Effect of Metal Oxide–Support Interactions on Ethylene Oligomerization over Nickel Oxide/Silica–Alumina Catalysts
Abstract
:1. Introduction
2. Results and Discussion
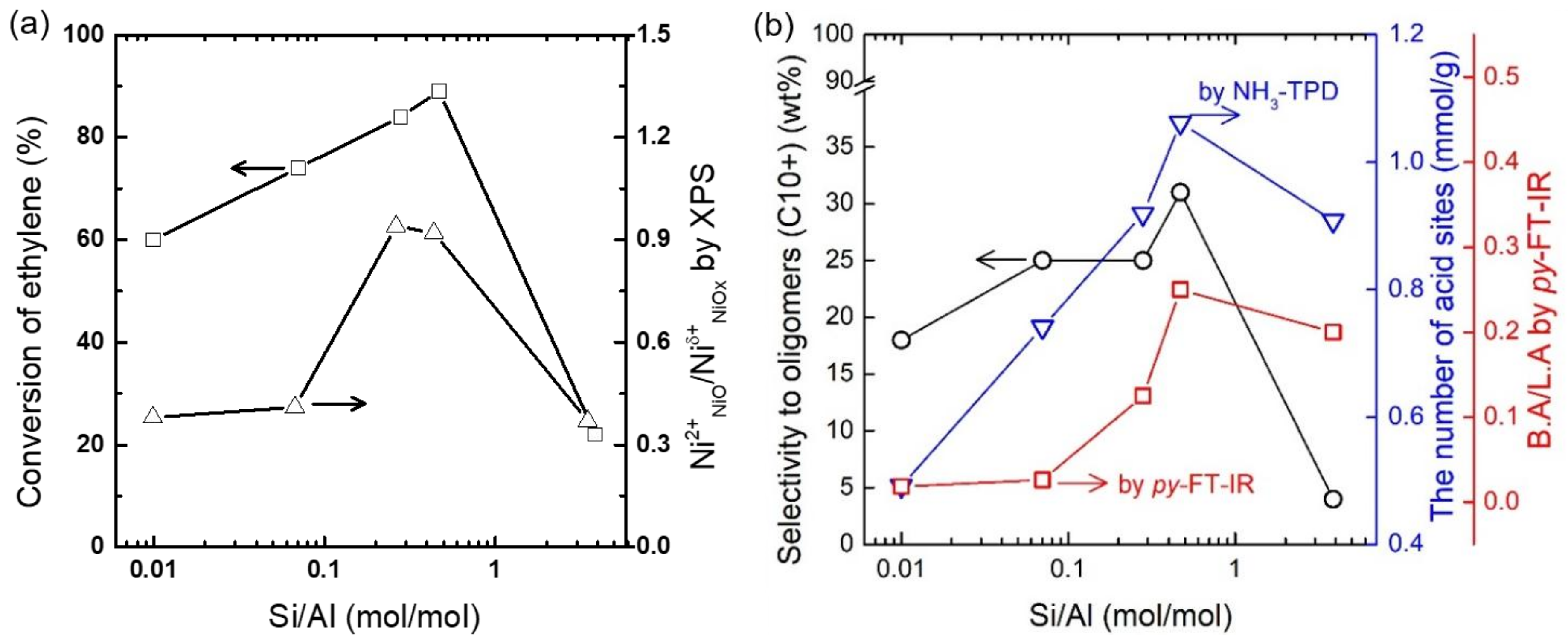
2.1. Structural and Electronic Properties of NiOx/SA Catalysts
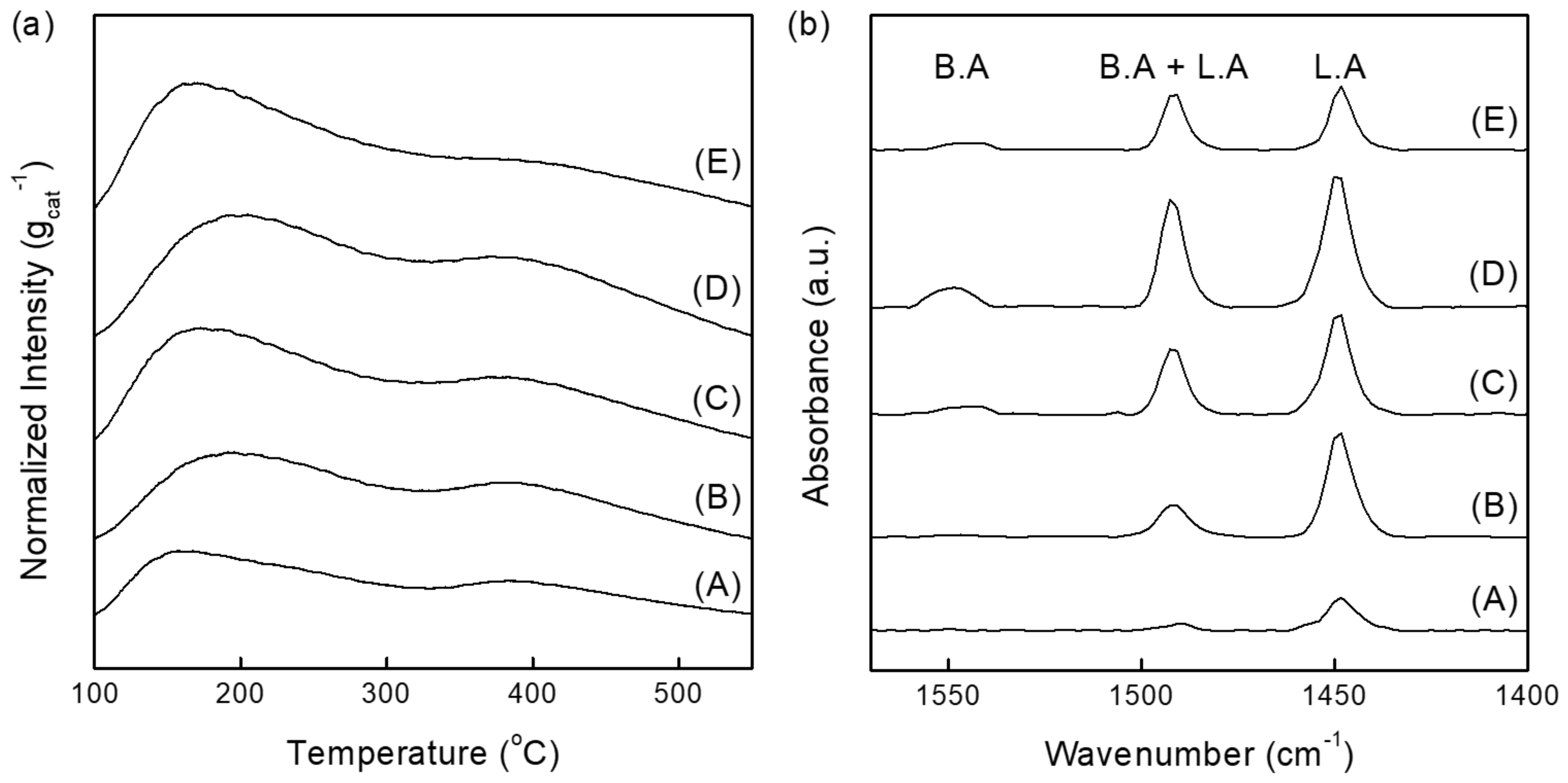
2.2. Acid Site Properties of NiOx/SA Catalysts
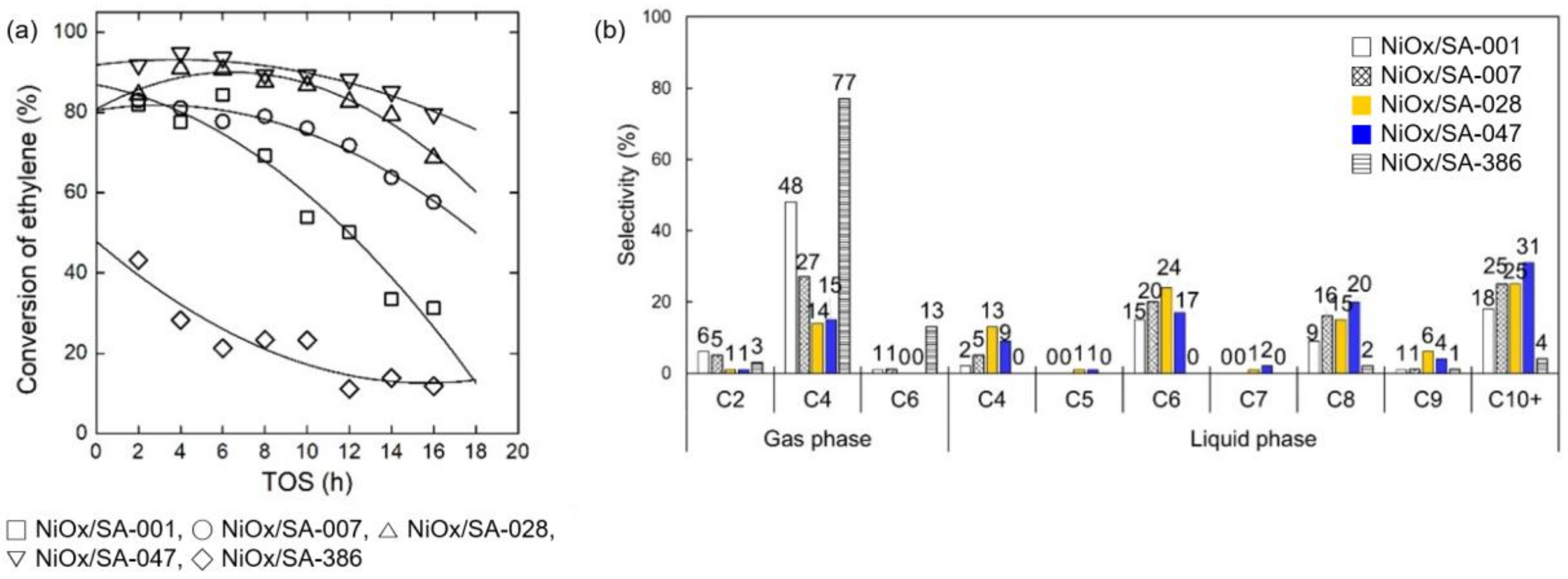
2.3. Ethylene Oligomerization over NiOx/SA Catalysts
3. Materials and Methods
3.1. Preparation of NiOx/SA Catalysts
3.2. Analytical Methods
3.3. Ethylene Oligomerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chae, H.-J.; Kim, T.-W.; Moon, Y.-K.; Kim, H.-K.; Jeong, K.-E.; Kim, C.-U.; Jeong, S.-Y. Butadiene production from bioethanol and acetaldehyde over tantalum oxide-supported ordered mesoporous silica catalysts. Appl. Catal. B Environ. 2014, 150–151, 596–604. [Google Scholar] [CrossRef]
- Babu, B.H.; Lee, M.; Hwang, D.W.; Kim, Y.; Chae, H.-J. An integrated process for production of jet-fuel range olefins from ethylene Ni-AlSBA-15 and Amberlyst-35 catalysts. Appl. Catal. A Gen. 2017, 530, 48–55. [Google Scholar] [CrossRef]
- Jiang, P.; Wu, X.; Zhu, L.; Jin, F.; Liu, J.; Xia, T.; Wang, T.; Li, Q. Production of jet fuel range paraffins by low temperature polymerization of gaseous light olefins using ionic liquid. Energy Convers. Manag. 2016, 120, 338–345. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Zhang, Y.; Lin, S.; Li, G.; Chen, L.; Fang, Y.; Xin, H.; Li, X. Ethylene oligomerization over heterogeneous catalysts. Energy Environ. Focus 2014, 3, 246–256. [Google Scholar] [CrossRef]
- Breuil, P.-A.R.; Magna, L.; Olivier-Bourbigou, H. Role of homogeneous catalysis in oligomerization of olefins: Focus on selected examples based on group 4 to group 10 transition metal complexes. Catal. Lett. 2015, 145, 173–192. [Google Scholar] [CrossRef]
- Huang, W.; Gong, F.; Fan, M.; Zhai, Q.; Hong, C.; Li, Q. Production of light olefins by catalytic conversion of lignocellulosic biomass with HZSM-5 zeolite impregnated with 6wt% lanthanum. Bioresour. Technol. 2012, 121, 248–255. [Google Scholar] [CrossRef]
- Ding, X.; Li, C.; Yang, C. Study on the oligomerization of ethylene in fluidized catalytic cracking (FCC) dry gas over metal-loaded HZSM-5 catalysts. Energy Fuels 2010, 24, 3760–3763. [Google Scholar] [CrossRef]
- Yamamura, M.; Chaki, K.; Wakatsuki, T.; Okado, H.; Fujimoto, K. Synthesis of ZSM-5 zeolite with small crystal size and its catalytic performance for ethylene oligomerization. Zeolites 1994, 14, 643–649. [Google Scholar] [CrossRef]
- Ying, L.; Zhu, J.; Cheng, Y.; Wang, L.; Li, X. Kinetic modeling of C2-C7 olefins interconversion over ZSM-5 catalyst. J. Ind. Eng. Chem. 2016, 33, 80–90. [Google Scholar] [CrossRef]
- Chen, C.S.H.; Bridger, R.F. Shape-selective oligomerization of alkenes to near-linear hydrocarbons by zeolite catalysis. J. Catal. 1996, 161, 687–693. [Google Scholar] [CrossRef]
- de Souza, M.O.; Mendes, F.M.T.; de Souza, R.F.; dos Santos, J.H.Z. XPS characterization of nickel-acetylacetonate impregnated in NaX and NaY zeolites. Microporous Mesoporous Mater. 2004, 69, 217–221. [Google Scholar] [CrossRef]
- Lallemand, M.; Finiels, A.; Fajula, F.; Hulea, V. Catalytic oligomerization of ethylene on Ni-containing dealuminated Y zeolites. Appl. Catal. A Gen. 2006, 301, 196–201. [Google Scholar] [CrossRef]
- Hulea, V.; Lallemand, M.; Finiels, A.; Fajula, F. Catalytic oligomerization of ethylene over Ni-containing MCM-22, MCM-41 and USY. Stud. Surf. Sci. Catal. 2005, 158, 1621–1628. [Google Scholar]
- Ng, F.T.T.; Creaser, D.C. Ethylene dimerization over modified nickel exchanged Y-zeolite. Appl. Catal. A Gen. 1994, 119, 327–339. [Google Scholar] [CrossRef]
- Martínez, A.; Arribas, M.A.; Concepción, P.; Moussa, S. New bifunctional Ni-H-beta catalysts for the heterogeneous oligomerization of ethylene. Appl. Catal. A Gen. 2013, 467, 509–518. [Google Scholar] [CrossRef]
- Lallemand, M.; Rusu, O.A.; Dumitriu, E.; Finiels, A.; Fajula, F.; Hulea, V. NiMCM-36 and NiMCM-22 catalysts for the ethylene oligomerization effect of zeolite texture and nickel cations/acid sites ratio. Appl. Catal. A Gen. 2008, 338, 37–43. [Google Scholar] [CrossRef]
- Lallemand, M.; Rusu, O.A.; Dumitriu, E.; Finiels, A.; Fajula, F.; Hulea, V. Ni-MCM-36 and Ni-MCM-22 catalysts for the ethylene oligomerization. Stud. Surf. Sci. Catal. 2008, 174, 1139–1142. [Google Scholar]
- Andrei, R.D.; Popa, M.I.; Fajula, F.; Hulea, V. Heterogeneous oligomerization of ethylene over highly active and stable Ni-AlSBA-15 mesoporous catalysts. J. Catal. 2015, 323, 76–84. [Google Scholar] [CrossRef]
- Lin, S.; Shi, L.; Zhang, H.; Zhang, N.; Yi, X.; Zheng, A.; Li, X. Tuning the pore structure of plug-containing Al-SBA-15 by post-treatment and its selectivity for C16 olefin in ethylene oligomerization. Microporous Mesoporous Mater. 2014, 184, 151–161. [Google Scholar] [CrossRef]
- de Souza, M.O.; Rodrigues, L.R.; Gauvin, R.M.; de Souza, R.F.; Pastore, H.O.; Gengembre, L.; Ruiz, J.A.C.; Gallo, J.M.R.; Milanesi, T.S.; Milani, M.A. Support effect in ethylene oligomerization mediated by heterogenized nickel catalysts. Catal. Commun. 2010, 11, 597–600. [Google Scholar] [CrossRef]
- Lallemand, M.; Finiels, A.; Fajula, F.; Hulea, V. Nature of the active sites in ethylene oligomerization catalyzed by Ni-containing molecular sieves: Chemical and IR spectral investigation. J. Phys. Chem. C 2009, 113, 20360–20364. [Google Scholar] [CrossRef]
- de Souza, M.O.; Rodrigues, L.R.; Pastore, H.O.; Ruiz, J.A.C.; Gengembre, L.; Gauvin, R.M.; de Souza, R.F. A nano-organized ethylene oligomerization catalyst: Characterization and reactivity of the Ni(MeCN)6(BF4)2/[Al]-MCM-41/AlEt3 system. Microporous Mesoporous Mater. 2006, 96, 109–114. [Google Scholar] [CrossRef]
- Hulea, V.; Fajula, F. Ni-exchanged AlMCM-41-An efficient bifunctional catalyst for ethylene oligomerization. J. Catal. 2004, 225, 213–222. [Google Scholar] [CrossRef]
- Moussa, S.; Arribas, M.A.; Concepción, P.; Martínez, A. Heterogeneous oligomerization of ethylene to liquids on bifunctional Ni-based catalysts: The influence of support properties on nickel speciation and catalytic performance. Catal. Today 2016, 277, 78–88. [Google Scholar] [CrossRef]
- Nicolaides, C.P.; Scurrell, M.S.; Semano, P.M. Nickel silica-alumina catalysts for ethene oligomerization-control of the selectivity to 1-alkene products. Appl. Catal. A Gen. 2003, 245, 43–53. [Google Scholar] [CrossRef]
- Heveling, J.; Nicolaides, C.P. Chain-length distributions obtained over nickel(II)-exchanged or impregnated silica-alumina catalysts for the oligomerization of lower alkenes. Catal. Lett. 2006, 107, 117–121. [Google Scholar] [CrossRef]
- Heveling, J.; Nicolaides, C.P.; Scurrell, M.S. Catalysts and conditions for the highly efficient, selective and stable heterogeneous oligomerization of ethylene. Appl. Catal. A Gen. 1998, 173, 1–9. [Google Scholar] [CrossRef]
- Espinoza, R.L.; Snel, R.; Korf, C.J.; Nicolaides, C.P. Catalytic oligomerization of ethene over nickel-exchanged amorphous silica-aluminas; effect of the acid strength of the support. Appl. Catal. 1987, 29, 295–303. [Google Scholar] [CrossRef]
- Espinoza, R.L.; Nicolaides, C.P.; Korf, C.J.; Snel, R. Catalytic oligomerization of ethene over nickel-exchanged amorphous silica-alumina; effect of the nickel concentration. Appl. Catal. 1987, 31, 259–266. [Google Scholar] [CrossRef]
- Espinoza, R.L.; Korf, C.J.; Nicolaides, C.P.; Snel, R. Catalytic oligomerization of ethene over nickel-exchanged amorphous silica-alumina; effect of the reaction conditions and modelling of the reaction. Appl. Catal. 1987, 29, 175–184. [Google Scholar] [CrossRef]
- Davydov, A.A.; Kantcheva, M.; Chepotko, M.L. FTIR spectroscopic study on nickel(II)-exchanged sulfated alumina: Nature of the active sites in the catalytic oligomerization of ethene. Catal. Lett. 2002, 83, 97–108. [Google Scholar] [CrossRef]
- Zhang, Q.; Kantcheva, M.; Lana, I.G.D. Oligomerization of ethylene in a slurry reactor using a nickel/sulfated alumina catalyst. Ind. Eng. Chem. Res. 1997, 36, 3433–3438. [Google Scholar] [CrossRef]
- Zhang, Q.; Lana, I.G.D. An analysis of mass transfer and kinetics during ethylene oligomerization over nickel/sulfated alumina catalyst in a slurry reactor. Chem. Eng. Sci. 1997, 52, 4187–4195. [Google Scholar] [CrossRef]
- Cai, T.; Zang, L.; Qi, A.; Wang, D.; Cao, D.; Li, L. Propene oligomerization catalyst derived from nickel sulfate supported on γ-alumina. Appl. Catal. 1991, 69, 1–13. [Google Scholar] [CrossRef]
- Lavrenov, A.V.; Buluchevskii, E.A.; Moiseenko, M.A.; Drozdov, V.A.; Arbuzov, A.B.; Gulyaeva, T.I.; Likholobov, V.A.; Duplyakin, V.K. Chemical composition optimization and characterization of the NiO/B2O3-Al2O3 system as a catalyst for ethylene oligomerization. Kinet. Catal. 2010, 51, 404–409. [Google Scholar] [CrossRef]
- Sohn, J.R.; Lee, S.Y. High catalytic activity of NiO-ZrO2 modified with WO3 for ethylene dimerization. Appl. Catal. A Gen. 1997, 164, 127–140. [Google Scholar] [CrossRef]
- Sohn, J.R.; Shin, D.C. New catalyst of NiO-ZrO2/WO3 for ethylene dimerization. J. Catal. 1996, 160, 314–316. [Google Scholar] [CrossRef]
- Sohn, J.R.; Kwon, S.H.; Shin, D.C. Spectroscopic studies on NiO supported on ZrO2 modified with MoO3 for ethylene dimerization. Appl. Catal. A Gen. 2007, 317, 216–225. [Google Scholar] [CrossRef]
- Sohn, J.R.; Kim, H.W.; Park, M.Y.; Park, E.H.; Kim, J.T.; Park, S.E. Highly active catalyst of NiO-ZrO2 modified with H2SO4 for ethylene dimerization. Appl. Catal. A Gen. 1995, 128, 127–141. [Google Scholar] [CrossRef]
- Sohn, J.R.; Kim, H.J. High catalytic activity of NiO-TiO2/SO42- for ethylene dimerization. J. Catal. 1986, 101, 428–433. [Google Scholar] [CrossRef]
- Al-Jarallah, A.M.; Anabtawi, J.A.; Siddiqui, M.A.B.; Aitani, A.M.; Al-Sa’doun, A.W. Part1 dimerization of ethylene to butane-1. Catal. Today 1992, 14, 1–121. [Google Scholar] [CrossRef]
- Brogaard, R.Y.; Olsbye, U. Ethene oligomerization in Ni-containing zeolites: Theoretical discrimination of reaction mechanisms. ACS Catal. 2016, 6, 1205–1214. [Google Scholar] [CrossRef]
- Yuen, S.; Kubsh, J.E.; Dumesic, J.A.; Topsoee, N.; Topsoee, H.; Chen, Y. Metal oxide-support interactions in silica-supported iron oxide catalysts probed by nitric oxide adsorption. J. Phys. Chem. 1982, 86, 3022–3032. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kubota, T.; Ohto, Y.; Nasu, S. Metal-oxide-support interactions in Fe/ZrO2 catalysts. J. Phys. Chem. B 2000, 104, 8462–8470. [Google Scholar] [CrossRef]
- Deo, G.; Wachs, I.E. Surface oxide-support interaction (SOSI) for surface redox sites. J. Catal. 1991, 129, 307–312. [Google Scholar] [CrossRef]
- Xu, M.; He, S.; Chen, H.; Cui, G.; Zheng, L.; Wang, B.; Wei, M. TiO2-x-modified Ni nanocatalyst with tunable metal-support interaction for water-gas shift reaction. ACS Catal. 2017, 7, 7600–7609. [Google Scholar] [CrossRef]
- Chen, P.; Khetan, A.; Yang, F.; Migunov, V.; Weide, P.; Stürmer, S.P.; Guo, P.; Kähler, K.; Xia, W.; Mayer, J.; et al. Experimental and theoretical understanding of nitrogen-doping-induced strong metal-support interactions in Pd/TiO2 catalysts for nitrobenzene hydrogenation. ACS Catal. 2017, 7, 1197–1206. [Google Scholar] [CrossRef]
- Wan, H.; Wu, B.; Xiang, H.; Li, Y. Fischer-Tropsch synthesis: Influence of support incorporation manner on metal dispersion, metal-support interaction, and activities of iron catalysts. ACS Catal. 2012, 2, 1877–1883. [Google Scholar] [CrossRef]
- Kim, P.; Kim, Y.; Kim, H.; Song, I.K.; Yi, J. Synthesis and characterization of mesoporous alumina with nickel incorporated for use in the partial oxidation of methane into synthesis gas. Appl. Catal. A Gen. 2004, 272, 157–166. [Google Scholar] [CrossRef]
- Delmon, B.; Delmon, B.; Thomas, J.M.; Bell, R.G.; Catlow, C.R.A.; Delmon, B.; Feijen, E.J.P.; Martens, J.A.; Jacobs, P.A.; Souverijns, W.; et al. Preparation of Solid Catalysts: Sections 2.2.2–2.3.3. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 264–365. [Google Scholar]
- Rao, G.R.; Rao, C.N.R. A study of strong metal-support interaction based on an electron spectroscopic investigation of nitrogen adsorption on simulated nickel/titania, nickel/alumina and related catalyst surfaces. J. Phys. Chem. 1990, 94, 7986–7991. [Google Scholar] [CrossRef]
- Tauster, S.J. Strong metal-support interactions. Acc. Chem. Res. 1987, 20, 389–394. [Google Scholar] [CrossRef]
- Lee, M.; Yoon, J.W.; Kim, Y.; Yoon, J.S.; Chae, H.-J.; Han, Y.-H.; Hwang, D.W. Ni/SIRAL-30 as a heterogeneous catalyst for ethylene oligomerization. Appl. Catal. A Gen. 2018, 562, 87–93. [Google Scholar] [CrossRef]
- Rynkowski, J.M.; Paryjczak, T.; Lenik, M. On the nature of oxidic nickel phases in NiO/γ-Al2O3 catalysts. Appl. Catal. A Gen. 1993, 106, 73–82. [Google Scholar] [CrossRef]
- Kim, P.; Kim, Y.; Kim, H.; Song, I.K.; Yi, J. Synthesis and characterization of mesoporous alumina for use as a catalyst support in the hydrodechlorination of 1,2-dichloropropane: Effect of preparation condition of mesoporous alumina. J. Mol. Catal. A Chem. 2004, 219, 87–95. [Google Scholar] [CrossRef]
- Boukha, Z.; Jiménez-González, C.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Synthesis, characterization and performance evaluation of spinel-derived Ni/Al2O3 catalysts for various methane reforming reactions. Appl. Catal. B Environ. 2014, 158–159, 190–201. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Thitsartarn, W.; Sutthiumporn, K.; Kawi, S. Inverse NiAl2O4 on LaAlO3-Al2O3: Unique catalytic structure for stable CO2 reforming of methane. J. Phys. Chem. C 2013, 117, 8120–8130. [Google Scholar] [CrossRef]
- Heracleous, E.; Lee, A.F.; Wilson, K.; Lemonidou, A.A. Investigation of Ni-based alumina-supported catalysts for the oxidative dehydrogenation of ethane to ethylene: Structural characterization and reactivity studies. J. Catal. 2005, 231, 159–171. [Google Scholar] [CrossRef]
- Mile, B.; Stirling, D.; Zammitt, M.A.; Lovell, A.; Webb, M. The location of nickel oxide and nickel in silica-supported catalysts: Two forms of “NiO” and the assignment of temperature-programmed reduction profiles. J. Catal. 1988, 114, 217–229. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Boukha, Z.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Behavior of coprecipitated NiAl2O4/Al2O3 catalysts for low-temperature methane steam reforming. Energy Fuels 2014, 28, 7109–7121. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni-Al2O3 catalysts prepared by solution combustion method for syngas methnation. Catal. Commun. 2012, 17, 34–38. [Google Scholar] [CrossRef]
- Gayán, P.; Dueso, C.; Abad, A.; Adanez, J.; de Diego, L.F.; García-Labiano, F. NiO/Al2O3 oxygen carriers for chemical-looping combustion prepared by impregnation and deposition-precipitation methods. Fuel 2009, 88, 1016–1023. [Google Scholar]
- Zou, X.; Wang, X.; Li, L.; Shen, K.; Lu, X.; Ding, W. Development of highly effective supported nickel catalysts for pre-reforming of liquefied petroleum gas under low steam to carbon molar ratios. Int. J. Hydrogen Energy 2010, 35, 12191–12200. [Google Scholar] [CrossRef]
- Wu, H.; Pantaleo, G.; La Parola, V.; Venezia, A.M.; Collard, X.; Aprile, C.; Liotta, L.F. Bi- and trimetallic Ni catalysts over Al2O3 and Al2O3-MOx (M = Ce or Mg) oxides for methane dry reforming: Au and Pt additive effects. Appl. Catal. B Environ. 2014, 156–157, 350–361. [Google Scholar] [CrossRef]
- Le Valant, A.; Bion, N.; Can, F.; Duprez, D.; Epron, F. Preparation and characterization of bimetallic Rh-Ni/Y2O3-Al2O3 for hydrogen production by raw bioethanol steam reforming: Influence of the addition of nickel on the catalyst performances and stability. Appl. Catal. B Environ. 2010, 97, 72–81. [Google Scholar] [CrossRef]
- Diskin, A.M.; Cunningham, R.H.; Ormerod, R.M. The oxidative chemistry of methane over supported nickel catalysts. Catal. Today 1998, 46, 147–154. [Google Scholar] [CrossRef]
- Al-Dalama, K.; Stanislaus, A. Temperature programmed reduction of SiO2-Al2O3 supported Ni, Mo and NiMo catalysts prepared with EDTA. Thermochim. Acta 2011, 520, 67–74. [Google Scholar] [CrossRef]
- Li, G.; Hu, L.; Hill, J.M. Comparison of reducibility and stability of alumina-supported Ni catalysts prepared by impregnation and co-precipitation. Appl. Catal. A Gen. 2006, 301, 16–24. [Google Scholar] [CrossRef]
- Le Page, J.F.; Avnir, D.; Taglauer, E.; Guisnet, M.; Moretti, G.; Che, M.; Bozon-Verduraz, F.; Anpo, M.; Roduner, E.; Knözinger, H. Characterization of Solid Catalysts: Sections 3.1.4–3.2.2. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 582–689. [Google Scholar]
- Singh, K.S.W.; Rouquerol, J.; Bergeret, G.; Gallezot, P.; Vaarkamp, M.; Koningsberger, D.C.; Datye, A.K.; Niemantsverdriet, J.W.; Butz, T.; Engelhardt, G.; et al. Characterization of Solid Catalysts: Sections 3.1.1–3.1.3. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 427–582. [Google Scholar]
- Metzger, E.D.; Brozek, C.K.; Comito, R.J.; Dincă, M. Selective dimerization of ethylene to 1-butene with a porous catalyst. ACS Cent. Sci. 2016, 2, 148–153. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, Y.; Ryu, J.; Kim, Y.-A.; Park, E.D.; Choi, J.-W.; Ha, J.-M.; Suh, D.J.; Lee, H. Production of high carbon number hydrocarbon fuels from a lignin-derived α-O-4 phenolic dimer, benzyl phenyl ether, via isomerization of ether to alcohols on high-surface-area silica-alumina aerogel catalysts. Appl. Catal. B Environ. 2013, 142–143, 668–676. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
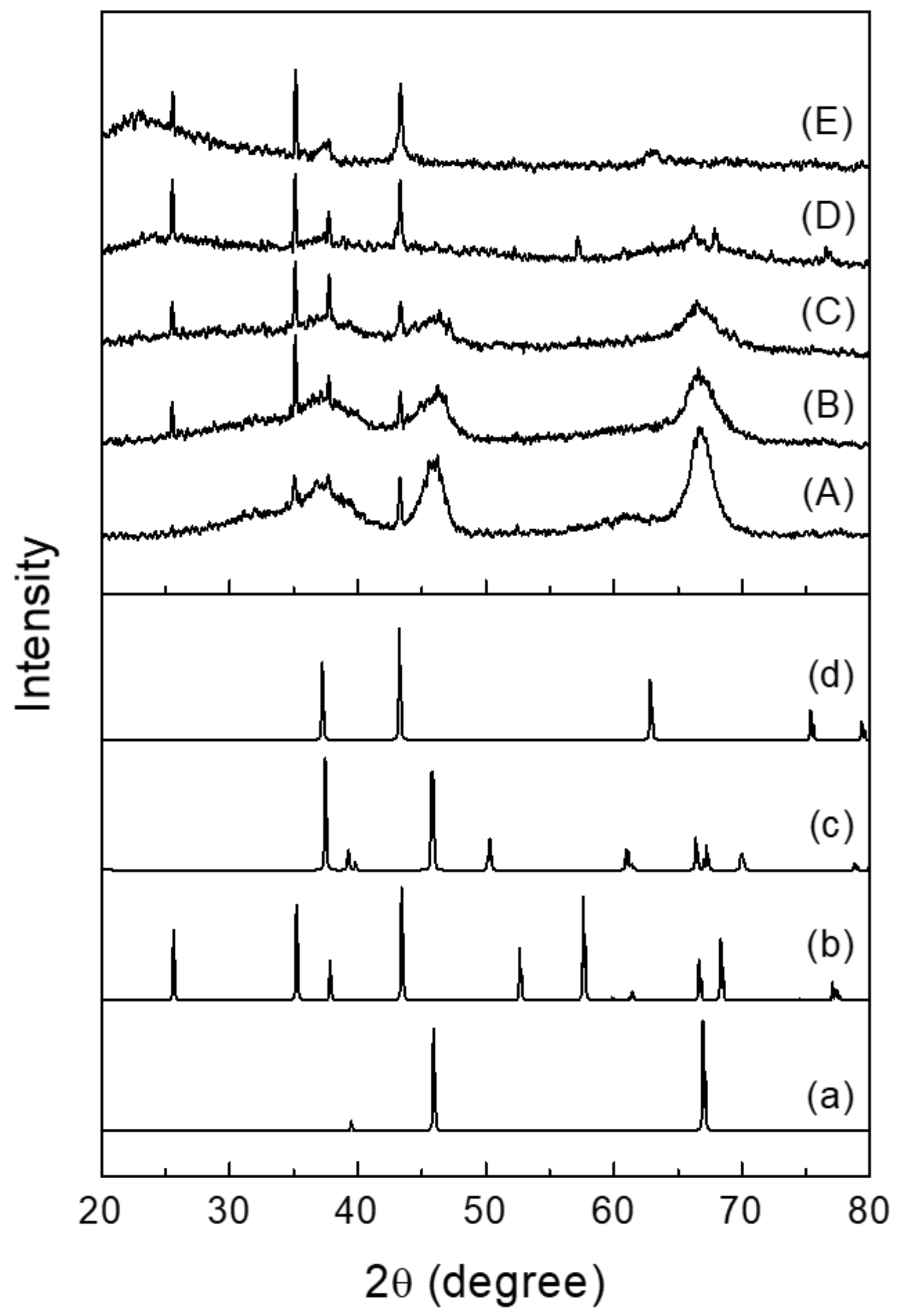
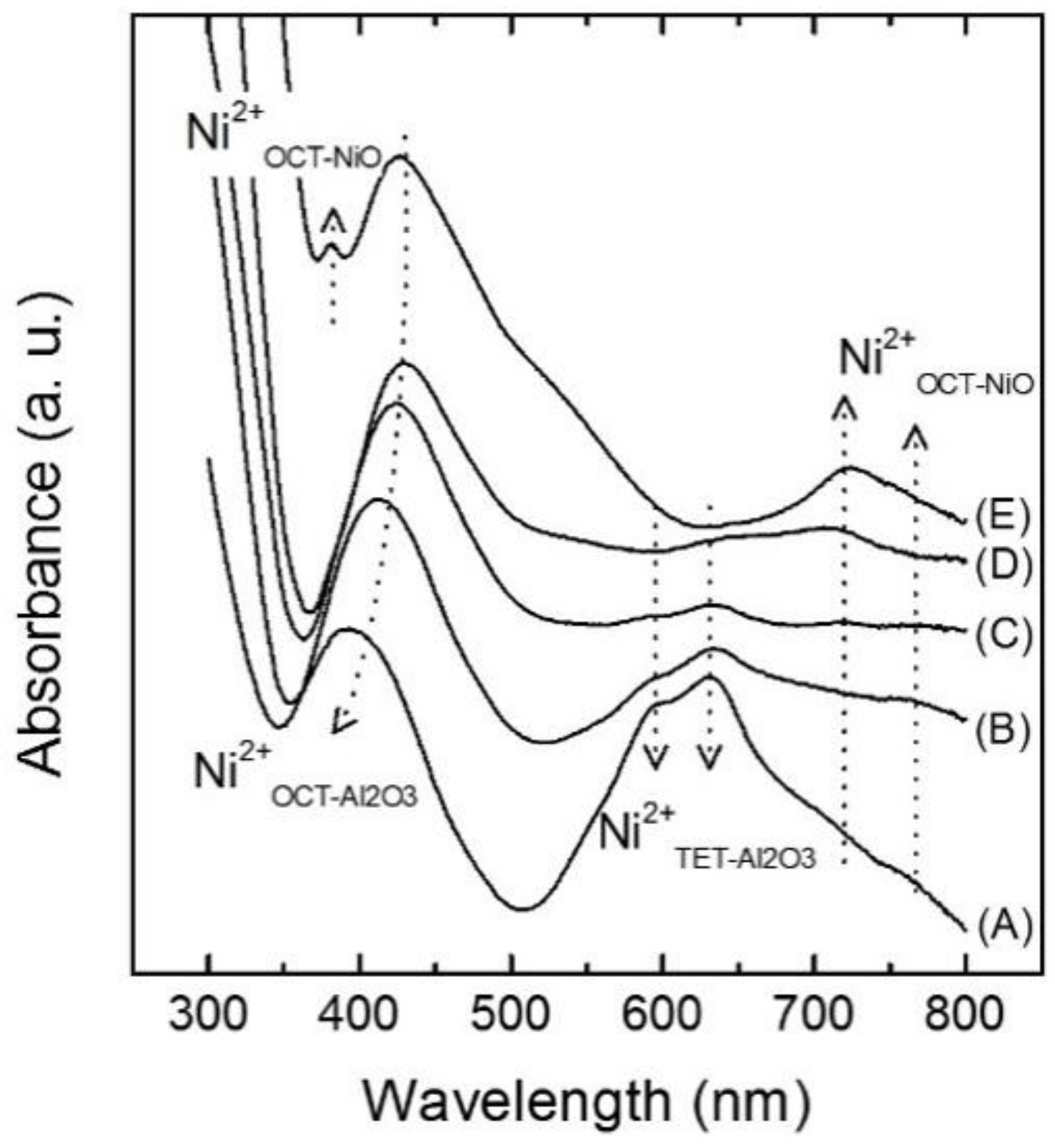
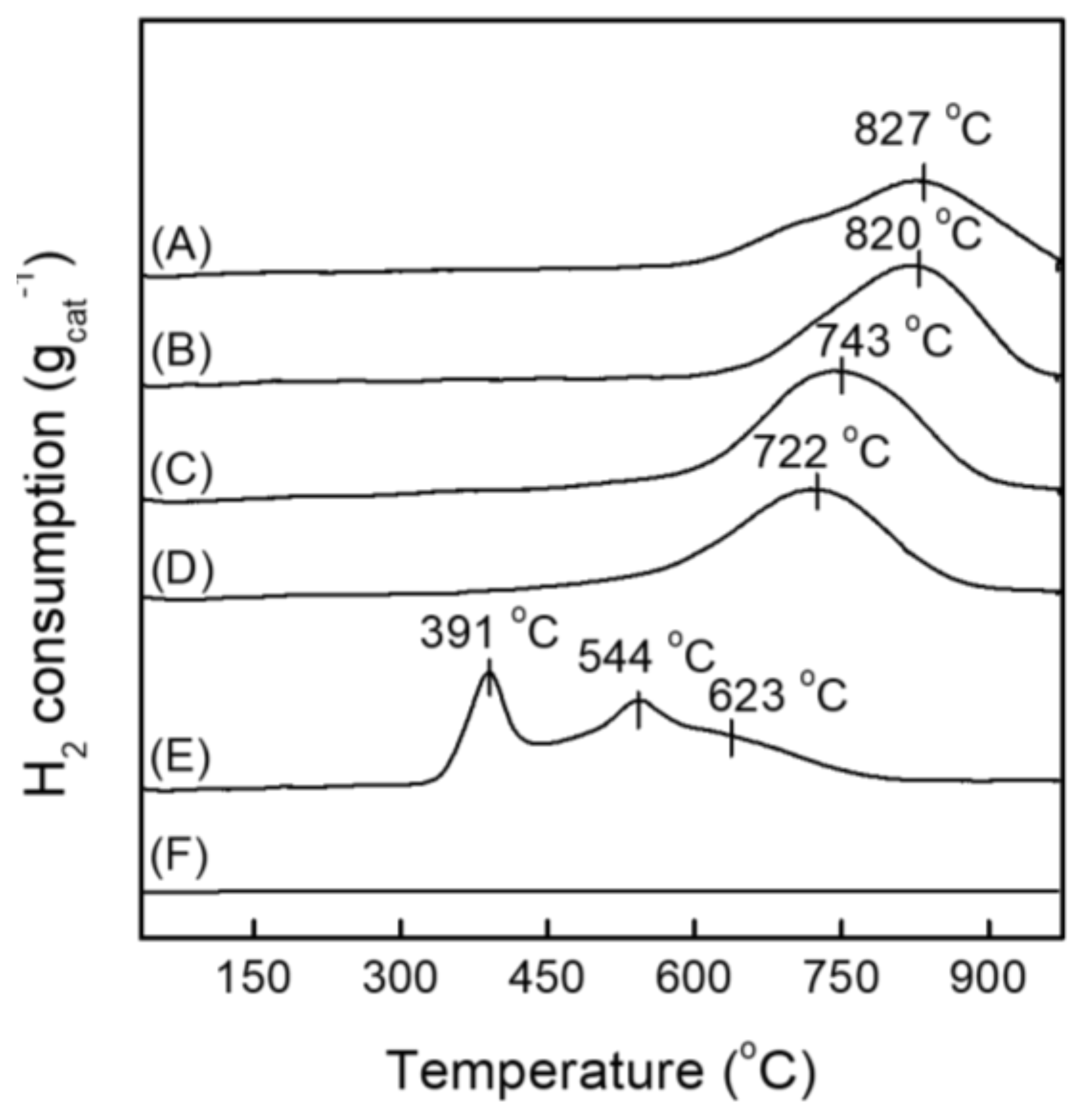
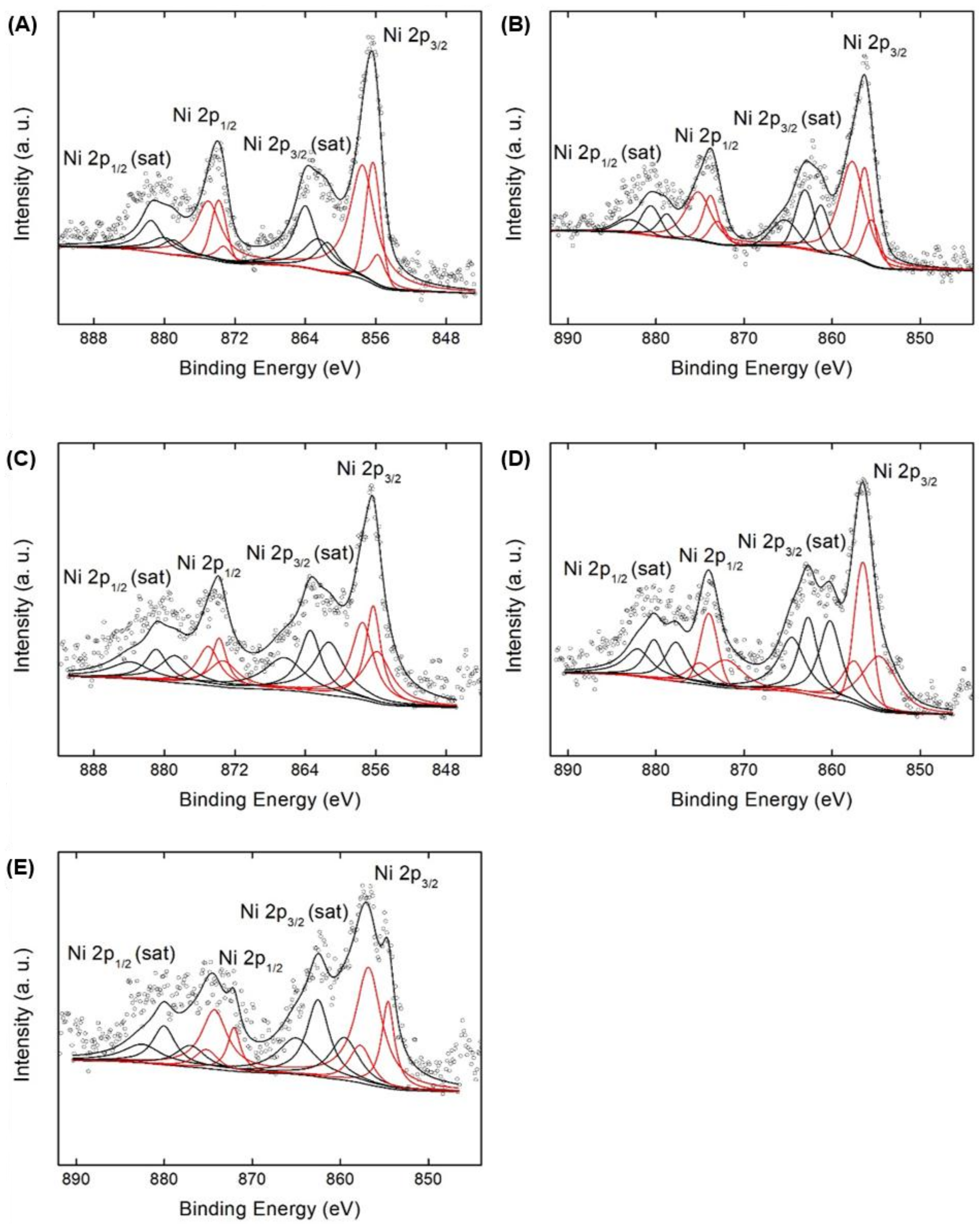
| Catalyst | Si/Al 1 | Ni Content (wt %) 1 | BET Surface Area (m2/g) 2 | Pore Volume (cm3/g) 4 | Pore Size (nm) 4 | ||
|---|---|---|---|---|---|---|---|
| Total | Microporous | External 3 | |||||
| NiOx/SA-001 | 0.01 | 3.81 | 145 | 19 | 126 | 0.4 | 13.0 |
| NiOx/SA-007 | 0.07 | 3.41 | 324 | 21 | 303 | 0.7 | 6.0 |
| NiOx/SA-028 | 0.28 | 3.94 | 321 | 87 | 234 | 0.7 | 8.0 |
| NiOx/SA-047 | 0.47 | 3.85 | 449 | 23 | 426 | 1.2 | 7.7 |
| NiOx/SA-386 | 3.86 | 3.80 | 484 | 0 | 484 | 0.7 | 4.9 |
| Catalyst | Total H2 Uptake (mmol/g) | Reducible Ni Species (mmol/g) 1 | ||
|---|---|---|---|---|
| NiOx → NiOL 2 | NiOL 2 → NiOS 3 | NiOS 3 → Ni | ||
| NiOx/SA-001 | 1.47 | - | - | 1.47 (827) |
| NiOx/SA-007 | 1.88 | - | - | 1.88 (820) |
| NiOx/SA-028 | 2.46 | - | - | 2.46 (743) |
| NiOx/SA-047 | 2.21 | - | - | 2.21 (722) |
| NiOx/SA-386 | 2.42 | 0.75 (391) | 1.03 (544) | 0.64 (623) |
| Catalyst | Ni2+ by NiO | Niδ+ by NiOx | Ni2+ by NiAl2O4 | Ni2+ from NiO/Niδ+ from NiOx | |
|---|---|---|---|---|---|
| NiOx/SA-001 | Binding energy (eV) | 855.8 | 856.3 | 857.5 | 0.38 |
| Area (a.u.) | 363.8 | 969.7 | 2206.6 | ||
| Area ratio (%) | 10.3 | 27.4 | 62.3 | ||
| NiOx/SA-007 | Binding energy (eV) | 855.5 | 856.3 | 857.7 | 0.41 |
| Area (a.u.) | 402.5 | 973.2 | 1817.1 | ||
| Area ratio (%) | 12.6 | 30.5 | 56.9 | ||
| NiOx/SA-028 | Binding energy (eV) | 855.8 | 856.3 | 857.5 | 0.94 |
| Area (a.u.) | 901.2 | 909.8 | 1063.5 | ||
| Area ratio (%) | 30.2 | 32.2 | 37.6 | ||
| NiOx/SA-047 | Binding energy (eV) | 854.6 | 856.5 | 857.5 | 0.92 |
| Area (a.u.) | 975.1 | 1057.8 | 583.2 | ||
| Area ratio (%) | 37.3 | 40.4 | 22.3 | ||
| NiOx/SA-386 | Binding energy (eV) | 854.6 | 856.8 | 857.7 | 0.37 |
| Area (a.u.) | 458.9 | 1241.0 | 307.6 | ||
| Area ratio (%) | 22.9 | 61.8 | 15.3 |
| Catalyst | NH3-TPD (mmol/gcat) | py-FT-IR | ||
|---|---|---|---|---|
| B.A (mmol/gcat) | L.A (mmol/gcat) | B.A/L.A | ||
| NiOx/SA-001 | 0.49 | 0.00 | 0.02 | 0.00 |
| NiOx/SA-007 | 0.74 | 0.00 | 0.06 | 0.00 |
| NiOx/SA-028 | 0.92 | 0.01 | 0.08 | 0.13 |
| NiOx/SA-047 | 1.06 | 0.03 | 0.12 | 0.25 |
| NiOx/SA-386 | 0.91 | 0.01 | 0.05 | 0.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.S.; Park, M.B.; Kim, Y.; Hwang, D.W.; Chae, H.-J. Effect of Metal Oxide–Support Interactions on Ethylene Oligomerization over Nickel Oxide/Silica–Alumina Catalysts. Catalysts 2019, 9, 933. https://doi.org/10.3390/catal9110933
Yoon JS, Park MB, Kim Y, Hwang DW, Chae H-J. Effect of Metal Oxide–Support Interactions on Ethylene Oligomerization over Nickel Oxide/Silica–Alumina Catalysts. Catalysts. 2019; 9(11):933. https://doi.org/10.3390/catal9110933
Chicago/Turabian StyleYoon, Ji Sun, Min Bum Park, Youngmin Kim, Dong Won Hwang, and Ho-Jeong Chae. 2019. "Effect of Metal Oxide–Support Interactions on Ethylene Oligomerization over Nickel Oxide/Silica–Alumina Catalysts" Catalysts 9, no. 11: 933. https://doi.org/10.3390/catal9110933
APA StyleYoon, J. S., Park, M. B., Kim, Y., Hwang, D. W., & Chae, H.-J. (2019). Effect of Metal Oxide–Support Interactions on Ethylene Oligomerization over Nickel Oxide/Silica–Alumina Catalysts. Catalysts, 9(11), 933. https://doi.org/10.3390/catal9110933





