The Steric Effect in Green Benzylation of Arenes with Benzyl Alcohol Catalyzed by Hierarchical H-beta Zeolite
Abstract
1. Introduction
2. Results and Discussion
2.1. Essential Characterization of Beta Zeolite Catalysts
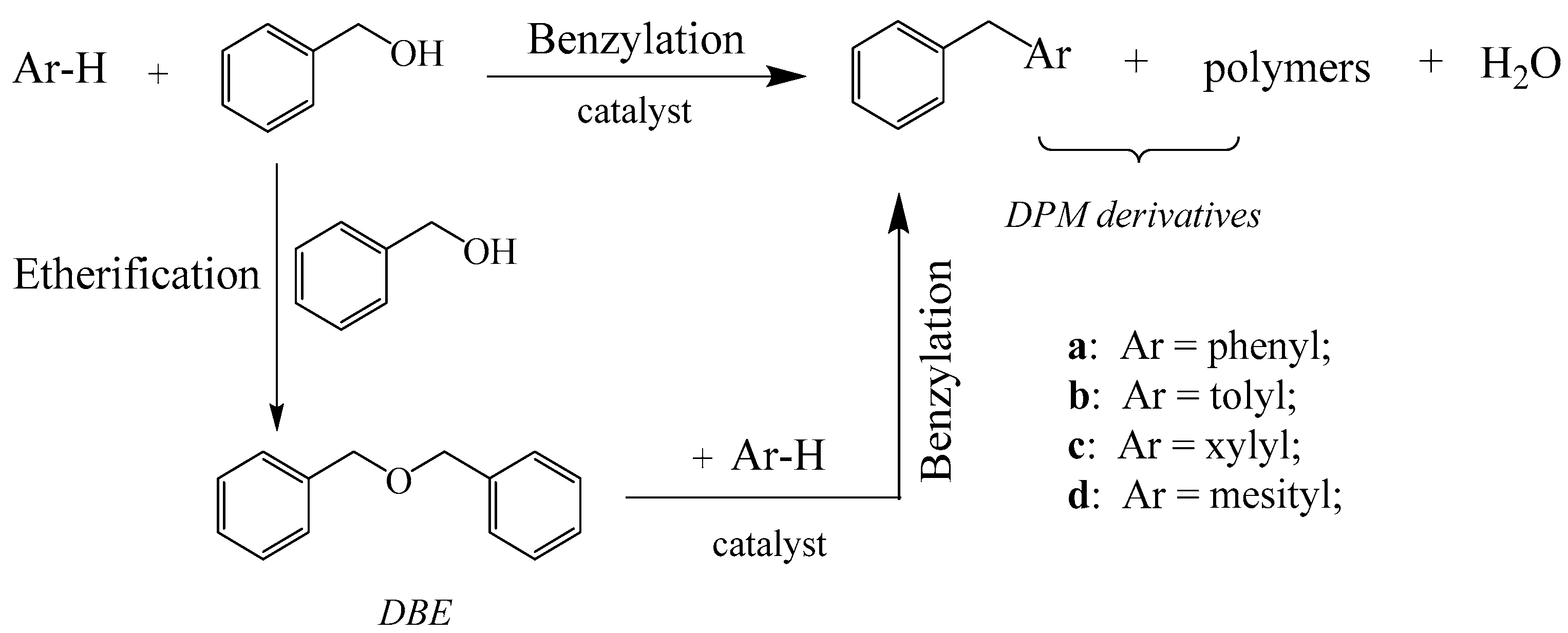
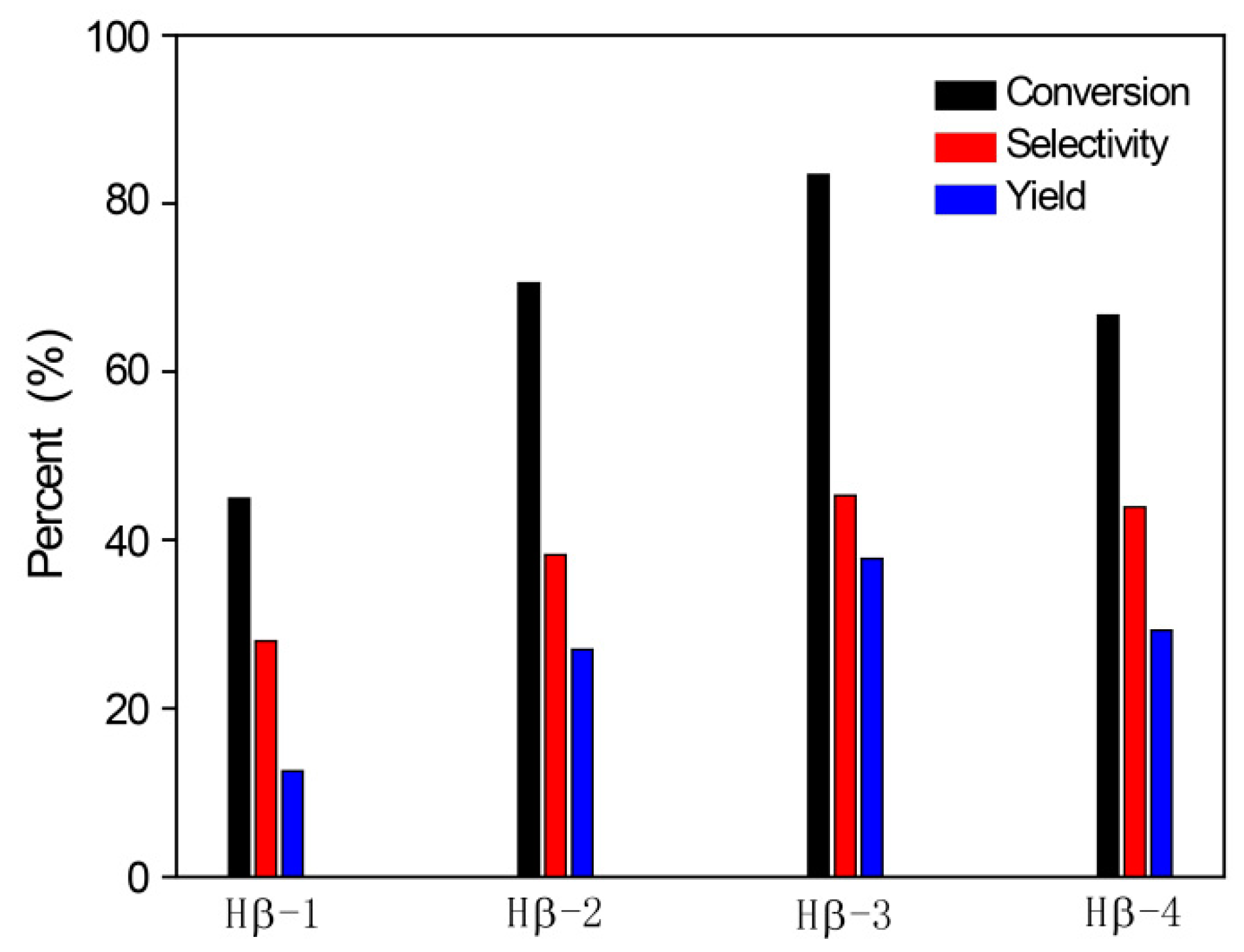
2.2. Benzylation of Arenes with Benzyl Alcohol
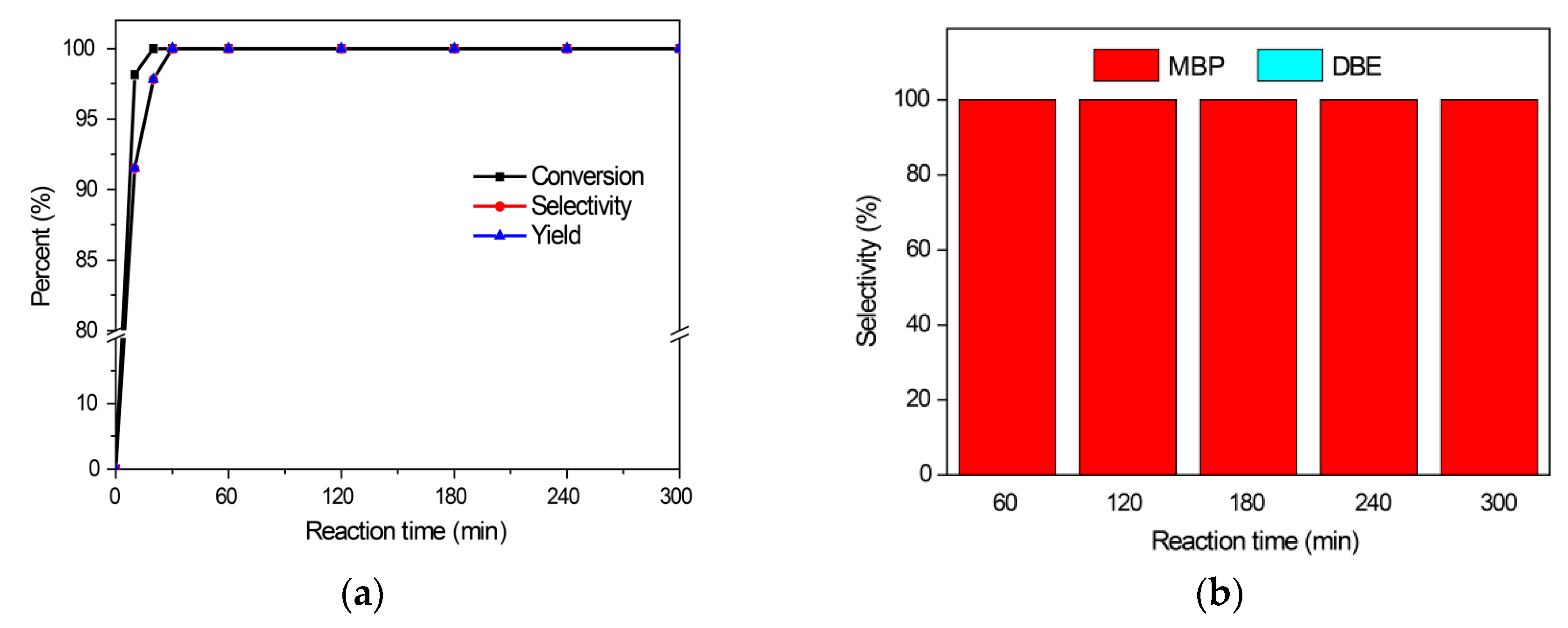
2.2.1. Benzylation of Benzene with Benzyl Alcohol
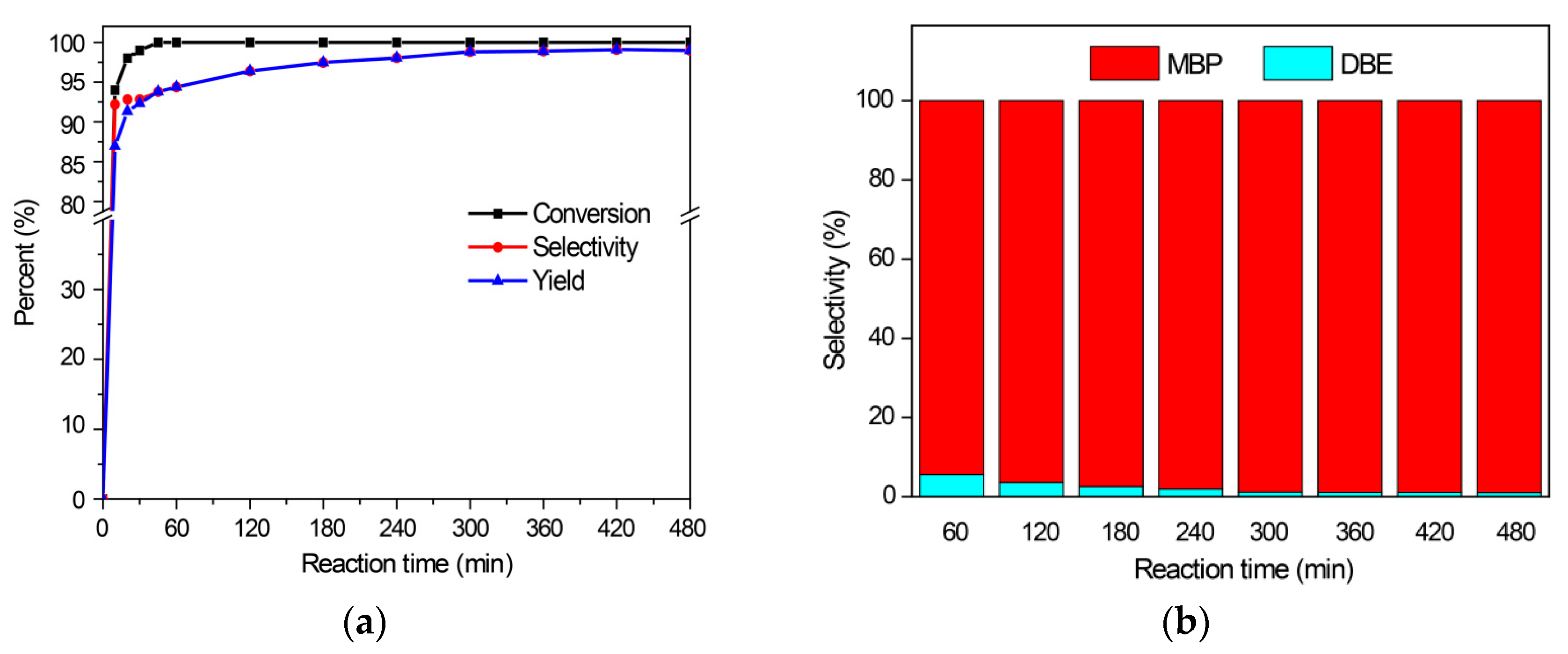
2.2.2. Benzylation of Toluene with Benzyl Alcohol
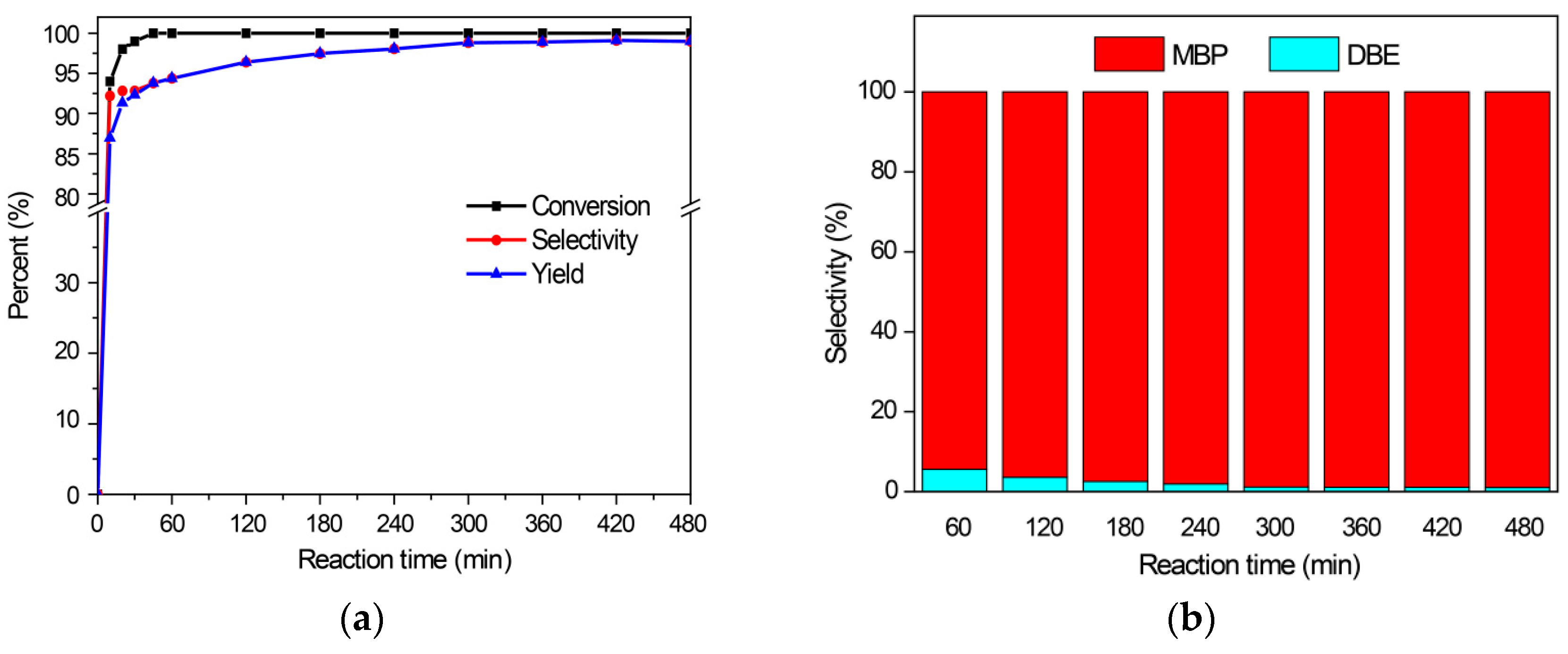
2.2.3. Benzylation of P-Xylene and Mesitylene with Benzyl Alcohol
2.2.4. The Reaction Activities of Different Arenes Substrates
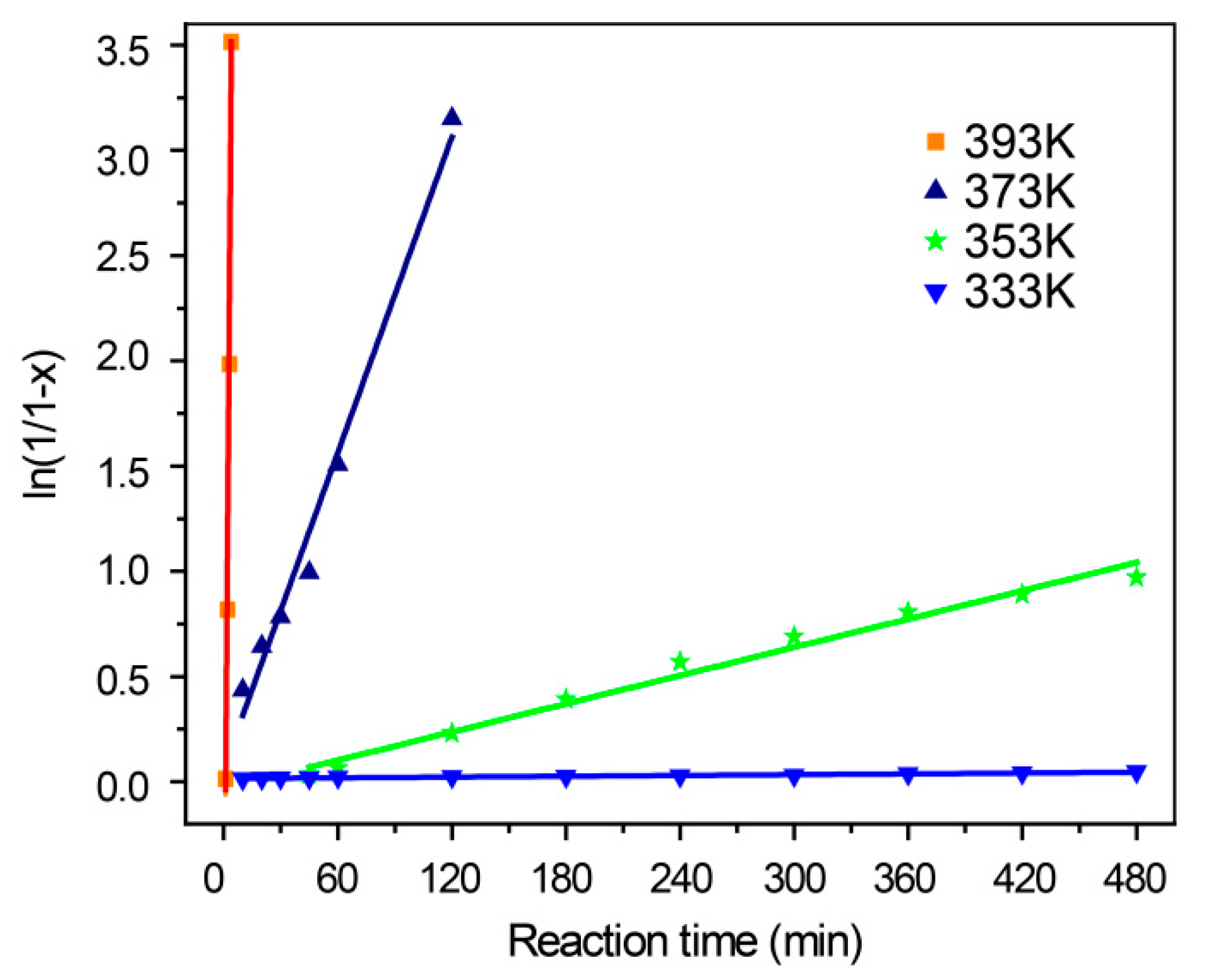
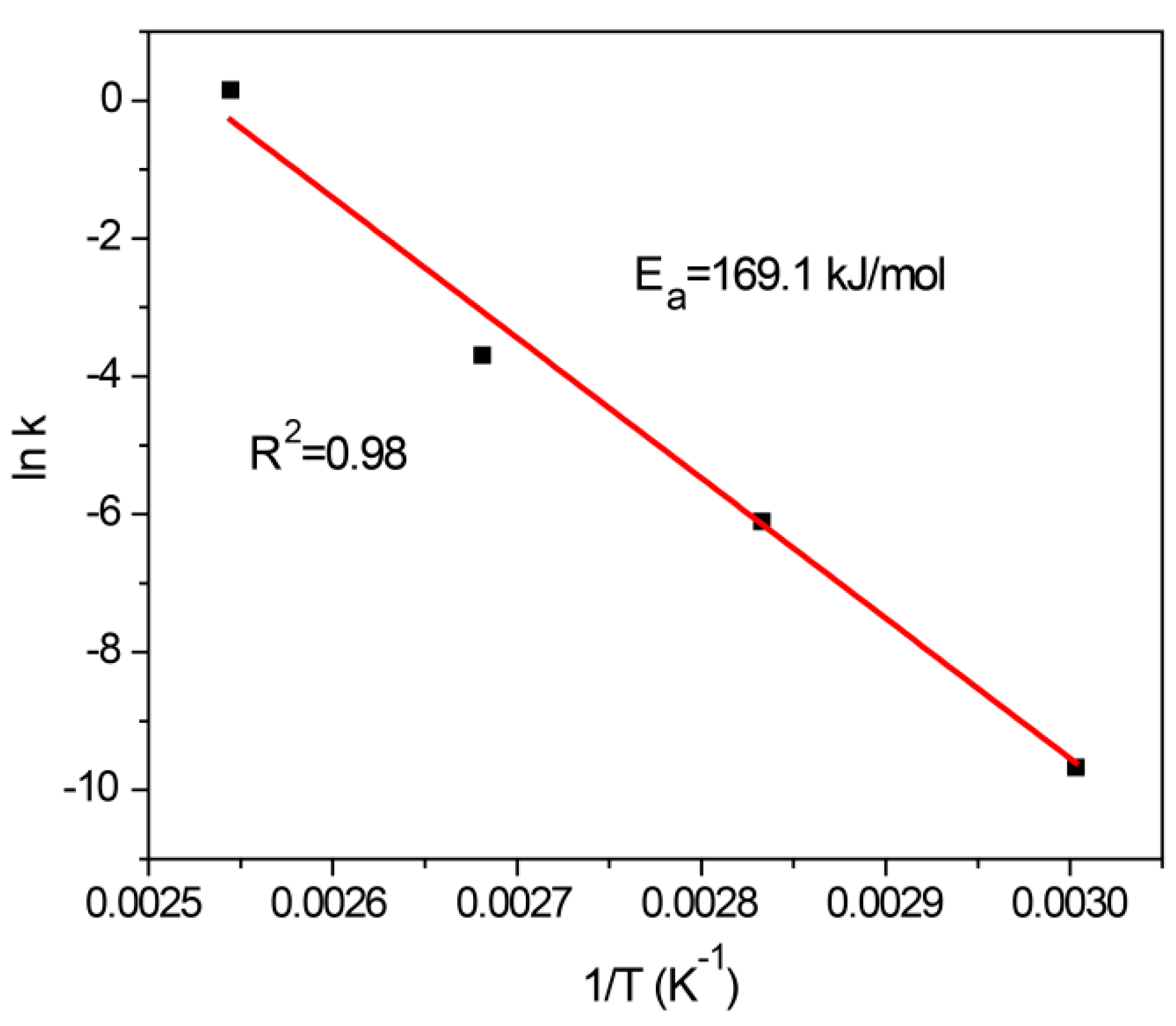
2.3. Effects of Reaction Temperature
2.4. Recycling of the Catalyst
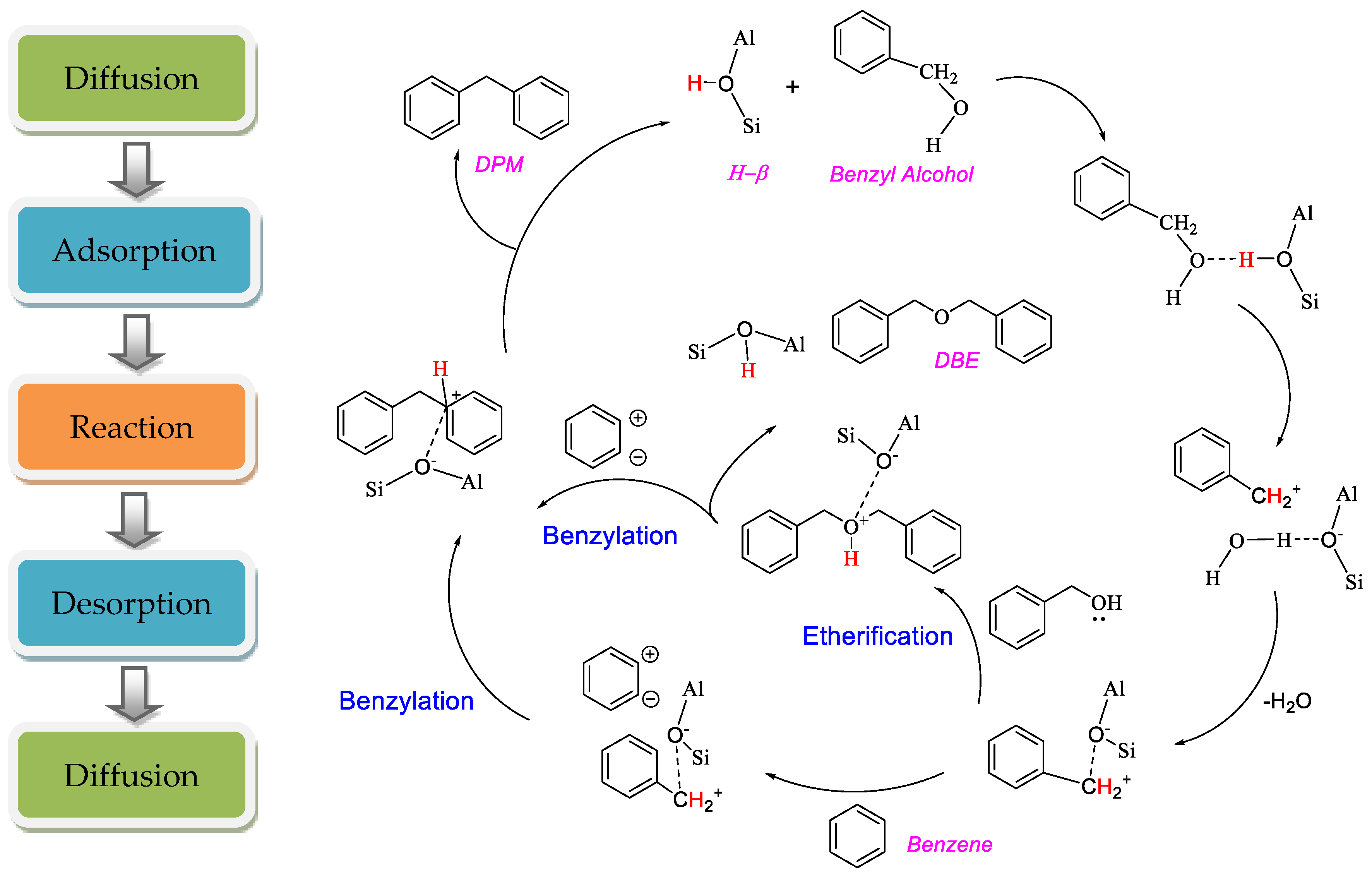
2.5. Mechanism Discussion
2.5.1. The Whole Heterogeneous Catalytic Process
2.5.2. Diffusion
2.5.3. Adsorption and Surface Reaction
3. Materials and Methods
3.1. Reagents
3.2. Catalyst Preparation
3.3. Characterizations
3.4. Benzylation of Arenes with Benzyl Alcohol
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olah, G.A. Friedel-Crafts and Related Reactions; Interscience Publishers: Geneva, Switzerland, 1963; Volume 1. [Google Scholar]
- Liu, Y.; Baráth, E.; Shi, H.; Hu, J.; Camaioni, D.M.; Lercher, J.A. Solvent-determined mechanistic pathways in zeolite-H-BEA-catalysed phenol alkylation. Nat. Catal. 2018, 1, 141. [Google Scholar] [CrossRef]
- Schultz, E.E.; Braffman, N.R.; Luescher, M.U.; Hager, H.H.; Balskus, E.P. Biocatalytic Friedel-Crafts Alkylation Using a Promiscuous Biosynthetic Enzyme. Angew. Chem. Int. Ed. 2019, 58, 3151–3155. [Google Scholar] [CrossRef]
- Narender, N.; Mohan, K.K.; Kulkarni, S.; Reddy, I.A.K. Liquid phase benzylation of benzene and toluene with benzyl alcohol over modified zeolites. Catal. Commun. 2006, 7, 583–588. [Google Scholar] [CrossRef]
- Candu, N.; Wuttke, S.; Kemnitz, E.; Coman, S.M.; Pârvulescu, V.I. Replacing benzyl chloride with benzyl alcohol in heterogeneous catalytic benzylation of aromatic compounds. Pure Appl. Chem. 2012, 84, 427–437. [Google Scholar] [CrossRef]
- Haixia, M.; Zhaoteng, X.; Jinghong, M.; ZHANG, Y.; Ruifeng, L. Nanoscale ZSM-5 zeolite for the benzylation of aromatic hydrocarbons. Chin. J. Catal. 2012, 33, 183–191. [Google Scholar]
- Hao, W.; Zhang, W.; Guo, Z.; Ma, J.; Li, R. Mesoporous Beta Zeolite Catalysts for Benzylation of Naphthalene: Effect of Pore Structure and Acidity. Catalysts 2018, 8, 504. [Google Scholar] [CrossRef]
- Pu, X.; Su, Y. Heterogeneous catalysis in microreactors with nanofluids for fine chemicals syntheses: Benzylation of toluene with benzyl chloride over silica-immobilized FeCl3 catalyst. Chem. Eng. Sci. 2018, 184, 200–208. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Hasanalian, J.; Najafian, H. Alumina-supported FeCl3, MnCl2, CoCl2, NiCl2, CuCl2, and ZnCl2 as catalysts for the benzylation of benzene by benzyl chloride. J. Mol. Catal. A Chem. 2004, 209, 209–214. [Google Scholar] [CrossRef]
- Ostovar, S.; Rodríguez-Padrón, D.; Saberi, F.; Balu, A.M.; Luque, R. Versatile Sulfathiazole-Functionalized Magnetic Nanoparticles as Catalyst in Oxidation and Alkylation Reactions. Catalysts 2019, 9, 348. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Zhou, Z.; Zhang, Y.; Li, H.; Stampfl, C.; Liang, C.; Huang, J. Benzylation of Arenes with Benzyl Chloride over H-Beta Zeolite: Effects from Acidity and Shape-Selectivity. J. Phys. Chem. C 2017, 121, 15248–15255. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Zhang, K.; Suh, J.M.; Choi, J.W.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Recent Advances in the Nanocatalysts-assisted NaBH4 Reduction of Nitroaromatics in water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef]
- Souza de Carvalho Filho, J.F.; Maciel Pereira, M.; Gomes Aranda, D.A.; Monnerat Araujo Ribeiro de Almeida, J.; Falabella Sousa-Aguiar, E.; Nothaft Romano, P. Application of Response Surface Methodology for Ethanol Conversion into Hydrocarbons Using ZSM-5 Zeolites. Catalysts 2019, 9, 617. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A.; Sprynskyy, M.; Nowak, I.; Jaroniec, M.; Buszewski, B. Application of novel hierarchical niobium-containing zeolites for synthesis of alkyl lactate and lactic acid. J. Colloid Interface Sci. 2018, 516, 379–383. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A.; Sprynskyy, M.; Nowak, I.; Buszewski, B. Catalytic Isomerization of Dihydroxyacetone to Lactic Acid and Alkyl Lactates over Hierarchical Zeolites Containing Tin. Catalysts 2018, 8, 31. [Google Scholar] [CrossRef]
- Zhao, D.; Prinsen, P.; Wang, Y.; Ouyang, W.; Delbecq, F.; Len, C.; Luque, R. Continuous flow alcoholysis of furfuryl alcohol to alkyl levulinates using zeolites. ACS Sustain. Chem. Eng. 2018, 6, 6901–6909. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Sampaio, M.J.; Da Silva, E.S.; Serp, P.; Faria, J.L.; Silva, C.G. Synthesis of selected aromatic aldehydes under UV-LED irradiation over a hybrid photocatalyst of carbon nanofibers and zinc oxide. Catal. Today 2019, 328, 286–292. [Google Scholar] [CrossRef]
- Sun, Y.; Prins, R. Friedel-Crafts alkylations over hierarchical zeolite catalysts. Appl. Catal. A Gen. 2008, 336, 11–16. [Google Scholar] [CrossRef]
- Yuan, B.; Li, Y.; Wang, Z.; Yu, F.; Xie, C.; Yu, S. A Novel Brønsted-Lewis acidic catalyst based on heteropoly phosphotungstates: Synthesis and catalysis in benzylation of p-xylene with benzyl alcohol. Mol. Catal. 2017, 443, 110–116. [Google Scholar] [CrossRef]
- Li, C.; Cho, H.J.; Wang, Z.; Gou, J.; Ren, Y.; Xi, H.; Fan, W. Effects of the Framework and Mesoporosity on the Catalytic Activity of Hierarchical Zeolite Catalysts in Benzyl Alcohol Conversion. ChemCatChem 2016, 8, 2406–2414. [Google Scholar] [CrossRef]
- Jin, H.; Ansari, M.B.; Jeong, E.-Y.; Park, S.-E. Effect of mesoporosity on selective benzylation of aromatics with benzyl alcohol over mesoporous ZSM-5. J. Catal. 2012, 291, 55–62. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Baxter, N.C.; Kuo, G.-T.; Wang, S. Synthesis of hierarchical ZSM-5 zeolites by solid-state crystallization and their catalytic properties. J. Catal. 2017, 349, 53–65. [Google Scholar] [CrossRef]
- Leng, K.; Wang, Y.; Hou, C.; Lancelot, C.; Lamonier, C.; Rives, A.; Sun, Y. Enhancement of catalytic performance in the benzylation of benzene with benzyl alcohol over hierarchical mordenite. J. Catal. 2013, 306, 100–108. [Google Scholar] [CrossRef]
- Saxena, S.K.; Viswanadham, N. Enhanced catalytic properties of mesoporous mordenite for benzylation of benzene with benzyl alcohol. Appl. Surf. Sci. 2017, 392, 384–390. [Google Scholar] [CrossRef]
- Kim, J.-C.; Cho, K.; Ryoo, R. High catalytic performance of surfactant-directed nanocrystalline zeolites for liquid-phase Friedel-Crafts alkylation of benzene due to external surfaces. Appl. Catal. A Gen. 2014, 470, 420–426. [Google Scholar] [CrossRef]
- Na, K.; Jo, C.; Kim, J.; Cho, K.; Jung, J.; Seo, Y.; Messinger, R.J.; Chmelka, B.F.; Ryoo, R. Directing zeolite structures into hierarchically nanoporous architectures. Science 2011, 333, 328–332. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Lancelot, C.; Lamonier, C.; Morin, J.-C.; Revel, B.; Delevoye, L.; Rives, A. Effect of post treatment on the local structure of hierarchical Beta prepared by desilication and the catalytic performance in Friedel-Crafts alkylation. Microporous Mater. 2015, 206, 42–51. [Google Scholar] [CrossRef]
- Candu, N.; Florea, M.; Coman, S.; Parvulescu, V. Benzylation of benzene with benzyl alcohol on zeolite catalysts. Appl. Catal. A Gen. 2011, 393, 206–214. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhu, L.; Rigutto, M.; van der Made, A.; Yang, C.; Pan, S.; Wang, L.; Zhu, L.; Jin, Y. Highly mesoporous single-crystalline zeolite beta synthesized using a nonsurfactant cationic polymer as a dual-function template. J. Am. Chem. Soc. 2014, 136, 2503–2510. [Google Scholar] [CrossRef]
- Chen, J.; Hua, W.; Xiao, Y.; Huo, Q.; Zhu, K.; Zhou, X. Tailoring the structure of hierarchically porous zeolite beta through modified orientated attachment growth in a dry gel system. Chem.—A Eur. J. 2014, 20, 14744–14755. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, L.; Zhu, Z.; Li, C.; Xi, H.; Qian, Y. Hierarchically structured Beta zeolites with intercrystal mesopores and the improved catalytic properties. Appl. Catal. A Gen. 2014, 470, 412–419. [Google Scholar] [CrossRef]
- Möller, K.; Yilmaz, B.; Jacubinas, R.M.; Müller, U.; Bein, T. One-step synthesis of hierarchical zeolite beta via network formation of uniform nanocrystals. J. Am. Chem. Soc. 2011, 133, 5284–5295. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Luo, C.; Liu, J.; Chen, B. Direct synthesis of hierarchical zeolites with oriented nanocrystals without adding extra templates. RSC Adv. 2013, 3, 6295–6298. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Mesoporosity—A new dimension for zeolites. Chem. Soc. Rev. 2013, 42, 3689–3707. [Google Scholar] [CrossRef] [PubMed]
- Al-Dughaither, A.S.; de Lasa, H. HZSM-5 Zeolites with Different SiO2/Al2O3Ratios. Characterization and NH3 Desorption Kinetics. Ind. Eng. Chem. Res. 2014, 53, 15303–15316. [Google Scholar] [CrossRef]
- Alejandro, S.; Valdes, H.; Manero, M.H.; Zaror, C.A. BTX abatement using Chilean natural zeolite: The role of Bronsted acid sites. Water Sci. Technol. 2012, 66, 1759–1765. [Google Scholar] [CrossRef]
- Vinu, A.; Sawant, D.P.; Ariga, K.; Hartmann, M.; Halligudi, S.B. Benzylation of benzene and other aromatics by benzyl chloride over mesoporous AlSBA-15 catalysts. Microporous Mater. 2005, 80, 195–203. [Google Scholar] [CrossRef]
- Shuai, Y.; Liu, Y.; Tian, H.; Dai, Z.; Yi, Z.; Han, Z.; Long, J. Study on Diffusion of Hydrocarbons in FAU and MFI Zeolites by Molecular Simulation and Related Experiments. China Pet. Process. Petrochem. Technol. 2012, 14, 1–8. [Google Scholar]
- Xu, S.; Zhang, X.; Cheng, D.G.; Chen, F.; Ren, X. Effect of hierarchical ZSM-5 zeolite crystal size on diffusion and catalytic performance of n -heptane cracking. Front. Chem. Sci. Eng. 2018, 12, 780–789. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A. Hierarchical zeolites: Synthesis and catalytic properties. Microporous Mater. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Perez-Ramirez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Roque-Malherbe, R.; Wendelbo, R.; Mifsud, A.; Corma, A. Diffusion of aromatic hydrocarbons in H-ZSM-5, H-Beta, and H-MCM-22 zeolites. J. Phys. Chem. 1995, 99, 14064–14071. [Google Scholar] [CrossRef]
- Song, A.; Ma, J.; Xu, D.; Li, R. Adsorption and Diffusion of Xylene Isomers on Mesoporous Beta Zeolite. Catalysts 2015, 5, 2098–2114. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Y.; Liu, D.; Wu, Y.; Xi, H.; Qian, Y. Molecular simulation and experimental studies of a mesoporous ZSM-5 type molecular sieve. Phys. Chem. 2013, 15, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Toda, J.; Sastre, G. Diffusion of Trimethylbenzenes, Toluene, and Xylenes in UWY Zeolite as a Catalyst for Transalkylation of Trimethylbenzenes with Toluene. J. Phys. Chem. C 2018, 122, 7885–7897. [Google Scholar] [CrossRef]
- Ercan, C.; Dautzenberg, F.M.; Yeh, C.Y.; Barner, H.E. Mass-Transfer Effects in Liquid-Phase Alkylation of Benzene with Zeolite Catalysts. Ind. Eng. Chem. Res. 1998, 37, 1724–1728. [Google Scholar] [CrossRef]
- Beltrame, P. Kinetics of the reaction of naphthalene with benzyl alcohol over a Nafion$minus;silica composite, as a function of temperature. Appl. Catal. A Gen. 2004, 268, 169–174. [Google Scholar] [CrossRef]
- Beltrame, P.; Zuretti, G. Kinetics of the reaction of toluene with benzyl alcohol over a Nafion-silica composite. Appl. Catal. A Gen. 2005, 283, 33–38. [Google Scholar] [CrossRef]
- Zhan, G.; Hong, Y.; Lu, F.; Ibrahim, A.-R.; Du, M.; Sun, D.; Huang, J.; Li, Q.; Li, J. Kinetics of liquid phase oxidation of benzyl alcohol with hydrogen peroxide over bio-reduced Au/TS-1 catalysts. J. Mol. Catal. A Chem. 2013, 366, 215–221. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Xu, D.; Asahina, S.; Cychosz, K.A.; Agrawal, K.V.; Al, W.Y.; Bhan, A.; Al, H.S.; Terasaki, O. Synthesis of self-pillared zeolite nanosheets by repetitive branching. Science 2012, 336, 1684–1687. [Google Scholar] [CrossRef]
- Đokić, M.; Kesić, Ž.; Krstić, J.; Jovanović, D.; Skala, D. Decrease of free fatty acid content in vegetable oil using silica supported ferric sulfate catalyst. Fuel 2012, 97, 595–602. [Google Scholar] [CrossRef]
- Beltrame, P.; Zuretti, G. Kinetic studies for processes of liquid-phase alkylation of aromatics over solid acid catalysts. Green Chem. 2004, 6, 7–13. [Google Scholar] [CrossRef]
- Derouane, E.G.; Crehan, G.; Dillon, C.J.; Bethell, D.; He, H.; Derouane-Abd Hamid, S.B. Zeolite Catalysts as Solid Solvents in Fine Chemicals Synthesis. J. Catal. 2000, 194, 410–423. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Singh, A.P. Sorption capacity and diffusion of pure liquids in ZSM-5 type zeolites. Zeolites 1986, 6, 206–208. [Google Scholar] [CrossRef]
- Van der Perre, S.; Van Assche, T.; Bozbiyik, B.; Lannoeye, J.; De Vos, D.E.; Baron, G.V.; Denayer, J.F. Adsorptive characterization of the ZIF-68 metal-organic framework: A complex structure with amphiphilic properties. Langmuir 2014, 30, 8416–8424. [Google Scholar] [CrossRef] [PubMed]
- Jae, J.; Tompsett, G.A.; Foster, A.J.; Hammond, K.D.; Auerbach, S.M.; Lobo, R.F.; Huber, G.W. Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal. 2011, 279, 257–268. [Google Scholar] [CrossRef]
- Bachari, K.; Millet, J.M.M.; Benaïchouba, B.; Cherifi, O.; Figueras, F. Benzylation of benzene by benzyl chloride over iron mesoporous molecular sieves materials. J. Catal. 2004, 221, 55–61. [Google Scholar] [CrossRef]
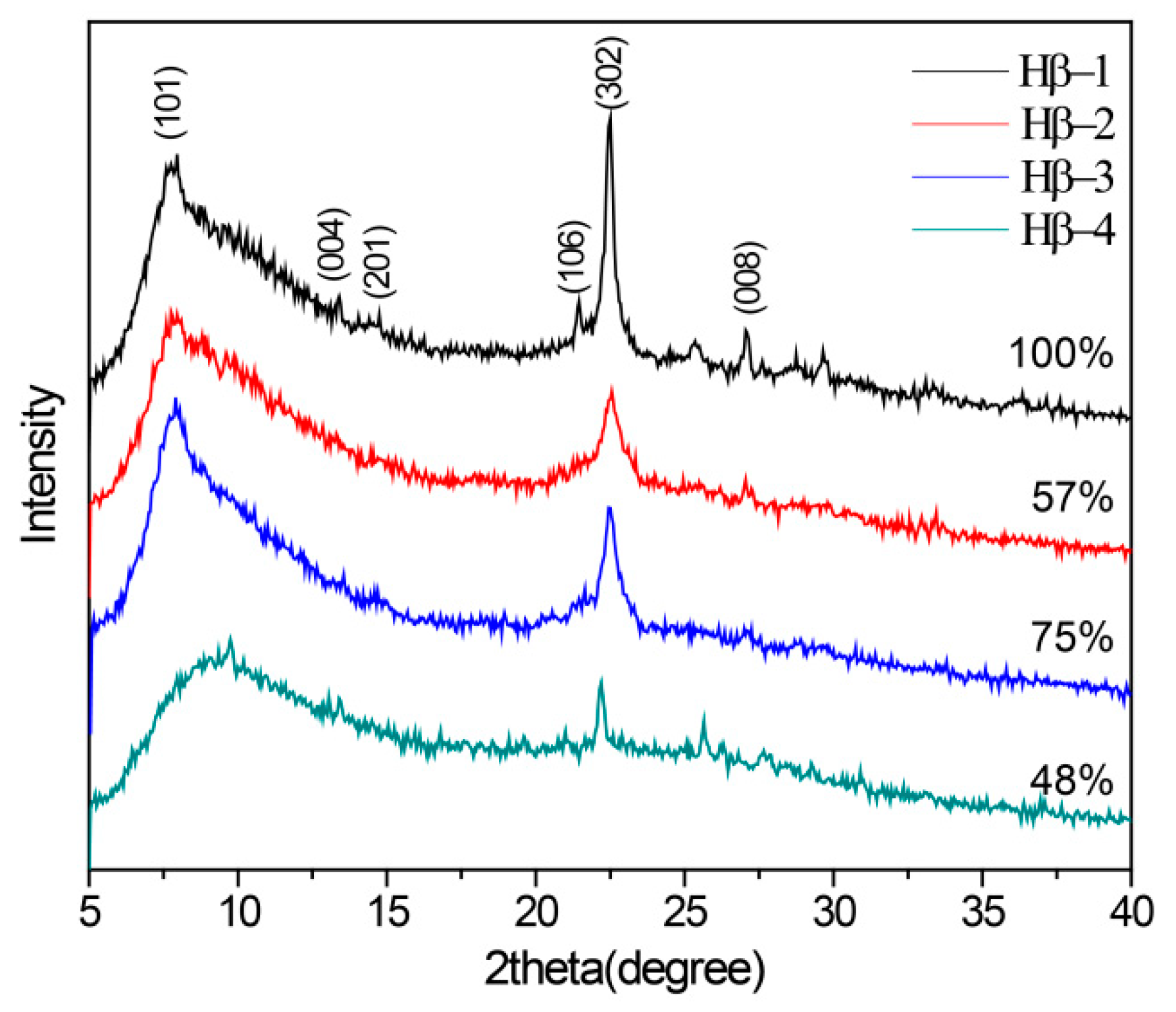
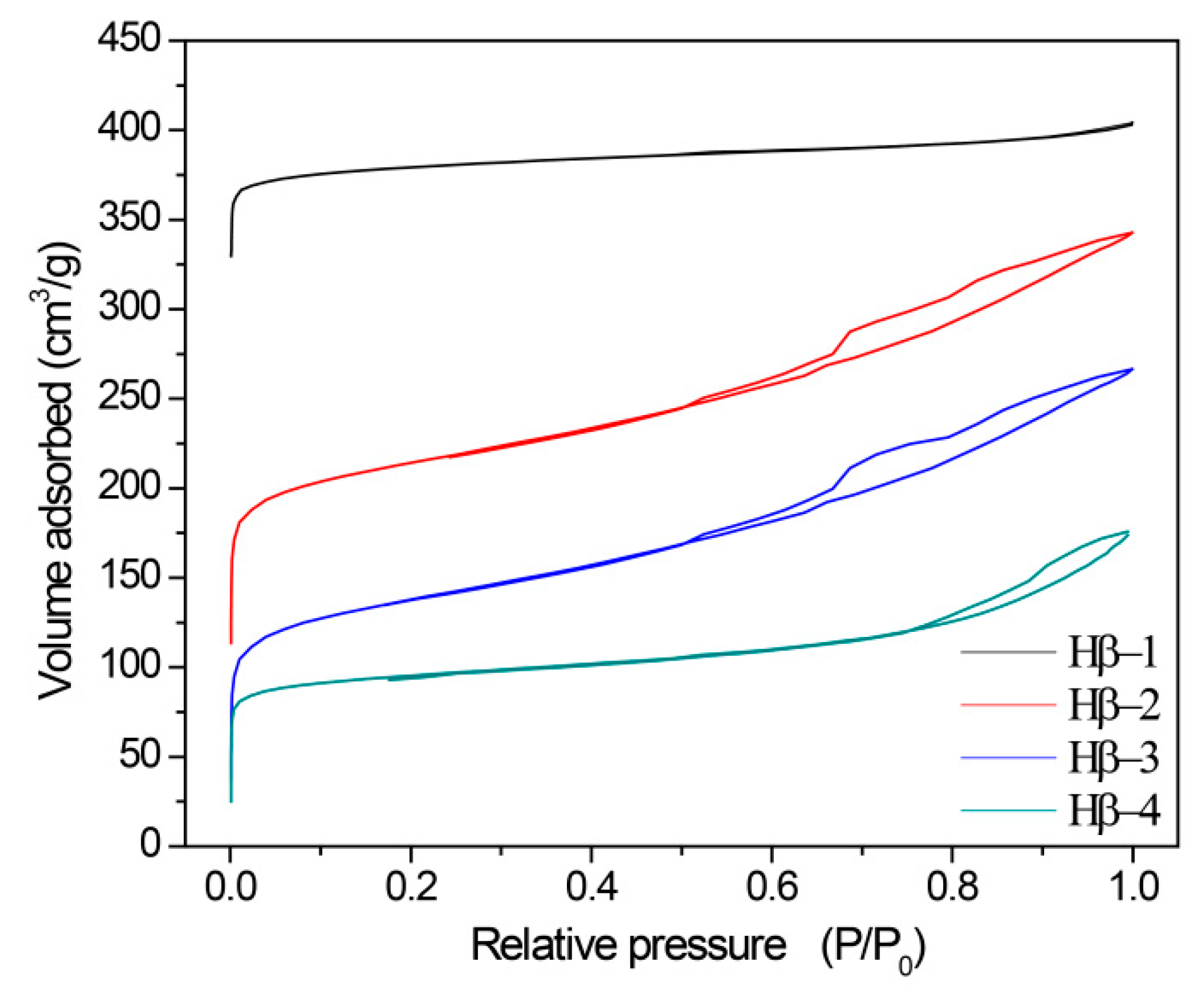





| Sample | BET Surface Area (m2/g) a | External Surface Area (m2/g) b | Micropore Volume (cm3/g) b | Mesopore Volume (cm3/g) c | Mesopore Diameter (nm) d | Si/Al e |
|---|---|---|---|---|---|---|
| Hβ-1 | 345.7 | 63.6 | 0.14 | 0.06 | - | 25.0 |
| Hβ-2 | 366.5 | 212.0 | 0.07 | 0.30 | 6.1 | 21.4 |
| Hβ-3 | 375.6 | 215.9 | 0.08 | 0.30 | 6.3 | 23.7 |
| Hβ-4 | 241.5 | 76.9 | 0.08 | 0.16 | 7.3 | 28.4 |
| Entry | Substrates | Temperature/K | a × b × c /nm × nm × nm [39] | Conversion/% | Selectivity/% | ka/min−1 |
|---|---|---|---|---|---|---|
| 1 |  | 353 | 0.341 × 0.666 × 0.729 | 100 | 100 | 0.3993 |
| 2 |  | 373 | 0.380 × 0.666 × 0.818 | 100 | 94 | 0.1191 |
| 3 |  | 373 | 0.390 × 0.666 × 0.919 | 98 | 89 | 0.0566 |
| 4 |  | 373 | 0.425 × 0.829 × 0.888 | 92 | 74 | 0.0477 |
| Items | Initial | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 |
|---|---|---|---|---|---|---|
| Conversion (%) | 98 | 97 | 96 | 94 | 90 | 88 |
| Selectivity (%) | 85 | 80 | 82 | 81 | 82 | 82 |
| Entry | Substrates | Kinetic Diameter [43,57] (Å) | KBzH/Kare | ka (min−1) | ka’ (mol·min−1g−1) |
|---|---|---|---|---|---|
| 1 |  | 5.8 | 2.7 | 0.3993 | 2.9/K2BzOH |
| 2 |  | 6.7 | 7.2 | 0.1191 | 6.2/K2BzOH |
| 3 |  | 6.8 | 9.4 | 0.0566 | 5.0/K2BzOH |
| 4 |  | 8.6 | 26.0 | 0.0477 | 32/K2BzOH |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Lu, M.; Wang, X.; Lu, J.; Yang, J. The Steric Effect in Green Benzylation of Arenes with Benzyl Alcohol Catalyzed by Hierarchical H-beta Zeolite. Catalysts 2019, 9, 869. https://doi.org/10.3390/catal9100869
Liu X, Lu M, Wang X, Lu J, Yang J. The Steric Effect in Green Benzylation of Arenes with Benzyl Alcohol Catalyzed by Hierarchical H-beta Zeolite. Catalysts. 2019; 9(10):869. https://doi.org/10.3390/catal9100869
Chicago/Turabian StyleLiu, Xinyu, Meihuan Lu, Xuan Wang, Juyou Lu, and Jianxin Yang. 2019. "The Steric Effect in Green Benzylation of Arenes with Benzyl Alcohol Catalyzed by Hierarchical H-beta Zeolite" Catalysts 9, no. 10: 869. https://doi.org/10.3390/catal9100869
APA StyleLiu, X., Lu, M., Wang, X., Lu, J., & Yang, J. (2019). The Steric Effect in Green Benzylation of Arenes with Benzyl Alcohol Catalyzed by Hierarchical H-beta Zeolite. Catalysts, 9(10), 869. https://doi.org/10.3390/catal9100869