Abstract
For enhancing the cetane number (CN) of diesel fraction, the selective oxidative ring opening method was applied to upgrade ring hydrocarbons. Organic acids, one of the main products from this oxidative reaction, being esterified by the phase transfer catalysis (PTC) approach were studied. Adipic acid, benzoic acid, and phthalic acid were used as model compounds. Reaction time, reaction temperature, the amount of water, and the amount of catalyst in the esterification process were investigated and optimized using orthogonal experimental design method. The kinetics of esterification process was then conducted under the optimal condition. The types of catalysts and organic acids, the amount of catalyst and water were also investigated. The PTC esterification was one rate controlling reaction on the interface between the aqueous phase and the oil phase. Hydrophobicity is a key factor for converting benzoic acid, adipic acid, and phthalic acid to the corresponding esters. It was found that around 5–8% water is the optimal quantity for the given reaction system. Two cases of esterification processes of PTC were proposed.
1. Introduction
Straight run gas oil and fluid catalytic cracking (FCC) light cycle oil (LCO) are known as two main contributors to diesel pool [1]. The former has a cetane number (CN) ranging from 30 to 55 with an aromatics content of 15–40%, and the latter has a CN ranging from 5 to 30 with an aromatics content of 60–85% [1,2]. High levels ring hydrocarbons (cyclic hydrocarbons and aromatics) in diesel fuel result in the increase of emissions, such as particulates, during burning.
Raising CN and lowering the ring hydrocarbon content of diesel fuel can reduce the impact of the combustion of diesel fuel on air quality, knock, and smoke. The increasingly stringent regulation was implemented in 2016: the CN tested by ASTM D 976 is not lower than 40, or the aromatics content tested by ASTM D 1319 is not higher than 35%, which are both required in the Standard Specification for Diesel Fuel Oils ASTM D 975-16a. This has resulted in an increased interest and demand for new or improved processes for enhancing the CN of diesel fuels.
Current mainstream technologies for the improvement of CN of diesel distillates are severe hydrogenation to open the rings of aromatics and cycloparaffins by using noble metal catalysts [3,4,5,6,7]. However, there are sulfur or nitrogen compounds in the feed of these deep hydro-ring opening processes, which readily pollute these noble metal catalysts [3,5,6]. In addition, the compositions of the feed and the blending strategies are other key factors that determine the CN of deep hydro-ring opening products. For example, raw materials with high aromatic hydrocarbon content, such as LCO, may not satisfy the CN requirement even after being treated by the hydro-ring opening; therefore, further upgrading is necessary [8]. The application of deep hydrotreating for higher CN will be subjected to higher cost due to the severe conditions and complicated process. Another popular method for enhancing CN is adding improver, which, however, only works in a very limited range and introduces the heteroatoms simultaneously [9]. Cyclohexane can be oxidized to generate adipic acid by air with the presence of hot nitric acid oxide [10]. The dual bonds of cyclic olefins are also prone to oxidation by potassium permanganate, and to being broken to form binary acids [10]. As early as 1932, the maleic anhydride by benzene oxidation has been produced in industrial scale [11]. Based on the above-mentioned information, oxidative ring-opening of cyclic ketones is proposed [12,13,14,15,16,17]; oxidative ring-opening of cyclic hydrocarbons [18,19,20,21] is also proposed for chain alkanes [22] or decreasing emissions [23]. These oxidative processes work with catalyst under a mild condition. We are the first to propose using this oxidation method to enhance the CN of diesel fuel [9]. This oxidation process of ring hydrocarbons has two steps: the oxidation of ring hydrocarbons to cyclic ketones, and that of cyclic ketone to acids. The former has made industrial scale into reality [13,17], and the latter has been being studied since then [15]. Vanadium modified sulphated ZrO2 was proven to be an effective catalyst for the oxidation of the cyclohexanes for enhancing the CN; FeCl3, oxovanadium (V) and iodine-cerium (IV) salts, and vanadium polyoxometalates, especially, vanadium-based heteropolyoxometalate acid, for the oxidative ring-opening of cyclic ketones.
As one of the main products from the aforementioned oxidative reaction [15], acids need to be converted into ester. Esters are produced if carboxylic acids are heated along with alcohols with the presence of an acid catalyst (usually concentrated sulphuric acid). The esterification reaction is slow and reversible. The traditional esterification process has many drawbacks, such as serious corrosion to the equipment due to the concentrated sulphuric acid and the byproduct generated from side reactions. Phase transfer catalysis (PTC) has been widely used in esterification because of its mild reaction conditions, high yield, high selectivity, and the simple operation process [24,25,26,27]. In addition, theoretically, the CN of ester is higher than the corresponding cyclo-hydrocarbon, so the CN of diesel feedstock are expected to increase after the oxidation and esterification treatment.
For enhancing the CN of diesel fraction, selective oxidative ring opening method was applied to upgrade ring hydrocarbons. This study introduces the approach of PTC that is used to esterify acids, the main products from this oxidative reaction, into esters. Adipic acid, benzoic acid, and phthalic acid were used as model compounds. Reaction time, reaction temperature, the amount of water, and the amount of catalyst during the esterification process were investigated and optimized using orthogonal experimental design method. The kinetics of esterification process was then conducted under the optimal condition. The types of catalysts and organic acids, and the amount of catalyst and water were also investigated.
2. Results and Discussion
2.1. Esterification of Adipic Acid, Phthalic Acid, and Benzoic Acid
Three organic acids—phthalic acid, adipic acid, and benzoic acid—have been esterified using Tetrabutyl Ammonium Bromide (TBAB) as catalyst through the approach of PTC. The orthogonal experimental results (shown in Supplementary Materials Tables S1–S3) showed the similar trend in the effects of temperature, time, water amount, and catalyst amount. It can be seen that the reaction temperature is the most significant among the factors that has an impact on the conversion of organic acids to esters, followed by the amount of water, the amount of catalyst, and reaction time, respectively.
In the esterification of organic acids by PTC, benzoic acid had the highest conversion ratio of 97% among the three acids. This may be due to the fact that benzoic acid is more hydrophobic than the other two; therefore, its anion is easy to transfer between aqueous phase and organic phase, leading to a higher esterification conversion ratio. Adipic acid is hydrophilic and it is difficult for its anion to transfer from aqueous phase to organic phase. Thus, relative low conversion ratio of 79% was obtained. Given the above reason, phthalic acid is expected to have a conversion ratio between that of adipic acid and benzoic acid, which was also evidenced by the experimental results (86%).
2.2. Kinetics Study of the Esterification Process
2.2.1. Effects of Temperature on the Esterification of Adipic Acid
Supplementary Materials Tables S4–S7 summarized the concentration of 1-bromobutane changing along with reaction time during the esterification process at the temperatures of 96 °C, 103 °C, 107 °C, and 110 °C. The consumption of 1-bromobutane indicates a pseudo first-order reaction during the esterification by PTC at all temperatures, as shown in Figure 1, Figure 2, Figure 3 and Figure 4.
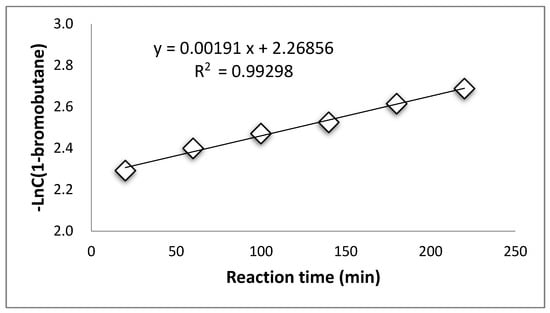
Figure 1.
Pseudo first-reaction order of 1-bromobutane at 96 °C.
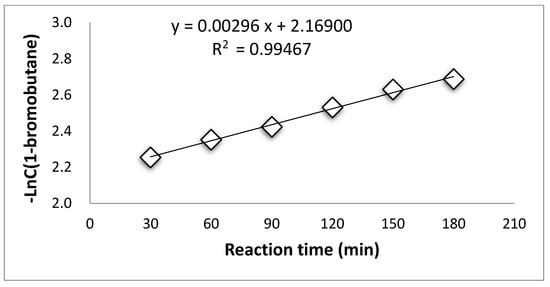
Figure 2.
Pseudo first-reaction order of 1-bromobutane at 103 °C.
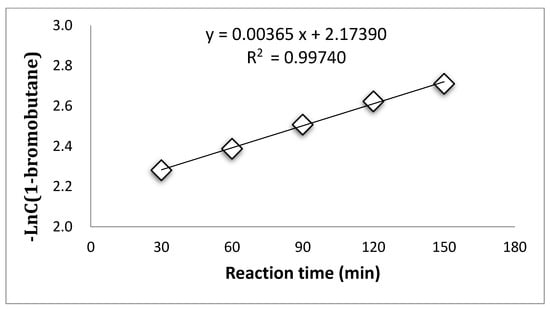
Figure 3.
Pseudo first-order reaction of 1-bromobutane at 107 °C.
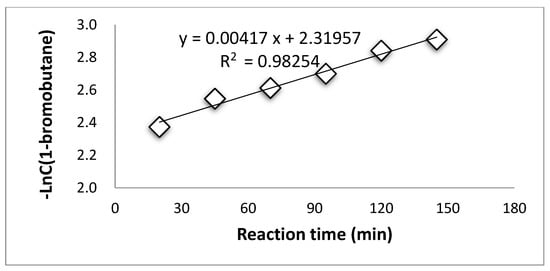
Figure 4.
Pseudo first-order reaction of 1-bromobutane at 110 °C.
These results suggest that the PTC esterification is the quasi—first order reaction.
The rate constants at different temperatures calculated from Figure 1, Figure 2, Figure 3 and Figure 4 are listed in Supplementary Materials Table S8.
Fitting to the Arrhenius equation,
where Kobs is the rate constant, Ea is the activation energy, R is the universal molar gas constant, T is absolute temperature (K) and A is the pre-exponential factor. Based on the Arrhenius equation, the activation energy of the esterification process, 15.88 kcal/mol, was obtained, as shown in Figure 5. According to literature, reactions with activation energy greater than 10 kcal/mol are the typical phase transfer catalytic reactions controlled by chemical reactions [28,29].
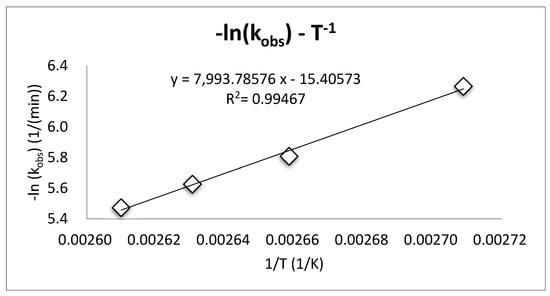
Figure 5.
The relationship between the rate constant of 1-bromobutane and the temperature during the phase transfer catalysis (PTC) esterification process.
2.2.2. Effects of the Amount of Water on the Esterification of Adipic Acid
Different amount of water were introduced to the reaction system: 0 mL, 1 mL, 3 mL, 5 mL, and 10 mL. The effects of the amount of water on the esterification of adipic acid are shown in Figure 6 and Supplementary Materials Figures S1–S5.
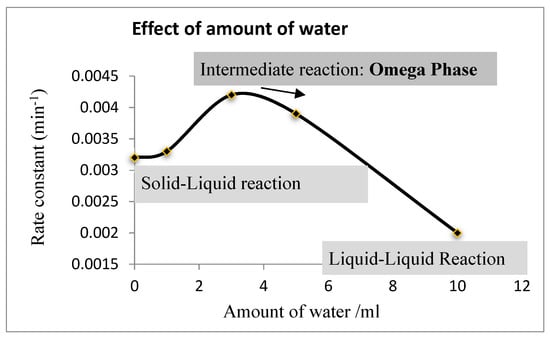
Figure 6.
Effect of amount of water on the esterification constant.
Figure 6 shows that 3–4 mL of water (5–8%) is the optimal amount for the given reaction system. This result coincided very well with the literature [30]. Water is essential for the reaction system since a thin layer of omega phase can be formed with the presence of water. However, excessive water (>5 mL in this reaction system) can reduce the effective concentration of NaX and Q+X− in the bulk aqueous phase and in turn, reduce the reaction rate. It also is interesting to observe that a faster reaction rate was obtained without water than with excessive water (10 mL).
2.2.3. Effects of the Amount of TBAB on the Esterification of Adipic Acid
The effects of TBAB amount (0–0.008 mol) on the kinetics of esterification reaction were studied and the results are shown in Figure 7 and Supplementary Materials Figures S6–S9.
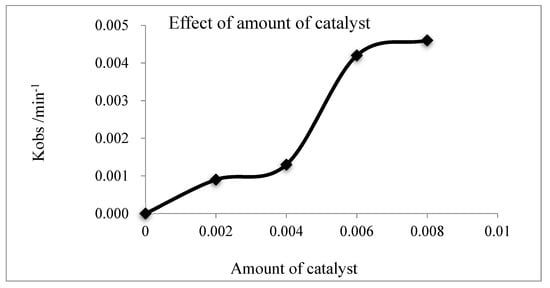
Figure 7.
Effect of amount of TBAB on the esterification kinetics.
In Figure 7, when the amount of TBAB catalyst increased from 0 to 0.004 mol, the rate constant indicated a slow increase from 0 to 0.0013 min−1. Afterward, the rate constant showed a sudden increase from 0.0013 to 0.0042 min−1, as the amount of TBAB increased from 0.004 to 0.006 mol. The increase rate dropped down as the amount of TBAB was beyond 0.006 mol. This phenomenon was the so-called Omega phase effect in the PTC process. In the range of 0 to 0.004 mol, the TBAB completely dissolved in the aqueous phase. When the TBAB increases from 0.004 to 0.006 mol, the aqueous phase was saturated with TBAB and Omega phase was formed [28]. The overall reaction rate is highly promoted. With the amount of TBAB increasing from 0.006 to 0.008 mol, the TBAB in the aqueous phase was in super-saturation state, but the Omega Phase remained constant; therefore, the reaction rate was nearly constant.
2.2.4. Effects of the Types of the Catalysts on the Esterification of Adipic Acid
Three phase transfer catalysts: TBAB, Tetrahexyl Ammonium Bromide (THAB), and Benzyltriethyl Ammonium Bromide (BTEAB) were studied in the esterification reaction. Their structures were shown in Figure 8. The effects of the types of catalyst on the esterification of adipic acid are shown in Supplementary Materials Figures S10–S12.
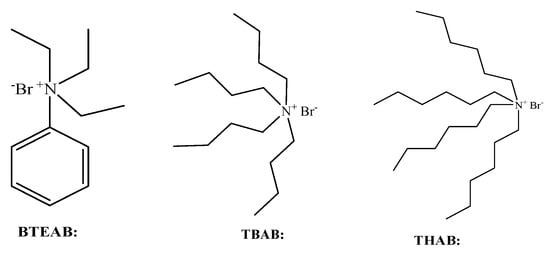
Figure 8.
Structure of three-phase transfer catalysts.
Table 1 shows the reaction rate constants of these three catalysts, which are in the following sequence: THAB > TBAB > BTEAB. The quantitative empirical parameter q introduced by Halpern was used to assess quaternary ammonium catalysts [28,31], as shown in Figure 9.

Table 1.
Rate constant of 1-bromobutane using different catalysts.
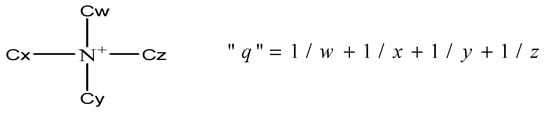
Figure 9.
Quantitative empirical parameter introduced by Halpern.
It is generally accepted that, for mass-transfer controlled reactions, the q is greater than 1; whereas for chemical-controlled reactions, the q is less than 1 [28,31]. The above results demonstrated that THAB has a better performance than the other two catalysts. The calculation shows that q value of THAB is less than 1, indicating that the esterification of adipic acid is a chemical-controlled reaction. Having a longer alkyl chain makes the solubility of THAB in organic phase higher and further makes its separation and reusability more difficult.
2.2.5. Effect of Different Organic Acids on the Esterification Process
The effects of organic acid types on the kinetics of esterification reactions were studied. Three organic acids: adipic acid, benzoic acid, and phthalic acid were used in the esterification reaction. The effects of the types of reactant acids on the esterification of adipic acid are shown in Supplementary Materials Figures S13–S15. The corresponding reaction rate constants were calculated and shown in Table 2.

Table 2.
Rate constants of 1-bromobutane for different organic acids.
The sequence of the rate constants was: Adipic acid (0.0042 min−1) < Phthalic acid (0.0046 min−1) < Benzoic acid (0.0055 min−1), which fell in the same trend of hydrophobicity of the organic acids. Mono-carboxyl and phenyl structure of benzoic acid suggests a greater hydrophobic property than that of adipic acid and phthalic acid; thereby it is capable of transferring from aqueous phase to oil phase, which results in a higher reaction rate.
2.2.6. Esterification Processes
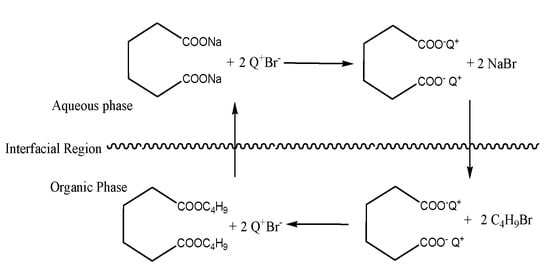
Figure 10.
Esterification process by PTC.
Phase transfer catalyzed esterification of adipic acid can be divided into the following processes:
- (1)
- mass transfer process: the anion of adipic acid combines with the cation of the catalyst to form the active ion pair, which is transferred from water phase to organic phase;
- (2)
- chemical reaction process in organic phase: reactive ions transferred to organic phase react with 1-bromobutane to form the final product dibutyl adipate.
The reactions taking place in the aqueous and oil phase can be expressed by the following two Equations (1a), (1b) and (2).
where k1 represents the reaction rate constant of ion pair formation in mass transfer catalytic reaction, and k−1 represents the decomposition rate constant of ion pair in mass transfer catalytic reaction. k2 is the reaction rate constant of this esterification reaction. The consumption rate of reactants and the formation rate of products can be described by Equation (3)
In this equation, [R − Y]org is the concentration of reactant at a certain moment in the esterification process. The expression of (Q+X−)org can be found in Equation (4).
The relative concentration of (Q+X−)org and (Q+Y−)org may be varied. But they must satisfy Equation (5).
If [Q+X−]org and [Q+Y−]org keeps stable, then the system has reached a steady state and,
When the amount of phase transfer catalyst in the organic phase remains constant, (Q)org can be expressed as follows:
Applying the steady state approximation, and adding to both side of Equation (4) and then,
Substitute (8) into (3), the following expression can be obtained to describe the rate of formation of products or that of the disappearance of reactants during the reaction,
Thus, in case (1), when K1[X−]aq + K−1 [Y−]aq >> k2[R − Y], Equation (9) can be simplified as:
Case 1: During this phase transfer catalyzed esterification process, the chemical reaction rate in organic phase is much slower than the mass transfer rate. Having a small amount of water in this reaction system, (NaX)aq is saturated in the aqueous phase throughout the reaction. Under this condition, the concentration of (Q+X−)org formed during the esterification process in the organic phase remains constant and rapidly reaches the steady state, so it is reasonable to estimate that the concentration of [Q+X−]org is in a steady state. When the right part of the equation is divided by k1[X−]aq, the following equation becomes (K = k1/k−1)
If K1[X−]aq >> K−1[Y−]aq + K2[R − Y] in case (2), Equation (9) can be simplified as:
Case 2: During this phase transfer catalyzed esterification process, the mass transfer process is very fast and much faster than the rate of esterification chemical reaction. Under this condition, in the organic phase, (Q+X−)org accumulates quickly and remains constant.
3. Experimental
3.1. Chemicals
After the oxidative ring-opening reaction, the single-ring and multi-ring of cyclic hydrocarbons and aromatics were expected to be converted into acids [9].
In this paper, TBAB, THAB, and BTEAB were used as phase transfer catalysts; adipic acid, benzoic acid, and phthalic acid as model compounds; 1-bromobutane as esterification reactants; and dodecane as model feed. All the chemicals used in the experiments in this paper were purchased from Sigma-Aldrich (Oakville, ON, Canada) and their purity is higher than 99%.
3.2. Experimental Design
Based on the preliminary experimental results, orthogonal experimental method was used to optimize the reaction conditions by investigating the contribution of each factor (such as the amount of water and phase transfer catalyst, reaction temperature, and reaction time, etc.) and each level of each factor on the conversion ratio into 1-bromobutane. The orthogonal results were summarized in Supplementary Materials Tables S1–S3. Herein K and k respectively stands for the sum and the average values of experimental results for each level of each factor, and R stands for the range of k.
Then based on the orthogonal experimental results, the effects of temperature, type of organic acids and catalysts, and the amount of catalyst and water, on the kinetics of the esterification reaction were investigated. Generally, in a typical PTC esterification process, the reaction parameters were set to adipic acid of 0.01 mol, water of 3 mL, sodium hydroxide of 0.02 mol, TBAB of 0.006 mol, 1-bromobutane of 0.04 mol, dodecane of 40 g, and temperature of 110 °C.
3.3. PTC Process Evaluation
During the PTC reaction, two phases, inorganic (aqueous) phase and organic (oil) phase, coexists in the reaction system. The adipic acid and sodium hydroxide were mixed to form sodium adipate. The phase transfer catalyst, TBAB, the solvent, dodecane, and 1-bromobutane were added into the above-mentioned solution, which was stirred and heated till reflux for different reaction times ranging from 20 to 240 min, as shown in the specific figure and table (Figure 1, Figure 2, Figure 3 and Figure 4, Supplementary Materials Tables S1–S7, and Figures S1–S15). In order to remove the Br− and to get the pure products, the organic phase was washed by deionized water in loops. Taking adipic acid as an example, the chemical reaction involved in this process is shown in Figure 11.
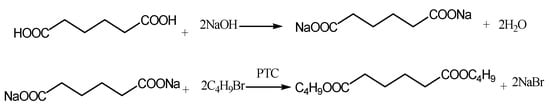
Figure 11.
Chemical reactions during adipic acid esterification process by PTC.
Firstly, adipic acid is converted into disodium adipate, and the anion of adipic acid is formed in aqueous phase. Then, disodium adipate reacts with the phase transfer catalysts and forms the intermediate of diquaternary ammonium adipate, which dissolves in both aqueous phase and oil phase. This intermediate enters oil phase and reacts with 1-bromobutane to produce butyl adipate, which is the target product ester, and the by-product, sodium bromide, is released simultaneously. Sodium bromide finally moves into the aqueous phase because it is an inorganic salt only dissolving in the aqueous. The unconverted 1-bromobutane and the resultant-butyl adipate were quantitatively tested by GC (FID) (SHIMADZU, Nakagyo-ku, Kyoto, Japan), and the detailed testing information is the same as the previous publication [33]. The conversion and the selectivity were calculated by the amount of unconverted 1-bromobutane and that of the resultant-butyl adipate, respectively.
4. Conclusions
Organic acids (adipic acid, benzoic acid, and phthalic acid), one of the main products from oxidative ring opening of ring hydrocarbons, being esterified into the corresponding esters by the PTC approach was studied.
The reaction temperature was found to be the most significant among the factors on the conversion of organic acid into ester, followed by the amount of water, amount of catalyst, and reaction time, respectively. Hydrophobicity is a key factor to the conversion of adipic acid, benzoic acid, and phthalic acid.
The studied PTC esterification was the quasi-first-order reaction. The activation energy for esterification of adipic acid is 15.88 kcal/mol. The Omega phase effect phenomenon was found when the TBAB increased from 0.004 mol to 0.006 mol in the PTC process. Around 5–8% water is the optimal quantity for the given reaction system.
Two cases of esterification processes of PTC were proposed.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/10/851/s1, Figure S1: First-order reaction curve without water, Figure S2: First-order reaction curve with 1 mL water, Figure S3: First-order reaction curve with 3 mL water, Figure S4: First-order reaction curve with 5 mL water, Figure S5: First-order reaction curve with 10 mL water, Figure S6: First-order reaction curve with 0.002 mol TBAB, Figure S7: First-order reaction curve with 0.004 mol TBAB, Figure S8: First-order reaction curve with 0.006 mol TBAB, Figure S9: First-order reaction curve with 0.008 mol TBAB, Figure S10: First-order reaction curve with THAB as PTC catalyst, Figure S11: First-order reaction curve with TBAB as PTC catalyst, Figure S12: First-order reaction curve with BTEAB as PTC catalyst, Figure S13: First-order reaction curve for adipic acid, Figure S14: First-order reaction curve for benzoic acid, Figure S15: First-order reaction curve for phthalic acid. Table S1: Orthogonal experimental factors and levels for the esterification of adipic acid, Table S2: Orthogonal experimental factors and levels for the esterification of phthalic acid, Table S3: Orthogonal experimental factors and levels for the esterification of benzoic acid, Table S4: Effects of reaction time on the concentration of 1-bromobutane at 96 °C, Table S5: Effects of reaction time on the concentration of 1-bromobutane at 103 °C, Table S6: Effects of reaction time on the concentration of 1-bromobutane at 107 °C, Table S7: Effects of reaction time on the concentration of 1-bromobutane at 110 °C, Table S8: The rate constant of 1-bromobutane at different temperatures.
Author Contributions
Conceptualization, H.L. and Y.Z.; Data curation, H.L., X.L. and S.N.; Formal analysis, H.L. and X.L.; Funding acquisition, H.W. and Y.Z.; Investigation, H.L. and X.L.; Methodology, H.W., H.L., Y.Z. and S.N.; Project administration, H.L.; Resources, H.L.; Software, H.L., H.W. and X.L.; Supervision, H.L., Y.Z. and J.Z.; Validation, H.L. and X.L.; Visualization, H.W., H.L. and X.L.; Writing—original draft, H.W., H.L. and X.L.; Writing—review & editing, H.W., R.R., J.P., H.Z. (Haiping Zhang), Y.Z., J.Z. and H.Z. (Hui Zhang).
Funding
The authors gratefully thank the Ring-Opening of Cycloalkanes for Increasing Cetane Number of Diesel Fuels, Supported by CanmetEnergy, NRCan, the Atlantic Innovation, and Jiaxing University (#70518034) for financial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Occelli, M.L. Fluid Catalytic Cracking VII: Materials, Methods and Process Innovations. In Studies in Surface Science and Catalysis; Elsevier Science: Amsterdam, The Netherlands, 2007; Volume 166, pp. 1–319. [Google Scholar]
- Rosinski, G.; Olsen, C. Cetane Improvement in Diesel Hydrotreating. In ART Catalagram® 106 Special Edition; Advanced Refining Technologies LLC.: Chicago, IL, USA, 2009; pp. 12–16. [Google Scholar]
- Chang, J.R.; Chang, S.L.; Lin, T.B. γ-Alumina-Supported Pt Catalysts for Aromatics Reduction: A Structural Investigation of Sulfur Poisoning Catalyst Deactivation. J. Catal. 1997, 169, 338–346. [Google Scholar] [CrossRef]
- Jacquin, M.; Jones, D.J.; Rozière, J.; López, A.J.; Rodríguez-Castellón, E.; Trejo Menayo, J.M.; Lenarda, M.; Storaro, L.; Vaccari, A.; Albertazzi, S. Cetane improvement of diesel with a novel bimetallic catalyst. J. Catal. 2004, 228, 447–459. [Google Scholar] [CrossRef]
- Sidhpuria, K.B.; Parikh, P.A.; Bahadur, P.; Jasra, R.V. Rhodium Supported Hβ Zeolite for the Hydrogenation of Toluene. Ind. Eng. Chem. Res. 2008, 47, 4034–4042. [Google Scholar] [CrossRef]
- Sidhpuria, K.B.; Patel, H.A.; Parikh, P.A.; Bahadur, P.; Bajaj, H.C.; Jasra, R.V. Rhodium nanoparticles intercalated into montmorillonite for hydrogenation of aromatic compounds in the presence of thiophene. Appl. Clay Sci. 2009, 42, 386–390. [Google Scholar] [CrossRef]
- Taillades-Jacquin, M.; Jones, D.J.; Rozière, J.; Moreno-Tost, R.; Jiménez-López, A.; Albertazzi, S.; Vaccari, A.; Storaro, L.; Lenarda, M.; Trejo-Menayo, J.-M. Novel mesoporous aluminosilicate supported palladium-rhodium catalysts for diesel upgrading: II. Catalytic activity and improvement of industrial diesel feedstocks. Appl. Catal. A-Gen. 2008, 340, 257–264. [Google Scholar] [CrossRef]
- Stanislaus, A.; Cooper, B.H. Aromatic Hydrogenation Catalysis: A Review. Catal. Rev. 1994, 36, 75–123. [Google Scholar] [CrossRef]
- Ren, R.; Yang, H.; Zheng, Y.; Ng, S.; Zhao, J. Oxidative Ring-Opening of 2-Methylcyclohexanone Catalysed by Supported Heteropolyacid Catalysts for Cetane Number Enhancement. Can. J. Chem. Eng. 2013, 91, 776–782. [Google Scholar] [CrossRef]
- Xu, C. Organic Chemistry, 2nd ed.; China Higher Education Press: Beijing, China, 2002. [Google Scholar]
- Sun, C.; Wang, X.; Chen, T.; Sun, Q.; Ma, L.; Sun, F.; Sun, Z.; Sun, X.; Li, Q.; Yu, G.; et al. Principle and Application of Organic Oxidation Reactions; Chemical Industry Press: Beijing, China, 2013. [Google Scholar]
- Ma, S.; Zhang, J. 2,3,4-or 2,3,5-Trisubstituted Furans: Catalyst-Controlled Highly Regioselective Ring-Opening Cycloisomerization Reaction of Cyclopropenyl Ketones. J. Am. Chem. Soc. 2003, 125, 12386–12387. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Ornstein, P.L. Ferric Salt Catalysed Oxygenation of Cycloalkanones to Oxo Esters by Molecular Oxygen. J. Org. Chem. 1983, 48, 1133–1135. [Google Scholar]
- Ali, B.E.; Bregeault, J.M.; Martin, J.; Martin, C. Oxidative Cleavage of Ketones by Vanadium Polymetalates and Dioxygen. New J. Chem. 1989, 13, 173–175. [Google Scholar]
- Ali, B.E.; Bregeault, J.M.; Mercier, J.; Martin, J.; Martin, C.; Convertb, O. The Oxidation of Ketones with a Heteropolyacid, H5[PMo10V2O40] and Dioxygen. J. Chem. Soc. Chem. Commun. 1989, 62, 825–826. [Google Scholar] [CrossRef]
- He, L.; Kanamori, M.; Horiuchi, C.A. Oxidation of 2-Alkylcycloalkanones with Iodine-Cerium (IV) Salts in Alcohols. J. Chem. Res. 1999, 2, 122–123. [Google Scholar] [CrossRef]
- Bregeault, J.M.; Launaya, F.; Atlamsani, A. Catalytic Oxidative Carbon–Carbon Bond Cleavage of Ketones with Dioxygen: Assessment of Some Metal Complexes. Acc. Acad. Sci. Ser. IIC Chem. 2001, 4, 11–26. [Google Scholar]
- Bloss, C.; Wagner, V.; Jenkin, M.E.; Volkamer, R.; Bloss, W.J.; Lee, J.D.; Heard, D.E.; Wirtz, K.; Martin-Reviejo, M.; Rea, G.; et al. Development of a detailed chemical mechanism (MCMv3.1) for the atmospheric oxidation of aromatic hydrocarbons. Atmos. Chem. Phys. 2005, 5, 641–664. [Google Scholar] [CrossRef]
- Greenberg, A. Exploration of Selected Pathways for Metabolic Oxidative Ring Opening of Benzene Based on Estimates of Molecular Energetics. In Active Oxygen in Biochemistry; Valentine, J.S., Foote, C.S., Greenberg, A., Liebman, J.F., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 401–432. [Google Scholar]
- Hoffmann, E.H.; Tilgner, A.; Wolke, R.; Boge, O.; Walter, A.; Herrmann, H. Oxidation of substituted aromatic hydrocarbons in the tropospheric aqueous phase: Kinetic mechanism development and modeling. Phys. Chem. Chem. Phys. 2018, 20, 10960–10977. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Boehman, A. Oxidation chemistry of cyclic hydrocarbons in a motored engine: Methylcyclopentane, tetralin, and decalin. Combust. Flame 2010, 157, 495–505. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Ma, H. Synthesis of Linear Alkane (C14–C28) from Cyclohexane with V-Promoted Sulfated ZrO2 Under Mild Conditions. Energy Fuel 2008, 22, 218–222. [Google Scholar] [CrossRef]
- Tailleur, R.G.; Caris, P.C. Selective Oxidation of a Hydrotreated Light Catalytic Gas Oil to Produce Low-Emission Diesel Fuel. Energy Fuel 2009, 23, 799–804. [Google Scholar] [CrossRef]
- Chidambaram, M.; Sonavane, S.U.; de la Zerda, J.; Sasson, Y. Didecyldimethylammonium bromide (DDAB): A universal, robust, and highly potent phase-transfer catalyst for diverse organic transformations. Tetrahedron 2007, 63, 7696–7701. [Google Scholar] [CrossRef]
- Leng, Y.; Wang, J.; Zhu, D.R.; Ren, X.Q.; Ge, H.Q.; Shen, L. Heteropolyanion-Based Ionic Liquids: Reaction-Induced Self-Separation Catalysts for Esterification. Angew. Chem. Int. Ed. 2009, 48, 168–171. [Google Scholar] [CrossRef]
- Yang, H.M.; Chen, C.H. Catalytic esterification of sodium salicylate in third-liquid phase under ultrasound-assisted tri-liquid phase-transfer catalysis. J. Mol. Catal. A-Chem. 2009, 312, 107–113. [Google Scholar] [CrossRef]
- Coleman, M.T. Use of diethoxymethane as a solvent for phase-transfer esterification of carboxylic acids. Synth. Commun. 2012, 42, 1911–1913. [Google Scholar] [CrossRef]
- Starks, C.M.; Liotta, C.L.; Halpen, M. Phase-Transfer Catalysis: Fundamentals, Applications, and Industrial Perspective; Chapman & Hall: New York, NY, USA, 1994. [Google Scholar]
- Zahalka, H.A.; Sasson, Y. Esterificantion of 1,4-dichlorobutane with sodium formate under solid-liquid phase transfer catalysis. A kinetic study. Can. J. Chem. 1989, 67, 245–249. [Google Scholar] [CrossRef]
- Yang, H.; Huang, C. Kinetics for benzoylation of sodium phenolide by liquid-liquid phase-transfer catalysis. Appl. Catal. A-Gen. 2006, 299, 258–265. [Google Scholar] [CrossRef]
- Rabinovitz, M.; Cohen, Y.; Halpern, M. Hydroxide ion initiated reactions under phase transfer catalysis conditions: Mechanism and implications. New synthetic methods. Angew. Chem. Int. Ed. 1986, 25, 960–970. [Google Scholar] [CrossRef]
- Makosza, M.; Krylowa, L. Remarks on the mechanism of phase-transfer catalyzed carbanion generation in two-phase systems. Tetrahedron 1999, 55, 6395–6402. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.; Feng, P.; Han, X.; Zheng, Y. Integration of catalytic cracking and hydrotreating technology for triglyceride deoxygenation. Catal. Today 2017, 291, 172–179. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).