Catal. A Gen. 2017, 529, 10–19. [Google Scholar] [CrossRef]
- Baubet, B.; Devers, E.; Hugon, A.; Leclerc, E.; Afanasiev, P. The influence of MoS2 slab 2D morphology and edge state on the properties of alumina-supported molybdenum sulfide catalysts. Appl. Catal. A Gen. 2014, 487, 72–81. [Google Scholar] [CrossRef]
- Dinter, N.; Rusanen, M.; Raybaud, P.; Kasztelan, S.; Da Silva, P.; Toulhoat, H. Temperature-programmed reduction of unpromoted MoS2-based hydrodesulfurization catalysts: First-principles kinetic Monte Carlo simulations and comparison with experiments. J. Catal. 2010, 275, 117–128. [Google Scholar] [CrossRef]
- Scheffer, B.; Dekker, N.J.J.; Magnus, P.J.; Mouljin, J.A. A temperature-programmed reduction study of sulfided Co-Mo/Al2O3 hydrodesulfurization catalysts. J. Catal. 1990, 121, 31–46. [Google Scholar] [CrossRef]
- Calais, C.; Matsubayashi, N.; Geantet, C.; Yoshimura, Y.; Shimada, H.; Nishijima, A.; Lacroix, M.; Breysse, M. Crystallite size determination of highly dispersed unsupported MoS2 catalysts. J. Catal. 1998, 174, 130–141. [Google Scholar] [CrossRef]
- Da Silva, P. Influence de la Taille des Particules de MoS2 Supportées sur l´Activité et la Sélectivité des Reactions d´Hydrotraitement; Thèse UMPC Paris VI—ENSPM. Ph.D. Thesis, Université Pierre-et-Marie-Curie, Paris, France, 1998. [Google Scholar]
- Yoosuka, B.; Tumnantong, D.; Prasassarakich, P. Unsupported MoS2 and CoMoS2 catalysts for hydrodeoxygenation of phenol. Chem. Eng. Sci. 2012, 79, 1–7. [Google Scholar] [CrossRef]
- Raybaud, P.; Hafner, J.; Kresse, G.; Kasztelan, S.; Toulhoat, H. Structure, Energetics, and Electronic Properties of the Surface of a Promoted MoS2 Catalyst: An ab Initio Local Density Functional Study. J. Catal. 2000, 190, 128–143. [Google Scholar] [CrossRef]
- Yoosuk, B.; Kim, J.H.; Song, C.; Ngamcharussrivichai, C.; Prasassarakich, P. Highly active MoS2, CoMoS2 and NiMoS2 unsupported catalysts prepared by hydrothermal synthesis for hydrodesulfurization of 4,6-dimethyldibenzothiophene. Catal. Today 2008, 130, 14–23. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Chai, Y.; Zhao, J.; Li, Y.; Liu, D.; Liu, Y.; Liu, C. Effect of sulfiding conditions on the hydrodesulfurization performance of the ex-situ presulfided CoMoS/γ-Al2O3 catalysts. Fuel 2018, 234, 1144–1153. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Chai, Y.; Zhao, J.; Li, Y.; Liu, Y.; Liu, C. Highly Active CoMoS/Al2O3 Catalysts ex Situ Presulfided with Ammonium Sulfide for Selective Hydrodesulfurization of Fluid Catalytic Cracking Gasoline. Ind. Eng. Chem. Res. 2018, 57, 2041–2049. [Google Scholar] [CrossRef]
- Kooyman, P.J.; Van Veen, J.A.R. The detrimental effect of exposure to air on supported MoS2. Catal. Today 2008, 130, 135–138. [Google Scholar] [CrossRef]
- Bremmer, G.M.; Van Haandel, L.; Hensen, E.J.M.; Frenken, J.W.M.; Kooyman, P.J. Instability of NiMoS2 and CoMoS2 hydrodesulfurization catalysts at ambient conditions: A quasi in situ high-resolution transmission electron microscopy and x-ray photoelectron spectroscopy study. J. Phys. Chem. C 2016, 120, 19204–19211. [Google Scholar] [CrossRef]
- Breysse, M.; Afanasiev, P.; Geantet, C.; Vrinat, M. Overview of support effects in hydrotreating catalysts. Catal. Today 2003, 86, 5–16. [Google Scholar] [CrossRef]
- Moya, S.A.; Escudey, M. Use of pH measurements for the characterization of MoO3/Al2O3 catalysts. J. Chem. Soc. Chem. Commun. 1994, 16, 1829–1830. [Google Scholar] [CrossRef]
- Nikulshina, M.; Kokliukhin, A.; Mozhaev, A.; Nikulshin, P. CoMo/Al2O3 hydrotreating catalysts prepared from single Co2Mo10-heteropolyacid at extremely high metal loading. Catal. Commun. 2019, 127, 51–57. [Google Scholar] [CrossRef]
- Song, W.; Lai, W.; Chen, Z.; Cao, J.; Wang, H.; Lian, Y.; Yang, W.; Jiang, X. Fabrication of 3D Porous Hierarchical NiMoS Flowerlike Architectures for Hydrodesulfurization Applications. ACS Appl. Nano Mater. 2018, 1, 442–454. [Google Scholar] [CrossRef]


| Oxidation | Atomic % | ||||||
|---|---|---|---|---|---|---|---|
| Time | O 1s | Al 2p | Mo 3d | S 2p | |||
| Mo4+ | MoSxOy | Mo6+ | S2− | S6+ | |||
| 0 min | 59.81 | 33.48 | 1.94 | 0.01 | 0.23 | 4.54 | 0 |
| 5 min | 59.16 | 33.80 | 2.03 | 0.04 | 0.23 | 4.73 | 0 |
| 10 min | 58.71 | 34.46 | 1.96 | 0.05 | 0.20 | 4.63 | 0 |
| 1 h | 59.04 | 34.13 | 1.96 | 0.07 | 0.25 | 4.57 | 0 |
| 2 h | 59.85 | 33.19 | 1.89 | 0.08 | 0.32 | 4.45 | 0.23 |
| 1 day | 59.59 | 33.75 | 1.76 | 0.08 | 0.38 | 4.10 | 0.35 |
| 6 days | 61.96 | 31.82 | 1.36 | 0.13 | 0.68 | 3.57 | 0.48 |
| 1 month | 62.63 | 31.09 | 1.34 | 0.18 | 0.76 | 3.28 | 0.72 |
| Support | Loading, wt.% (±0.1) | SBET, m2g−1 | Promotional Effect PE (± 1.0) | ||||
|---|---|---|---|---|---|---|---|
| Mo | Co | Co | (±5) | (PE = kEBCoMo/kEBMo) | |||
| WAS a | WASox a | WASsulph | WASsulph | CIMox b | WASox | WASsulph | |
| Al-77 | 4.5 | 0.5 | 0.8 | 85 | 5.6 | 8.4 | 9.8 |
| Al-78 | 4.1 | 0.4 | 0.8 | 81 | 4.5 | 5.7 | 7.8 |
| Al-103 | 6.3 | 0.6 | 0.8 | 109 | 5.2 | 6.4 | 11.4 |
| Al-127 | 7.4 | 1.0 | 1.2 | 131 | 5.4 | 7.6 | 13.7 |
| Al-212 | 12.3 | 1.0 | 1.7 | 215 | 3.6 | 5.5 | 10.2 |
| Al-262 | 14.3 | 2.0 | 2.4 | 244 | 3.4 | 6.3 | 11.8 |
| Al-262-e c | 14.3 | - | 2.4 | 249 | - | - | 11.1 |
| Zr-108 | 6.0 | 1.3 | 2.0 | 101 | 3.2 | 4.1 | 8.0 |
| Ti-140 | 7.9 | 2.0 | 2.8 | 130 | 4.0 | 4.1 | 6.3 |
| CoMo Catalysts | Atomic % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| O 1s | Al 2p | Co | Mo 3d | S 2p | |||||
| Co2+ox | Co2+sulph | Mo4+ | MoSxOy | Mo6+ | S2− | S6+ | |||
| WASsulph/Al-262 | 59.84 | 30.89 | 0.56 | 1.32 | 1.69 | 0.52 | 0.24 | 5.99 | 0 |
| WASsulph/Al-212 | 57.96 | 32.99 | 0.55 | 1.19 | 1.65 | 0.47 | 0.26 | 5.06 | 0 |
| WASsulph/Al-127 | 57.24 | 33.05 | 0.37 | 0.95 | 0.82 | 0.33 | 0.16 | 4.02 | 0 |
| WASox/Al-262 | 57.72 | 32.96 | 0.74 | 0.33 | 1.60 | 0.56 | 0.37 | 5.70 | 0 |
| WASox/Al-212 | 58.02 | 34.13 | 0.50 | 0.23 | 1.56 | 0.50 | 0.27 | 4.80 | 0 |
| WASox/Al-127 | 58.73 | 35.50 | 0.47 | 0.33 | 0.75 | 0.38 | 0.17 | 3.27 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaluža, L.; Koštejn, M.; Gulková, D. Deposition of Co onto Supported MoS2 by Water-Assisted Spreading: Increased Activity Promotion in Hydrodesulphurization of 1-Benzothiophene. Catalysts 2019, 9, 987. https://doi.org/10.3390/catal9120987
Kaluža L, Koštejn M, Gulková D. Deposition of Co onto Supported MoS2 by Water-Assisted Spreading: Increased Activity Promotion in Hydrodesulphurization of 1-Benzothiophene. Catalysts. 2019; 9(12):987. https://doi.org/10.3390/catal9120987
Chicago/Turabian StyleKaluža, Luděk, Martin Koštejn, and Daniela Gulková. 2019. "Deposition of Co onto Supported MoS2 by Water-Assisted Spreading: Increased Activity Promotion in Hydrodesulphurization of 1-Benzothiophene" Catalysts 9, no. 12: 987. https://doi.org/10.3390/catal9120987
APA StyleKaluža, L., Koštejn, M., & Gulková, D. (2019). Deposition of Co onto Supported MoS2 by Water-Assisted Spreading: Increased Activity Promotion in Hydrodesulphurization of 1-Benzothiophene. Catalysts, 9(12), 987. https://doi.org/10.3390/catal9120987