Abstract
In this study, a set of enantiomerically pure aziridines bearing a phosphine oxide moiety were prepared in high yields and tested as chiral catalysts in the direct asymmetric Mannich reaction of hydroxyacetone, an amine (p-anisidine), and various aromatic aldehydes. The appropriate Mannich adducts were formed in chemical yields from moderate to good with a high level of enantio- and diastereoselectivity. The best results were obtained using the catalysts bearing a free NH-aziridine subunit.
1. Introduction
A comprehensive literature search concerning asymmetric organocatalysis [1,2] revealed a great number of various interesting catalysts that are prone to efficiently catalyze enantioselective transformations. These are, for example, proline and its derivatives, cinchona alkaloids, and thiourea systems [2]. Moreover, organocatalytic reactions can be carried out not only under standard chemical conditions, but also by invoking other techniques like ultrasounds [3] or mechanochemistry [4]. Over the last decade, the numerous compounds exhibiting various biological activities such as indole alkaloids [5] and other alkaloids [6] have been synthesized through the use of one or more organocatalytic approaches. Additionally, in recent years, enormous progress concerning organocatalytic cascade (domino) reactions [7,8] has been observed.
The organocatalyzed enantioselective Mannich reaction [9] constitutes one of the ways of constructing nitrogen-containing chiral systems. Apart from simple, common molecules like proline, more sophisticated systems have been tested in this transformation, for example, chiral ammonium betaine [10], D-glucosamine derivatives [11], metal-based complexes [12], or protease [13]. The Mannich reaction can serve for the formation of natural products [14] exhibiting various biological activities [15], for the formation of the quaternary carbon stereogenic centers [16,17], or for the synthesis of other β-amino ketones [18].
The chiral phosphorus-functionalized aziridines have been marginally mentioned in the chemical literature [19,20,21,22,23]. Very recently, an enantioselective Michael addition promoted by chiral phosphinoyl-aziridines was published by our group [24]. Taking into account our knowledge in the area of asymmetric organocatalysis [25,26,27] as well as with the Mannich reaction [3] and with regard to all of the above facts, we decided to prepare a series of chiral phosphorus-functionalized aziridines in order to test their catalytic activity in the direct asymmetric organocatalytic Mannich reaction of amine (p-anisidine), hydroxyacetone, and aromatic aldehydes. In our opinion, a successful realization of the present research task may enrich the existing catalyst library with chiral heteroorganic P,N-systems relatively simple in synthesis. Moreover, we hope that their catalytic activity will be exhibited in other asymmetric transformations with the exception of the Michael and Mannich reactions.
2. Results and Discussion
2.1. Synthesis of the Chiral Aziridines 1–9 and 14
Ten chiral enantiomerically pure aziridines 1–9 and 14 bearing the phosphine oxide moiety (Figure 1) were prepared.
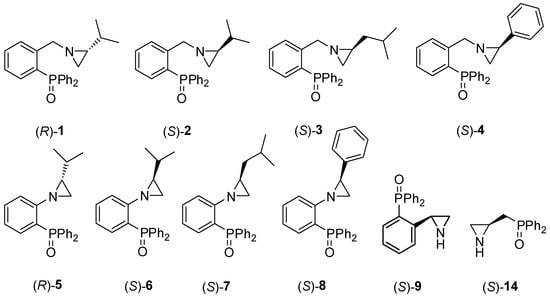
Figure 1.
Chiral aziridines 1–9 and 14 functionalized by the P=O group.
Compounds 1–8 were synthesized according to procedures recently reported by our group [24] starting from either 2-(diphenylphosphino)benzoic acid (catalysts 1–4) or from o-bromoanisole (systems 5–8). Chiral catalyst 9, containing the free NH group, was obtained in a one-step process comprising a treatment of (S)-2-phenylaziridine with two equivalents of sec-BuLi and one equivalent of diphenylphosphinic chloride at −78 °C in anhydrous tetrahydrofurane (Scheme 1). The corresponding (S)-2-diphenylphosphinoyl-2-phenylaziridine 9 was afforded in a 69% yield after column chromatography on silica gel.
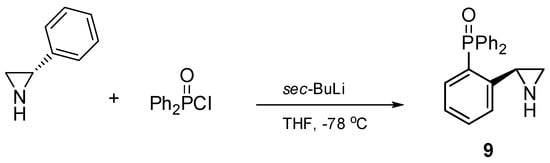
Scheme 1.
One-step synthesis of chiral aziridine 9.
In turn, aziridine 14 was obtained from N-trityl aziridine alcohol 10 [28] via a four-step synthetic pathway (Scheme 2). First, alcohol 10 was reacted with tosyl anhydride in the presence of triethylamine in dry dichloromethane to afford the corresponding tosylate 11 in a 79% yield. In the next step, tosyl derivative 11 was converted into the phosphine 12 using diphenylphosphine potassium salt in the presence of Et3N in acetonitrile in a 62% yield. Phosphine 12 was then oxidized with hydrogen peroxide to phosphine oxide 13 in a 79% yield. Finally, compound 13 was treated with the mixture of sulfuric acid, water, and methanol to remove the N-trityl block, giving the catalyst 14 in a 22% yield.
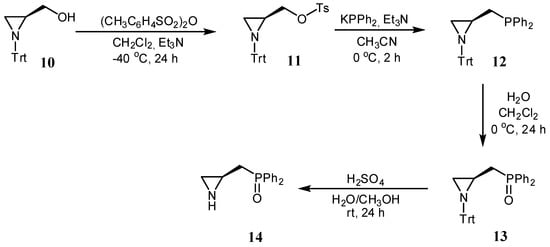
Scheme 2.
Synthesis of organocatalyst 14.
2.2. Asymmetric Three-Component Mannich Reaction Promoted by Aziridines 1–9, 14
In order to check the catalytic efficiency of chiral aziridines 1–9 and 14, asymmetric direct three-component Mannich reactions of p-anisidine, hydroxyacetone, and p-nitrobenzaldehyde were performed in the presence of the aforementioned chiral catalysts 1–9 and 14 (Scheme 3). The testing reactions were performed in dimethyl sulfoxide at room temperature.
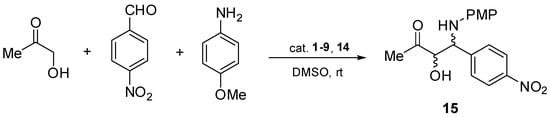
Scheme 3.
Asymmetric direct Mannich reaction catalyzed by chiral aziridines 1–9, 14.
The results of all of the asymmetric Mannich processes are presented in Table 1.

Table 1.
Three-component asymmetric Mannich reaction in the presence of aziridines 1–9, 14.
In the first set of experiments, the chiral catalysts 1–4 containing a methylene linker between the aromatic ring and an aziridinyl nitrogen atom were applied in the title reaction (Table 1, entries 1–4). In all cases, the corresponding Mannich products were formed in moderate chemical yields (27–43%) and moderate stereoselectivities (32–40% of ee, 8:1–10:1 of dr). It should also be noted that the use of catalysts with an opposite absolute configuration at the carbon atom of the aziridine subunit (Table 1, entries 1–2) led to the formation of both enantiomers of product 15, which is consistent with our earlier studies [3,24], showing that configuration at the carbon next to the amine moiety is crucial for the stereochemistry of the reaction.
Based on our findings on the asymmetric Michael reaction [24], analogous systems 5–8 without the methylene group were tested in terms of catalytic activity in the asymmetric Mannich reaction (Table 1, entries 5–8). As anticipated, chiral product 15 was created in higher yields (60–69%) and with higher stereoselectivity. Moreover, as previously mentioned, the application of both enantiomeric aziridines 5 and 6 resulted in the formation of the products having opposite absolute configurations (Table 1, entries 5 and 6).
Finally, catalysts 9 and 14 with a free NH-aziridine subunit were tested in the title reaction. The corresponding product 15 was formed in high chemical yields (up to 90%) and with excellent enantio- and diastereoselectivities (96% of ee, 20:1 of dr, respectively) (Table 1, entries 9 and 10).
2.3. Asymmetric Mannich Reaction in the Presence of Catalyst 9
With the most effective aziridine 9 in hand, we decided to extend the spectrum of aromatic aldehydes used as substrates in the asymmetric Mannich reaction under the same conditions (Scheme 4). All results are summarized in Table 2.
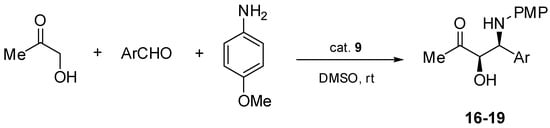
Scheme 4.
Enantioselective Mannich reaction catalyzed by aziridine 9.

Table 2.
Asymmetric Mannich reaction in the presence of aziridine 9.
A close inspection of Table 2 reveals that chiral aziridine phosphine oxide 9 is prone to efficiently promote the Mannich reaction of aromatic aldehydes with hydroxyacetone and p-anisidine, leading to the desired products in high chemical yields and with satisfactory levels of enantio- and diastereoselectivity. Only in the case of the use of o-substituted aldehyde (Table 2, entry 2) were the values of yield and selectivity somewhat lower.
Basing on the aforementioned results, a tentative transition-state model explaining the absolute configuration of Mannich products was proposed (Figure 2). It should be mentioned that a similar model was described by us recently [24]. After deprotonation of an acidic proton of the ketone, complex A is generated. Such a formed enolate is oriented in such a way that its methyl group is located opposite the unsubstituted carbon atom of the aziridine ring. In this situation, considering the (R)-configuration at the C2 atom of aziridine, only the Si-face of the enolate is possible to attack (the Re-face is crowded by the side chain R of aziridine) (complex A, Figure 2). Next, strong H-bonds between NH and the oxygen atom of enolate causes a situation where the enolate may attack the double bond of imine formed from p-nitrobenzaldehyde and p-anisidine. The most favorable orientation of the imine in the transition state is the one in which the double bonds of imine and enolate are in the same plane, thus, the p-methoxy-substituent of imine and the methyl group of the enolate are on two sides of this plane. Finally, this leads to the formation of the syn-Mannich product with the (S,R)-configuration (Figure 2, B).
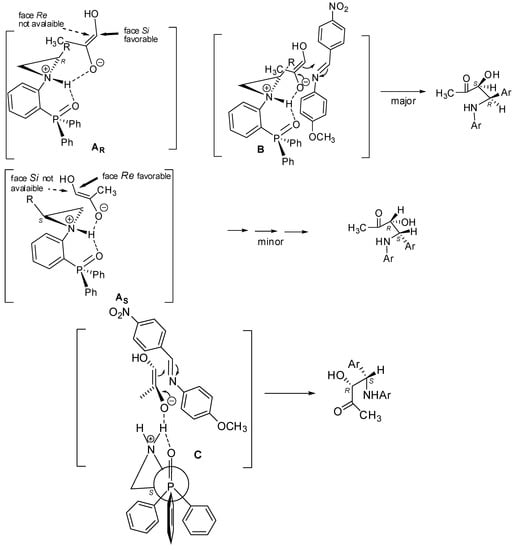
Figure 2.
Tentative transition state model.
In the case of the (S)-configured aziridine (Figure 2, C), the orientation of the enolate and its further interaction of the imine leads to the formation of the (R,S)-configured Mannich product.
In order to investigate the necessity of the presence of the P=O group in the catalyst structure, two additional experiments were performed (Scheme 5).
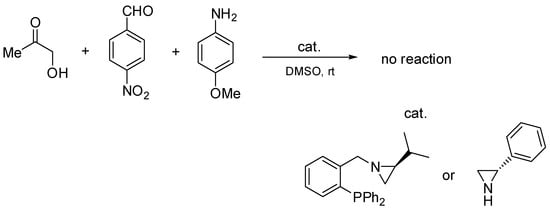
Scheme 5.
Additional Mannich reactions with phosphine and aziridine.
In these additional Mannich reactions, the corresponding phosphine and (S)-2-phenylaziridine were used as catalysts. In both cases, the formation of the Mannich product was not observed.
3. Materials and Methods
3.1. Materials
Tetrahydrofurane was dried from sodium benzophenone ketyl radical. Dichloromethane was distilled over calcium hydride. 1H, 13C, and 31P NMR spectra were recorded on a Bruker Avance III instrument at 600 MHz and 150 MHz, respectively, with CDCl3 as the solvent and TMS as the internal standard. Data are reported as s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, and br. s = broad singlet. Optical rotations were measured on a Anton Paar MCP500 or on a Perkin-Elmer 241 MC polarimeter with a sodium lamp at room temperature (c 0.5). Column chromatography was performed using Merck 60 silica gel. TLC was performed on Merck 60 F254 silica gel plates. Visualization was accomplished with UV light (254 nm) or using iodine vapors. The enantiomeric excess (ee) values were determined by chiral HPLC (Chiralcel AD-H column). Chiral aziridines 1–8 were synthesized according to the general procedures described recently [24].
3.2. Methods
3.2.1. Synthesis of (S)-2-diphenylphosphinoyl-2-phenylaziridine 9
Sec-BuLi (1.43 mL, 2 mmol, 1.4 M solution in cyclohexane) was added dropwise to a solution of (S)-2-phenylaziridine (119 mg, 1 mmol) in anhydrous tetrahydrofurane (10 mL) at −78 °C under argon. The resulting brown solution was stirred at this temperature for 2 h and diphenylphosphinic chloride (237 mg, 1 mmol) was added. The mixture was stirred at −78 °C for 2 h and then warmed up to room temperature. The reaction was quenched by the addition of saturated aqueous NH4Cl (5 mL). The mixture was poured into water and extracted with Et2O (3 × 10 mL). The combined organic layers were dried over Na2SO4 and concentrated in vacuo. The crude product was purified via column chromatography on silica gel (hexane:ethyl acetate 50:50).
(S)-2-diphenylphosphinoyl-2-phenylaziridine: slightly yellow solid, 220 mg, 69% yield; m.p. 77.6–79.0 °C; [α]D20 + 18.0 (c 0.5, CHCl3);
1H-NMR (600 MHz, CDCl3): δ = 1.84 (br s, 1H), 2.24 (dd, J = 2.0 Hz, 13.0 Hz, 1H), 2.95 (dd, J = 6.0 Hz, 17.9 Hz, 1H), 3.78 (dq, J = 3.2 Hz, 6.0 Hz, 15.6 Hz, 1H), 7.30–7.33 (m, 1H), 7.35-7.40 (m, 5H), 7.44–7.48 (m, 1H), 7.49–7.53 (m, 2H), 7.54–7.57 (m, 1H), 7.87–7.92 (m, 2H), 7.98–8.03 (m, 2H);
13C-NMR (150 MHz, CDCl3): δ = 33.0 (d, J = 7.4 Hz, CH2N), 37.0 (d, J = 5.0 Hz, CHN) 126.2 (Car), 127.8 (Car), 128.4 (Car), 128.5 (2 Car), 128.6 (Car), 131.5 (Car), 131.6 (2 Car), 131.7 (Car), 131.9 (d, J = 3.2 Hz, Car), 132.0 (d, J = 2.1 Hz, Car), 132.1 (Car), 132.4 (Car), 132.9 (Car), 133.3 (Car,) 137.6 (Cq ar), 137.7 (Cq ar) ppm;
31P-NMR (150 MHz, CDCl3): δ = 32.9 ppm; Anal. Calcd. for C20H18NOP C 75.20, H 5.70, N 4.40; found C 75.28, H 5.89, N 4.60.
3.2.2. Synthesis of [(S)-1-Tritylaziridin-2-yl]methyl-4-methylbenzenesulfonate 11
N-Trityl aziridine alcohol 10 was synthesized according to the protocol described previously (Jarzyński et al., 2015). A solution of p-toluenesulfonic anhydride (2.66 mmol) in CH2Cl2 (4 mL) was added dropwise to a solution of aziridine alcohol 10 (561 mg, 1.78 mmol) and triethylamine (0.5 mL) in anhydrous CH2Cl2 (5 mL) at −40 °C. The solution was stirred at room temperature for 24 h and then the reaction was quenched by the addition of water (5 mL). The mixture was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried over Na2SO4 and concentrated in vacuo. The crude product was purified via column chromatography on silica gel (hexane:ethyl acetate 80:20).
[(S)-1-Tritylaziridin-2-yl]methyl-4-methylbenzenesulfonate: colorless oil, 658 mg, 79% yield; 1H-NMR (600 MHz, CDCl3): δ = 1.15 (d, J = 6.0 Hz, 1H), 1.51–1.56 (m, 1H), 1.73 (d, J = 2.8 Hz, 1H), 2.49 (s, 3H), 4.13–4.18 (m, 1H), 4.30-4.33 (m, 1H), 7.21–7.30 (m, 9H), 7.34 (d, J = 8.1 Hz, 1H), 7.40–7.43 (m, 6H), 7.76 (d, J = 8.3 Hz, 1H).
3.2.3. Synthesis of Diphenyl-[[(S)-1-tritylaziridin-2-yl]methyl]phosphine 12
A solution of diphenylphosphine potassium salt (0.5 M, 3.5 mL in THF) was added dropwise to a solution of tosylate 11 (680 mg, 1.45 mmol) and triethylamine (0.4 mL) in anhydrous acetonitrile (26 mL) at 0 °C under argon. The solution was stirred at room temperature for 2 h. The mixture was concentrated in vacuo and the crude product was purified via column chromatography on silica gel (hexane:ethyl acetate 90:10).
Diphenyl-[[(S)-1-tritylaziridin-2-yl]methyl]phosphine: colorless oil, 436 mg, 62% yield; 1H-NMR (600 MHz, CDCl3): δ = 1.04 (d, J = 6.2 Hz, 1H), 1.27–1.34 (m, 1H), 1.53 (d, J = 3.2 Hz, 1H), 2.20 (dd, J = 3.5 Hz, 8.7 Hz, 1H), 2.78 (dd, J = 3.5 Hz, 13.5 Hz, 1H), 7.19–7.23 (m, 3H), 7.23–7.27 (m, 6H), 7.28–7.37 (m, 10H), 7.44–7.45 (m, 6H); 31P-NMR (150 MHz, CDCl3): δ = −21.5 ppm.
3.2.4. Synthesis of (S)-2-(Diphenylphosphinoylmethyl)-1-trityl-aziridine 13
A solution of hydrogen peroxide (20 mL, 30% in water) was added dropwise to a solution of 12 (483 mg, 1 mmol) in dichloromethane (8 mL). The mixture was stirred at 0 °C for 24 h. The reaction was poured into water and extracted with CH2Cl2 (3 × 3 mL). The combined organic layers were dried over MgSO4 and concentrated in vacuo to provide 13 as a colorless oil.
(S)-2-(Diphenylphosphinoylmethyl)-1-trityl-aziridine: colorless oil, 395 mg, 79% yield; 1H-NMR (600 MHz, CDCl3): δ = 1.06 (d, J = 5.9 Hz, 1H), 1.55–1.59 (m, 2H), 2.34–2.40 (m, 1H), 3.12-3.17 (m, 1H), 7.19–7.26 (m, 10H), 7.40–7.41 (m, 6H), 7.42–7.47 (m, 4H), 7.52–7.54 (m, 2H), 7.67–7.69 (m, 3H). 31P-NMR (150 MHz, CDCl3): δ = 30.7 ppm.
3.2.5. Synthesis of (S)-2-(Diphenylphosphinoylmethyl)aziridine 14
To a solution of compound 13 (395 mg, 0.8 mmol), the mixture of MeOH:H2O:H2SO4 (3:8:60, 14 mL) was added and sonicated for 5 min. Then, the mixture was stirred at rt over 24 h. After that, 10 mL of H2O was added and the solution was basified to pH 10. Next, the mixture was extracted with EtOAc (3 × 10 mL) and organic extracts were washed with NaHCO3 and NaCl. The combined organic layers were dried over MgSO4 and concentrated in vacuo and the crude product was purified via column chromatography on silica gel (hexane:ethyl acetate 50:50).
(S)-2-(Diphenylphosphorylmethyl)aziridine: colorless solid, 45 mg, 22% yield; m.p. 81.3–82.0 °C; [α]D20 + 25.0 (c 0.5, CHCl3); 1H-NMR (600 MHz, CDCl3): δ = 0.86–0.92 (m, 1H), 2.18 (dd, J = 3.7 Hz, 12.0 Hz, 1H), 2.36 (dd, J = 3.7 Hz, 12.4 Hz, 1H), 2.47–2.52 (m, 1H), 2.79–2.85 (m, 1H), 7.47–7.53 (m, 5H), 7.71–7.75 (m, 2H), 7.78–7.82 (m, 3H); 13C-NMR (150 MHz, CDCl3): δ = 33.9 (s, CH2N), 34.4 (s, CHN), 57.1 (s, CH2), 126.2 (Car), 127.8 (Car), 127.9 (Car), 128.4 (Car), 128.5 (Car), 128.6 (Car), 130.6 (Car), 130.9 (Car), 131.5 (d, J = 3.2 Hz, Car), 131.6 (d, J = 2.1 Hz, Car), 145.9 (Cq ar), 146.9 (Cq ar) ppm; 31P-NMR (150 MHz, CDCl3): δ = 29.4 ppm; Anal. Calcd. for C15H16NOP C 70.03, H 6.27, N 5.44; found C 70.04, H 6.26, N 5.44.
3.2.6. Organocatalytic Asymmetric Mannich Reaction–General Protocol
An aldehyde (0.2 mmol), p-anisidine (27 mg, 0.22 mmol), hydroxyacetone (0.43 g, 0.4 mL, 5.82 mmol) and the chiral catalyst (0.07 mmol) were dissolved in DMSO (1.6 mL) in a round-bottom flask. The mixture was stirred at room temperature overnight. Next, the reaction was quenched by the addition of water and extracted with ethyl acetate (3x). The combined organic layers were dried over anhydrous MgSO4 and the solvent was evaporated in vacuo. The crude product was subjected to column chromatography (silica gel, hexane:ethyl acetate from 80:20 to 70:30) affording the corresponding Mannich adducts 15–19. Chemical yields, optical rotations, enantiomeric excess, and diastereomeric ratios of these chiral products are presented in Table 1. 1H and 13C NMR spectra of the corresponding Mannich adducts are in accordance with the literature [3]. Their HPLC chromatograms are included in the Supplementary Materials.
4. Conclusions
The chiral aziridines functionalized by the phosphinoyl group have proven to be efficient promoters in catalyzing asymmetric the direct three-component Mannich reaction of p-anisidine, hydroxyacetone, and various aromatic aldehydes affording chiral products in high yields and with satisfactory levels of enantio- and diastereoselectivity. Furthermore, using both enantiomeric catalysts, each enantiomer of the Mannich product may be achieved. The highest catalytic activity in the asymmetric Mannich reaction was achieved using catalysts with a free NH-aziridine moiety.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/10/837/s1, copies of NMR spectra of compounds 9 and 14 and selected HPLC chromatograms for the Mannich adducts.
Author Contributions
Conceptualization and methodology, S.L. and M.R.; Software, A.M.P., A.Z., and M.R.; Investigation, A.B., A.Z., J.A., A.M.P., and M.R.; Writing—original draft preparation, M.R.; Writing—review and editing, S.L. and M.R.; Supervision, M.R. and S.L.
Funding
This research was funded by the National Science Centre (NCN) (Grant No. 2016/21/B/ST5/00421 for M.R.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Seayad, J.; List, B. Asymmetric organocatalysis. Org. Biomol. Chem. 2005, 3, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Da Gama Oliveira, V.; do Carmo Cardoso, M.F.; da Silva Magalhães Forezi, L. Organocatalysis: A brief overview on its evolution and application. Catalysts 2018, 8, 605. [Google Scholar] [CrossRef]
- Rachwalski, M.; Leenders, T.; Kaczmarczyk, S.; Kiełbasiński, P.; Leśniak, S.; Rutjes, F.P.J.T. Efficient catalysts for asymmetric Mannich reactions. Org. Biomol. Chem. 2013, 11, 4207–4213. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Chimni, S.S. Mechanochemistry assisted asymmetric organocatalysis: A sustainable approach. Beilstein J. Org. Chem. 2012, 8, 2132–2141. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, D.-F.; Gong, L.-Z. Recent progress in organocatalytic asymmetric total syntheses of complex indole alkaloids. Natl. Sci. Rev. 2017, 4, 381–396. [Google Scholar] [CrossRef]
- Hui, C.; Pu, F.; Xu, J. Metal-catalyzed asymmetric Michael addition in natural product synthesis. Chem. Eur. J. 2017, 23, 4023–4036. [Google Scholar] [CrossRef]
- Chanda, T.; Zhao, J.C.-G. Recent progress in organocatalytic asymmetric domino transformations. Adv. Synth. Catal. 2018, 360, 2–79. [Google Scholar] [CrossRef]
- Szöllösi, G. Asymmetric one-pot reactions using heterogeneous chemical catalysis: Recent steps towards sustainable processes. Catal. Sci. Technol. 2018, 8, 389–422. [Google Scholar] [CrossRef]
- Verkade, J.M.M.; van Hemert, L.J.C.; Quaedflieg, P.J.L.M.; Rutjes, F.P.J.T. Organocatalysed asymmetric Mannich reactions. Chem. Soc. Rev. 2008, 37, 29–41. [Google Scholar] [CrossRef]
- Torii, M.; Kato, K.; Uraguchi, D.; Ooi, T. Chiral ammonium betaine-catalyzed asymmetric Mannich-type reaction of oxindoles. Beilstein J. Org. Chem. 2016, 12, 2099–2103. [Google Scholar] [CrossRef]
- Sharma, A.; Peddinti, R.K. Direct asymmetric Mannich reaction catalyzed by a D-glucosamine-derived organocatalyst. Synlett 2018, 29, 630–634. [Google Scholar] [CrossRef]
- Karimi, B.; Enders, D.; Jafari, E. Recent advances in metal-catalyzed asymmetric Mannich reactions. Synthesis 2013, 45, 2769–2812. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Xiang, Y.; He, Y.-H.; Guan, Z. Anti-selective direct asymmetric Mannich reaction catalyzed by protease. Tetrahedron Lett. 2019, 60, 1066–1071. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Q.; Gao, S. Recent advances in the intramolecular Mannich reaction in natural products total synthesis. Org. Chem. Front. 2018, 5, 1049–1066. [Google Scholar] [CrossRef]
- Bai, S.; Zhu, Y.; Wu, Q. Asymmetric Mannich reaction: Synthesis of novel chiral 5-(substituted aryl)-1,3,4-thiadiazole derivatives with anti-plant-virus potency. Heterocycl. Commun. 2019, 25, 47–51. [Google Scholar] [CrossRef]
- Trost, B.M.; Saget, T.; Hung, C.-I. Direct catalytic asymmetric Mannich reactions for the construction of quaternary carbon stereocenters. J. Am. Chem. Soc. 2016, 138, 3659–3662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, L.; Hu, Y.; Zha, Z.; Wang, Z.; Loh, T.-P. Bifunctional amino sulfonohydrazide catalyzed direct asymmetric Mannich reaction of cyclic ketimines with ketones: Highly diastereo- and enantioselective construction of quaternary carbon stereocenters. Org. Lett. 2015, 17, 1050–1053. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Nie, J.; Cai, H.; Ma, J.-A. Cyclic aldimines as superior electrophiles for Cu-catalyzed decarboxylative Mannich reaction of β-ketoacids with a broad scope and high enantioselectivity. Org. Lett. 2014, 16, 2542–2545. [Google Scholar] [CrossRef]
- Doğan, Ö.; Çağli, E. PFAM catalyzed enantioselective diethylzinc addition to imines. Turk. J. Chem. 2015, 39, 290–296. [Google Scholar] [CrossRef]
- Dogan, Ö.; Tan, D. Enantioselective direct aldol reactions promoted by phosphine oxide aziridinyl phosphonate organocatalysts. Tetrahedron Asymmetry 2015, 26, 1348–1353. [Google Scholar] [CrossRef]
- Eröksüz, S.; Dogan, Ö.; Garner, P.P. A new chiral phosphine oxide ligand for enantioselective 1,3-dipolar cycloaddition reactions of azomethine ylides. Tetrahedron Asymmetry 2010, 21, 2535–2541. [Google Scholar] [CrossRef]
- Dogan, Ö.; Isci, M.; Aygun, M. New phosphine oxide aziridinyl phosphonates as chiral Lewis bases for the Abramov-type phosphonylation of aldehydes. Tetrahedron Asymmetry 2013, 24, 562–567. [Google Scholar] [CrossRef]
- Dogan, Ö.; Bulut, A.; Ali Tecimer, M. Chiral phosphine oxide aziridinyl phosphonate as a Lewis base catalyst for enantioselective allylsilane addition to aldehydes. Tetrahedron Asymmetry 2015, 26, 966–969. [Google Scholar] [CrossRef]
- Wujkowska, Z.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Phosphinoyl-aziridines as a new class of chiral catalysts for enantioselective Michael addition. Tetrahedron 2019, 75, 230–235. [Google Scholar] [CrossRef]
- Rachwalski, M.; Leśniak, S.; Kiełbasiński, P. Highly enantioselective aza-Henry reaction promoted by amine-functionalized tridentate sulfinyl ligands. Tetrahedron Asymmetry 2011, 22, 1087–1089. [Google Scholar] [CrossRef]
- Rachwalski, M.; Leśniak, S.; Sznajder, E.; Kiełbasiński, P. Highly enantioselective Henry reaction catalyzed by chiral tridentate heteroorganic ligands. Tetrahedron Asymmetry 2009, 20, 1547–1549. [Google Scholar] [CrossRef]
- Rachwalski, M.; Leśniak, S.; Kiełbasiński, P. Highly enantioselective asymmetric direct aldol reaction catalyzed by amine-functionalized tridentate sulfinyl ligands. Tetrahedron Asymmetry 2011, 22, 1325–1327. [Google Scholar] [CrossRef]
- Jarzyński, S.; Leśniak, S.; Pieczonka, A.M.; Rachwalski, M. N-Trityl-aziridinyl alcohols as highly efficient chiral catalysts in asymmetric additions of organozinc species to aldehydes. Tetrahedron Asymmetry 2015, 26, 35–40. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).