Facile Green Preparation of Rhodium Nanoclusters Supported Nano-Scaled Graphene Platelets for Sonogashira Coupling Reaction and Reduction of p-Nitrophenol
Abstract
:1. Introduction
2. Results and Discussion
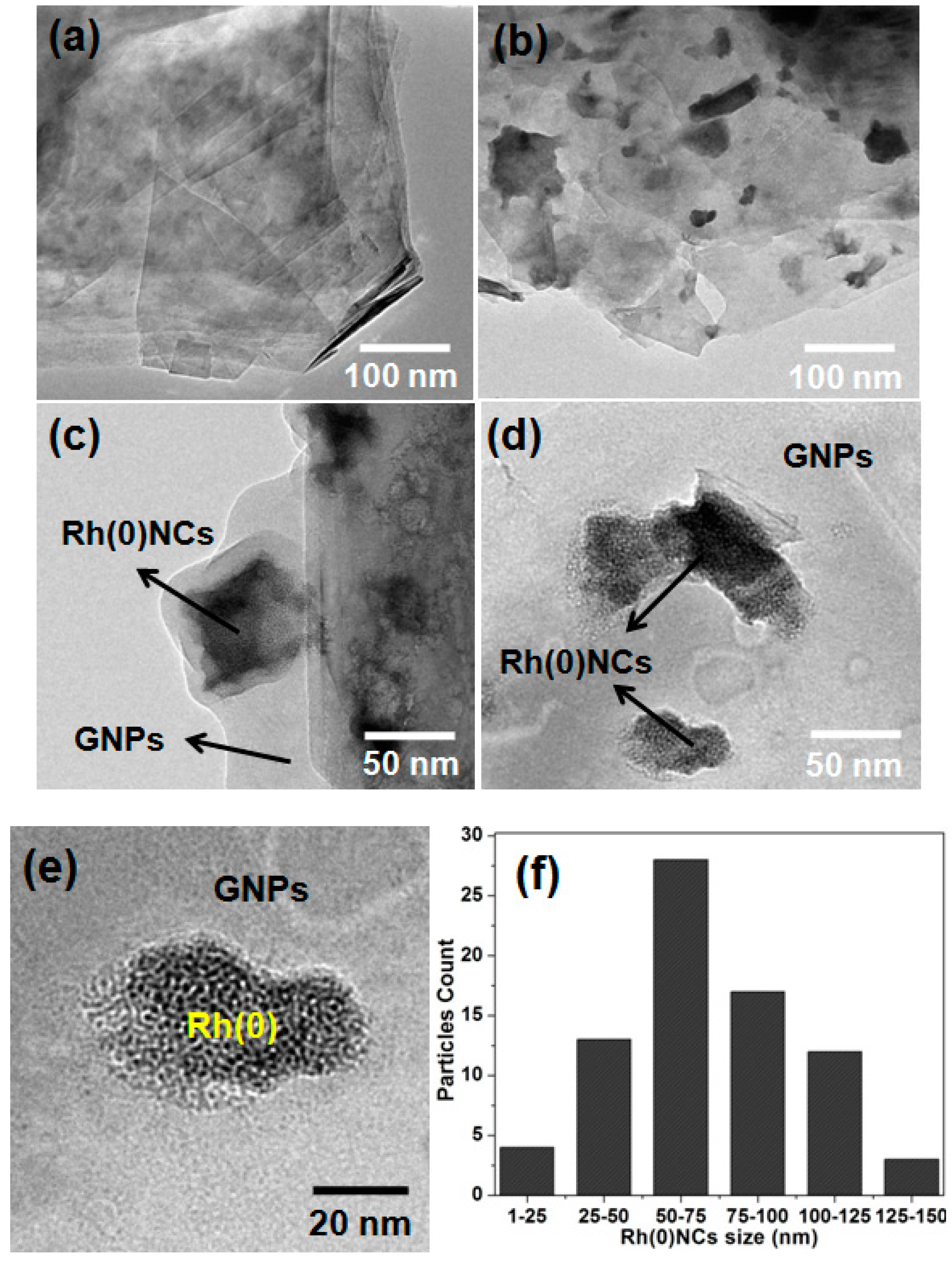
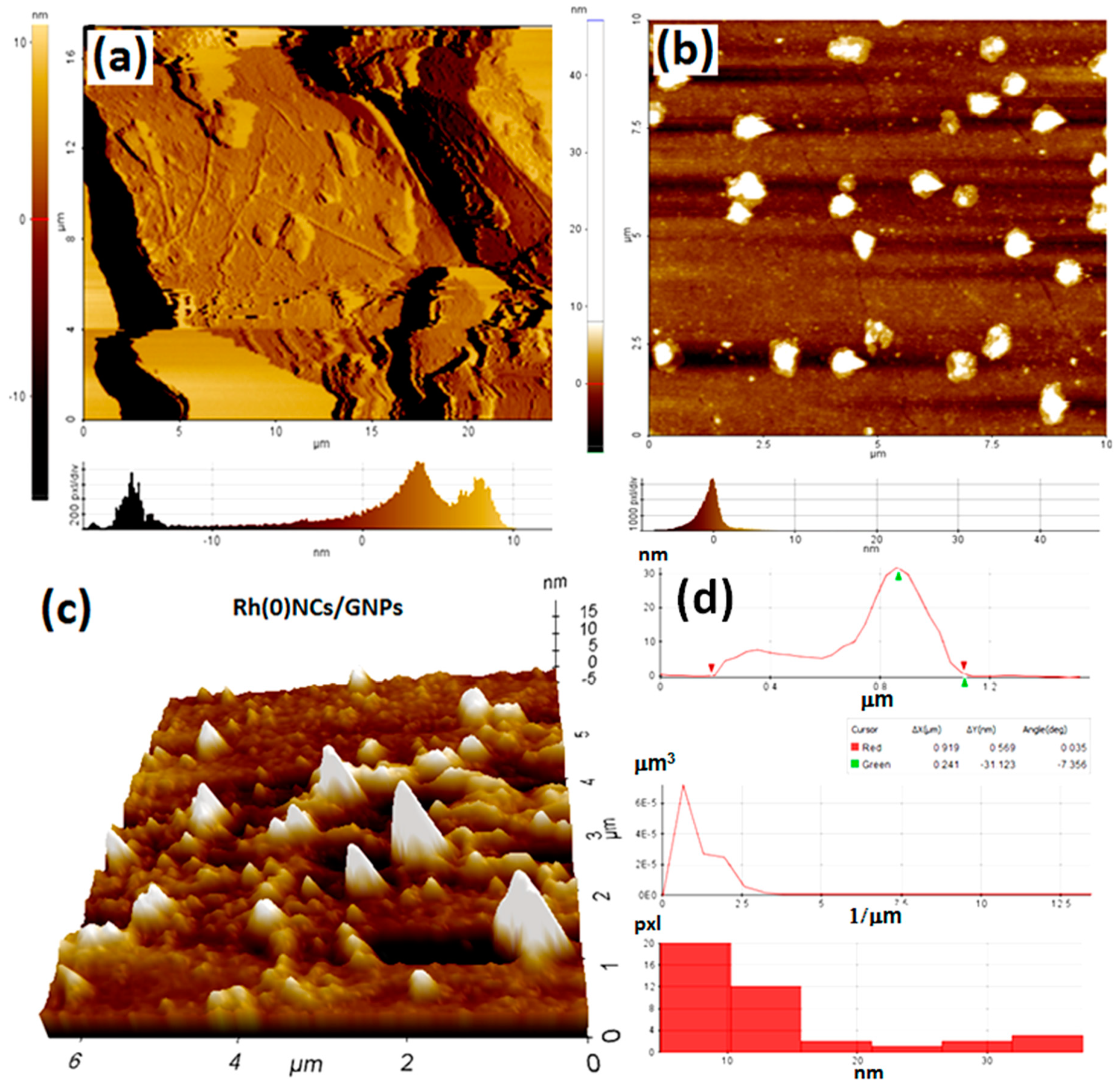
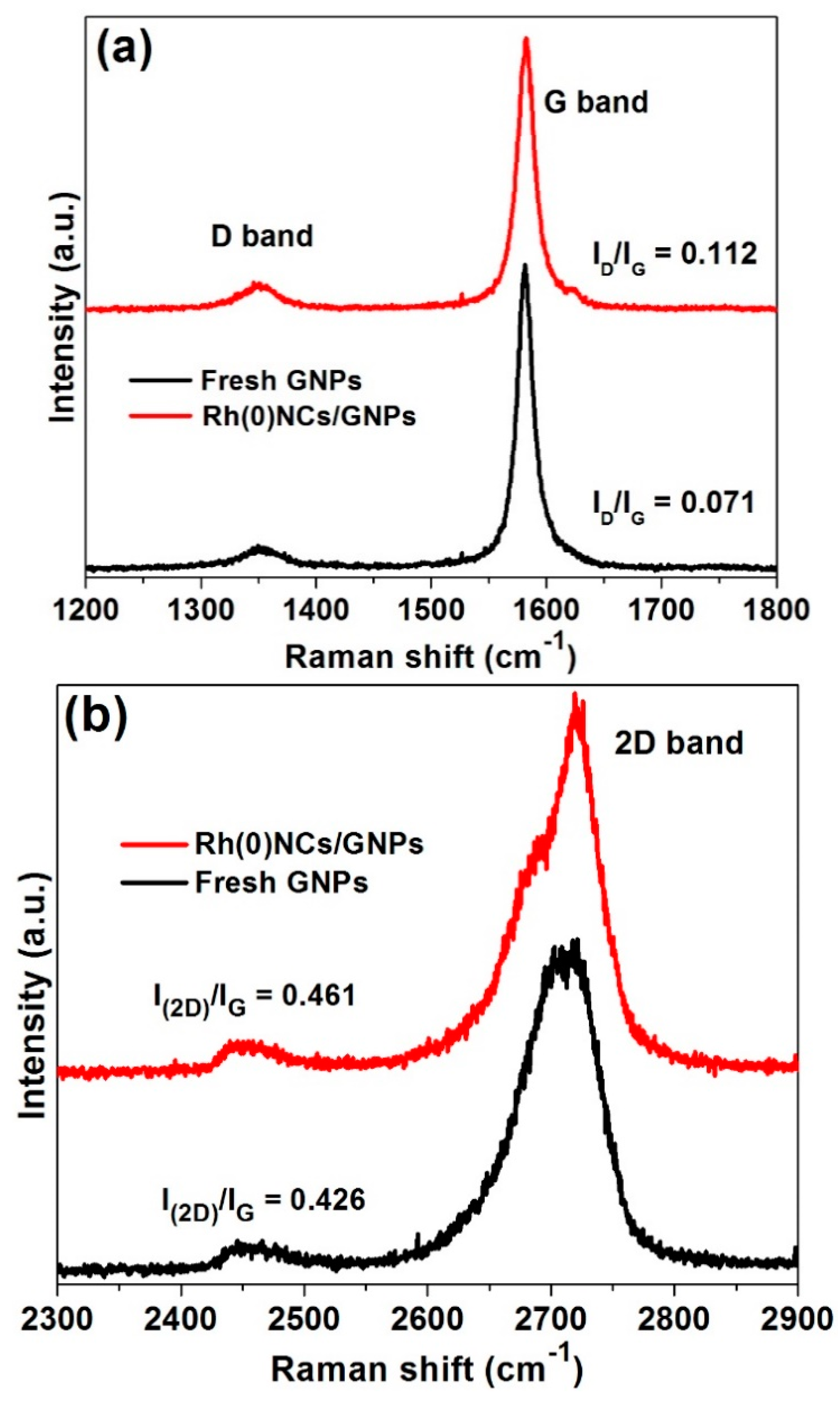
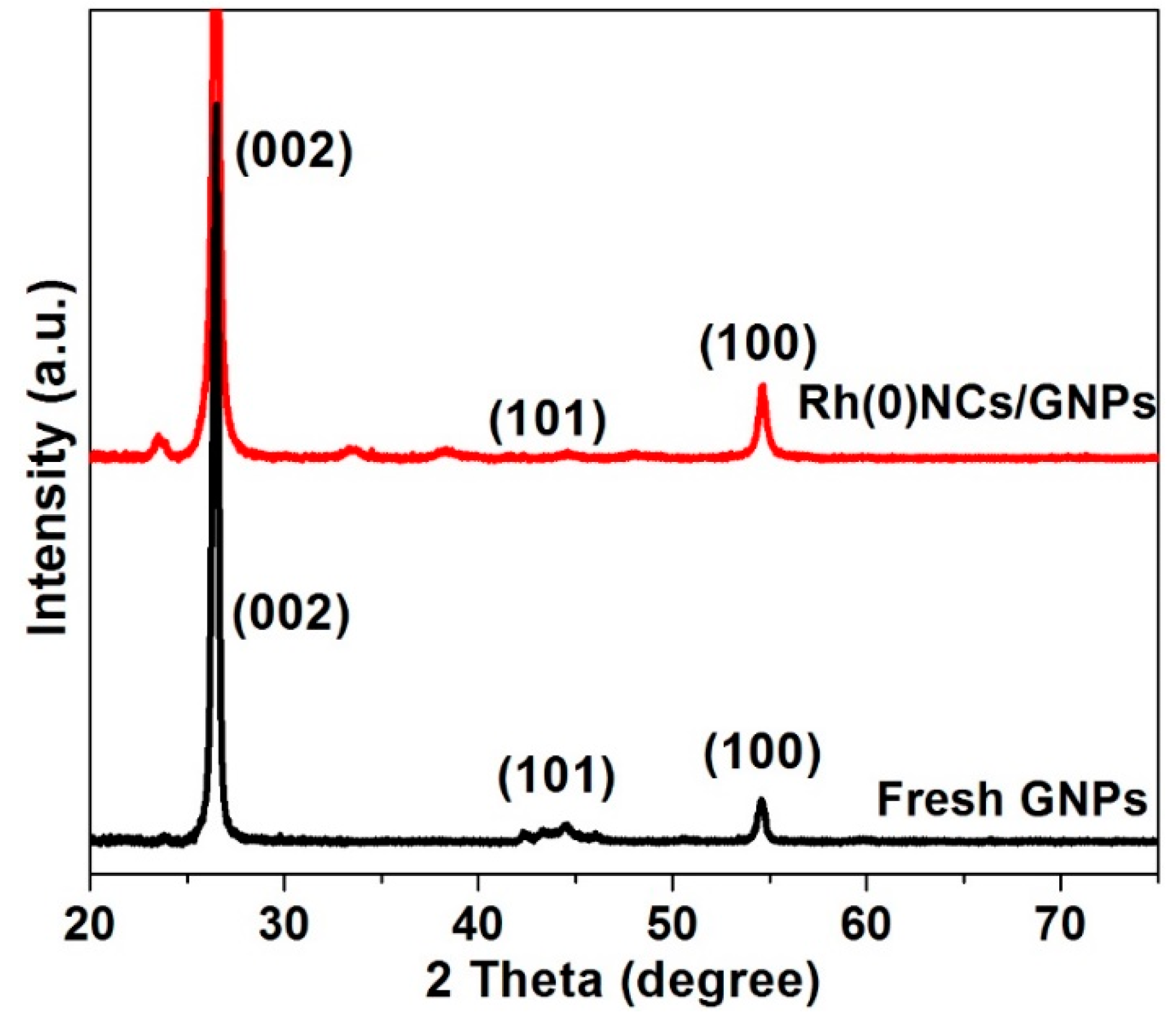
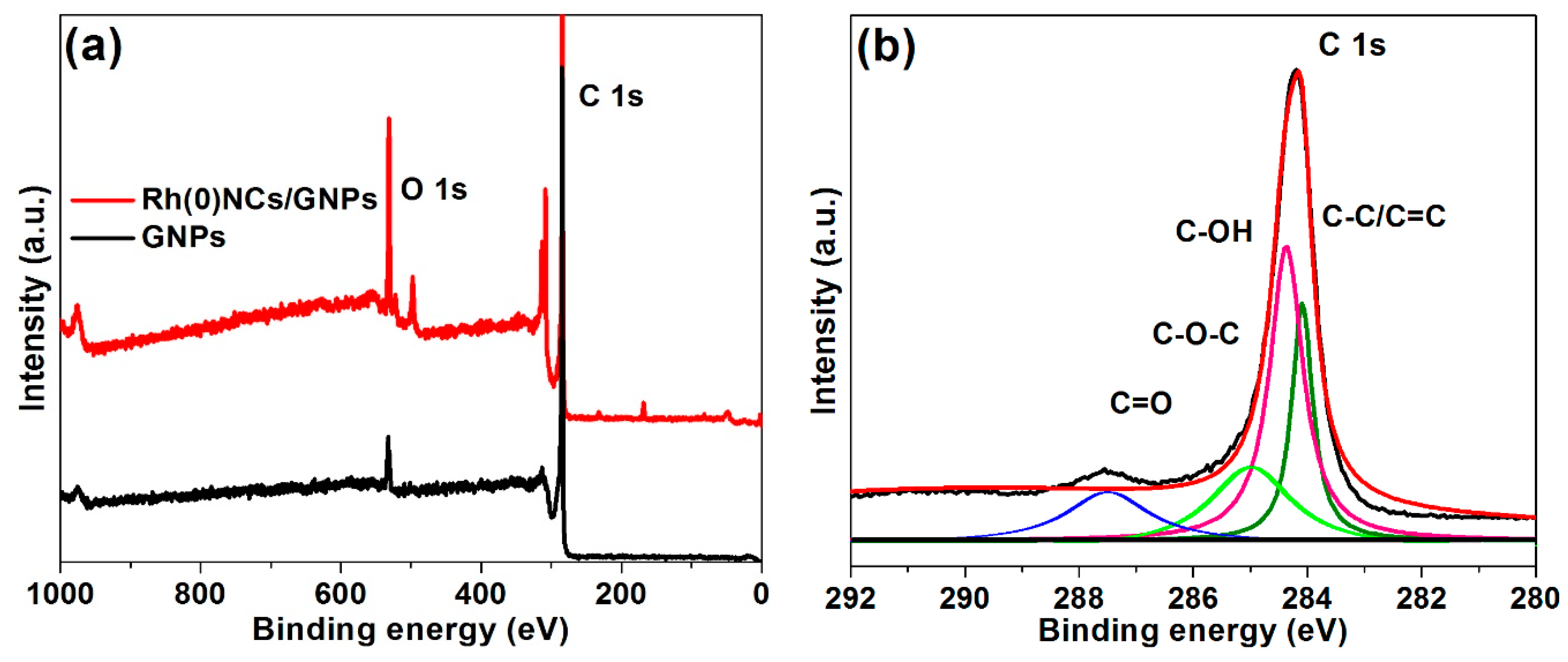
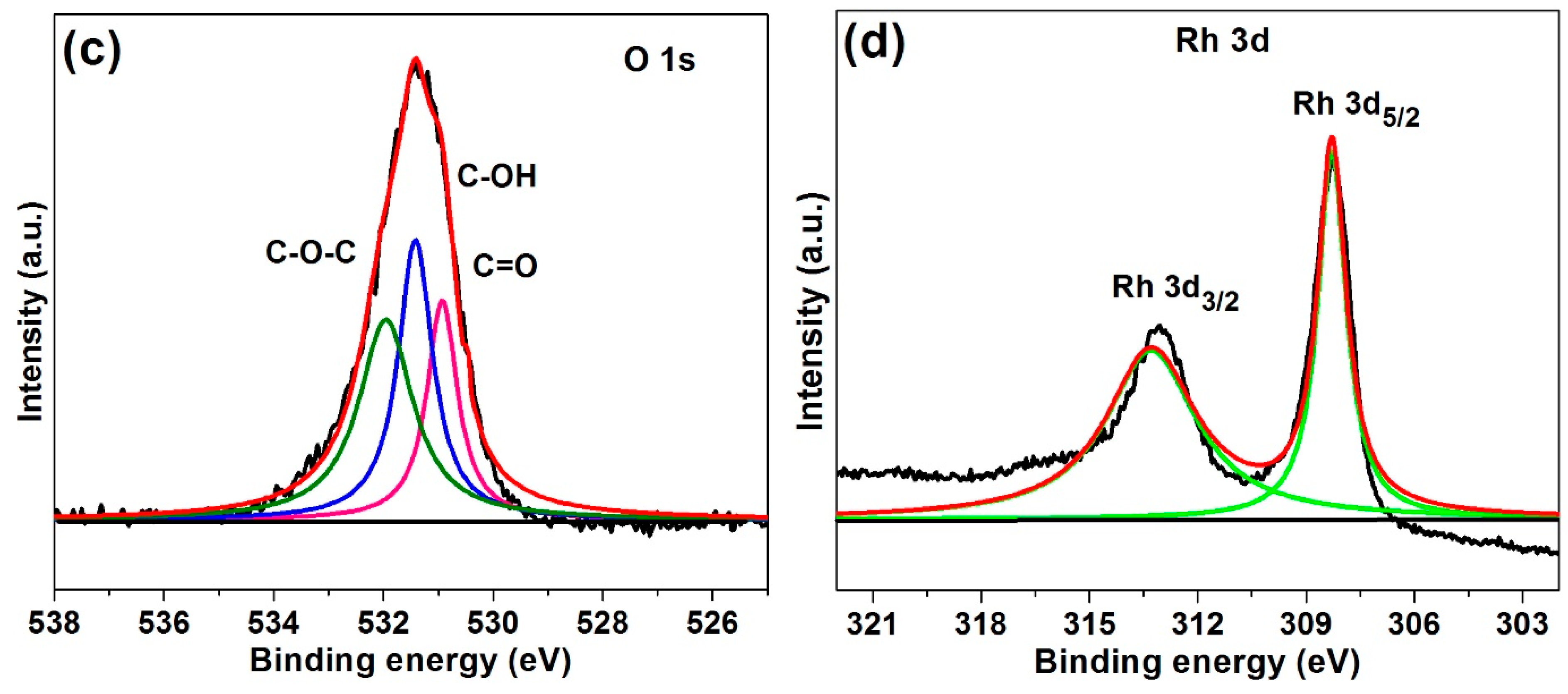
2.1. Characterization of Rh(0)NCs/GNPs Catalyst
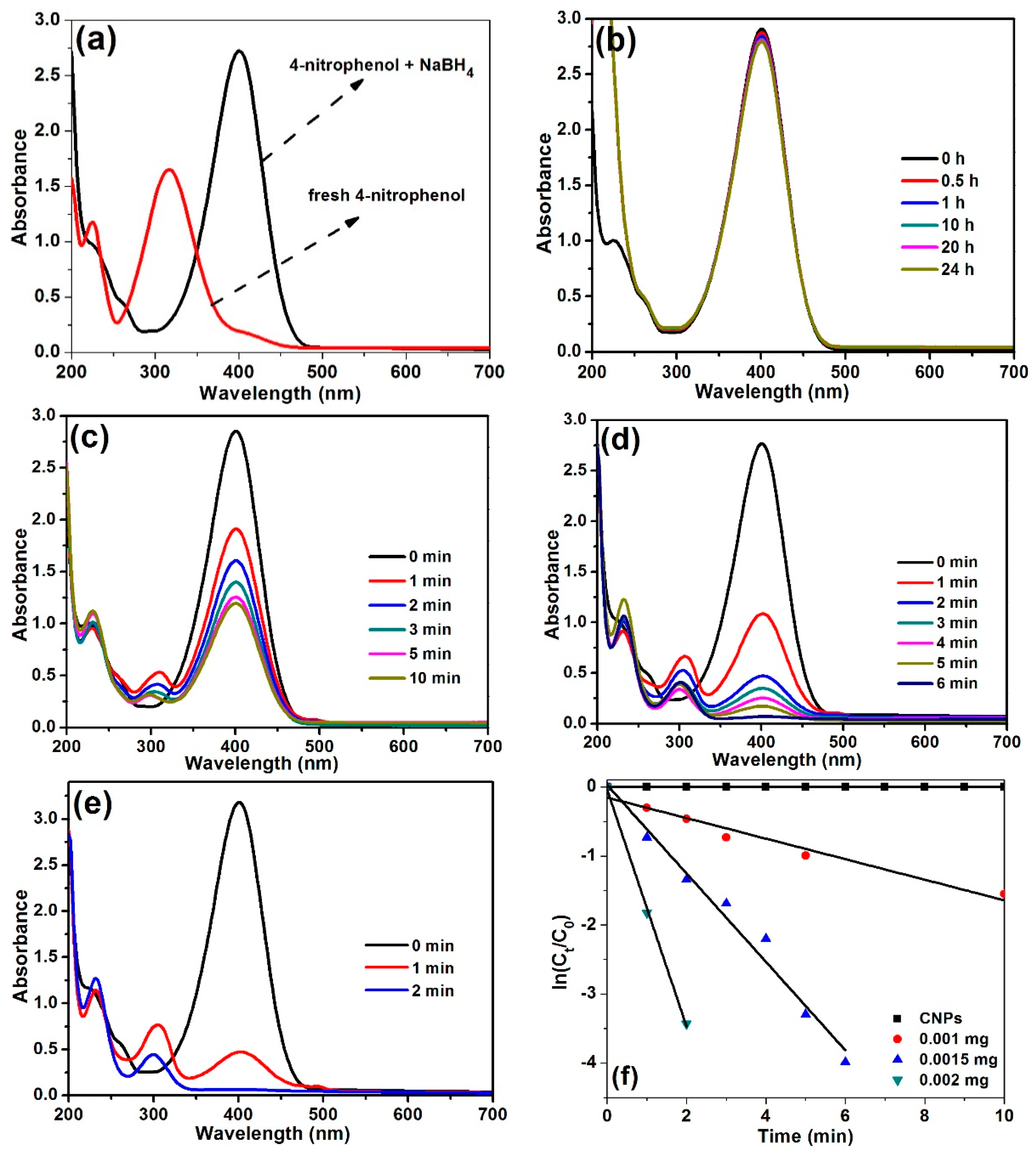
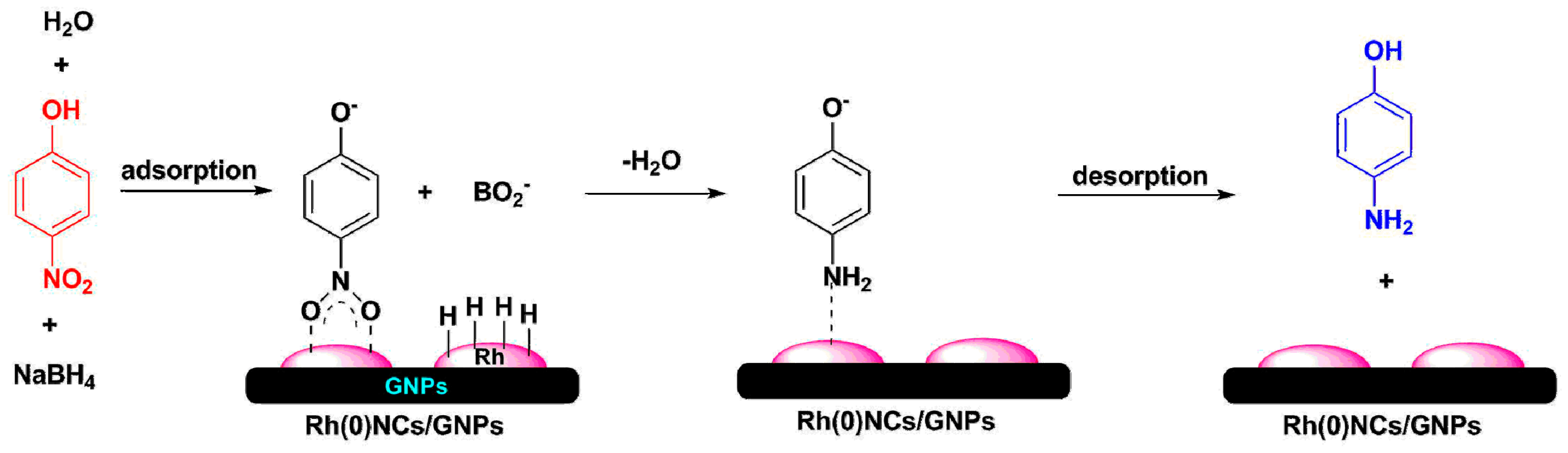
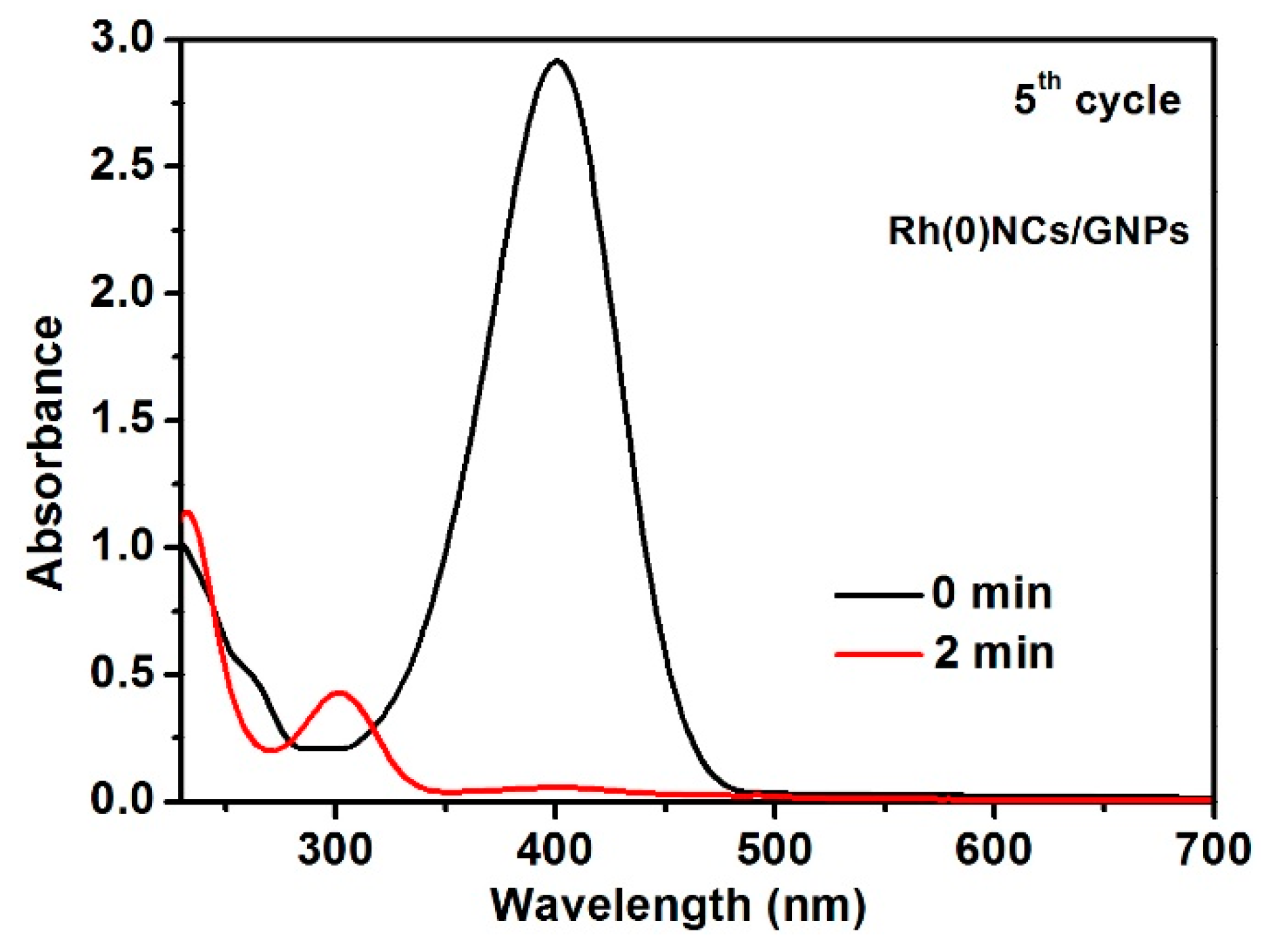
2.2. Reduction of 4-Nitrophenol
2.3. Sonogashira Coupling Reaction
3. Experimental Section
3.1. Materials
3.2. Preparation of Rh(0)NCs/GNPs
3.3. Characterization
3.4. Procedure for Sonogashira Coupling Reaction
3.5. Procedure for 4-Nitrophenol Reduction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhiman, M.; Chalke, B.; Polshettiwar, V. Efficient Synthesis of Monodisperse Metal (Rh, Ru, Pd) Nanoparticles Supported on Fibrous Nanosilica (KCC-1) for Catalysis. ACS Sustain. Chem. Eng. 2015, 3, 3224–3230. [Google Scholar] [CrossRef]
- Jia, C.J.; Schuth, F. Colloidal Metal Nanoparticles as a Component of Designed Catalyst. Phys. Chem. Chem. Phys. 2011, 13, 2457–2487. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhattacherjee, D.; Das, P. Supported Rhodium Nanoparticles Catalyzed Reduction of Nitroarenes, Arylcarbonyls and Aryl/Benzyl Sulfoxides using Ethanol/Methanol as In Situ Hydrogen Source. Adv. Synth. Catal. 2018, 360, 2131–2137. [Google Scholar] [CrossRef]
- Jiang, B.; Li, C.; Dag, O.; Abe, H.; Takei, T.; Imai, T.; Hossain, M.S.A.; Islam, M.T.; Wood, K.; Henzie, J.; et al. Mesoporous Metallic Rhodium Nanoparticles. Nat. Commun. 2017, 8, 15581. [Google Scholar] [CrossRef]
- Xie, S.; Liu, X.Y.; Xia, Y. Shape-controlled Syntheses of Rhodium Nanocrystals for the Enhancement of their Catalytic Properties. Nano Res. 2015, 8, 82–96. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Barreda, A.; Gutierrez, Y.; Gonzalez, F.; Moreno, F.; Everitt, H.O.; Liu, J. Size-Tunable Rhodium Nanostructures for Wavelength-Tunable Ultraviolet Plasmonics. NanoscaleHoriz 2016, 1, 75–80. [Google Scholar] [CrossRef]
- Kang, S.; Shin, W.; Choi, M.H.; Ahn, M.; Kim, Y.K.; Kim, S.; Min, D.H.; Jang, H. Morphology-Controlled Synthesis of Rhodium Nanoparticles for Cancer Phototherapy. ACS Nano 2018, 12, 6997–7008. [Google Scholar] [CrossRef]
- Siebels, M.; Schlusener, C.; Thomas, J.; Xiao, Y.X.; Yang, X.Y.; Janiak, C. Rhodium Nanoparticles Supported on Covalent Triazine-based Frameworks as Reusable Catalyst for Benzene Hydrogenation and Hydrogen Evolution Reaction. J. Mater. Chem. A 2019, 7, 11934–11943. [Google Scholar] [CrossRef]
- Motoyama, Y.; Takasaki, M.; Yoon, S.H.; Mochida, I.; Nagashima, H. Rhodium Nanoparticles Supported on Carbon Nanofibers as an Arene Hydrogenation Catalyst Highly Tolerant to a Coexisting Epoxido Group. Org. Lett. 2009, 11, 5042–5045. [Google Scholar] [CrossRef]
- Huang, C.; Li, C.; Shi, G. Graphene Based Catalysts. Energy Environ. Sci. 2012, 5, 8848–8868. [Google Scholar] [CrossRef]
- Gopiraman, M.; Chung, I.M. Highly Active and Cost-effective CuO-based Carbon Nanocomposite with Unique Morphology for Catalytic Synthesis of Imines under Solvent-free Conditions. J. Taiwan. Inst. Chem. Eng. 2017, 81, 455–464. [Google Scholar] [CrossRef]
- Qiao, F.; Yang, J.; Liang, Q.; Wang, X.; Xu, Q.; Wang, Q. Fabrication of 3D Graphene/CdTe Quantum Dots Composite through Electrophoretic Deposition and its Electrical Properties. J. Mater. Sci. Mater. Electron. 2017, 28, 15333–15337. [Google Scholar] [CrossRef]
- Zeng, M.; Shah, S.A.; Huang, D.; Parviz, D.; Yu, Y.H.; Wang, X.; Green, M.J.; Cheng, Z. Aqueous Exfoliation of Graphite into Graphene Assisted by SulfonylGraphene Quantum Dots for Photonic Crystal Applications. ACS Appl. Mater. Interfaces 2017, 9, 30797–30804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Fan, Y.; Pei, Y.; Qiao, M. Graphene-supported Metal/metal Oxide Nanohybrids: Synthesis and Applications in Heterogeneous Catalysis. Catal. Sci. Technol. 2015, 5, 3903–3916. [Google Scholar] [CrossRef]
- Lin, Y.; Watson, K.A.; Fallbach, M.J.; Ghose, S.; Smith, J.G., Jr.; Delozier, D.M.; Cao, W.; Crooks, R.E.; Connell, J.W. Rapid, Solventless, Bulk Preparation of Metal Nanoparticle-Decorated Carbon Nanotubes. ACS Nano 2009, 3, 871–884. [Google Scholar] [CrossRef]
- Gopiraman, M.; Karvembu, R.; Kim, I.S. Highly Active, Selective, and Reusable RuO2/SWCNT Catalyst for Heck Olefination of Aryl Halides. ACS Catal. 2014, 4, 2118–2129. [Google Scholar] [CrossRef]
- Gopiraman, M.; Deng, D.; Ganesh Babu, S.; Hayashi, T.; Karvembu, R.; Kim, I.S. Sustainable and Versatile CuO/GNS Nanocatalyst for Highly Efficient Base Free Coupling Reactions. ACS Sustain. Chem. Eng. 2015, 3, 2478–2488. [Google Scholar] [CrossRef]
- Gopiraman, M.; Babu, S.G.; Karvembu, R.; Kim, I.S. Nanostructured RuO2 on MWCNTs: Efficient Catalyst for Transfer Hydrogenation of Carbonyl Compounds and Aerial Oxidation of Alcohols. Appl. Catal. A 2014, 484, 84–96. [Google Scholar] [CrossRef]
- Gopiraman, M.; Babu, S.G.; Khatri, Z.; Kai, W.; Kim, Y.A.; Endo, M.; Karvembu, R.; Kim, I.S. An Efficient, Reusable Copper-oxide/carbon-nanotube Catalyst for N-Arylation of Imidazole. Carbon 2013, 62, 135–148. [Google Scholar] [CrossRef]
- Gopiraman, M.; Ganesh Babu, S.; Khatri, Z.; Kai, W.; Kim, Y.A.; Endo, M.; Kim, I.S. Dry Synthesis of Easily Tunable Nano Ruthenium Supported on Graphene: Novel Nanocatalysts for Aerial Oxidation of Alcohols and Transfer Hydrogenation of Ketones. J. Phys. Chem. C 2013, 117, 23582–23596. [Google Scholar] [CrossRef]
- Gopiraman, M.; Saravanamoorthy, S.; Deng, D.; Ilangovan, A.; Kim, I.S.; Chung, I.M. Facile Mechanochemical Synthesis of Nickel/Graphene Oxide Nanocomposites with Unique and Tunable Morphology: Applications in Heterogeneous Catalysis and Supercapacitors. Catalysts 2019, 9, 486. [Google Scholar] [CrossRef]
- Somasundaram, S.; Ill-Min, C.; Vanaraj, R.; Ramaganthan, B.; Mayakrishnan, G. Highly Active and Reducing Agent-free Preparation of Cost-effective NiO-based Carbon Nanocomposite and its Application in Reduction Reactions under Mild Conditions. J. Ind. Eng. Chem. 2018, 60, 91–101. [Google Scholar] [CrossRef]
- Gopiraman, M.; Bang, H.; Babu, S.G.; Wei, K.; Karvembu, R.; Kim, I.S. Catalytic N-oxidation of Tertiary Amines on RuO2 NPs Anchored GrapheneNanoplatelets. Catal. Sci. Technol. 2014, 4, 2099–2106. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic Concepts and Recent Advances in Nitrophenol Reduction by Gold-and other Transition Metal Nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Liori, A.A.; Stamatopoulos, I.K.; Papastavrou, A.T.; Pinaka, A.; Vougioukalakis, G.C. A Sustainable, User-Friendly Protocol for the Pd-Free Sonogashira Coupling Reaction. Eur. J. Org. Chem. 2018, 2018, 6134–6139. [Google Scholar] [CrossRef]
- Kanuru, V.K.; Humphrey, S.M.; Kyffin, J.M.; Jefferson, D.A.; Burton, J.W.; Armbruster, M.; Lambert, R.M. Evidence for Heterogeneous Sonogashira Coupling of Phenylacetylene and Iodobenzene Catalyzed by Well Defined Rhodium Nanoparticles. Dalton Trans. 2009, 37, 7602–7605. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yan, N.; Dyson, P.J. Advances in the Rational Design of Rhodium Nanoparticle Catalysts: Control via Manipulation of the Nanoparticle Core and Stabilizer. ACS Catal. 2012, 2, 1057–1069. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Defected Graphene Nano-platelets for Enhanced Hydrophilic Nature and Visible Light-induced Photoelectrochemical Performances. J. Phys. Chem. Solids 2017, 104, 233–242. [Google Scholar] [CrossRef]
- Sujith, R.; Chauhan, P.K.; Gangadhar, J.; Maheshwari, A. GrapheneNanoplatelets as Nanofillers in Mesoporous Silicon Oxycarbide Polymer Derived Ceramics. Sci. Rep. 2018, 8, 17633. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-free Synthesis of Nitrogen-doped Graphene via Thermal Annealing Graphite Oxide with Melamine and its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Dong, L.; Yu, W.; Liu, M.; Liu, Y.; Shao, Q.; Li, A.; Yan, W. Novel Composite Electrode of the Reduced Graphene Oxide Nanosheets with Gold Nanoparticles Modified by Glucose Oxidase for Electrochemical Reactions. Catalysts 2019, 9, 764. [Google Scholar] [CrossRef]
- Jin, C.; Xia, W.; Nagaiah, T.C.; Guo, J.; Chen, X.; Bron, M.; Schuhmann, W.; Muhler, M. On the Role of the Thermal Treatment of Sulfided Rh/CNT Catalysts Applied in the Oxygen Reduction Reaction. Electrochim. Acta 2009, 54, 7186–7193. [Google Scholar] [CrossRef]
- Nancy, P.; Nair, A.K.; Antoine, R.; Thomas, S.; Kalarikkal, N. In Situ Decoration of Gold Nanoparticles on Graphene Oxide via Nanosecond Laser Ablation for Remarkable Chemical Sensing and Catalysis. Nanomaterials 2019, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Kim, H.S. Fast and Fully-scalable Synthesis of Reduced Graphene Oxide. Sci. Rep. 2015, 5, 10160. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.W.; Zheng, L. Microwave-assisted Synthesis of Hexagonal Gold Nanoparticles Reduced by Organosilane (3-Mercaptopropyl) trimethoxysilane. Materials 2019, 12, 1680. [Google Scholar] [CrossRef]
- Sun, L.; Xiang, X.; Wu, J.; Cai, C.; Ao, D.; Luo, J.; Tian, C.; Zu, X. Bi-Metal Phosphide NiCoP: An Enhanced Catalyst for the Reduction of 4-Nitrophenol. Nanomaterials 2019, 9, 112. [Google Scholar] [CrossRef]
- Morales, M.V.; Rocha, M.; Freire, C.; Asedegbega-Nieto, E.; Gallegos-Suarez, E.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Development of Highly Efficient Cu versus Pd Catalysts Supported on Graphitic Carbon Materials for the Reduction of 4-Nitrophenol to 4-Aminophenol at Room Temperature. Carbon 2017, 111, 150–161. [Google Scholar] [CrossRef]
- Vilian, A.E.; Choe, S.R.; Giribabu, K.; Jang, S.C.; Roh, C.; Huh, Y.S.; Han, Y.K. PdNanospheres Decorated Reduced Graphene Oxide with Multi-functions: Highly Efficient Catalytic Reduction and Ultrasensitive Sensing of Hazardous 4-Nitrophenol Pollutant. J. Hazard. Mater. 2017, 333, 54–62. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhao, Y.H.; Zhou, Y.; Guo, X.L.; Chen, Z.T.; Zhang, W.J.; Zhang, Y.; Chen, J.; Wang, Z.M.; Sun, L.T.; et al. High-efficient Catalytic Reduction of 4-Nitrophenol based on Reusable Ag Nanoparticles/graphene-loading Loofah Sponge Hybrid. Nanotechnology 2018, 29, 315702. [Google Scholar] [CrossRef]
- Sareen, S.; Mutreja, V.; Pal, B.; Singh, S. Synthesis of Bimetallic Au-Ag Alloyed Mesocomposites and their Catalytic Activity for the Reduction of Nitroaromatics. Appl. Surf. Sci. 2018, 435, 552–562. [Google Scholar] [CrossRef]
- Gopiraman, M.; Deng, D.; Saravanamoorthy, S.; Chung, I.M.; Kim, I.S. Gold, Silver and Nickel Nanoparticle Anchored Cellulose Nanofiber Composites as Highly Active Catalysts for the Rapid and Selective Reduction of Nitrophenols in Water. RSC Adv. 2018, 8, 3014–3023. [Google Scholar] [CrossRef]
- Wang, C.; Ciganda, R.; Yate, L.; Moya, S.; Salmon, L.; Ruiz, J.; Astruc, D. RhAg/rGONanocatalyst: Ligand-controlled Synthesis and Superior Catalytic Performances for the Reduction of 4-Nitrophenol. J. Mater. Sci. 2017, 52, 9465–9476. [Google Scholar] [CrossRef]
- Lv, J.J.; Wang, A.J.; Ma, X.; Xiang, R.Y.; Chen, J.R.; Feng, J.J. One-pot synthesis of porous Pt–Au nanodendrites supported on reduced graphene oxide nanosheets toward catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2015, 3, 290–296. [Google Scholar] [CrossRef]
- Hareesh, K.; Joshi, R.P.; Sunitha, D.V.; Bhoraskar, V.N.; Dhole, S.D. Anchoring of Ag-Au Alloy Nanoparticles on Reduced Graphene Oxide Sheets for the Reduction of 4-Nitrophenol. Appl. Surf. Sci. 2016, 389, 1050–1055. [Google Scholar]
- Yang, Y.; Ren, Y.; Sun, C.; Hao, S. Facile Route Fabrication of Nickel based Mesoporous Carbons with High Catalytic Performance towards 4-Nitrophenol Reduction. Green Chem. 2014, 16, 2273–2280. [Google Scholar] [CrossRef]
- Zhao, F.; Kong, W.; Hu, Z.; Liu, J.; Zhao, Y.; Zhang, B. Tuning the Performance of Pt–Ni Alloy/reduced Graphene Oxide Catalysts for 4-Nitrophenol Reduction. RSC Adv. 2016, 6, 79028–79036. [Google Scholar] [CrossRef]
- Vellaichamy, B.; Periakaruppan, P.; Thomas, J. Synthesis of AuNPs@RGONanosheets for Sustainable Catalysis toward Nitrophenols Reduction. Ultrason. Sonochem. 2018, 48, 362–369. [Google Scholar] [CrossRef]
- Mohamed, M.J.S.; Denthaje, K.B. Novel RGO-ZnWO4-Fe3O4Nanocomposite as an Efficient Catalyst for Rapid Reduction of 4-Nitrophenol to 4-Aminophenol. Ind. Eng. Chem. Res. 2016, 55, 7267–7272. [Google Scholar] [CrossRef]
- Gopiraman, M.; Saravanamoorthy, S.; Chung, I.M. Highly Active Human-hair-supported Noble Metal (Ag or Ru) Nanocomposites for Rapid and Selective Reduction of p-Nitrophenol to p-Aminophenol. Res. Chem. Intermed. 2017, 43, 5601–5614. [Google Scholar] [CrossRef]
- Gopiraman, M.; Muneeswaran, M.; Kim, I.S. Highly Porous Ru/C and Cu/C Nanocatalysts derived from Custard Apple for Rapid and Selective Reduction of p-Nitrophenol. Nano Prog. 2019, 1, 30–36. [Google Scholar] [CrossRef]
- Saravanamoorthy, S.; Vijayakumar, E.; Jemimah, S.; Ilangovan, A. Catalytic Reduction of p-Nitrophenol and Carbonyl Compounds by NiO-nanoparticles Fastened Graphene Oxide. Chem. Sci. Eng. Res. 2019, 1, 1–7. [Google Scholar]
- Chinchilla, R.; Najera, C. Recent Advances in Sonogashira Reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef] [PubMed]
- Karami, K.; Abedanzadeh, S.; Hervés, P. Synthesis and Characterization of Functionalized Titania-supported Pd Catalyst deriving from New Orthopalladated Complex of Benzophenone Imine: Catalytic Activity in the Copper-free Sonogashira Cross-Coupling Reactions at Low Palladium Loadings. RSC Adv. 2016, 6, 93660–93672. [Google Scholar] [CrossRef]
- Sonogashira, K. Development of Pd–Cu Catalyzed Cross-Coupling of Terminal Acetylenes with sp2-carbon Halides. J. Organomet. Chem. 2002, 653, 46–49. [Google Scholar] [CrossRef]
- Diyarbakir, S.; Can, H.; Metin, O. Reduced Graphene Oxide-supported CuPd Alloy Nanoparticles as Efficient Catalysts for the Sonogashira Cross-coupling Reactions. ACS Appl. Mater. Interfaces 2015, 7, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Nakamura, T.; Yorimitsu, H.; Oshima, K. Cobalt-catalyzed Heck-type Reaction of Alkyl Halides with Styrenes. J. Am. Chem. Soc. 2002, 124, 6514–6515. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Gholinejad, M.; Hoseini, J. Magnetite (Fe3O4) Nanoparticles-catalyzed Sonogashira–Hagihara Reactions in Ethylene Glycol under Ligand-free Conditions. Adv. Synth. Catal. 2011, 353, 125–132. [Google Scholar] [CrossRef]



| S. No | Catalyst(Amount Used, mg) | Reactant | kapp(× 10−3 s−1) | k’(× 10−3 mg−1s−1) | TOF(s−1) | References |
|---|---|---|---|---|---|---|
| 10 | Au-Ag/r-GO (0.1) | 4-NP | 3.47 | 34.7 | 0.042 | [42] |
| 7 | Ni/MC-750 (3) | 4-NP | 6.26 | 20.9 | 1.44 | [43] |
| 4 | Ni/GO-2 (0.75) | 4-NP | 35.4 | 47.2 | 25.33 | [19] |
| 3 | Ni/GO-1 (0.75) | 4-NP | 28.1 | 14.0 | 31.66 | [19] |
| 9 | Pt–Ni/RGO (3) | 4-NP | 3.70 | 1.23 | 110.9 | [44] |
| 12 | AuNPs-RGO (0.05) | 4-NP | 28.37 | 11.2 | 0.222 | [45] |
| 7 | RhAg0.5/rGO | 4-NP | 14.8 | 1415 | - | [40] |
| 8 | Rh(0)NCs/GNPs | 4-NP | 62.07 | 31035 | 112.5 | This work |
| 15 | RGO-ZnWO4-Fe3O4 | 4-NP | 176.8 | 353.6 | - | [46] |
| 10 | Ag NPs/RGO-LS | 4-NP | 32.0 | 0.4 | - | [39] |
| 11 | Ni/GNP | 4-NP | 42.0 | 2.1 | 0.38 | [22] |
| 12 | Ru/HHP | 4-NP | 62.1 | 31.1 | - | [49] |
| 13 | Cu/C | 4-NP | 0.3 | 0.13 | 0.15 | [50] |
| 14 | Ru/C | 4-NP | 1.3 | 0.52 | 0.29 | [50] |
| 15 | Ni-oxide/GOSs | 4-NP | 60.8 | 60.8 | 0.73 | [51] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayakrishnan, G.; Somasundaram, S.; Ullah, S.; Andivelu, I.; Ick Soo, K.; Ill Min, C. Facile Green Preparation of Rhodium Nanoclusters Supported Nano-Scaled Graphene Platelets for Sonogashira Coupling Reaction and Reduction of p-Nitrophenol. Catalysts 2019, 9, 908. https://doi.org/10.3390/catal9110908
Mayakrishnan G, Somasundaram S, Ullah S, Andivelu I, Ick Soo K, Ill Min C. Facile Green Preparation of Rhodium Nanoclusters Supported Nano-Scaled Graphene Platelets for Sonogashira Coupling Reaction and Reduction of p-Nitrophenol. Catalysts. 2019; 9(11):908. https://doi.org/10.3390/catal9110908
Chicago/Turabian StyleMayakrishnan, Gopiraman, Saravanamoorthy Somasundaram, Sana Ullah, Ilangovan Andivelu, Kim Ick Soo, and Chung Ill Min. 2019. "Facile Green Preparation of Rhodium Nanoclusters Supported Nano-Scaled Graphene Platelets for Sonogashira Coupling Reaction and Reduction of p-Nitrophenol" Catalysts 9, no. 11: 908. https://doi.org/10.3390/catal9110908
APA StyleMayakrishnan, G., Somasundaram, S., Ullah, S., Andivelu, I., Ick Soo, K., & Ill Min, C. (2019). Facile Green Preparation of Rhodium Nanoclusters Supported Nano-Scaled Graphene Platelets for Sonogashira Coupling Reaction and Reduction of p-Nitrophenol. Catalysts, 9(11), 908. https://doi.org/10.3390/catal9110908









