Production of 5-Hydroxymethylfurfural from Glucose in Water by Using Transition Metal-Oxide Nanosheet Aggregates
Abstract
1. Introduction
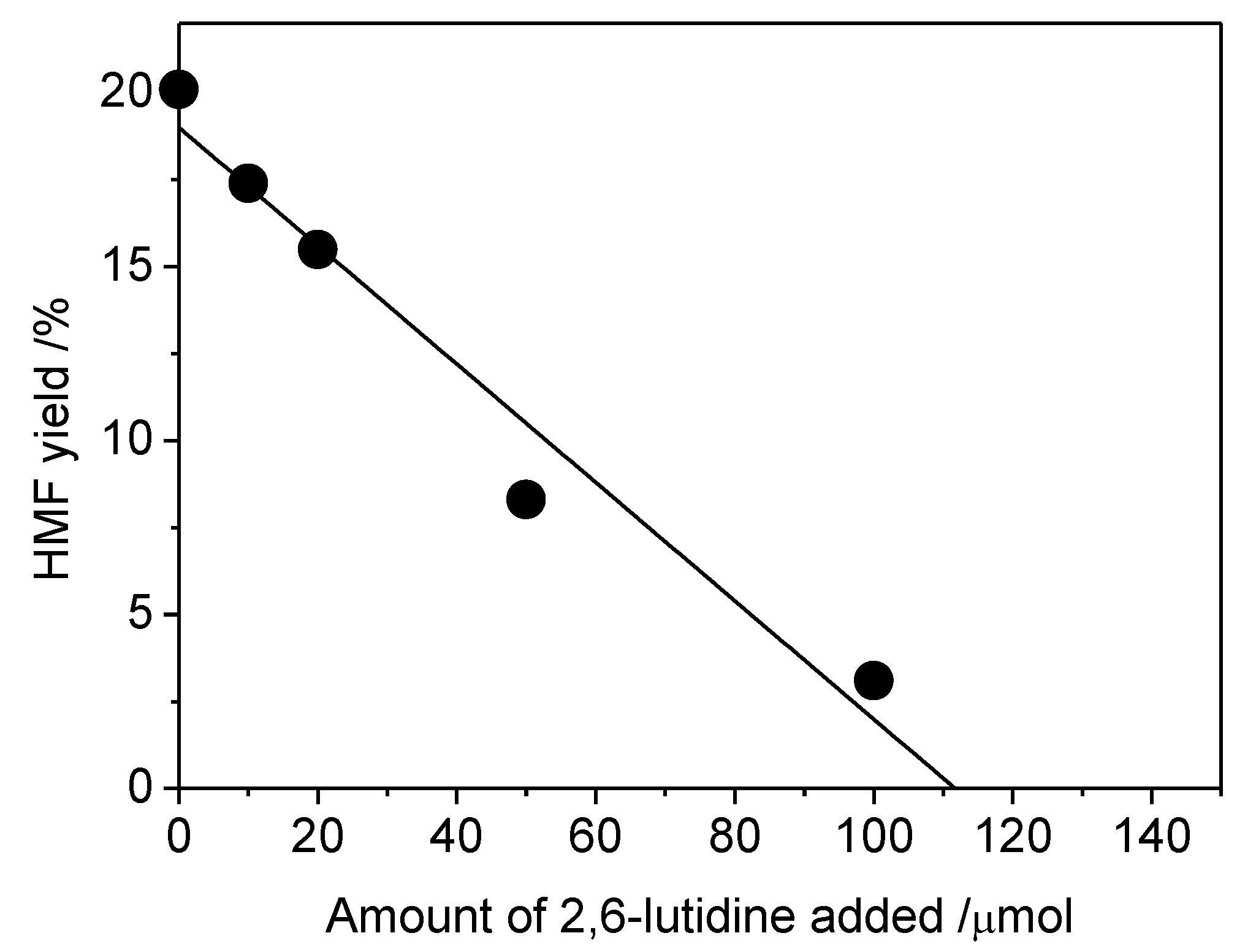
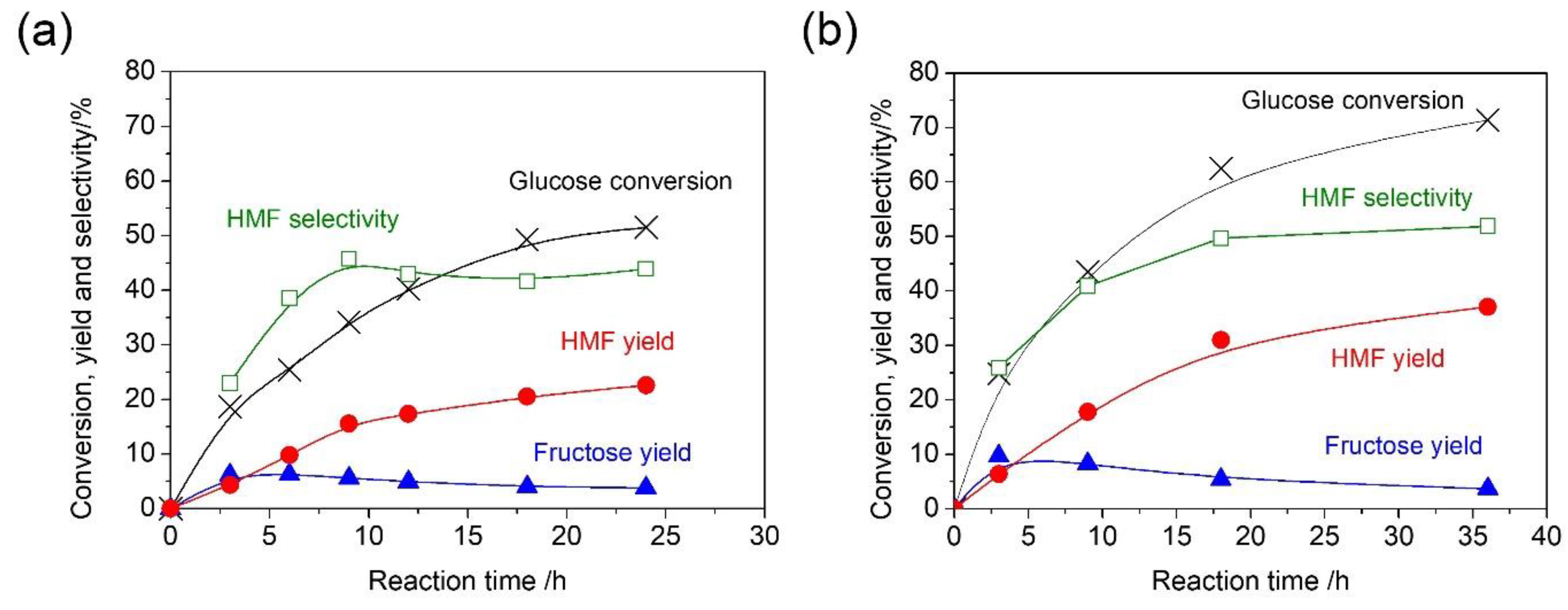
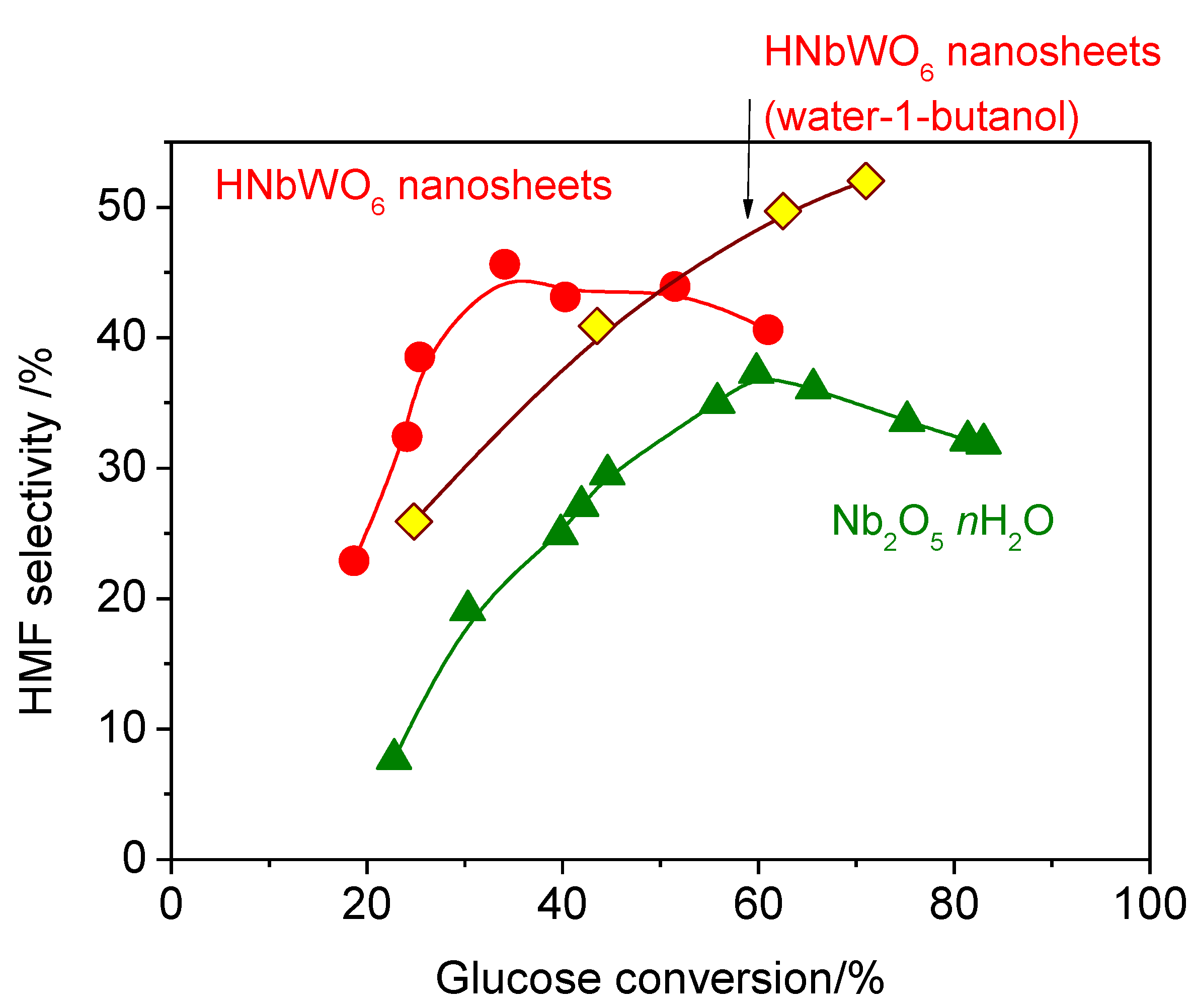
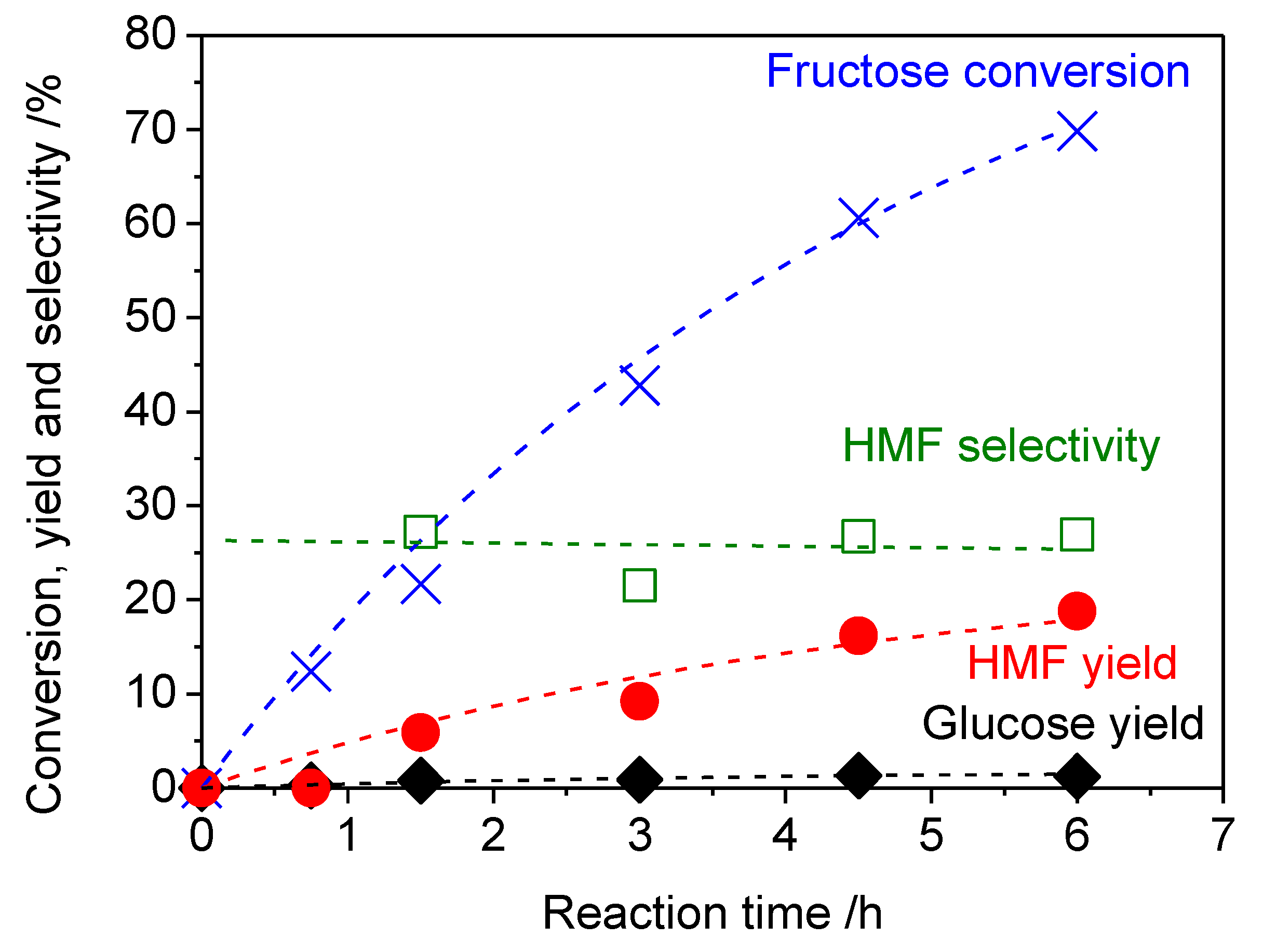
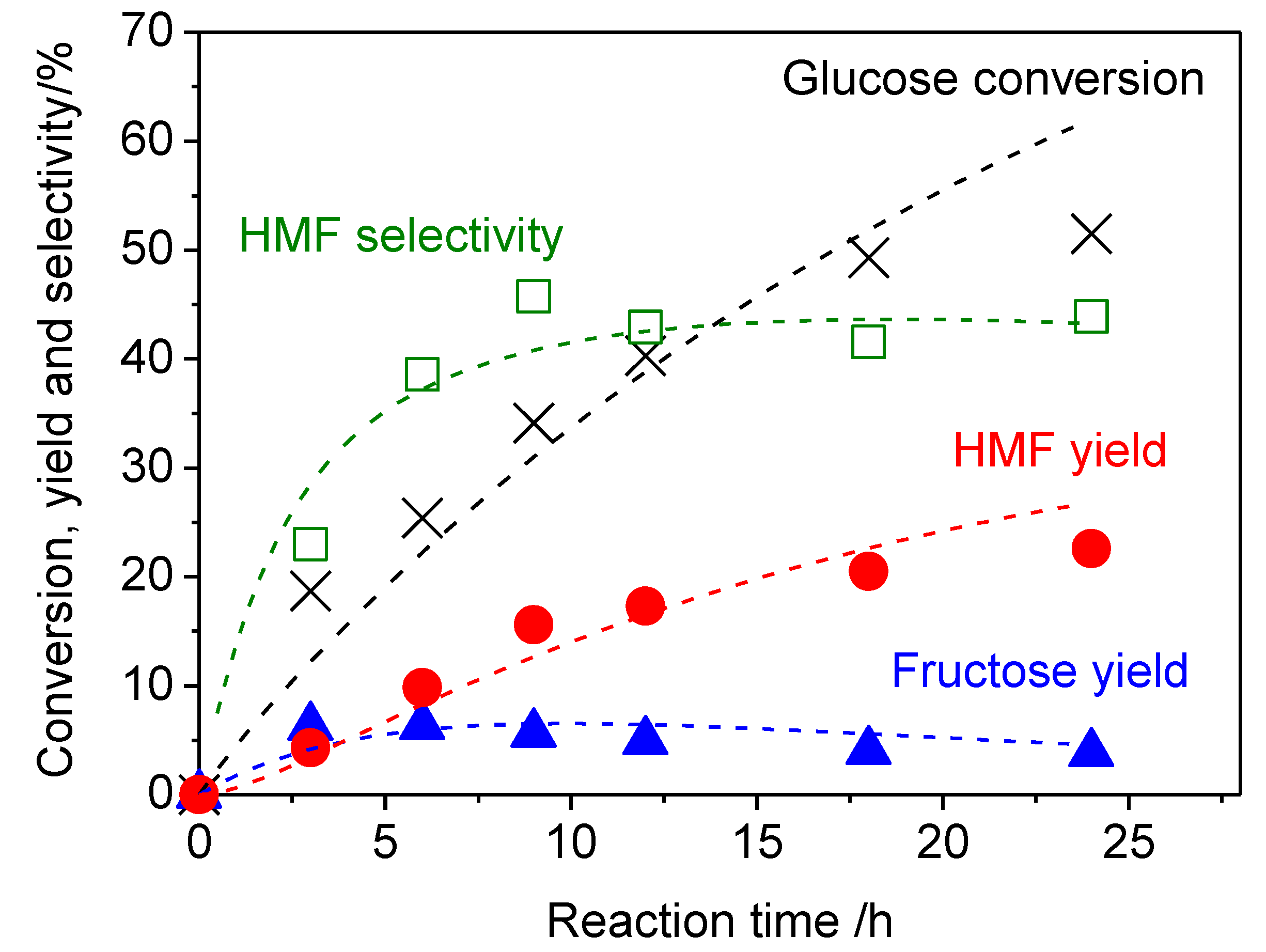
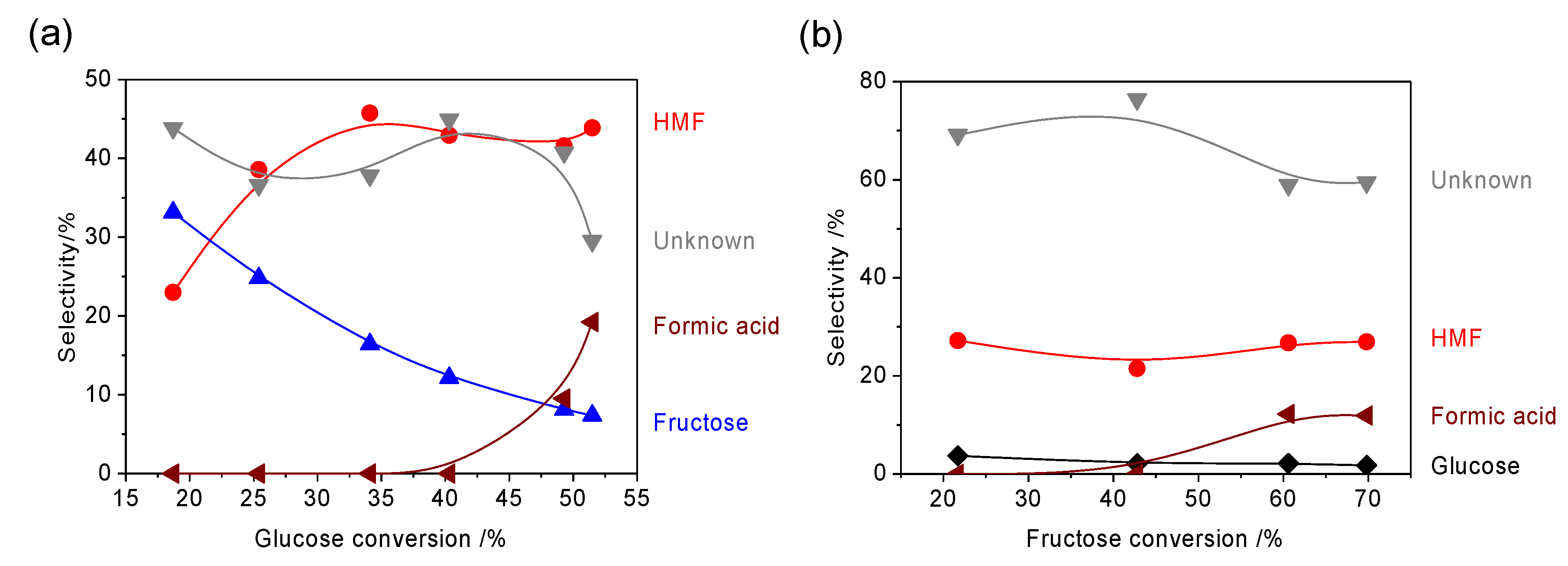
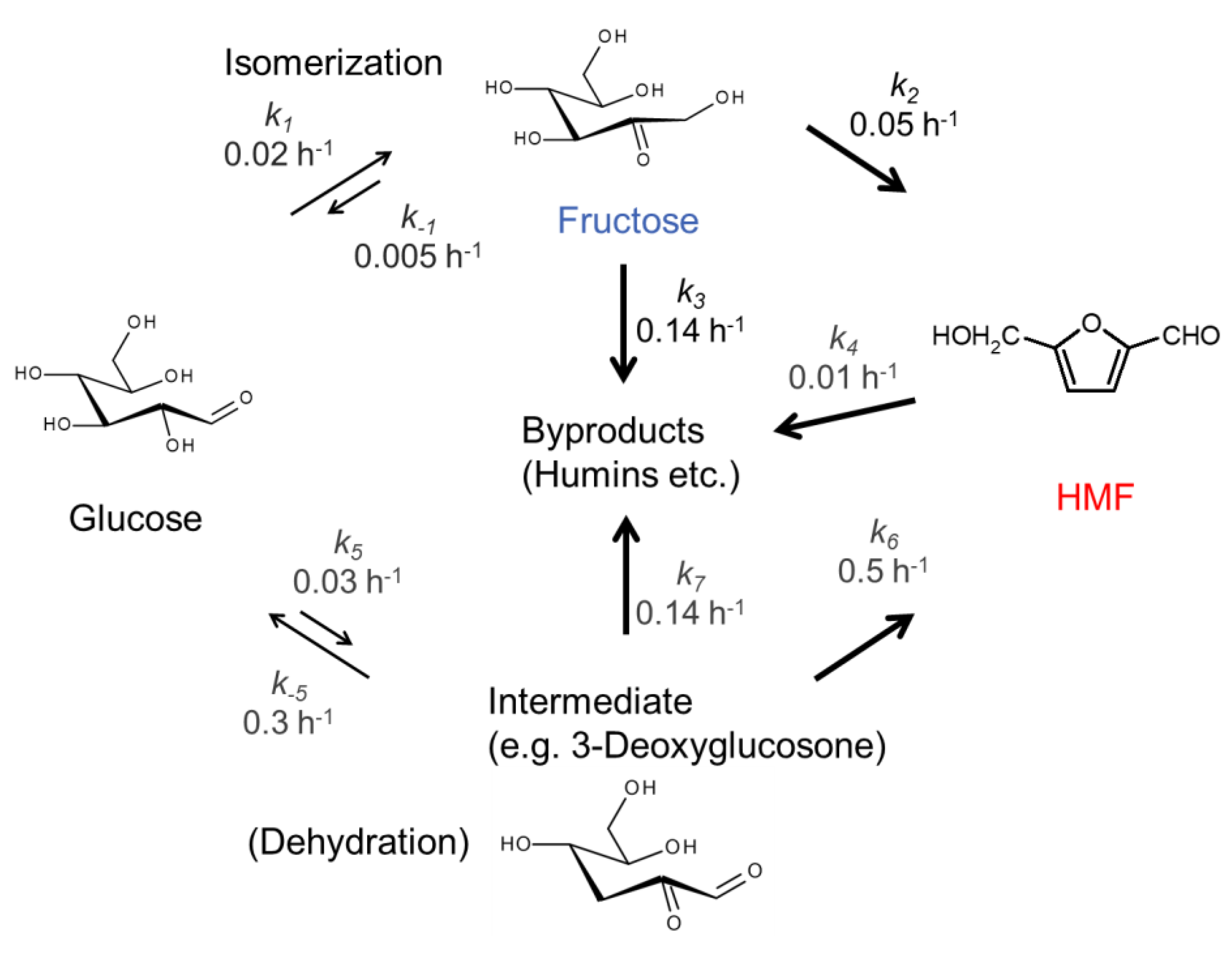
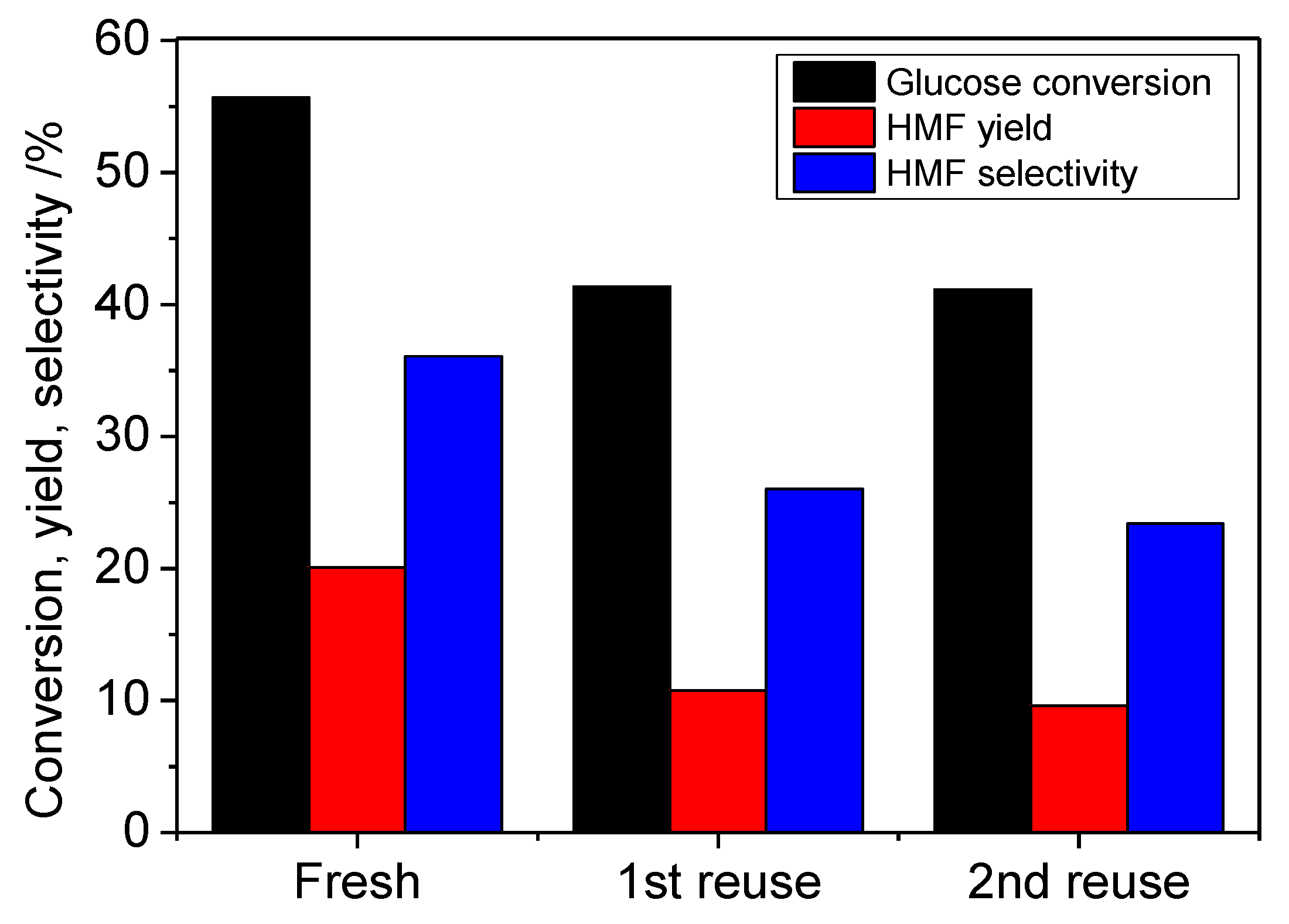
2. Results and Discussion
3. Experimental
3.1. Chemicals
3.2. Catalyst Synthesis
3.3. Characterization
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Takagaki, A.; Takahashi, M.; Nishimura, S.; Ebitani, K. One-Pot Synthesis of 2,5-Diformylfuran from Carbohydrate Derivatives by Sulfonated Resin and Hydrotalcite-Supported Ruthenium Catalysts. ACS Catal. 2011, 1, 1562–1565. [Google Scholar] [CrossRef]
- Gupta, N.K.; Nishimura, S.; Takagaki, K.; Ebitani, K. Hydrotalcite supported gold-nanoparticle-catalyzed highly efficient aqueous oxidation of 5-hydroxymethyl-2-furfural into 2,5-furandicarboxylic acid using molecular oxygen. Green Chem. 2011, 13, 824–827. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Tian, Z.; Weng, Y.; Wang, C.; Ma, J.; Zhu, C.; Li, W.; Liu, Q.; Ma, L. Selective oxidation of 5-hydroxymethylfurfural to 2,5-furanedicarboxylic acid over Au/CeO2 catalysts: The morphology effect of CeO2. Catal. Sci. Technol. 2019, 9, 1570–1580. [Google Scholar] [CrossRef]
- Hayashi, E.; Yamaguchi, Y.; Kamata, K.; Tsunoda, N.; Kumagai, Y.; Oba, F.; Hara, M. Effect of MnO2 Crystal Structure on Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic acid. J. Am. Chem. Soc. 2019, 141, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, P.; Chou, H.-J.; Lee, L.-C.; Chung, P.-W. Chemical Transformation for 5-Hydroxymethylfurfural Production from Saccharides Using Molten Salt System. ACS Sustain. Chem. Eng. 2018, 6, 5712–5717. [Google Scholar] [CrossRef]
- Takagaki, A.; Ohara, M.; Nishimura, S.; Ebitani, K. A one-pot reaction for biorefinery: Combination of solid acid and base catalysts for direct production of 5-hydroxymethylfurfural from saccharides. Chem. Commun. 2009, 6276–6278. [Google Scholar] [CrossRef]
- Tuteja, J.; Nishimura, S.; Ebitani, K. One-Pot Synthesis of Furans from Various Saccharides Using a Combination of Solid Acid and Base Catalysts. Bull. Chem. Soc. Jpn. 2012, 85, 275–281. [Google Scholar] [CrossRef]
- Otomo, R.; Yokoi, T.; Kondo, J.N.; Tatsumi, T. Dealuminated Beta zeolite as effective bifunctional catalyst for direct transformation of glucose to 5-hydroxymethylfurfural. Appl. Catal. A Gen. 2014, 470, 318–326. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Sakurada, T.; Ogasawara, Y.; Mizuno, N. Tin-Tungsten Mixed Oxide as Efficient Heterogeneous Catalyst for Conversion of Saccharides to Furan Derivatives. Chem. Lett. 2011, 40, 542–543. [Google Scholar] [CrossRef]
- Huang, F.; Li, W.; Liu, Q.; Zhang, T.; An, S.; Li, D.; Zhu, X. Sulfonated tobacco stem carbon as efficient catalyst for dehydration of C6 carbohydrate to 5-hydrxymethylfurfural in γ-valerolactone/water. Fuel Process. Technol. 2018, 181, 294–303. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Hu, F.; Wu, Y.; Wang, H.; Chen, Y.; Yuan, H.; Gao, L.; Xiao, G. Highly efficient Cr/β zeolite catalyst for conversion of carbohydrates into 5-hydroxymethylfurfural: Characterization and performance. Fuel Process. Technol. 2019, 190, 38–46. [Google Scholar] [CrossRef]
- Wiesfeld, J.J.; Gaquere, R.; Hensen, E.J.M. Mesoporous Doped Tungsten Oxide for Glucose Dehydration to 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2019, 7, 7552–7562. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Wu, Y.; Song, X.; Gao, L.; Li, W.; Das, L.; Xiao, G. Efficient production of furfural from xylose and wheat straw by bifunctional chromium phosphate catalyst in biphasic systems. Fuel Process. Technol. 2018, 175, 90–96. [Google Scholar] [CrossRef]
- Jadhav, H.; Pedersen, C.M.; Sølling, T.; Bols, M. 3-Deoxy-glucosone in an intermediate in the formation of furfurals from D-glucose. ChemSusChem 2011, 4, 1049–1051. [Google Scholar] [CrossRef]
- Takagaki, A.; Sugisawa, M.; Lu, D.; Kondo, J.N.; Hara, M.; Domen, K.; Hayashi, S. Exfoliated Nanosheets as a New Strong Solid Acid Catalyst. J. Am. Chem. Soc. 2003, 125, 5479–5485. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Lu, D.; Kondo, J.N.; Hara, M.; Hayashi, S.; Domen, K. Exfoliated HNb3O8 Nanosheets as a Strong Protonic Solid Acid. Chem. Mater. 2005, 17, 2487–2489. [Google Scholar] [CrossRef]
- Tagusagawa, C.; Takagaki, A.; Hayashi, S.; Domen, K. Characterization of HNbWO6 and HTaWO6 Metal Oxide Nanosheet Aggregates as Solid Acid Catalysts. J. Phys. Chem. C 2009, 113, 7831–7837. [Google Scholar] [CrossRef]
- Takagaki, A.; Tagusagawa, C.; Hayashi, S.; Hara, M.; Domen, K. Nanosheets as highly active solid acid catalysts for green chemical syntheses. Energy Environ. Sci. 2010, 3, 82–93. [Google Scholar] [CrossRef]
- Dias, A.S.; Kima, S.; Carriazo, D.; Rives, V.; Pillinger, M.; Valente, A.A. Exfoliated titanate niobite and titanoniobate nanosheets as solid acid catalysts for the liquid-phase dehydration of D-xylose into furfural. J. Catal. 2006, 244, 230–237. [Google Scholar] [CrossRef]
- Wu, Q.; Yan, Y.; Zhang, Q.; Lu, J.; Yang, Z.; Zhang, Y.; Tang, Y. Catalytic Dehydration of Carbohydrates on in Situ Exfoliatable Layered Niobic Acid in an Aqueous System under Microwave Irradiation. ChemSusChem 2013, 6, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Nakato, T.; Okuhara, T. Water-tolerant solid acid catalysis of Cs2.5H0.5PW12O40 for hydrolysis of esters in the presence of excess water. Appl. Catal. A Gen. 1997, 165, 227–240. [Google Scholar] [CrossRef]
- Li, L.; Yoshinaga, T.; Okuahra, T. Water-tolerant catalysis by Mo-Zr mixed oxides calcined at high temperatures. Phys. Chem. Chem. Phys. 1999, 1, 4913–4918. [Google Scholar] [CrossRef]
- Zheng, A.; Liu, S.-B.; Deng, F. 31P NMR Chemical Shifts of Phosphorus Probes as Reliable and Practical Acidity Scales for Solid and Liquid Catalysts. Chem. Rev. 2017, 117, 12475–12531. [Google Scholar] [CrossRef] [PubMed]
- Okuhara, T. Water-Tolerant Solid Acid Catalysts. Chem. Rev. 2002, 102, 3641–3666. [Google Scholar] [CrossRef]
- Tagusagawa, C.; Takagaki, A.; Hayashi, S.; Domen, K. Evaluation of strong acid properties of layered HNbMoO6 and catalytic activity for Friedel-Crafts alkylation. Catal. Today 2009, 142, 267–271. [Google Scholar] [CrossRef]
- Kao, H.-M.; Yu, C.-Y.; Yeh, M.-C. Detection of the inhomogeneity of Brønsted acidity in H-mordenite and H-β zeolites: A comparative NMR study using trimethylphosphine and trimethylphosphine oxide as 31P NMR probes. Micropor. Mesopor. Mater. 2002, 53, 1–12. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, W.-H.; Huang, S.-J.; Wu, Y.-C.; Lee, H.-K.; Liu, S.-B. Discernment and Quantification of Internal and External Acid Sites on Zeolites. J. Phys. Chem. B 2002, 106, 14462–14469. [Google Scholar] [CrossRef]
- Nakajima, K.; Baba, Y.; Noma, R.; Kitano, M.; Kondo, J.N.; Hayashi, S.; Hara, M. Nb2O5·nH2O as a Heterogeneous Catalyst with Water-Tolerant Lewis Acid Sites. J. Am. Chem. Soc. 2011, 133, 4224–4227. [Google Scholar] [CrossRef]
- Takagaki, A.; Ebitani, K. Glucose to Value-added Chemicals: Anhydroglucose Formation by Selective Dehydration over Solid Acid Catalysts. Chem. Lett. 2009, 38, 650–651. [Google Scholar] [CrossRef]
- Takagaki, A.; Tagusagawa, C.; Domen, K. Glucose production from saccharides using layered transition metal oxide and exfoliated nanosheets as a water-tolerant solid acid catalyst. Chem. Commun. 2008, 5363–5365. [Google Scholar] [CrossRef] [PubMed]
- Tagusagawa, C.; Takagaki, A.; Takanabe, K.; Ebitani, K.; Hayashi, S.; Domen, K. Layered and nanosheet tantalum molybdate as strong solid acid catalysts. J. Catal. 2010, 270, 206–212. [Google Scholar] [CrossRef]
- Lanziano, C.A.S.; Moya, S.F.; Barrett, D.H.; Teixeira-Neto, E.; Guirardello, R.; de Souto da Silva, F.; Rinaldi, R.; Rodella, C.B. Hybrid Organic-Inorganic Anatase as a Bifunctional Catalyst for Enhanced Production of 5-Hydroxyethylfurfural from Glucose in Water. ChemSusChem 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Li, X.; Liu, X.; Xia, Y.; Hu, B.; Lu, G.; Wang, Y. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurfural over water-tolerant niobium-based catalysts. Fuel 2015, 139, 301–307. [Google Scholar] [CrossRef]
- Saravanan, K.; Park, K.S.; Jeon, S.; Bae, J.W. Aqueous Phase Synthesis of 5-Hydroxymethylfurfural from Glucose over Large Pore Mesoporous Zirconium Phosphates: Effect of Calcination Temperature. ACS Omega 2018, 3, 808–820. [Google Scholar] [CrossRef]
- Candu, N.; Fergani, M.E.; Verziu, M.; Cojocaru, B.; Jurca, B.; Apostol, N.; Teodorescu, C.; Pavulescu, V.I.; Coman, S.M. Efficient glucose dehydration to HMF onto Nb-BEA catalysts. Catal. Today 2019, 325, 109–116. [Google Scholar] [CrossRef]
- Takagaki, A.; Jung, J.C.; Hayashi, S. Solid Lewis acidity of boehmite γ-AlO(OH) and its catalytic activity for transformation of sugars in water. RSC Adv. 2014, 4, 43785–43791. [Google Scholar] [CrossRef]
- Marianou, A.A.; Michailof, C.M.; Pineda, A.; Iliopoulou, E.F.; Triantafyllidis, K.S. Effect of Lewis and Brønsted acidity on glucose conversion to 5-HMF and lactic acid in aqueous and organic media. Appl. Catal. A Gen. 2018, 555, 75–87. [Google Scholar] [CrossRef]
- Romo, J.E.; Wu, T.; Huang, X.; Lucero, J.; Irwin, J.L.; Bond, J.Q.; Carreon, M.A.; Wettstein, S.G. SAPO-34/5A Zeolite Bead Catalysts for Furan Production from Xylose and Glucose. ACS Omega 2018, 3, 16253–16259. [Google Scholar] [CrossRef]
- Lopes, M.; Dussan, K.; Leahy, J.J.; da Silva, V.T. Conversion of D-Glucose to 5- hydroxymethylfurfural using Al2O3-promoted sulphated tin oxide as catalyst. Catal. Today 2017, 279, 233–243. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Li, W.; Shen, P.; Wu, Y.; Song, X.; Zhu, Y.; Xu, N.; Gao, L.; Xiao, G. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurfural using an efficient and inexpensive manganese phosphate catalyst. Fuel Proc. Technol. 2018, 181, 199–206. [Google Scholar] [CrossRef]
- Benesi, H.A. Determination of proton acidity of solid catalysts by chromatographic adsorption of sterically hindered amines. J. Catal. 1973, 28, 176–178. [Google Scholar] [CrossRef]
- Jacobs, P.A.; Heylen, C.F. Active sites in zeolites. III. Selective poisoning of Brønsted sites on synthetic Y zeolites. J. Catal. 1974, 34, 267–274. [Google Scholar] [CrossRef]

| Catalyst | SBET /m2 g−1 | Acid Amount /mmol g−1 a | NH3-TPD Peak Position /K b | 31P MAS NMR Peal Position /ppm c |
|---|---|---|---|---|
| HNbWO6 nanosheets | 66 | 0.34 | 560 | 71 |
| HNb3O8 nanosheets | 101 | 0.28 | 550 | 70 |
| HTiNbO5 nanosheets | 153 | 0.24 | 535 | 63 |
| Nb2O5·nH2O | 128 | 0.30d | 550e | 65f |
| Amberlyst-15 | 50 | 4.8 | N.A. | 81 |
| Nafion NR50 | <0.1 | 0.9 | N.A. | N.A. |
| H-Beta g | 420 | 1.0 | 600 | 78h |
| H-ZSM5 i | 326 | 0.2 | 580 | 86j |
| Entry | Catalyst | Glucose Conversion /% | HMF Yield /% | HMF Selectivity /% | Fructose Yield /% | Formic Acid Yield /% | Unknown Yield /% b |
|---|---|---|---|---|---|---|---|
| 1 | HNbWO6 nanosheets | 56 | 20 | 36 | 5 | 5 | 25 |
| 2 | KNbWO6 nanosheets | 36 | 9 | 25 | 7 | N.D. | 21 |
| 3 | HNb3O8 nanosheets | 43 | 14 | 32 | 4 | N.D. | 26 |
| 4 | HTiNbO5 nanosheets | 55 | 11 | 21 | 2 | 10 | 31 |
| 5 | Nb2O5·nH2O | 63 | 20 | 31 | 2 | 4 | 37 |
| 6 | Amberlyst-15 | 7 | 0 | — | 0 | 0 | 7 |
| 7 | Nafion NR50 | 7 | 0 | — | 0 | 0 | 7 |
| 8 | H-Beta c | Trace | 0 | — | 0 | 0 | — |
| 9 | H-ZSM5 d | Trace | 0 | — | 0 | 0 | — |
| Entry | Catalyst | HMF Yield (Selectivity) /% | RS/C a | Temp. /K | Time /h | Reference |
|---|---|---|---|---|---|---|
| 1 | HNbWO6 nanosheets | 23 (44) | 1 | 393 | 24 | This study |
| 2 | HNbWO6 nanosheets | 20 (36) | 1 | 413 | 3 | This study |
| 3 | HNb3O8 nanosheets | 14 (34) | 50 | 428 | 2.5 | [22] |
| 4 | Nb2O5·nH2O | 12 (12) | 0.1 | 393 | 3 | [30] |
| 5 | meso-Nb2O5 | 18 (36) | 1 | 413 | 1 | [35] |
| 6 | Nb-BEA | 17 (41) | 6 | 453 | 24 | [37] |
| 7 | γ-AlO(OH) | 17 (18) | 1 | 443 | 24 | [38] |
| 8 | SnO2/γ-Al2O3 | 12 (14) | 1 | 423 | 1 | [39] |
| 9 | SAPO-34/5A | 20 (N.A.) | 1.7 | 463 | 3 | [40] |
| 10 | H-ZSM-5 (Si/Al = 90) | 0 (0) | 0.1 | 413 | 3 | [30] |
| 11 | H-mordenite (Si/Al = 90) | 0 (0) | 0.1 | 413 | 3 | [30] |
| 12 | SO42-/Al2O3-SnO2 | 19 (53) | 2 | 393 | 6 | [41] |
| 13 | Hybrid-TiO2 | 45 (60) | 1.2 | 403 | 7 | [34] |
| 14 | H3PO4/Nb2O5·nH2O | 48 (53) | 0.1 | 413 | 3 | [30] |
| 15 | meso-NbP | 34 (49) | 1 | 413 | 1 | [35] |
| 16 | meso-ZrP | 47 (56) | 1.6 | 428 | 6 | [36] |
| 17 | MnPO4 | 18 (25) | 2.5 | 433 | 1.5 | [42] |
| 18 | Amberlyst-15 | 0 (0) | 0.1 | 413 | 3 | [30] |
| 19 | Nafion NR50 | 0 (0) | 0.1 | 413 | 3 | [30] |
| Entry | Solvent | Temp./ K | Time /h | Glucose Conversion /% | HMF Yield /% | HMF Selectivity /% | Fructose Yield /% | Formic Acid Yield /% | Unknown Yield /% |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Water 1.5 mL | 393 | 3 | 27 | 11 | 40 | 0 | 0 | 16 |
| 2 | Water 1.5 mL | 403 | 3 | 37 | 14 | 38 | 0 | 0 | 23 |
| 3 | Water 1.5 mL | 413 | 3 | 56 | 20 | 36 | 5 | 5 | 25 |
| 4 | Water 1.5 mL | 423 | 3 | 70 | 24 | 35 | 4 | 6 | 36 |
| 5 | Water 3 mL | 393 | 24 | 52 | 23 | 44 | 4 | 5 | 31 |
| 6 | Water 3 mL + 1-butanol 3 mL | 393 | 36 | 71 | 37 | 52 | 4 | 0 | 31 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagaki, A. Production of 5-Hydroxymethylfurfural from Glucose in Water by Using Transition Metal-Oxide Nanosheet Aggregates. Catalysts 2019, 9, 818. https://doi.org/10.3390/catal9100818
Takagaki A. Production of 5-Hydroxymethylfurfural from Glucose in Water by Using Transition Metal-Oxide Nanosheet Aggregates. Catalysts. 2019; 9(10):818. https://doi.org/10.3390/catal9100818
Chicago/Turabian StyleTakagaki, Atsushi. 2019. "Production of 5-Hydroxymethylfurfural from Glucose in Water by Using Transition Metal-Oxide Nanosheet Aggregates" Catalysts 9, no. 10: 818. https://doi.org/10.3390/catal9100818
APA StyleTakagaki, A. (2019). Production of 5-Hydroxymethylfurfural from Glucose in Water by Using Transition Metal-Oxide Nanosheet Aggregates. Catalysts, 9(10), 818. https://doi.org/10.3390/catal9100818





