Preparation of Chloromethylated Pitch–Based Hyper–Crosslinked Polymers and An Immobilized Acidic Ionic Liquid as A Catalyst for the Synthesis of Biodiesel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of HCPpitch–CH2–Cl and [HCPpitch–Im–Pros][Tos]
2.2. Influence of Chloromethylation Parameters on Chlorine Content of HCPpitch–CH2–Cl
2.3. Influence of Esterification Parameters on the Biodiesel Yield and Reusability of the Catalyst
3. Materials and Methods
3.1. Materials
3.2. Preparation of Catalysis
3.2.1. Preparation of HCPpitch
3.2.2. Preparation of HCPpitch–CH2–Cl
3.2.3. Preparation of [HCPpitch–Im–Pros][Tos]
3.3. Determination of Grafting Ratio of Chloromethyl
3.4. Characterization
3.5. Preparation of Biodiesel
3.6. GC Analysis and Biodiesel Yield
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aghbashlo, M.; Hosseinpour, S.; Tabatabaei, M.; Mojarab Soufiyan, M. Multi-objective exergetic and technical optimization of a piezoelectric ultrasonic reactor applied to synthesize biodiesel from waste cooking oil (WCO) using soft computing techniques. Fuel 2019, 235, 100–112. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Tabatabaei, M.; Aghbashlo, M.; Khanali, M.; Demirbas, A. A comprehensive review on the environmental impacts of diesel/biodiesel additives. Energy Convers. Manag. 2018, 174, 579–614. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L. Biodiesel Production (FAEEs) by Heterogeneous Combi-Lipase Biocatalysts Using Wet Extracted Lipids from Microalgae. Catalysts 2019, 9, 296. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, W.; Zhou, W.; Liu, Y.; Han, Y.; Zheng, Y.; Liu, Y. Biodiesel Production by Esterification Reaction on K+ Modified MgAl-Hydrotalcites Catalysts. Catalysts 2019, 9, 742. [Google Scholar] [CrossRef]
- Shamun, S.; Belgiorno, G.; Di Blasio, G.; Beatrice, C.; Tunér, M.; Tunestål, P. Performance and emissions of diesel-biodiesel-ethanol blends in a light duty compression ignition engine. Appl. Therm. Eng. 2018, 145, 444–452. [Google Scholar] [CrossRef]
- Qiu, T.; Guo, X.; Yang, J.; Zhou, L.; Li, L.; Wang, H.; Niu, Y. The synthesis of biodiesel from coconut oil using novel Brønsted acidic ionic liquid as green catalyst. Chem. Eng. J. 2016, 296, 71–78. [Google Scholar] [CrossRef]
- Yang, Z.; Cui, X.; Jie, H.; Yu, X.; Zhang, Y.; Feng, T.; Liu, H.; Song, K. Kinetic Study and Process Simulation of Transesterification of Methyl Acetate and Isoamyl Alcohol Catalyzed by Ionic Liquid. Ind. Eng. Chem. Res. 2015, 54, 1204–1215. [Google Scholar] [CrossRef]
- Li, J.; Peng, X.; Luo, M.; Zhao, C.-J.; Gu, C.-B.; Zu, Y.-G.; Fu, Y.-J. Biodiesel production from Camptotheca acuminata seed oil catalyzed by novel Brönsted–Lewis acidic ionic liquid. Appl. Energy 2014, 115, 438–444. [Google Scholar] [CrossRef]
- Han, M.J.; Li, Y.; Gu, Z.; Shi, H.L.; Chen, C.; Wang, Q.; Wan, H.; Guan, G.F. Immobilization of thiol-functionalized ionic liquids onto the surface of MIL-101(Cr) frameworks by S-Cr coordination bond for biodiesel production. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 593–600. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Zhang, H.; Wang, A.; Yang, S. Acidic ionic liquid-functionalized mesoporous melamine-formaldehyde polymer as heterogeneous catalyst for biodiesel production. Fuel 2019, 239, 886–895. [Google Scholar] [CrossRef]
- Lisboa, M.C.; Rodrigues, C.A.; Barbosa, A.S.; Mattedi, S.; Freitas, L.S.; Mendes, A.A.; Dariva, C.; Franceschi, E.; Lima, A.S.; Soares, C.M.F. New perspectives on the modification of silica aerogel particles with ionic liquid used in lipase immobilization with platform in ethyl esters production. Process Biochem. 2018, 75, 157–165. [Google Scholar] [CrossRef]
- Xie, W.L.; Wan, F. Immobilization of polyoxometalate-based sulfonated ionic liquids on UiO-66-2COOH metal-organic frameworks for biodiesel production via one-pot transesterification-esterification of acidic vegetable oils. Chem. Eng. J. 2019, 365, 40–50. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Z.; Yang, X.; Wang, L.; Gu, Y.; Tan, B. Acid and base coexisted heterogeneous catalysts supported on hyper-crosslinking polymers for one-pot cascade reactions. J. Catal. 2017, 348, 168–176. [Google Scholar] [CrossRef]
- Bhunia, S.; Banerjee, B.; Bhaumik, A. A new hypercrosslinked supermicroporous polymer, with scope for sulfonation, and its catalytic potential for the efficient synthesis of biodiesel at room temperature. Chem. Commun. 2015, 51, 5020–5023. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Y.; Ma, D.; Shi, Z.; Ma, S. Mercury nano-trap for effective and efficient removal of mercury(II) from aqueous solution. Nat. Commun. 2014, 5, 5537. [Google Scholar] [CrossRef]
- Xu, S.; Luo, Y.; Tan, B. Recent development of hypercrosslinked microporous organic polymers. Macromol. Rapid Commun. 2013, 34, 471–484. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
- Wang, X.; Mu, P.; Zhang, C.; Chen, Y.; Zeng, J.; Wang, F.; Jiang, J.X. Control Synthesis of Tubular Hyper-Cross-Linked Polymers for Highly Porous Carbon Nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 20779–20786. [Google Scholar] [CrossRef]
- Teixeira, V.G.; Coutinho, F.M.B. Morphological study on the reactivity of styrene-divinylbenzene copolymers in a chloromethylation reaction. J. Appl. Polym. Sci. 2010, 26, 14849–14866. [Google Scholar] [CrossRef]
- Wu, J.; Gao, Y.; Zhang, W.; Tang, A.; Tan, Y.; Men, Y.; Tang, B. New imidazole-type acidic ionic liquid polymer for biodiesel synthesis from vegetable oil. Chem. Eng. Process. Process Intensif. 2015, 93, 61–65. [Google Scholar] [CrossRef]
- Karimi, B.; Vafaeezadeh, M. SBA-15 functionalized sulfonic acid containing a confined hydrophobic and acidic ionic liquid: A highly efficient catalyst for solvent-free thioacetalization of carbonyl compounds at room temperature. RSC Adv. 2013, 3, 23207. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, H.; Miao, J.; Han, M.; Yang, C.; Guan, G. Reusable and efficient polystyrene-supported acidic ionic liquid catalyst for esterifications. J. Mol. Catal. Chem. 2010, 332, 152–157. [Google Scholar] [CrossRef]
- Dragan, E.S.; Avram, E.; Axente, D.; Marcu, C. Ion-exchange resins. III. Functionalization-morphology correlations in the synthesis of some macroporous, strong basic anion exchangers and uranium-sorption properties evaluation. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2451–2461. [Google Scholar] [CrossRef]
- Chen, Z.B.; Kang, L.; Di, D.L.; Dong, F.; Yu, H. Chloromethylation Research of LX-1180 Macroporous Adsorption Resin in Ultrasonic Environment. Adv. Mater. Res. 2011, 233–235, 2893–2897. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, X.; Zhong, S.; Yi, W.-J.; Li, L.-J. A new chloromethylation method based on polystyrene–divinylbenzene. Chem. Pap. 2019, 73, 2183–2188. [Google Scholar] [CrossRef]
- Qi, J.; Lin, J.; Fu, H. One-step production of biodiesel from waste cooking oil catalysed by SO3H-functionalized quaternary ammonium ionic liquid. Curr. Sci. 2016, 110, 2129–2134. [Google Scholar] [CrossRef]
- Gao, H.; Ding, L.; Bai, H.; Liu, A.; Li, S.; Li, L. Pitch-based hyper-cross-linked polymers with high performance for gas adsorption. J. Mater. Chem. A 2016, 4, 16490–16498. [Google Scholar] [CrossRef]

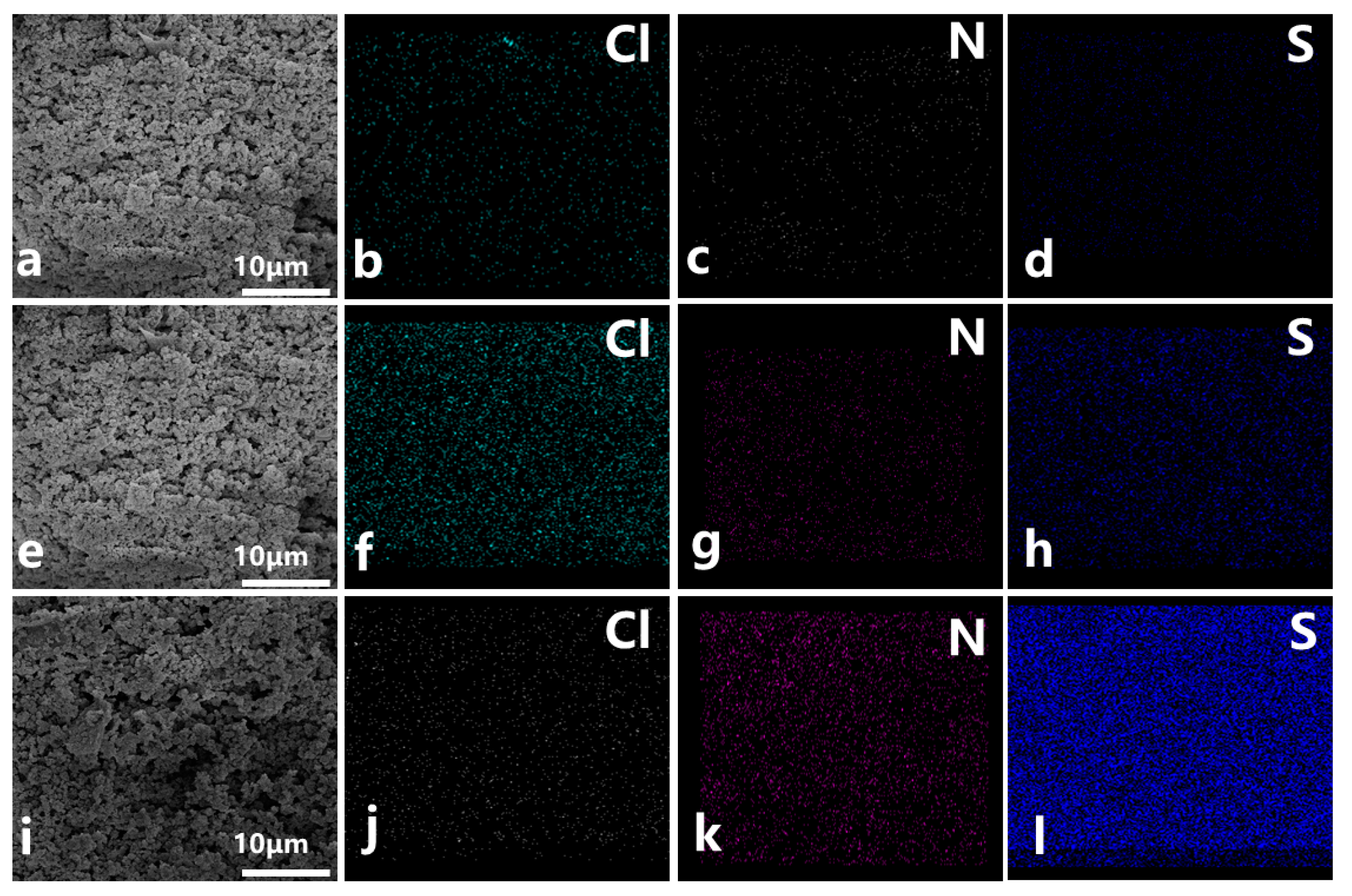

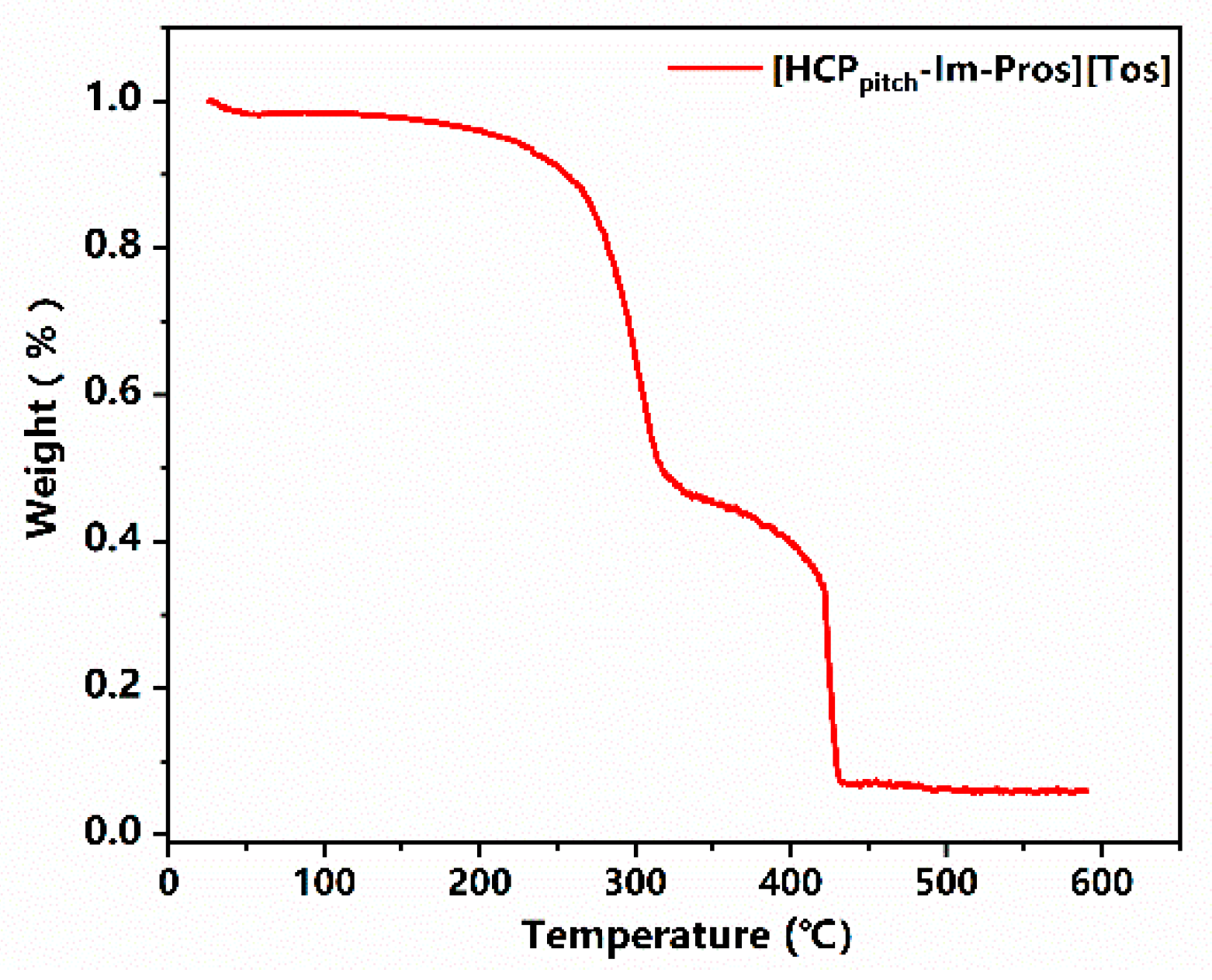
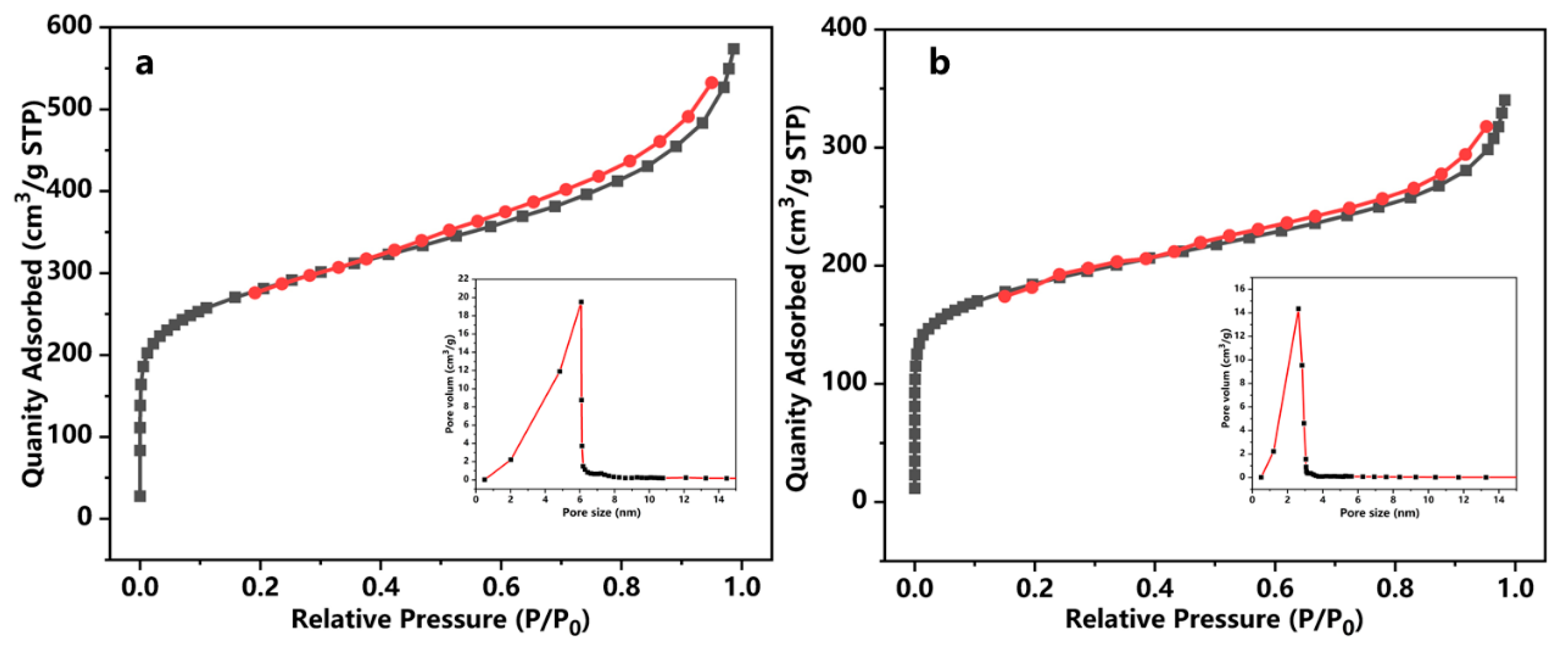
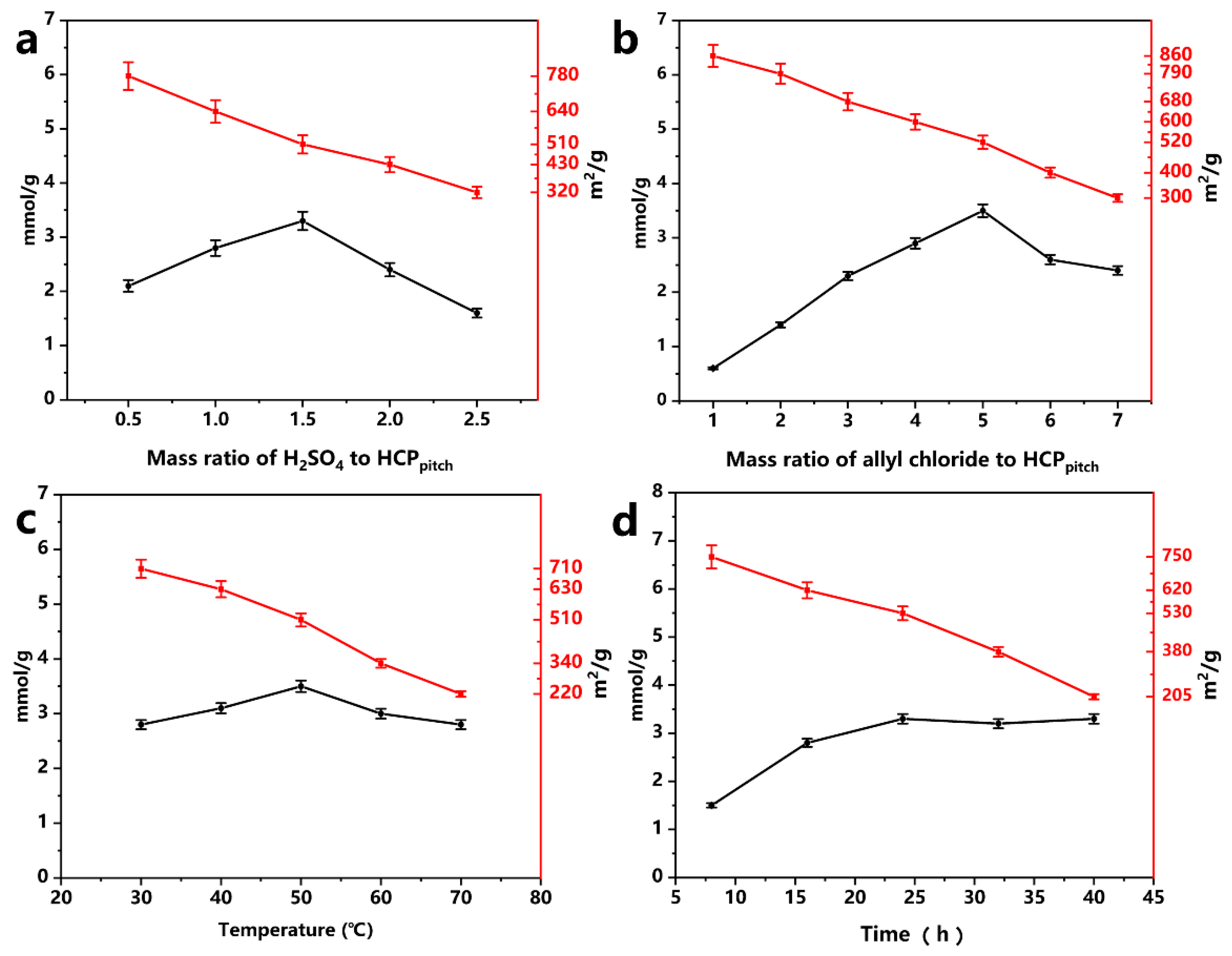


| Sample | Percent 1 N (wt%) | Percent 2 S (wt%) | Grafting Degree (mmol/g) |
|---|---|---|---|
| HCPpitch | 0.21 | 2.21 | - |
| HCPpitch–CH2–Cl | 0.17 | 3.70 | - |
| [HCPpitch–Im–Pros][Tos] | 4.89 | 11.6 | 3.0 |
| Sample | SBET a m2/g | SL b m2/g | M.A. c m2/g | PV d cm3/g | MPV e cm3/g |
|---|---|---|---|---|---|
| HCPpitch | 1153 | 1291 | 672 | 1.89 | 0.22 |
| HCPpitch–CH2–Cl | 530 | 613 | 310 | 0.47 | 0.19 |
| [HCPpitch–Im–Pros][Tos] | 380 | 425 | 240 | 0.21 | 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, B.; Xiang, X.; Liu, T.; Li, D.; Zhao, C.; Qiu, R.; Chen, X.; Lin, J.; Luo, X. Preparation of Chloromethylated Pitch–Based Hyper–Crosslinked Polymers and An Immobilized Acidic Ionic Liquid as A Catalyst for the Synthesis of Biodiesel. Catalysts 2019, 9, 963. https://doi.org/10.3390/catal9110963
Pei B, Xiang X, Liu T, Li D, Zhao C, Qiu R, Chen X, Lin J, Luo X. Preparation of Chloromethylated Pitch–Based Hyper–Crosslinked Polymers and An Immobilized Acidic Ionic Liquid as A Catalyst for the Synthesis of Biodiesel. Catalysts. 2019; 9(11):963. https://doi.org/10.3390/catal9110963
Chicago/Turabian StylePei, Baoyou, Xiaoyan Xiang, Ting Liu, Dongliang Li, Chaoyang Zhao, Rongxing Qiu, Xiaoyan Chen, Jinqing Lin, and Xiaoyan Luo. 2019. "Preparation of Chloromethylated Pitch–Based Hyper–Crosslinked Polymers and An Immobilized Acidic Ionic Liquid as A Catalyst for the Synthesis of Biodiesel" Catalysts 9, no. 11: 963. https://doi.org/10.3390/catal9110963
APA StylePei, B., Xiang, X., Liu, T., Li, D., Zhao, C., Qiu, R., Chen, X., Lin, J., & Luo, X. (2019). Preparation of Chloromethylated Pitch–Based Hyper–Crosslinked Polymers and An Immobilized Acidic Ionic Liquid as A Catalyst for the Synthesis of Biodiesel. Catalysts, 9(11), 963. https://doi.org/10.3390/catal9110963




