Efficient Degradation of Norfloxacin and Simultaneous Electricity Generation in a Persulfate-Photocatalytic Fuel Cell System
Abstract
:1. Introduction
2. Results and Discussion
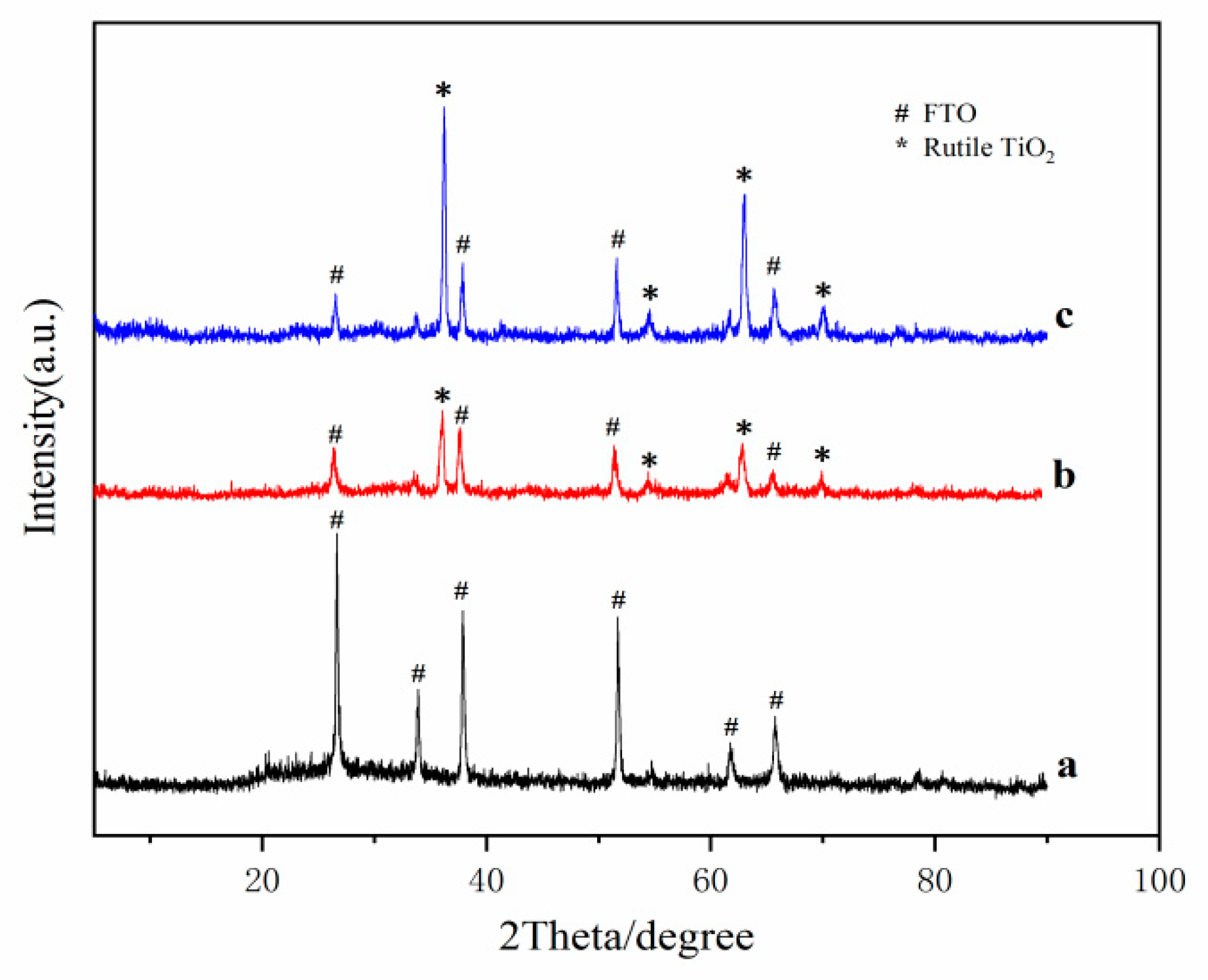
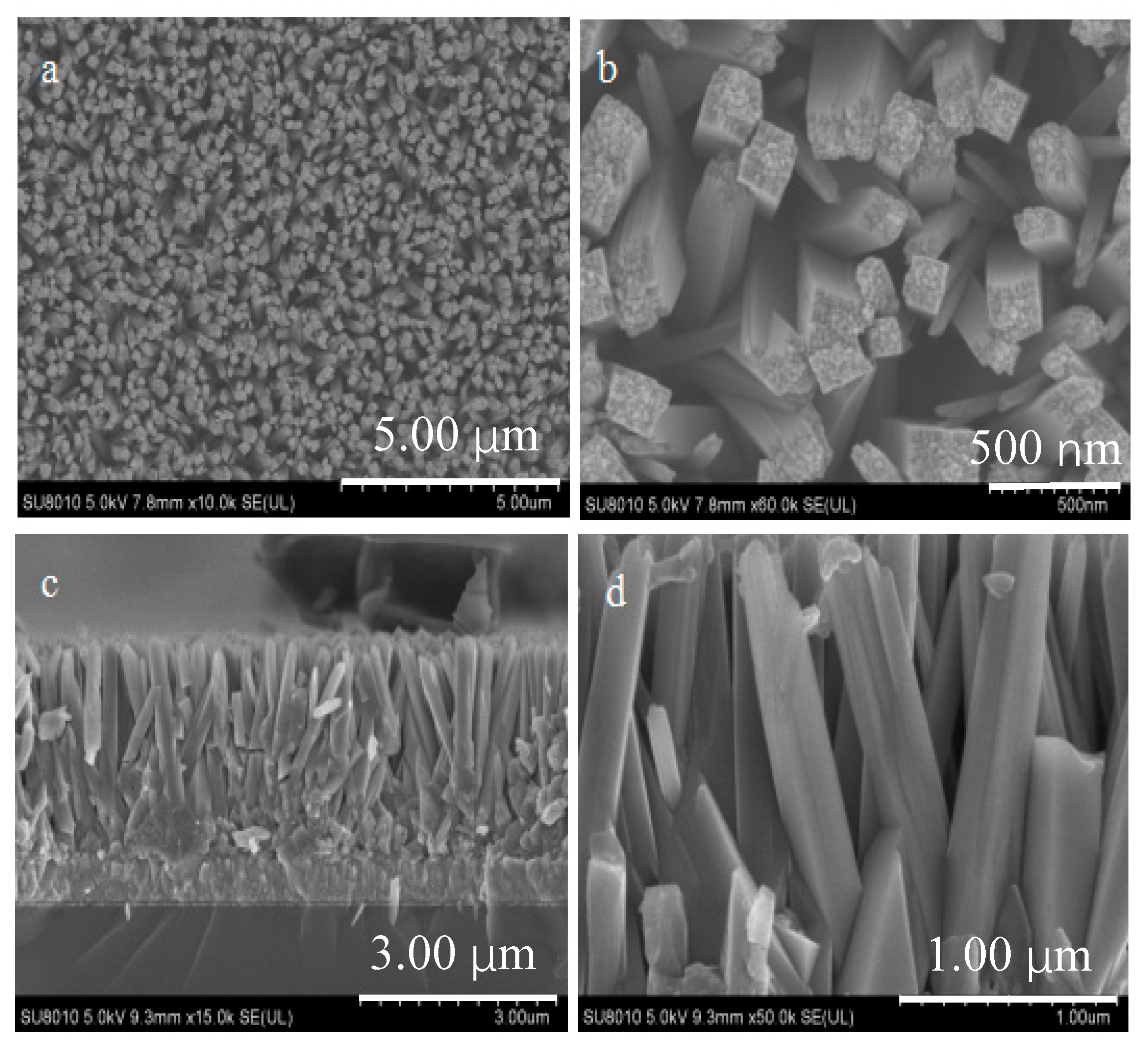
2.1. Crystalline Phase and Morphology of TiO2 Nanorods
2.2. Performance Comparison of Different Systems
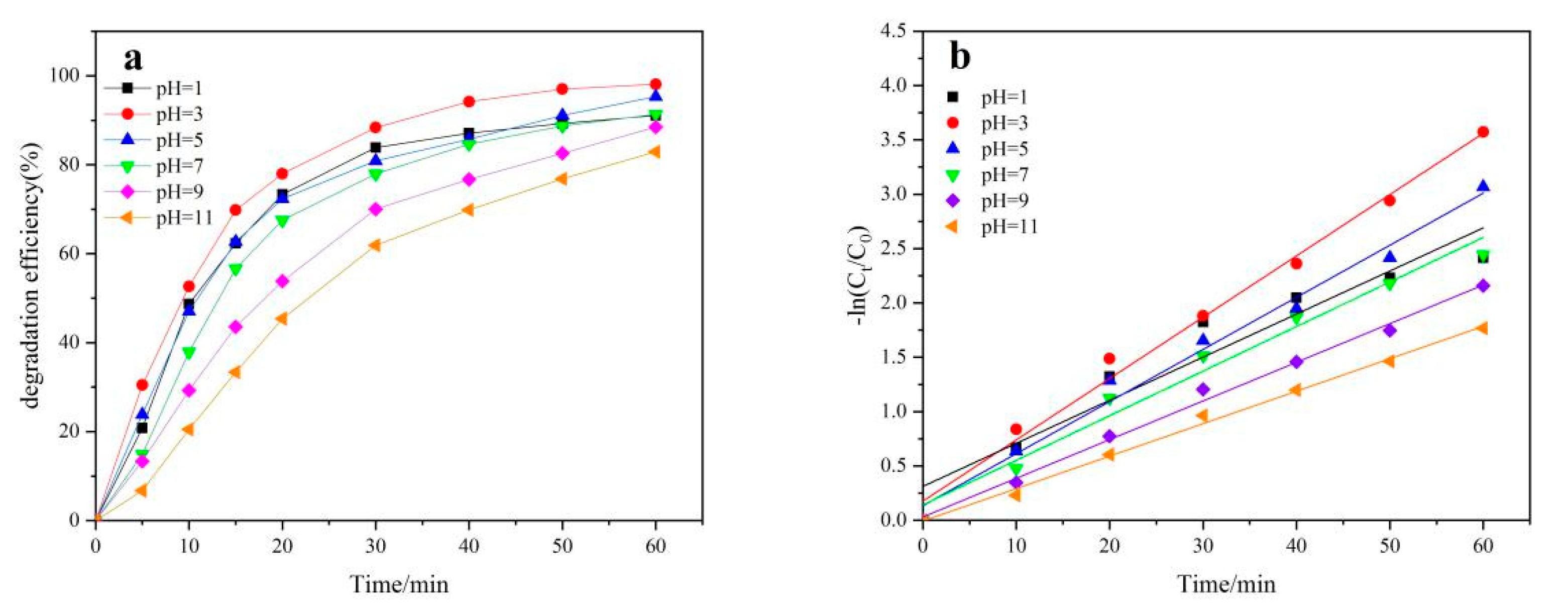
2.3. Effect of pH
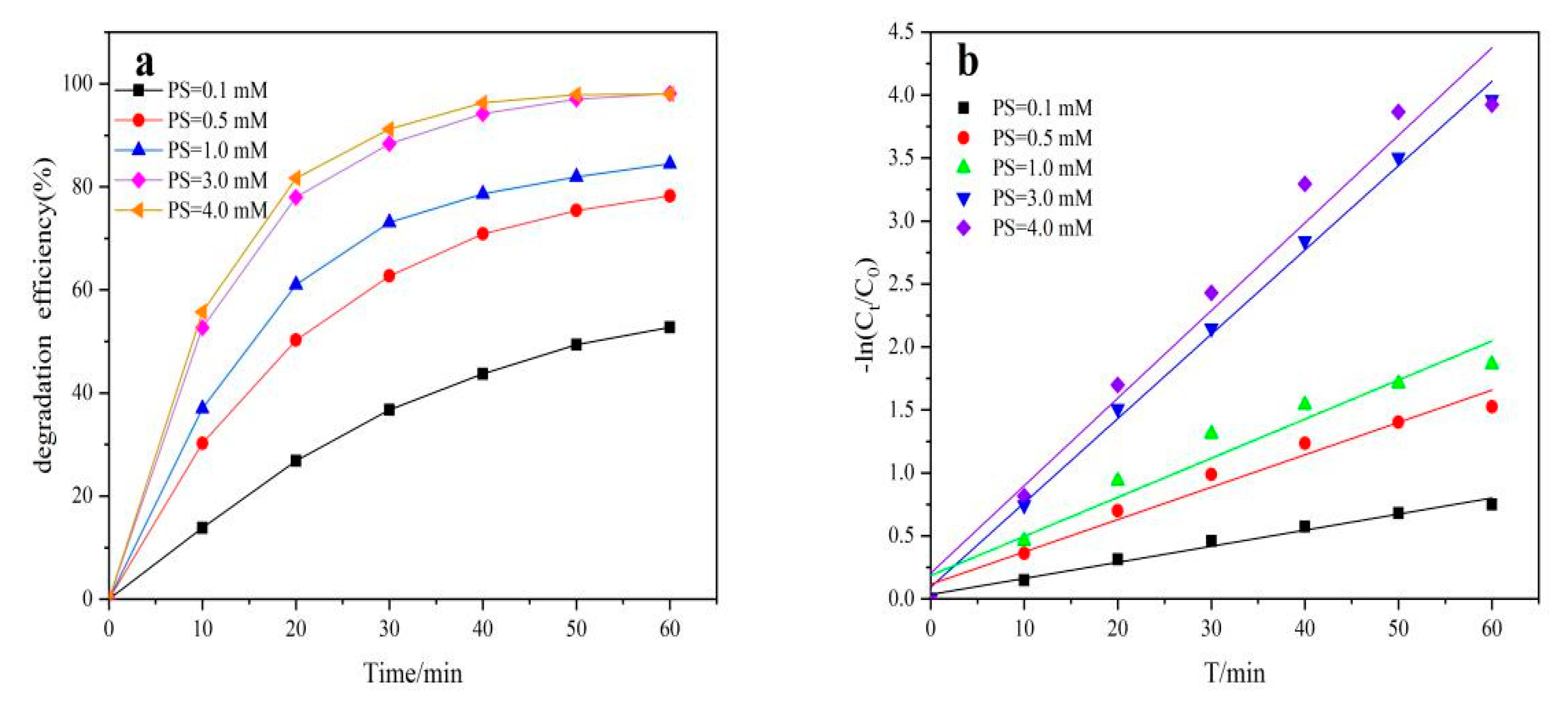
2.4. Effect of the PS Concentration
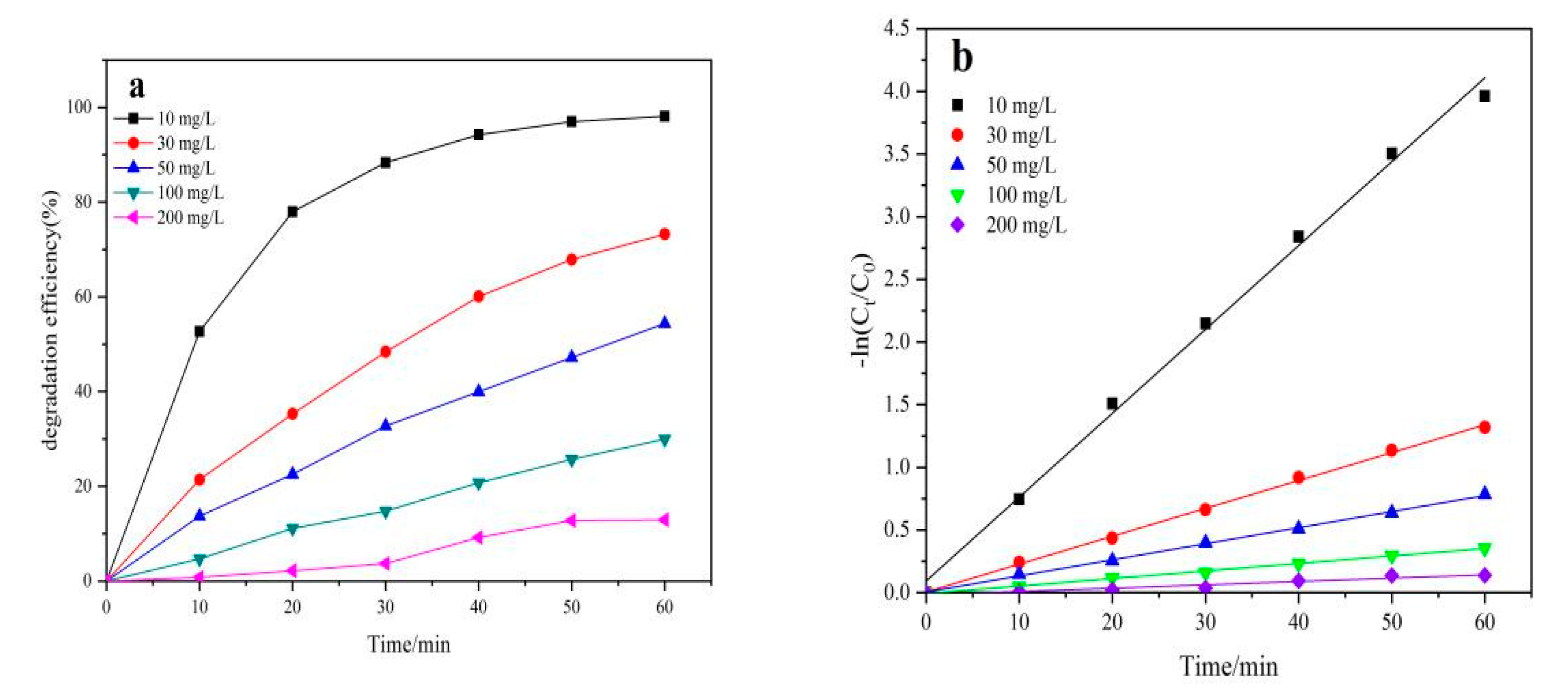
2.5. Effect of Initial NOR Concentration
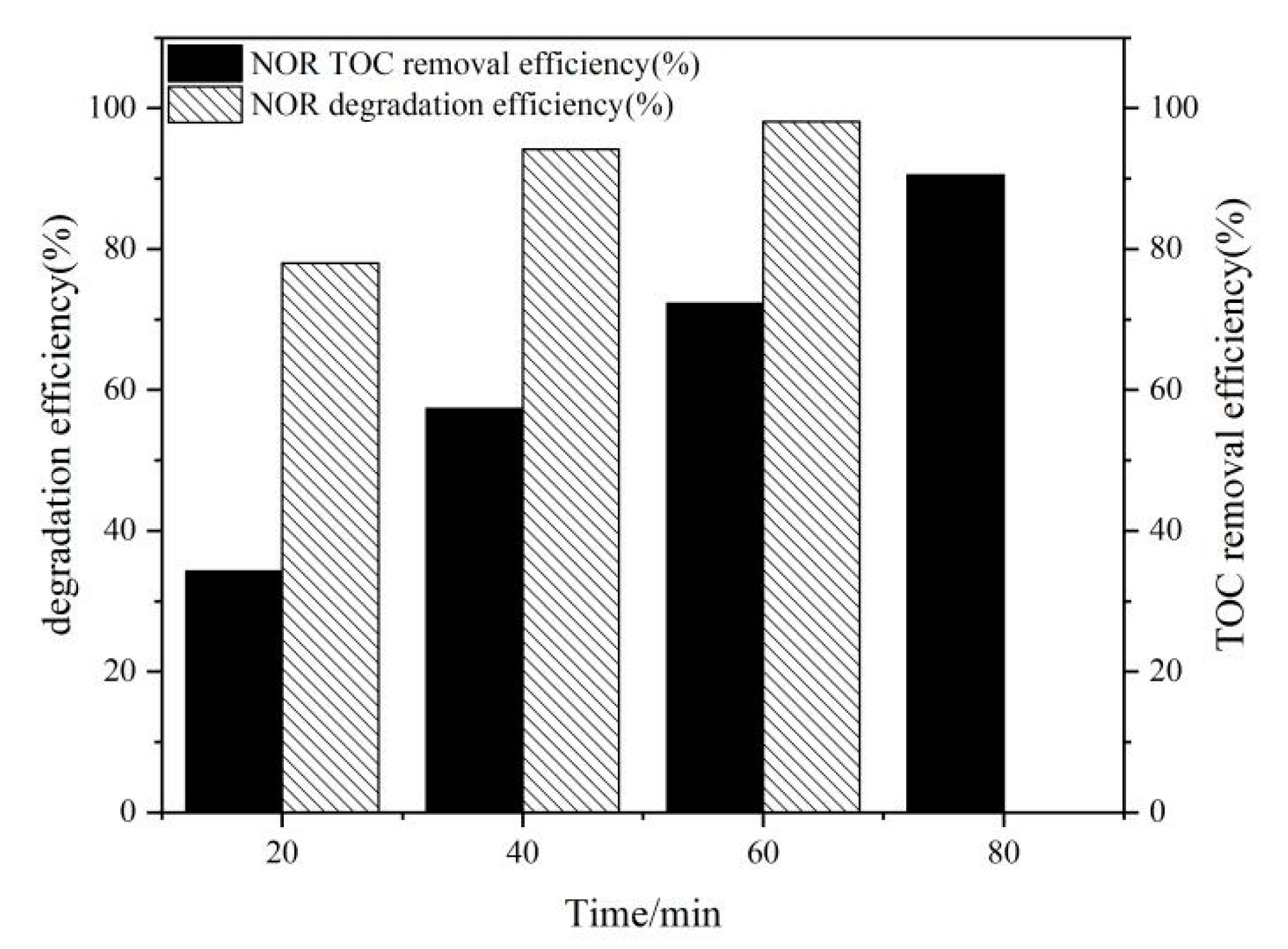
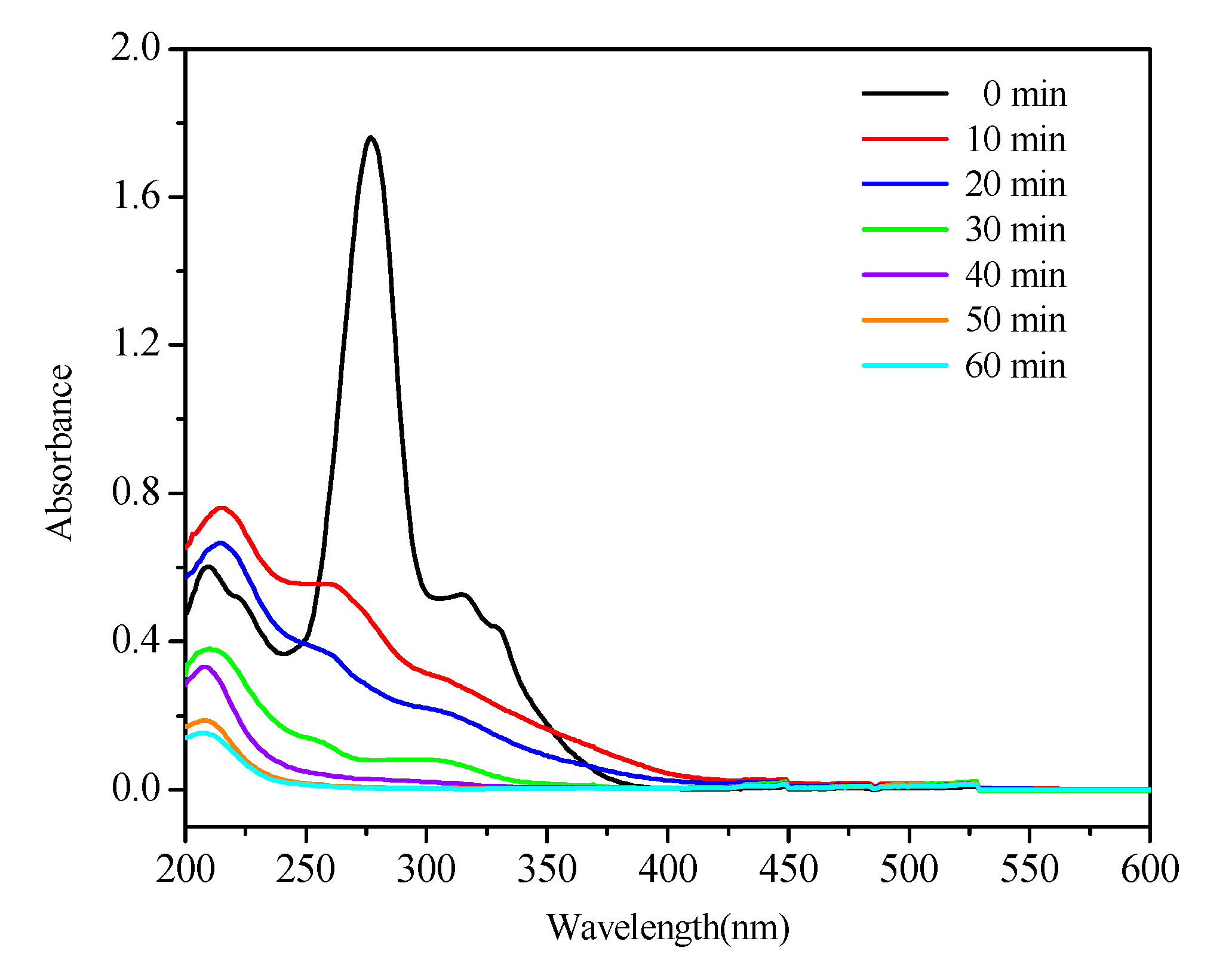
2.6. The Mineralization of NOR in PFC/PS System
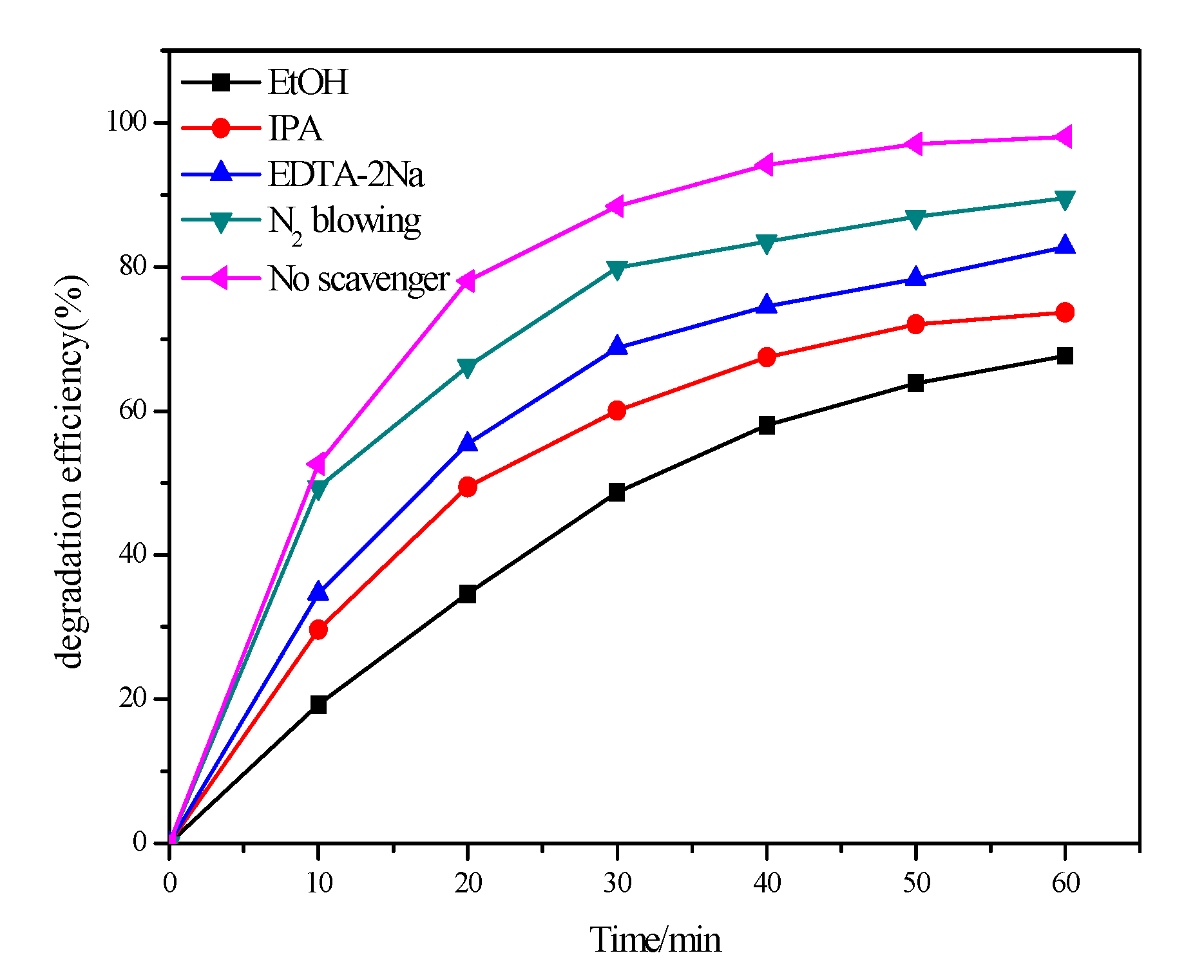
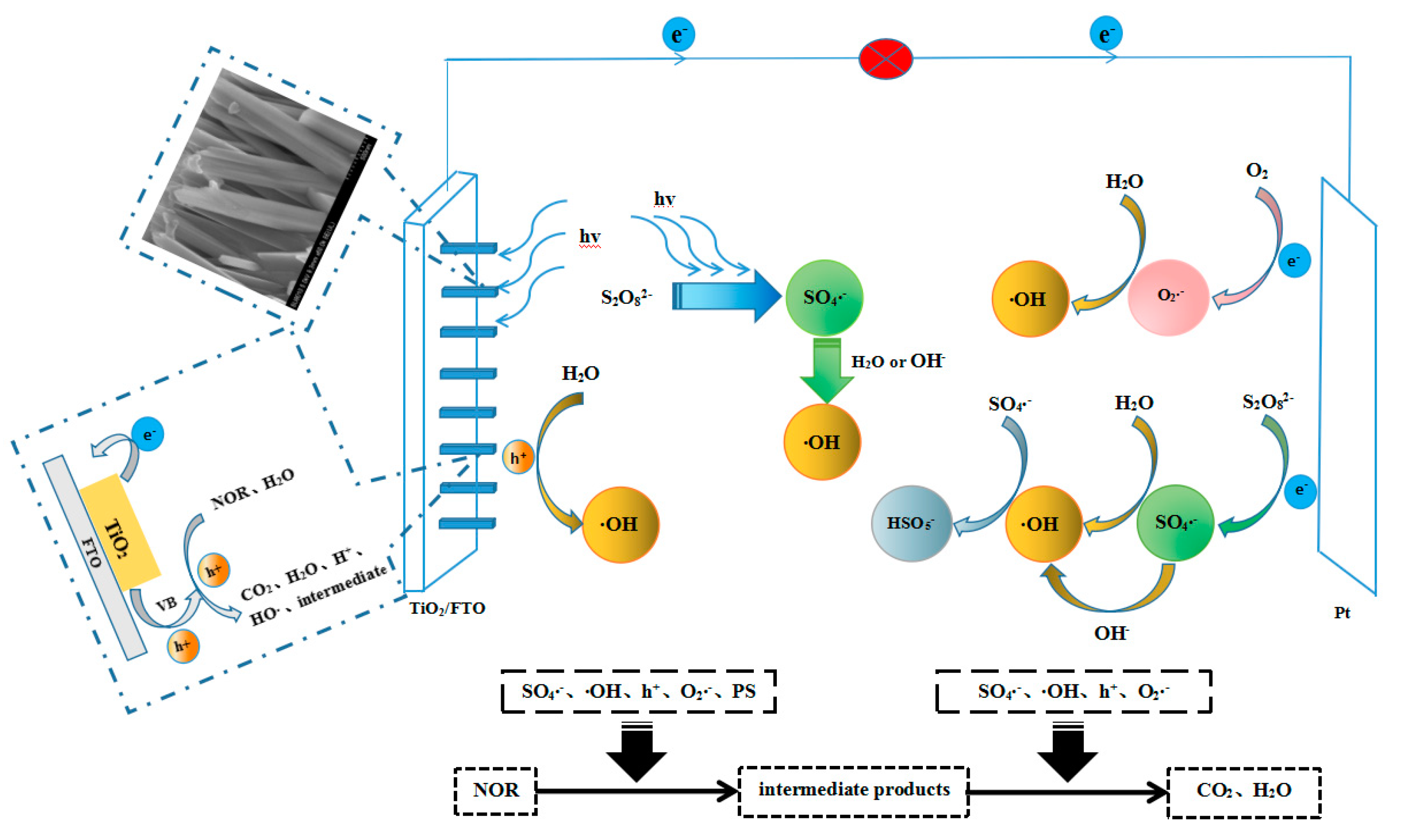
2.7. Roles of Different Reactive Radical Species in PFC/PS System
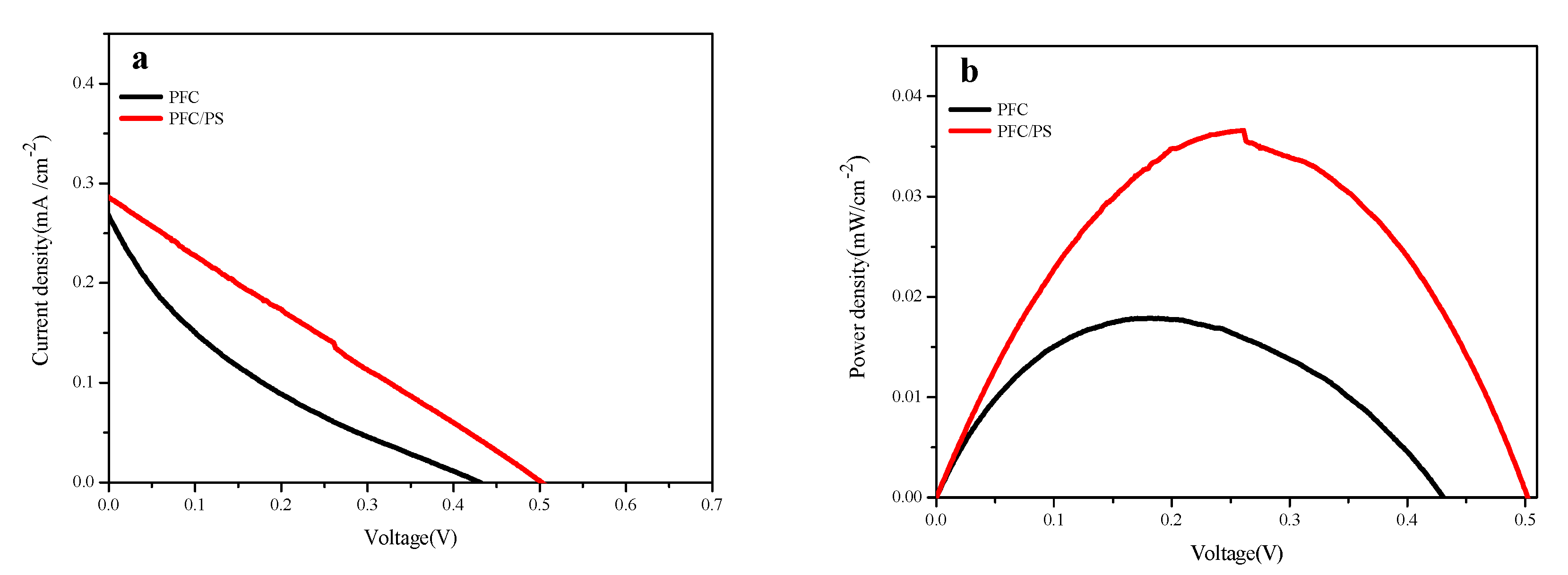
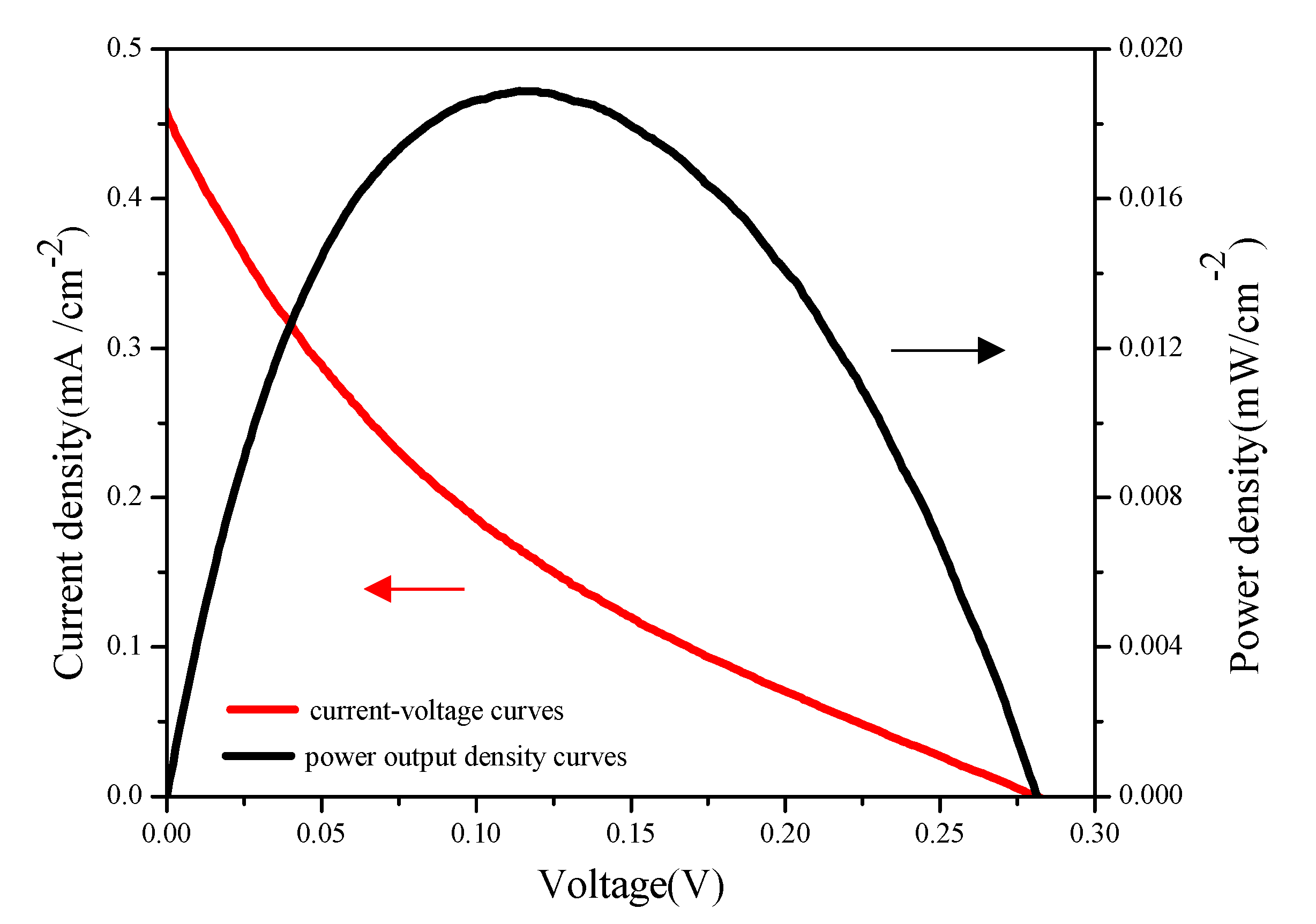
2.8. NOR Degradation and Simultaneous Electricity Generation in Actual Sample
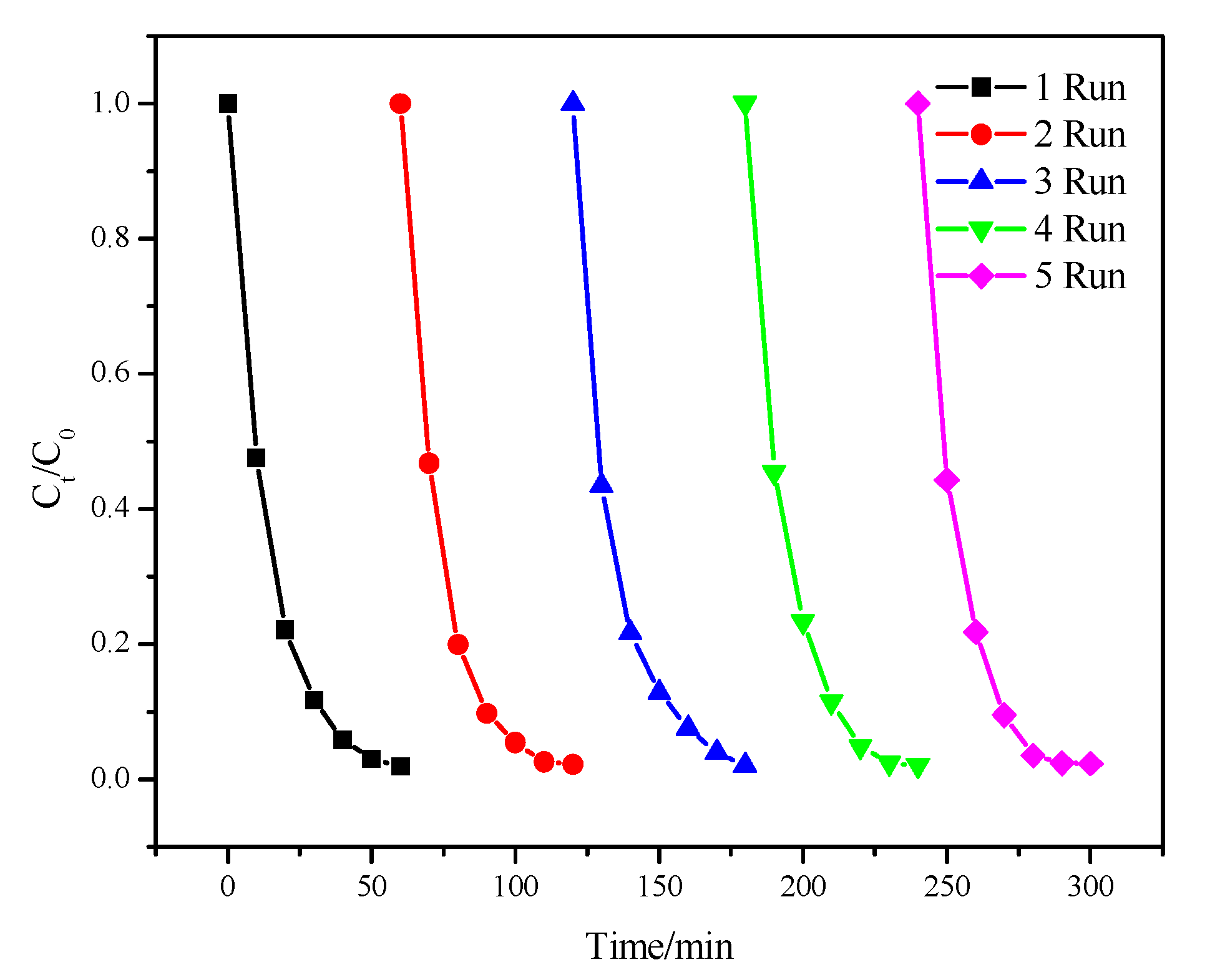
2.9. The Stability of PFC/PS System
3. Experimental
3.1. Materials
3.2. Preparation of TiO2 Nanorods
3.3. Characterization of TiO2 Nanorods
3.4. NOR Degradation Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, A.Y.; Wang, W.; Chen, J.J.; Liu, C.; Zhang, X.; Li, W.W.; Si, Y.; Yu, H.Q. Epitaxial facet junction on TiO2 single crystals for efficient photocatalytic water splitting. Energy Environ. Sci. 2018, 11, 1444–1448. [Google Scholar] [CrossRef]
- Taraka, T.P.Y.; Gautam, A.; Jain, S.L.; Bojja, S.; Pal, U. Controlled addition of Cu/Zn in hierarchical CuO/ZnO p-n heterojunction photocatalyst for high photoreduction of CO2 to MeOH. J. CO2 Util. 2019, 31, 207–214. [Google Scholar] [CrossRef]
- Cardoso, J.; Stulp, S.; De Brito, J.; Flor, J.; Frem, R.; Zanoni, M. MOFs based on ZIF-8 deposited on TiO2 nanotubes increase the surface adsorption of CO2 and its photoelectrocatalytic reduction to alcohols in aqueous media. Appl. Catal. B Environ. 2018, 225, 563–573. [Google Scholar] [CrossRef]
- Kaneco, S.; Shimizu, Y.; Ohta, K.; Mizuno, T. Photocatalytic reduction of high pressure carbon dioxide using TiO2 powders with a positive hole scavenger. J. Photochem. Photobiol. A Chem. 1998, 115, 223–226. [Google Scholar] [CrossRef]
- Urbain, F.; Murcia-López, S.; Nembhard, N.; Vázquez-Galván, J.; Flox, C.; Smirnov, V.; Welter, K.; Andreu, T.; Finger, F.; Morante, J.R. Solar vanadium redox-flow battery powered by thin-film silicon photovoltaics for efficient photoelectrochemical energy storage. J. Phys. D Appl. Phys. 2019, 52, 044001. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Curtis, T.P.; Dolfing, J.; Curtis, T. Determination of the Internal Chemical Energy of Wastewater. Environ. Sci. Technol. 2011, 45, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Yang, Q.; Chen, H.; Chen, X.; Jiao, T.; Peng, Q. Distinguished Cr(VI) capture with rapid and superior capability using polydopamine microsphere: Behavior and mechanism. J. Hazard. Mater. 2018, 342, 732–740. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Zhou, A.; Wang, A.; Wu, Z.; Lv, L.; Li, X.; Zhang, K.; Zhu, T. Removal of azo dye in an up-flow membrane-less bioelectrochemical system integrated with bio-contact oxidation reactor. Chem. Eng. J. 2017, 326, 454–461. [Google Scholar] [CrossRef]
- Ter Heijne, A.; Strik, D.P.B.T.B.; Hamelers, H.V.M.; Buisman, C.J.N. Cathode Potential and Mass Transfer Determine Performance of Oxygen Reducing Biocathodes in Microbial Fuel Cells. Environ. Sci. Technol. 2010, 44, 7151–7156. [Google Scholar] [CrossRef]
- Xia, L.; Bai, J.; Li, J.; Zeng, Q.; Li, X.; Zhou, B. A highly efficient BiVO 4 /WO 3 /W heterojunction photoanode for visible-light responsive dual photoelectrode photocatalytic fuel cell. Appl. Catal. B Environ. 2016, 183, 224–230. [Google Scholar] [CrossRef]
- Kan, L.; Yunlan, X.; Yi, H.; Chen, Y.; Yalin, W.; Jinping, J. Photocatalytic fuel cell (PFC) and dye self-photosensitization photocatalytic fuel cell (DSPFC) with BiOCl/Ti photoanode under UV and visible light irradiation. Environ. Sci. Technol. 2013, 47, 3490–3497. [Google Scholar]
- Wang, B.; Zhang, H.; Lu, X.-Y.; Xuan, J.; Leung, M.K. Solar photocatalytic fuel cell using CdS–TiO2 photoanode and air-breathing cathode for wastewater treatment and simultaneous electricity production. Chem. Eng. J. 2014, 253, 174–182. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Chen, Q.; Bai, J.; Zhou, B. Converting hazardous organics into clean energy using a solar responsive dual photoelectrode photocatalytic fuel cell. J. Hazard. Mater. 2013, 262, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wu, Z.; Zhao, G.; Zhang, Y.; Liu, J. A solar-driven photocatalytic fuel cell with dual photoelectrode for simultaneous wastewater treatment and hydrogen production. J. Mater. Chem. A 2015, 3, 3416–3424. [Google Scholar]
- Chen, Q.; Li, J.; Li, X.; Huang, K.; Zhou, B.; Cai, W.; Shangguan, W. Visible-Light Responsive Photocatalytic Fuel Cell Based on WO3/W Photoanode and Cu2O/Cu Photocathode for Simultaneous Wastewater Treatment and Electricity Generation. Environ. Sci. Technol. 2012, 46, 11451–11458. [Google Scholar] [CrossRef] [PubMed]
- Zarei, E.; Ojani, R. Fundamentals and some applications of photoelectrocatalysis and effective factors on its efficiency: A review. J. Solid State Electrochem. 2017, 12, 305–336. [Google Scholar] [CrossRef]
- Zhao, K.; Zeng, Q.; Bai, J.; Li, J.; Xia, L.; Chen, S.; Zhou, B. Enhanced organic pollutants degradation and electricity production simultaneously via strengthening the radicals reaction in a novel Fenton-photocatalytic fuel cell system. Water Res. 2017, 108, 293–300. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.-H.; Park, N.B. Chemistry of persulfates for the oxidation of organic contaminants in water. Membr. Water Treat. 2018, 9, 405–419. [Google Scholar]
- Yang, S.; Wang, P.; Yang, X.; Wei, G.; Zhang, W.; Shan, L. A novel advanced oxidation process to degrade organic pollutants in wastewater: Microwave-activated persulfate oxidation. J. Environ. Sci. 2009, 21, 1175–1180. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, D.; Rao, Y.; Na, L.; Qi, J.; Cheng, T.; Sun, Z.; Gu, J.; Huang, H. Persulfate activation in gas phase surface discharge plasma for synergetic removal of antibiotic in water. Chem. Eng. J. 2017, 337, 446–454. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, N.; Guo, W.; Wang, Y.; Huang, X.; Wu, P.; Dang, Z.; Zhang, X.; Xian, J. Simultaneous electricity production and antibiotics removal by microbial fuel cells. J. Environ. Manag. 2018, 217, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-H.; Ying, G.-G.; Su, H.-C.; Stauber, J.L.; Adams, M.S.; Binet, M.T. Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata. Environ. Toxicol. Chem. 2008, 27, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, M.; Qiu, Z.-G.; Guo, C.; Chen, Z.-L.; Shen, Z.-Q.; Wang, X.-W.; Li, J.-W. A survey of drug resistance bla genes originating from synthetic plasmid vectors in six chinese rivers. Environ. Sci. Technol. 2012, 46, 13448–13454. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.; Shah, L.A.; Khan, J.A.; Shah, N.S.; Nisar, J.; Khan, H.M.; Zhang, P.; Khan, A.R. Efficient photocatalytic degradation of norfloxacin in aqueous media by hydrothermally synthesized immobilized TiO2/Ti films with exposed {001} facets. J. Phys. Chem. A 2016, 120, 9916–9931. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ding, D.; Liu, C.; Cai, H.; Qu, Y.; Yang, S.; Gao, Y.; Cai, T. Degradation of norfloxacin by CoFe2O4-GO composite coupled with peroxymonosulfate: A comparative study and mechanistic consideration. Chem. Eng. J. 2018, 334, 273–284. [Google Scholar] [CrossRef]
- Kim, K.J.; Benkstein, K.D.; Lagemaat, J.V.D.; Frank, A.J. Characteristics of low-temperature annealed TiO2 films deposited by precipitation from hydrolyzed TiCl4 solutions. Chem. Mater. 2002, 14, 1042–1047. [Google Scholar] [CrossRef]
- Park, N.G.; Lagemaat, J.V.D.; Frank, A.J. Comparison of Dye-Sensitized Rutile- and Anatase-Based TiO2 Solar Cells. J. Phys. Chem. B 2000, 104, 8989–8994. [Google Scholar] [CrossRef]
- Ohno, T.; Tokieda, K.; Higashida, S.; Matsumura, M. Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal. A Gen. 2003, 224, 383–391. [Google Scholar] [CrossRef]
- Chenju, L.; Guo, Y. Mass transfer and chemical oxidation of naphthalene particles with zerovalent iron activated persulfate. Environ. Sci. Technol. 2010, 44, 8203–8208. [Google Scholar]
- Khalik, W.F.; Ho, L.-N.; Ong, S.-A.; Voon, C.-H.; Wong, Y.-S.; Yusoff, N.; Lee, S.-L.; Yusuf, S.Y. Optimization of degradation of Reactive Black 5 (RB5) and electricity generation in solar photocatalytic fuel cell system. Chemosphere 2017, 184, 112–119. [Google Scholar] [CrossRef]
- Cai, T.; Liu, Y.; Wang, L.; Zhang, S.; Zeng, Y.; Yuan, J.; Ma, J.; Dong, W.; Liu, C.; Luo, S. Silver phosphate-based Z-Scheme photocatalytic system with superior sunlight photocatalytic activities and anti-photocorrosion performance. Appl. Catal. B Environ. 2017, 208, 1–13. [Google Scholar] [CrossRef]
- Yi, L.Q.; Yi, F.Z.; Hao, Y.Y.; Xu, C.S. Enhanced visible light photocatalytic activity of AgBr on {001} facets exposed to BiOCl. J. Alloys Compd. 2017, 712, 535–542. [Google Scholar]
- Wang, K.; Pang, J.; Li, L.; Zhou, S.; Li, Y.; Zhang, T. Synthesis of hydrophobic carbon nanotubes/reduced graphene oxide composite films by flash light irradiation. Front. Chem. Sci. Eng. 2018, 12, 376–382. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ke, J.; Wang, S.; Wang, L.; Xiao, H. Black NiO-TiO2 nanorods for solar photocatalysis: Recognition of electronic structure and reaction mechanism. Appl. Catal. B Environ. 2018, 224, 705–714. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Z.; Deng, Q.; Zhu, L.; Luo, S.; Li, H. Paired photoelectrocatalytic reactions of glucose driven by a photoelectrochemical fuel cell with assistance of methylene blue. Electrochim. Acta 2016, 210, 38–44. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, S.; Chai, B.; Cao, D.; Wang, Y.; Zhao, X. Enhanced Photoelectrocatalytic Decomplexation of Cu-EDTA and Cu Recovery by Persulfate Activated by UV and Cathodic Reduction. Environ. Sci. Technol. 2016, 50, 6459–6466. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Liu, J.; Sun, H.; Zhang, H.; Duan, X.; Liang, P.; Li, X.; Tade, M.O.; Liu, S.; Wang, S. Facile assembly of Bi2O3 Bi2S3 /MoS2 n-p heterojunction with layered n -Bi2O3 and p -MoS2 for enhanced photocatalytic water oxidation and pollutant degradation. Appl. Catal. B Environ. 2017, 200, 47–55. [Google Scholar] [CrossRef]
- Li, N.; Tang, S.; Rao, Y.; Qi, J.; Zhang, Q.; Yuan, D. Peroxymonosulfate enhanced antibiotic removal and synchronous electricity generation in a photocatalytic fuel cell. Electrochim. Acta 2019, 298, 59–69. [Google Scholar] [CrossRef]
- Li, N.; Tang, S.; Rao, Y.; Qi, J.; Wang, P.; Jiang, Y.; Huang, H.; Gu, J.; Yuan, D. Improved dye removal and simultaneous electricity production in a photocatalytic fuel cell coupling with persulfate activation. Electrochim. Acta 2018, 270, 330–338. [Google Scholar] [CrossRef]
- Lau, T.K.; Chu, W.; Graham, N.J.D. The Aqueous Degradation of Butylated Hydroxyanisole by UV/S2O82-: Study of Reaction Mechanisms via Dimerization and Mineralization. Environ. Sci. Technol. 2007, 41, 613–619. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, X.; Chen, H.; Dong, W.; Zhao, J. Photodegradation of 4-tert-butylphenol in aqueous solution by UV-C, UV/H2O2 and UV/S2O82− system. J. Environ. Sci. Health Part A 2016, 51, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Lai, M.-C. Trichloroethylene Degradation by Zero Valent Iron Activated Persulfate Oxidation. Environ. Eng. Sci. 2008, 25, 1071–1078. [Google Scholar] [CrossRef]
- Liang, C.; Bruell, C.J.; Albert, M.F.; Paul, E.; Ryan, D.K. Evaluation of reverse osmosis and nanofiltration for in situ persulfate remediated groundwater. Desalination 2007, 208, 238–259. [Google Scholar] [CrossRef]
- Kermani, M.; Mohammadi, F.; Kakavandi, B.; Esrafili, A.; Rostamifasih, Z. Simultaneous catalytic degradation of 2,4-D and MCPA herbicides using sulfate radical-based heterogeneous oxidation over persulfate activated by natural hematite (α-Fe2O3/PS). J. Phys. Chem. Solids 2018, 117, 49–59. [Google Scholar] [CrossRef]
- Weng, C.H.; Tsai, K.L. Ultrasound and heat enhanced persulfate oxidation activated with Fe0 aggregate for the decolorization of C.I. Direct Red 23. Ultrason. Sonochem. 2016, 29, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Shi, Y.; Liu, Y.; Chen, F.; Wang, H.; Wu, X.-L.; Lin, H.; Chen, J. Enhanced catalytic degradation of bisphenol A by hemin-MOFs supported on boron nitride via the photo-assisted heterogeneous activation of persulfate. Sep. Purif. Technol. 2019, 229, 115822. [Google Scholar] [CrossRef]
- Jiang, Y.R.; Chou, S.Y.; Chang, J.L.; Huang, S.T.; Lin, H.P.; Chen, C.C. Hydrothermal synthesis of bismuth oxybromide-bismuth oxyiodide composites with highly visible light photocatalytic performance for the degradation of CV and phenol. Rsc Adv. 2015, 5, 30851–30860. [Google Scholar] [CrossRef]
- Song, W.; Ge, P.; Ke, Q.; Sun, Y.; Chen, F.; Wang, H.; Shi, Y.; Wu, X.-L.; Lin, H.; Chen, J.; et al. Insight into the mechanisms for hexavalent chromium reduction and sulfisoxazole degradation catalyzed by graphitic carbon nitride: The Yin and Yang in the photo-assisted processes. Chemosphere 2019, 221, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Y.; Yang, L.; Shang, Q.; Wang, D.; Guo, T.; Guo, Y. Novel photocatalytic system Fe-complex/TiO2 for efficient degradation of phenol and norfloxacin in water. Sci. Total Environ. 2019, 656, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A.; Tuqan, A.M.; Kibbi, N. Naproxen abatement by thermally activated persulfate in aqueous systems. Chem. Eng. J. 2015, 279, 861–873. [Google Scholar] [CrossRef]
- Tang, S.; Xue, L.I.; Zhang, C.; Liu, Y.; Zhang, W.; Yuan, D. Strengthening decomposition of oxytetracycline in DBD plasma coupling with Fe-Mn oxide-loaded granular activated carbon. Engl. Ed. Plasma Sci. Technol. 2019, 21, 86–92. [Google Scholar] [CrossRef]
- Ding, D.; Liu, C.; Ji, Y.; Yang, Q.; Chen, L.; Jiang, C.; Cai, T. Mechanism insight of degradation of norfloxacin by magnetite nanoparticles activated persulfate: Identification of radicals and degradation pathway. Chem. Eng. J. 2017, 308, 330–339. [Google Scholar] [CrossRef]
- Bin, L.; Aydil, E.S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar]
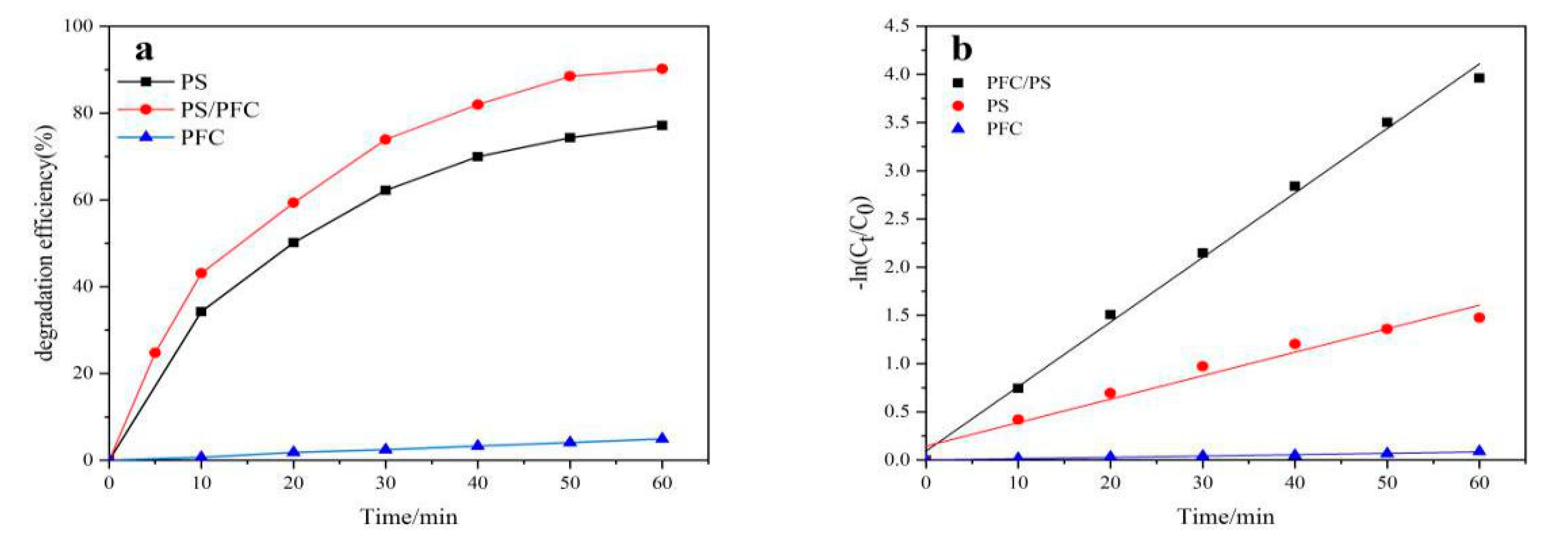
| Process | Apparent Rate Constant, k (min−1) | R2 |
|---|---|---|
| Photocatalytic fuel cell (PFC) | 1.40 × 10−3 | 0.9821 |
| Persulfate (PS) | 2.44 × 10−2 | 0.9654 |
| PFC/PS | 6.69 × 10−2 | 0.9962 |
| Initial pH value | Apparent Rate Constant, k (min−1) | R2 |
|---|---|---|
| pH = 1 | 3.96 × 10−2 | 0.9259 |
| pH = 3 | 5.64 × 10−2 | 0.9908 |
| pH = 5 | 4.79 × 10−2 | 0.9863 |
| pH = 7 | 4.10 × 10−2 | 0.9782 |
| pH = 9 | 3.56 × 10−2 | 0.9947 |
| pH = 11 | 2.99 × 10−2 | 0.9957 |
| PS Concentration (mM) | Apparent Rate Constant, k (min−1) | R2 |
|---|---|---|
| 0.1 | 1.28 × 10−2 | 0.9850 |
| 0.5 | 2.57 × 10−2 | 0.9716 |
| 1.0 | 3.10 × 10−2 | 0.9509 |
| 3.0 | 6.69 × 10−2 | 0.9962 |
| 4.0 | 6.95 × 10−2 | 0.9703 |
| Process, CNOR/(mg/L) | Apparent Rate Constant, k(min−1) | R2 |
|---|---|---|
| 10 | 6.69 × 10−2 | 0.9962 |
| 30 | 2.22 × 10−2 | 0.9986 |
| 50 | 1.28 × 10−2 | 0.9988 |
| 100 | 6.00 × 10−3 | 0.9971 |
| 200 | 2.70 × 10−3 | 0.9243 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, R.; Zou, L.; Liu, X. Efficient Degradation of Norfloxacin and Simultaneous Electricity Generation in a Persulfate-Photocatalytic Fuel Cell System. Catalysts 2019, 9, 835. https://doi.org/10.3390/catal9100835
Li J, Li R, Zou L, Liu X. Efficient Degradation of Norfloxacin and Simultaneous Electricity Generation in a Persulfate-Photocatalytic Fuel Cell System. Catalysts. 2019; 9(10):835. https://doi.org/10.3390/catal9100835
Chicago/Turabian StyleLi, Jiawen, Ruizhen Li, Luomei Zou, and Xingyong Liu. 2019. "Efficient Degradation of Norfloxacin and Simultaneous Electricity Generation in a Persulfate-Photocatalytic Fuel Cell System" Catalysts 9, no. 10: 835. https://doi.org/10.3390/catal9100835
APA StyleLi, J., Li, R., Zou, L., & Liu, X. (2019). Efficient Degradation of Norfloxacin and Simultaneous Electricity Generation in a Persulfate-Photocatalytic Fuel Cell System. Catalysts, 9(10), 835. https://doi.org/10.3390/catal9100835






