2. Results and Discussion
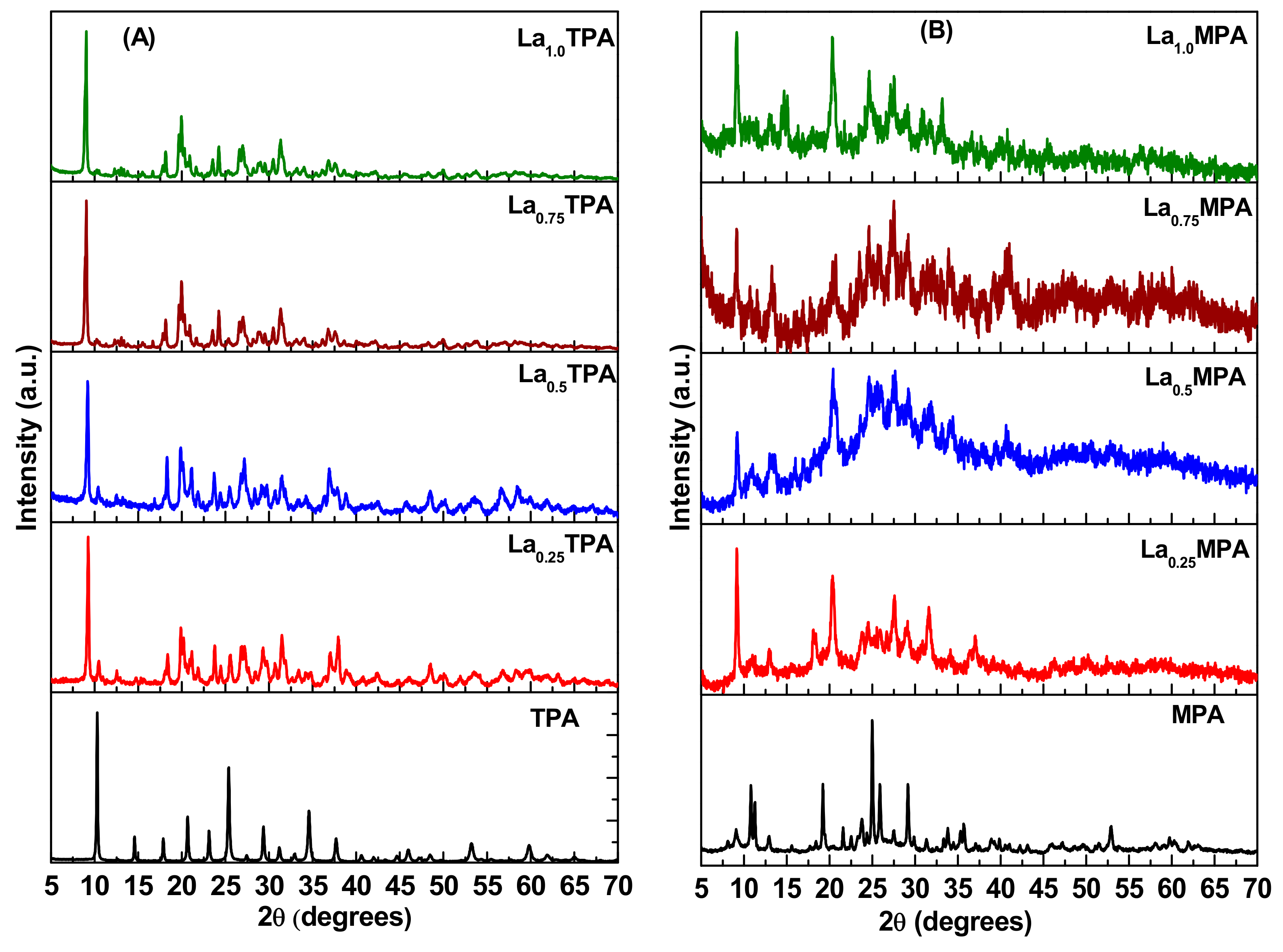
The phase composition and crystal structure of the synthesized materials were studied using a powder XRD technique. The XRD patterns of both La
xTPA and La
xMPA samples are shown in
Figure 1. The pure TPA sample, which was calcined at 300 °C, showed all the reflections corresponding to a cubic Pn3m space group [JCPDS PDF#50-0657]. The XRD pattern of La
0.25TPA sample exhibited a new set of sharp reflections. The reflections corresponding to pure TPA disappeared as the La atom composition increased beyond 0.50 (La
0.75TPA and La
1.0TPA samples). The shift in 2θ positions of reflections towards lower angles in the La
xTPA samples is consistent with the previously reported results [
16,
17]. The XRD patterns of calcined MPA and La
xMPA samples are also shown in
Figure 1.
The reflections of the calcined MPA sample are similar to the characteristics of the monoclinic Keggin structure. After incorporating the La, the reflections due to MPA disappeared and new reflections due to lanthanum salt of MPA appeared in all La exchanged MPA samples. It is interesting to note that the XRD patterns of La
xMPA samples also exhibited few broad humps due to the presence of an unknown amorphous material, which was not observed in the case of La
xTPA samples. This is probably due to the fact that MPA thermal stability is lower than TPA and a slight amount of Keggin compound was decomposed into oxides when La
xMPA samples were used. The sharp reflections observed in all the materials revealed that synthesized La
xTPA and La
xMPA are highly crystalline. The most intense XRD reflections for bulk TPA, MPA, and La exchanged salts were used to determine the crystallite size by applying the Scherrer equation, and the results are presented in
Table 1. The results show that the crystallite size decreased from 50 nm to 20 nm as the La composition increased to La
0.25TPA, and the crystallite size increased to 25 nm for La
1.0TPA material, demonstrating the formation of agglomerates in the case of La
1.0TPA.
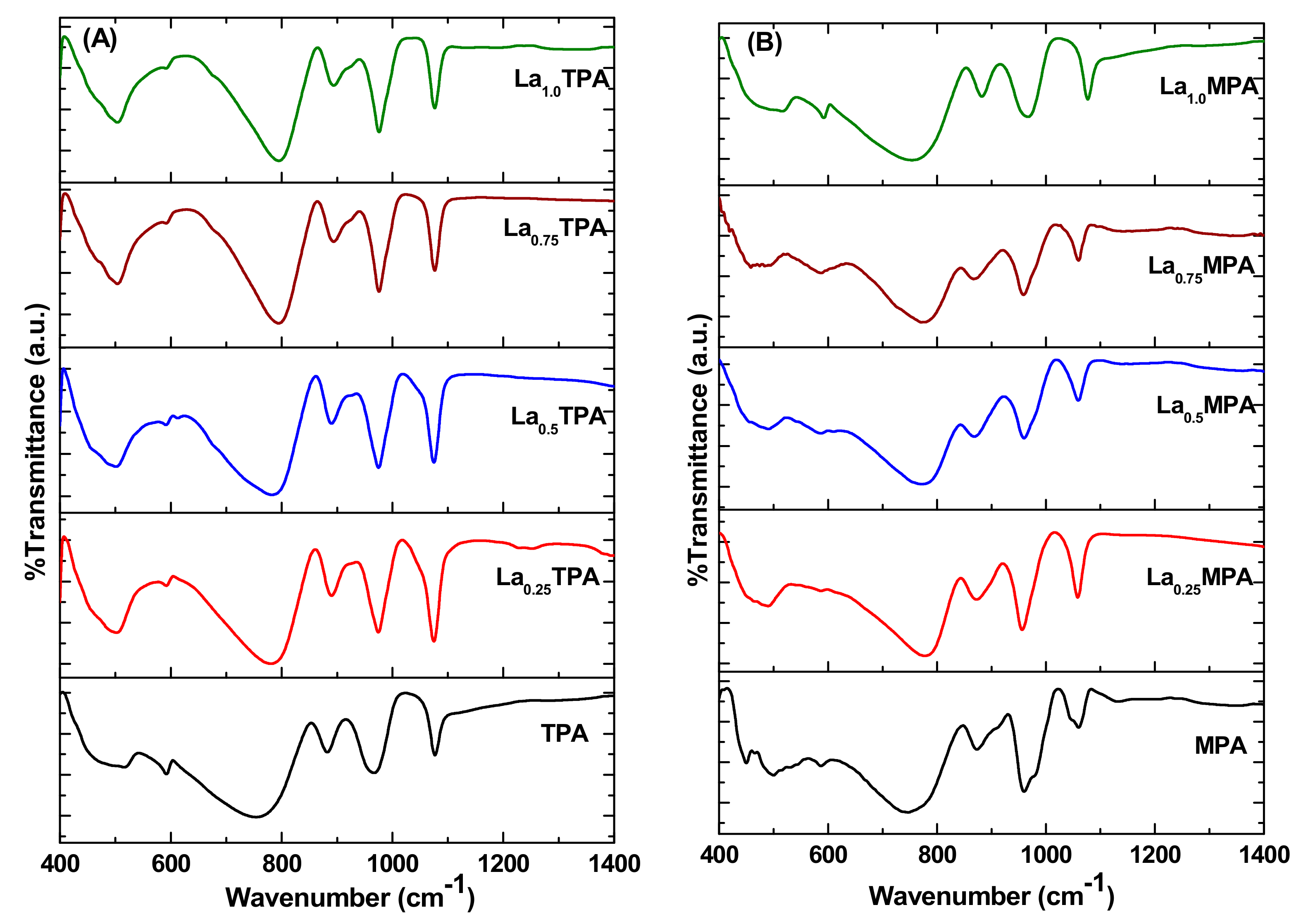
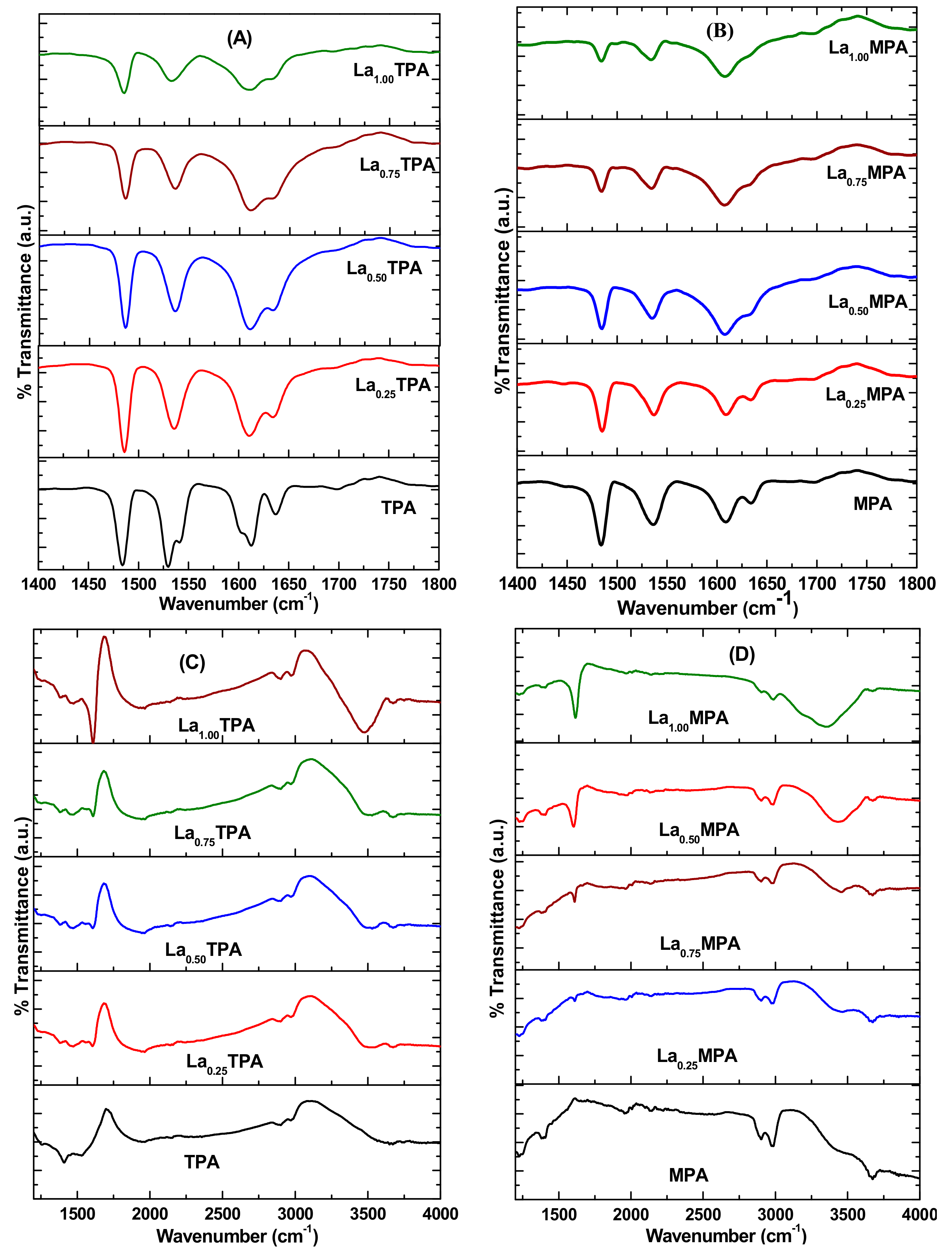
To study the structural features of synthesized samples, FT-IR analysis was also performed. The FT-IR spectra of La
xTPA and La
xMPA samples are shown in
Figure 2. The FT-IR spectrum of the TPA sample showed Keggin structure characteristic bands at 1080 cm
−1 (P-O
i), 965 cm
−1 (W-O
t), 885 cm
−1 (W-O
c-W), and 755 cm
−1 (W-O
e-W), where
i,
t,
c, and
e represent the precise position of oxygen atoms (internal, terminal, corner, and edge-shared) in the Keggin structure [
12]. It is clear that all the La
xTPA samples exhibited characteristic bands of the Keggin structure, which is an indication that the original structure was intact after the La exchange. However, it is interesting to note that position of the band corresponding to W-O
e-W stretching mode shifted from 755 cm
−1 to 782 cm
−1, 786 cm
−1, 790 cm
−1, and 800 cm
−1 for La
0.25TPA, La
0.5TPA, La
0.75TPA, and La
1.0TPA, respectively.
The Keggin ion in pure calcined MPA sample shows FT-IR bands at 1060 cm
−1, 960 cm
−1, 870 cm
−1, and 750 cm
−1 corresponds to P-O
i, Mo-O
t, Mo-O
c-Mo, and Mo-O
e-Mo bonds, respectively [
18]. All the FT-IR bands corresponding to Keggin ion appeared in La
xMPA samples, and also a shift in the peak position of Mo-O
e-Mo stretching mode was observed in these samples. The interaction between the Keggin anion [PW
12O
40]
3− or [PMo
12O
40]
3− and the Lewis acid La
3+ ions forms La-O-W bonds. This synergistic effect could change some characteristic peak positions. However, it is important to understand the location of La ions. It is well known that incorporated foreign metal ion in the Keggin structure could induce variations in the FT-IR spectra of samples [
19]. It was previously reported that incorporated metal ion reduced the symmetry of [PO
4]
3− ion, which resulted in a split in the P-O stretching band [
18]. The FT-IR spectra of La
xTPA and La
xMPA materials did not exhibit any peaks at 1080 cm
−1 or 1060 cm
−1 after La exchange. These results revealed that La atoms did not replace the W or Mo in peripheral positions of the Keggin ion, but they do act as cations in the secondary structure.
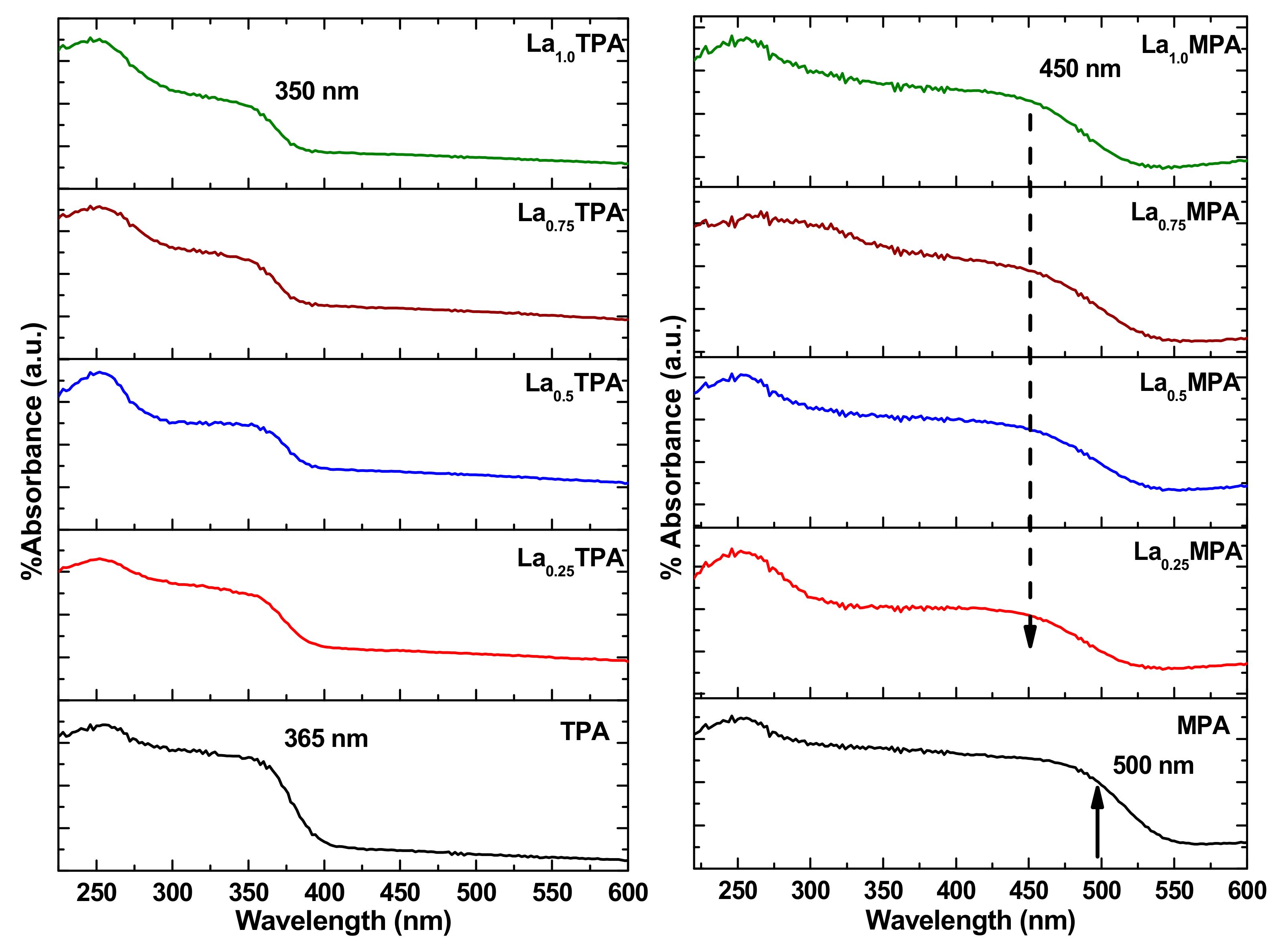
The structural aspects of the synthesized La exchanged TPA and MPA samples were investigated further with the DR UV-vis technique. The DR UV-vis spectra of La
xTPA and La
xMPA materials are presented in
Figure 3. The edge energy values were determined from the wavelength of the UV-vis absorption edge. The pure TPA and MPA samples clearly showed a representative weak absorption band at 260 nm (4.76 eV), which could be assigned to an O-W charge transfer in the Keggin ion [
20]. A wide peak centered at 365 nm (3.39 eV) was also observed which was due to the ligand-metal charge transfer from O to W
6+ in the Keggin anion; these observations are in agreement with a previous research report [
17]. The La
xTPA samples exhibited similar absorption peaks to that of the bulk TPA sample. However, the adsorption edge for La
1.00TPA samples was shifted to 3.54 eV, indicating that incorporation of La ions resulted in the shifting of the edge energy towards the visible-light region. Also, an increase of the La content resulted in an increase in the peak intensity, indicating that La concentration affected the optical absorption properties. The La
1.00TPA sample showed better optical absorption ability compared to La
0.25TPA and other synthesized samples. The DR UV-vis absorption spectra of MPA and La
xMPA samples were similar to that of TPA and La
xTPA samples. The wide absorption peak (due to LMCT) wavelength value of MPA and La
xMPA was higher than for TPA and La
xTPA samples. It was observed that the bulk MPA exhibited an energy edge at 2.47 eV which was shifted to 2.75 eV for the La
xMPA samples. These results indicated that the La
xMPA samples possessed an energy edge in the visible light region.
The morphology and microstructure of the investigated samples were studied by SEM analysis.
Figure S1 exhibits the SEM micrographs of investigated samples. The SEM images of TPA and La
0.25TPA samples revealed that these samples consisted of nanoparticles with an average pore width of around 35 nm. An increase of the La content resulted in an increase of particle size. It was observed that the large size irregular particles were formed by the aggregation of smaller particles. The largest size particles (with an average particle size of 115 nm) were observed for the La
1.00TPA sample. The XRD results are in good agreement that the reflection that La exchanged TPA samples are sharper compared to the TPA sample. The SEM micrographs of MPA and La
xMPA samples revealed that these samples are composed of spherical particles. Large-sized particles were observed in the case of La
xMPA samples which were similar to that of La
xTPA samples.
It is clear that the amount of La had a significant influence on the HPA particle size, however similar morphology of La
xTPA and La
xMPA samples indicated that the La content did not change the morphology of the samples. The SEM analysis results indicated that La
xTPA and La
xMPA materials were formed due to the self-assembly of several smaller HPA particles. The textural characteristics of the La
xTPA and La
xMPA samples were obtained from the N
2 physisorption results. The nitrogen adsorption-desorption isotherms of the investigated samples are depicted in
Figure S2. The nitrogen adsorption-desorption isotherms of TPA and La
xTPA samples are similar, and both show a slight increase with any increases of P/P
0. The isotherms clearly belong to Type II isotherms [
21], which are generally due to a single to multi-layer reversible adsorption process occurring on non-porous or macroporous solid surfaces. The observed results indicate that the samples possessed macro size pores, consistent with the measurement of the pore size.
Similar results were observed in the case of MPA and La
xMPA samples, as shown in
Figure S2 in the Supplementary Materials. The S
BET values increased from 2.5 m
2g
−1 (for TPA) to 36 m
2g
−1 (for the La
1.00TPA sample). The observed results are in accordance with morphological changes of the samples, and previous reports also reported similar observations [
22] suggesting that metal salts of TPA have higher surface areas compared to TPA. The pore size distribution measurements revealed an increase in the average pore width from 50 Å to 62 Å with an increase of the La amount (
Table 1). It was reported that large voids exist among the micro crystallites of the metal exchanged HPA salts [
23], as closely packed particles aggregates could form mesoporous voids. Inter particle spaces between micro crystallites are responsible for the increase in the average pore width for the La exchanged TPA and MPA samples.
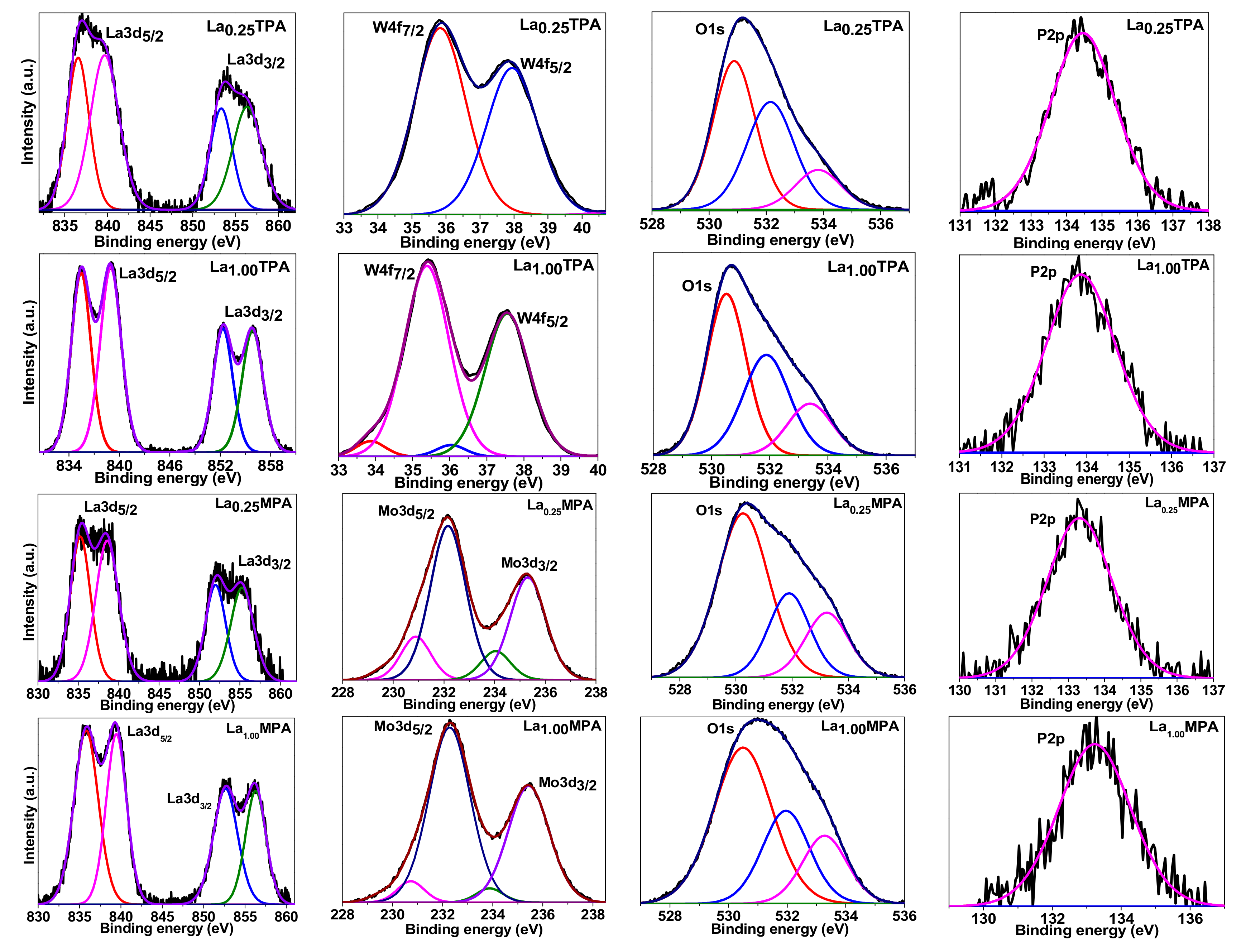
Figure 4 represents the deconvoluted XP spectra of representative samples. The La
3d photoelectron spectra for La
0.25TPA, La
1.00TPA, La
0.25MPA, and La
1.00MPA samples can be seen in Figure 6. It was reported that La
3d core spectrum splits into
3d5/2 and
3d3/2 lines because of spin-orbit interaction. The La exchanged HPA samples showed XPS peaks at around 836.6 eV, 839.7 eV, 853.3 eV, and 856.3 eV related to La
3d5/2 and La
3d3/2 components, respectively. And the two peaks in each line could be assigned to main and satellite peaks due to
3d0 4f0 and
3d0 4f1L configurations [
24]. It was observed that different types of La compounds exhibit satellite peaks at different binding energies [
25]. It was previously observed that the pure La
2O
3 displayed peaks at 835.8 eV and 839.3 eV related to La
3d5/2 components (La
+3 species) [
26]. From the XP spectra, it is clear that La
3d5/2 and La
3d3/2 peaks were observed at higher binding energy values, which is mainly due to the strong interaction of La
3+ with O moieties from the Keggin anion.
The W
4f XP peaks for La
0.25TPA sample appeared at 35.8 eV and 37.9 eV and were related to W
4f7/2 and W
4f5/2 contributions. It was reported that pure TPA sample (W
6+) exhibits W
4f7/2 and W
4f5/2 peaks at 35.8 eV and 37.8 eV [
27], respectively. Therefore, the surface characteristics of La
0.25TPA could be very similar to the TPA sample, as a very small amount of La was incorporated into the HPA structure. On other hand, the La
1.00TPA sample exhibited two different W
4f7/2 and W
4f5/2 contributions corresponding to W
6+ and W
5+ species (33.9 eV and 36.0 eV). This mainly due to the presence of some oxygen defect sites in La
1.00TPA, so some part of W
6+ is reduced to W
5+. It was observed that both La
0.25MPA and La
1.00MPA materials show two sets of Mo
3d5/2 and Mo
3d3/2 species corresponding to Mo
6+ and Mo
5+, while Mo
3d5/2 peaks observed at 232.2 eV and 231.0 eV can be attributed to Mo
6+ and Mo
5+ species of the HPA [
28]. It is well known that Mo based HPAs are more easily reducible than the W based HPAs, while electron localization occurs in a reduced Mo or W Keggin structure.
A single broad P
2p XP peak was observed in case of all the investigated La exchanged HPA samples. It was previously reported that in a well crystallized HPA structure consists of a single XP peak between 133–134 eV [
28] and also the FWHM of peaks appears to be almost the same. These observations suggest that there is no collapse of the Keggin structure of the HPA. The XP spectra of the samples showed three O
1s peaks at 531.2 eV, 532.6 eV, and 534.1 eV, respectively. The peak at 531.2 eV could be assigned to W-O-W or Mo-O-Mo species [
29]. The peak at 532.6 eV is attributed to W-O-P or Mo-O-P species. The third peak at 534 eV could be attributed to O-La species on the surface of the sample. There is a clear possibility for the presence of water molecules to seen on the sample surface, however these samples are calcined at 300 °C and a previous XPS study revealed that the binding energy of O
1s in water appears at around 535.5 eV [
30].
The bulk and surface composition of La
xTPA and La
xMPA materials were studied by ICP-AES and XPS techniques, respectively. A good agreement between theoretical and actual La concentration was observed across the investigated range La
0.25–La
1.00 (
Table 2). The surface La concentration is marginally lesser than the bulk concentration, indicating that the surface of catalyst has La-depletion. It was observed that there is a considerable difference between the surface and bulk La/W(Mo) ratios in the case of La
0.25TPA and La
0.25MPA samples. The observed deviation could be due to the formation of La exchanged salt, during which La
xPW
12O
40/La
xPMo
12O
40 crystals were covered by a surface layer of TPA or MPA, while a similar phenomenon was observed in the case of potassium salt from the heteropolyacid catalyst [
31]. In order to understand the type of acidic sites presented in the investigated catalysts, the samples were analyzed by the pyridine infrared adsorption method, and the results are shown in
Figure 5. It is well known that there are three major bands with respect to pyridine adsorption over the surface acid sites. The band that appeared at 1450 cm
−1 was due to the coordinately bonded pyridine over the Lewis (L) acid sites and the band that appeared at 1545 cm
−1 was mainly caused by pyridinium ion over the Brönsted (B) acid sites. The third IR band that appeared at 1505 cm
−1 was due to the pyridine molecules that bonded with both L and B acid sites [
32]. From
Figure 5, it is clear that La
xTPA and La
xMPA materials possessed both B and L acid sites. The B acid sites of the samples are created by the dissociation of H
2O molecules over La
3+ HPA structure; [La(H
2O)
n]
3+[PW
12O
40] or [La(OH)(H
2O)
n-1]
2+H
+[PW
12O
40].
The pure TPA and MPA possess the largest number of acid sites compared to the La exchanged samples and increase of La content led to a decrease in both B and L acid sites. The reason for the differences in the total number of acid sites of La
xTPA and La
xMPA samples and bulk HPAs was that the La exchanged samples possessed extra L acid sites due to the presence of La
3+ and also unsaturated Mo
6+ or W
6+ cations, whereas the B acid sites had unexchanged protons [
33]. To evaluate the basic properties of the La exchanged TPA and MPA samples, the H-donor pyrrole was used as a reacting molecule. The main features of FT-IR spectra of pyrrole adsorbed samples are presented in
Figure 5. Researchers utilized the ν(NH) stretching frequencies in the NH-O hydrogen bridge to measure the basic strength of zeolites and metal oxides [
32]. It was previously reported that the pyrrole adsorbed samples exhibits different types of narrow and broad bands due to the pyrrole adsorbed on basic sites [
34]. The La exchanged TPA and MPA samples exhibited five different new bands at 3678 cm
−1 (small), 3470 cm
−1 (broad), 1620 cm
−1 (sharp), 1450 cm
−1 (small), and 1375 cm
−1 (small) compared to bulk TPA and MPA samples. It was well reported that the several peaks appear in the range of 3560–2850 cm
−1 as combination bands, which are associated with strong basic sites [
35]. The bands that appeared at 1630 cm
−1 and 1450 cm
−1 are very similar for liquid pyrrole and are suggestive of pyrrole being chemisorbed on basic sites [
36]. The results from the figure clearly indicated that the intensity of the peaks due to basic sites increased with an increase of the La content in the HPA structure. The total acidic and basic sites per unit surface area values were calculated and presented in
Table S1. As expected, the La
1.0TPA sample possessed a large number of basic sites per unit surface area.
Transesterification of glyceryl tributyrate with methanol is a reversible chemical reaction to produce methyl-esters (also known as biodiesel) and glycerin, and this process comprises three different elementary reactions [
12]. In the first elementary step, a reaction between triglycerides and methanol yields methyl-esters and diglycerides. In the subsequent step [Equation (1)], the reaction between diglycerides and methanol obtains methyl-esters and monoglycerides. In the last step, the monoglycerides turn into methyl-esters and glycerin.
Catalytic transesterification activity measurements over LaxTPA and LaxMPA materials were performed to obtain the proper reaction conditions by changing different parameters such as the reaction temperature, reaction time, amount of catalyst, and the methanol/glyceryl tributyrate molar ratio. To evaluate the effect of La3+ ions in HPA structure on the transesterification activity, both LaxTPA and LaxMPA samples were tested for their reactions.
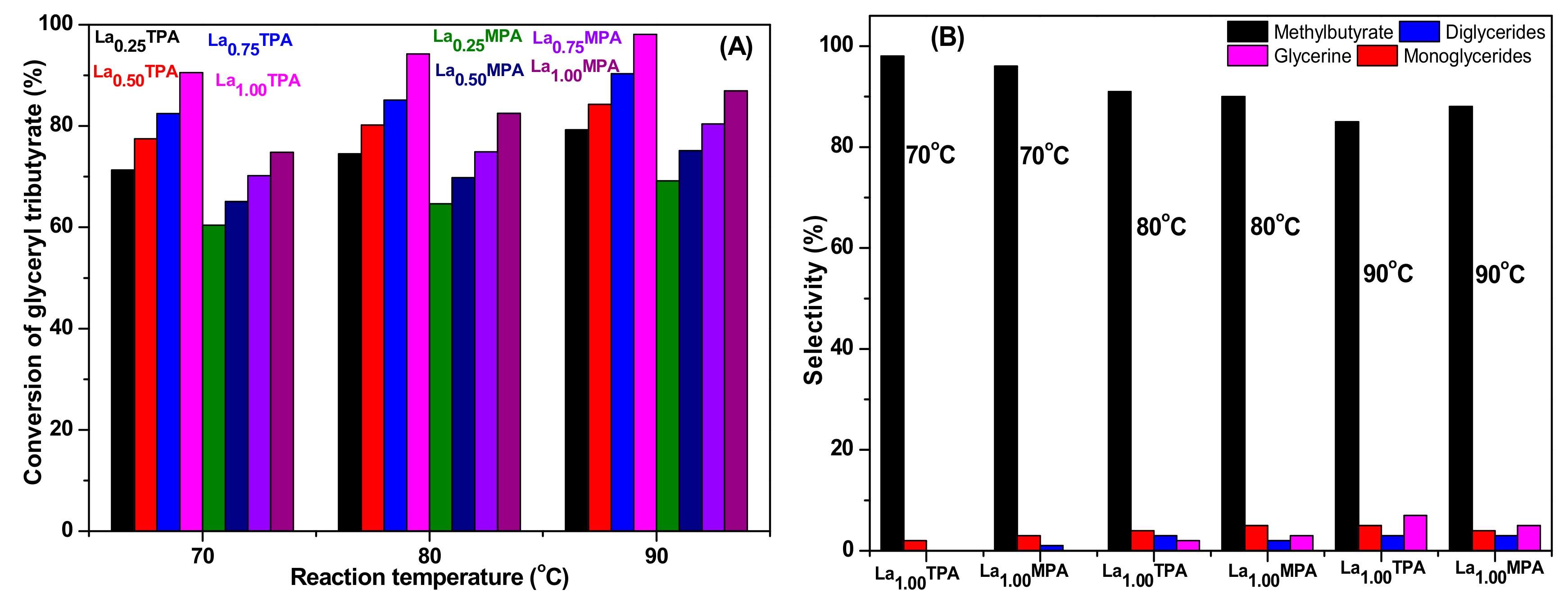
Figure 6 represents the conversion of glyceryl tributyrate and selectivities of different products obtained at three different reaction temperatures (70, 80, and 90 °C) using La
xTPA and La
xMPA materials. The obtained results clearly indicated that La
xTPA samples are efficient catalysts for transesterification compared to La
xMPA samples. The conversion of glyceryl tributyrates were enhanced with an increase of the reaction temperature in all catalyst samples. The La
1.00TPA sample exhibited 90.5%, 94.2%, and 98.1% of glyceryl tributyrate conversion at 70 °C, 80 °C, and 90 °C, respectively, after 150 min of reaction time. On other hand, the La
1.00MPA sample exhibited 74.8%, 82.5%, and 86.9% under same reaction conditions. The selectivities of transesterification products were also changed when the reaction temperature was altered for both series of La
xTPA and La
xMPA samples.
The selectivities of the products observed are in accordance with different reaction steps of transesterification of glyceryl tributyrate, as shown in Equations (1)–(3). It was clear that initially the triglycerides converts into diglycerides, and subsequently into monoglycerides and glycerin. At low conversions, the selectivity to methyl butyrate is between 96% and 98% in the case of highly active La1.00TPA and La1.00MPA catalysts. The selectivity to methyl butyrate was slightly lowered at high reaction temperatures, due to the formation of more glycerin. Low selectivity to monoglyceride was observed due to the fact that monoglyceride has a tendency to convert into methyl butyrate and glycerin. It was observed that a maximum of 7% selectivity to glycerin occurred for La1.0TPA sample.
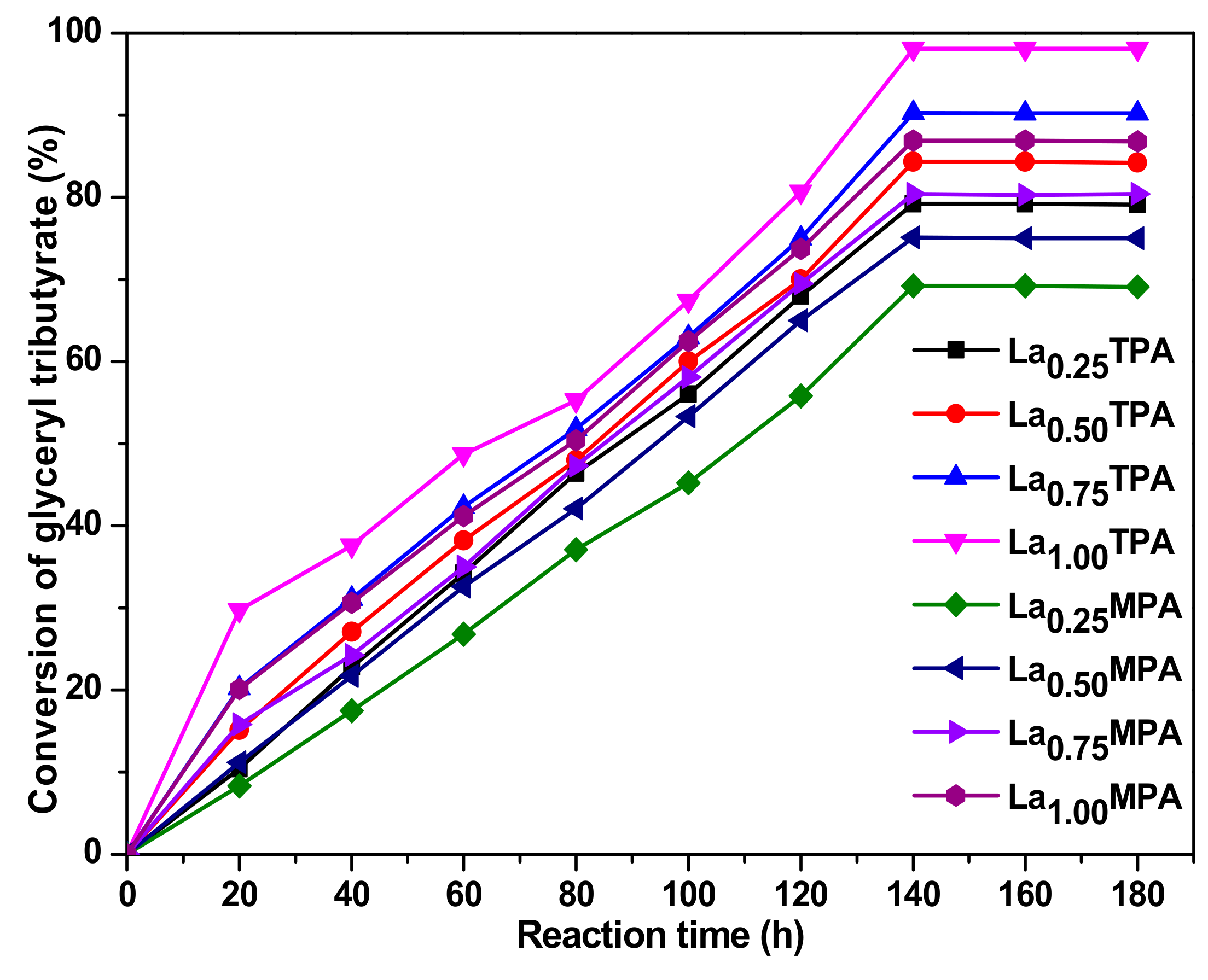
The influence of a reaction time over the conversion of glyceryl tributyrate and selectivity towards methyl butyrate for all the synthesized catalysts was studied at 90 °C and the results are shown in
Figure 7. Initially, the glyceryl tributyrate conversion levels increased linearly until they reached 120 min and reached a peak at 140 min. A further increase of the reaction time has not affected the conversion levels, indicating that the equilibrium was established at 120 min. This is mainly due to the fact that the transesterification continues via a stepwise reaction of the triglycerides, which led to the production of intermediate diglycerides and monoglycerides, which finally transformed into biodiesel and glycerin.
As observed in the FT-IR spectral analysis of pyrrole adsorbed La
xHPA samples, any increase in the La
3+ concentration (from 0.25 to 1.00) resulted in an increase in the number of basic sites of La
xHPA samples, which subsequently influenced the transesterification activity.
Figure 6 and
Figure 7 indicate that an increase of the La
3+ ion composition from 0.25 to 1.00 led to continuous enhancing of the activity, as the highest activity was found in the case of the catalyst that contained a La
3+ composition of 1.00. However, we have not increased the La
3+ composition beyond the stoichiometry of Keggin ion, due to the fact that incorporation of more counter cations could decompose the Keggin ion structure to form its oxides. The observed results are in accordance with the previous findings that basic sites of catalysts play a crucial role in transesterification. Among the synthesized catalysts, La
1.00TPA was found to exhibit the highest catalytic activity towards the transesterification under optimized reaction conditions. The superior performance of the La
1.0TPA sample could be related to its superior physico-chemical characteristics such as high surface area and a large number of basic sites per unit surface area.
The role of the methanol to glyceryl tributyrate molar ratio on the transesterification activity of the most active La
1.00TPA catalyst was also studied (
Figure S3). The transesterification reactions were conducted by changing the methanol to glyceryl tributyrate molar ratios of 3:1, 6:1, 9:1, and 12:1 for 1 h. The conversion of glyceryl tributyrate increased from 24% to 98% after increasing the methanol to glyceryl tributyrate ratio from 3:1 to 18:1. This result is consistent with a previous observation that the utilization of large molar ratios of methanol to glyceryl tributyrate is advantageous to obtain higher methyl ester yields [
4,
5]. This mainly due to the fact that excess alcohol does enhance the rate of transesterification and also assists in desorption of product molecules from the surface of the catalyst.
Further, we also studied the effect of loading of catalyst into the reaction mixture (
Figure S4). Several transesterification experiments were performed at 90 °C for 140 min using a methanol to glyceryl tributyrate 12:1 molar ratio in the presence of different loadings of La
1.00TPA catalyst (1, 3, 5, and 7 wt.%). It is clear that glyceryl tributyrate conversion was enhanced with an increase of the catalyst amount. A complete conversion of glyceryl tributyrate was observed in 150 min, when 5 wt.% catalyst loading was used, and longer reaction times were required when 1 and 3 wt.% loading was utilized. However, higher catalyst loading (greater than 5 wt.%) resulted in a minor diminution in glyceryl tributyrate conversion. This is mainly because of the fact that the reaction mixture becomes viscous at higher catalyst loading, which could lower the rate of diffusion of reactants and products.
Having demonstrated that the LaxTPA samples possessed both acid and base sites, we performed simultaneous transesterification and esterification of palmitic acid reaction over La1.00TPA catalyst to determine whether glyceryl tributyrate transesterification could take place in the presence of free fatty acid. This aspect is important as many biodiesel feedstocks consist of free fatty acids and the catalyst should able to work for one pot transesterification of triglycerides and esterification of free fatty acids.
The TOF values for La exchanged TPA and MPA samples along with bulk TPA and MPA catalysts are depicted in
Table 3. The bulk TPA and MPA samples showed lower activity for transesterification of glyceryl tributyrate. Incorporation of low amounts of La (La
0.25 and La
0.50) in TPA or MPA resulted in an increase in activity for tributyrate transesterification. It is interesting to note that further La incorporation (La
0.75 and La
1.00) resulted in enhanced performance with the highest TOF obtained in the case of a La
1.00TPA sample. The highest transesterification rate of 12.8 mmol h
−1 g
−1 obtained in the La
1.00TPA sample coincides with the conventional basic MgO catalyst, which offered 15 mmol h
−1 g
−1 under similar reaction conditions. However, the La
1.00TPA catalyst underperformed compared to the previously reported Li-doped CaO and Mg-Al layered double hydroxides catalysts [
37]. It is well reported that the transesterification activity of solid base catalysts depends on the strength of the basic sites. Formation of stronger and more basic sites in La
1.00TPA due to maximum La incorporation could be the responsible factor for the higher activity of La
1.00TPA. Moreover, HPAs are known to possess a very good resistance towards water and did not lose activity, in contrast to pure alkali and alkaline metal oxides.
It was also observed that the transesterification conversion was not affected due to the presence of palmitic acid, as the catalyst effectively converted the palmitic acid into its methyl ester in just 30 min of reaction time at a reaction temperature of 90 °C. It was reported that HPAs have a unique characteristic property where they could absorb the molecules into their bulk [
38], which allows the reactions to happen in the bulk as well as on the surface [
39]. The synthesized La
xTPA samples clearly possessed the Keggin HPA structure with balanced acid-base centers. Therefore, the reactant molecules could be migrating on the surface and bulk of HPA to undergo transesterification and/or esterification, with the subsequent products transported to the surface to desorb.
It is also known that some HPAs could dissolve in polar and non-solvents, therefore we performed a leaching test to determine whether any soluble HPAs were dissolved in the reactant components. As anticipated, the HPA samples which contained less La (La
0.25 and La
0.50) showed slight dissolution in hot methanol during the reaction. However, the samples which contained more La ions (La
0.75 and La
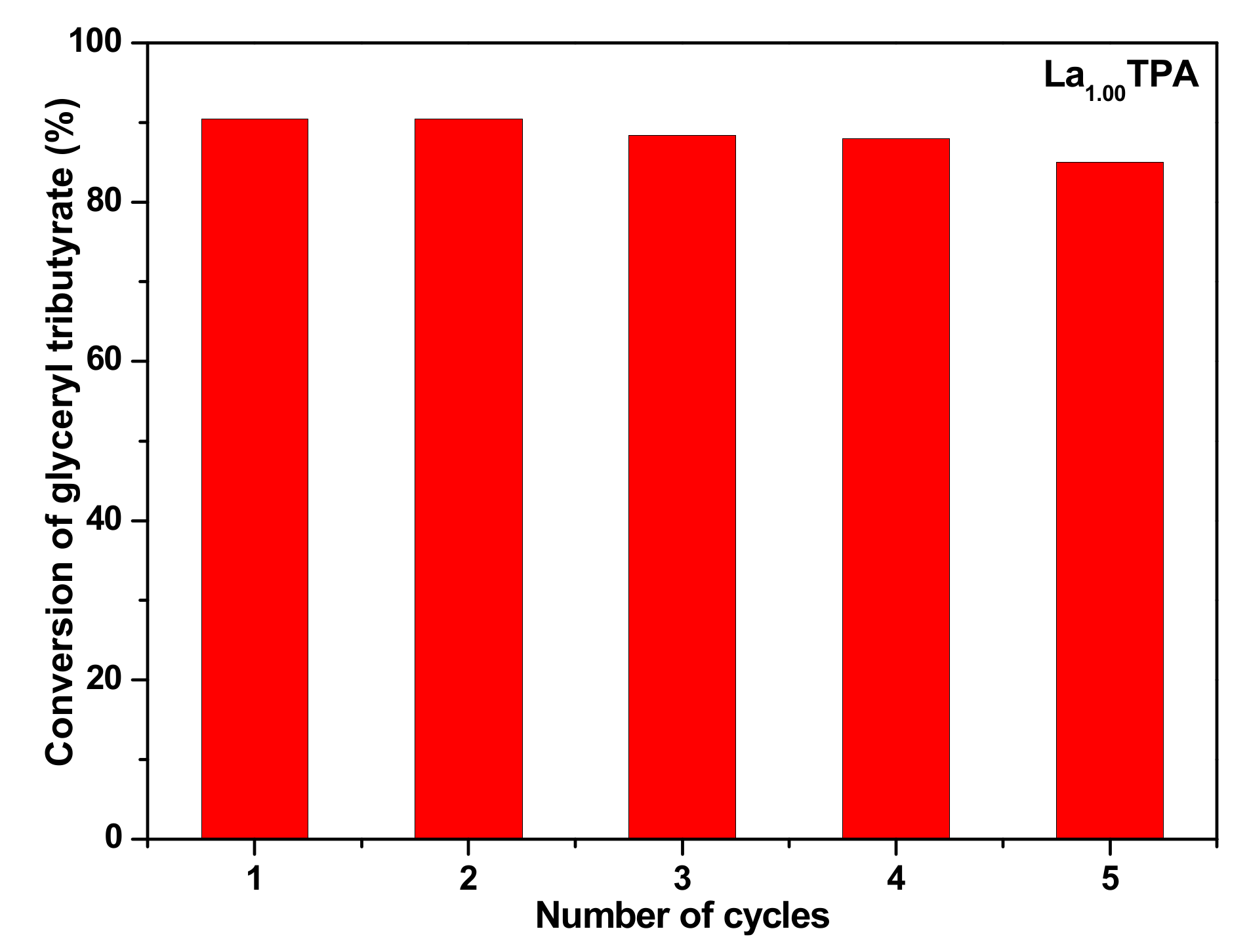
1.00) were stable during methanol reflux. Further, we studied the reusability of the synthesized La
xHPA catalysts. The transesterification of glyceryl tributyrate was carried out under studied reaction conditions. After the each cycle, catalyst was centrifuged and dried at 110 °C. The dried La
1.00TPA sample was used for five continual cycles under the identical reaction parameters described in the experimental section. The data obtained from recycle experiments was presented in
Figure 8. It was observed that the La
1.00TPA sample offered a very similar conversion of glyceryl tributyrate without any significant loss. However, after the fourth cycle there was a slight decrease in the conversion of glyceryl tributyrate, this is possibly because of a loss of catalyst amount throughout the recovery and regeneration steps.