Abstract
The physicochemical properties of biologically important benzalkonium chlorides (BKCs) and the effects of its structure on the de-chlorination of allyl chloride was studied by electrogenerated [Co(I)(bipyridine)3]+ (Co(I)) using an electrochemical technique. The results of [Co(II)(bipyridine)3]2+ (Co(II)) cyclic voltammetry in the presence of BKC demonstrates Co(II)/Co(III) redox couple for physicochemical analysis of BKC and Co(II)/Co(I) redox couple for catalytic application. Cyclic voltammetry over a range of scan rates and BKC concentrations revealed the BKC-bound Co(II)/Co(III) micelles showed that the identification of cmc and association of the probe Co(II) species, associated more in the hydrophobic region. In addition, change in diffusion coefficient value of Co(II)/Co(III) with BKC concentration demonstrates the association of Co(II) in micellar hydrophobic region. The beneficial effects of BKC could be accounted for by considering the benzyl headgroup-Co (II) precatalyst-volatile organic compounds (VOCs) (allyl chloride here) substrate interaction. Chromatography/mass spectroscopy (GC/MS) revealed 100% complete de-chlorination of allyl chloride accompanied by three non-chloro products. This is the first report of benzyl headgroup-induced micellar enhancement by an electrochemical method, showing that it is possible to use hydrophobic benzyl headgroup-substitution to tune the properties of micelles for various applications.
1. Introduction
Benzalkonium chloride (BKC) is used primarily as a preservative and antimicrobial agent, as well as a surfactant. The compound has potent biocidal activity, killing microorganisms, and inhibiting their growth [1,2], but is friendlier to human skin, which is why it is used as an ingredient in antibacterial hand wipes, antiseptic creams, and anti-itch ointments. Other than the medicinal field, BKC has a beneficiary role as a surfactant particularly in the corrosion resistance of carbon steel because of its strong adsorption and hydrophobicity [3]. To stabilize and increase the porosity during the preparation of porous carbon, BKC is used greatly as porosity stabilizer [4]. In solubilized media, BKC is used as the supporting electrolyte during the electrochemical reduction of CO2 reduction in methanol at room temperature [5,6]. Benzalkonium chloride has been applied to remove biofilms on marine-based sensors or optical components [7,8], which highlights the need to determine the diffusion coefficient for BKC in water. Smith et al. [9] developed a membrane diffusion method and derived the diffusion coefficient for BKC with C12 and C14 in water. Ultrafiltration or ultracentrifugation methods have been adopted to determine the partition coefficient of BKC and the free BKC surfactant concentration in micellar solutions [10,11]. In the biological and chemical fields, both solubilization and stabilization play important roles in their specific applications. A detailed mechanistic aspect to understanding BKC micelles is still lacking, particularly in the electrochemical field.
With the exception of BKC, many other self-aggregated surfactant monomers have been used to solubilize redox species in solution, and they have been analyzed by the electrode surface [12,13]. Advantageous of this phenomena used to harvest the physicochemical properties of the various surfactant solutions by single potential step chronoamperometry [14,15] and diffusion limiting current at micro-electrodes techniques [16,17]. Despite the interesting physicochemical identification of surfactants in electrochemistry, the mediated catalytic reduction of halo compounds, such as 4-bromobiphenyl, 4,4’-dichlorobiphenyl, allyl chloride, and PCB (Polychlorinated biphenyls) mixtures, catalyzed by different organic and inorganic mediators, e.g., 9-phenylanthracene [18], [Co(bpy)3]2+ (where bpy = 2,2’-bipyridine) [19,20], zinc phthalocyanine [21], etc., have been observed in aqueous solutions of various surfactants, such as CTAB (Cetyltrimethylammonium bromide), SDS (Sodiumdodecyl sulfate), and film-forming dodecyl dimethyl ammonium bromide. In a recent study, the cobalt polypyridine complex-mediated de-chlorination of several chlorinated pesticides with significantly enhanced catalytic rates was achieved in a surfactant-containing aqueous solution [22]. In addition, our previous study reported the electrocatalytic dehalogenation of allyl chloride and its methyl-substituted derivative β-methyl allylchloride mediated by electrogenerated [Co(I)(bpy)3]+ at a glassy carbon electrode in aqueous solutions of cationic micelles [23,24,25].
In the present investigation, physicochemical analysis of BKC with an alkyl chain length of C16 was analyzed using an electrochemical method. For this purpose, in the first part of the study, the [Co(II)(bpy)3]2+/[Co(III)(bpy)3]3+ redox reaction in BKC surfactant was studied by CV (Cyclic voltammetry) and some key parameters, such as the cmc (critical micellar concentration), micellar diffusion coefficient, and relative association constants of the oxidized species were estimated, as done for other surfactants [26,27,28,29,30,31]. Following this, the effects of BKC on allyl chloride catalysis were examined by product analysis and discussed.
2. Results and Discussion
2.1. Selection of Redox Couple
According to Figure 1, two redox couples for [Co(II)(bpy)3]2+ redox system were found. One was related to Co(II) to Co(III) oxidation and another one was for Co(II) to Co(I) reduction. One mM of [Co(II)(bpy)3]2+ in 0.1 M Na2SO4 medium shows highly reversible redox behavior of Co(II) to Co(III) (A1 and C1) oxidation (Figure1, curve a), but complicated redox peaks were observed in the case of Co(II) to Co(I) reduction (C2 and A2). At the same time, addition of 10 mM BKC led to the reversible Co(II)/Co(III) redox couple becoming quasi-reversible or diminished according to the BKC concentration. Surprisingly, the reduction couple Co(II)/Co(I) became reversible without any complications (Figure1, curve b).
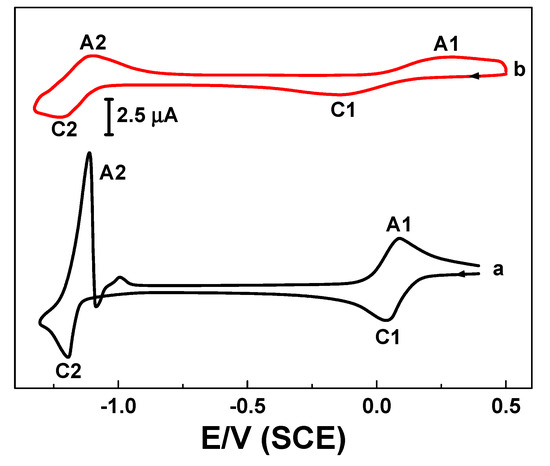
Figure 1.
Voltammetry responses of 1 mM [Co(II)(bpy)3]2+ on a GCE (Glassy carbon electrode) in 0.1 M Na2SO4 without (a) benzalkonium chloride (BKC) and (b) with 10 mM BKC at scan rate of 25 mVs−1.
It is more realistic that the Co(II)/Co(III) redox couple can be considered for the BKC aggregates and its physicochemical analysis due to effective change in peak current and potential with BKC concentration, Equation (1). Because of the reversible redox behavior in presence of BKC, the Co(II)/Co(I) redox couple can be considered for catalytic reduction analysis, as the reversibility is one of the mediated electrocatalytic processes [32] and solubilities of the VOCs in the micellar medium (Equation (2)).
[Co(II)(bpy)3]2+ + BKC ⇌ M-[Co(II)(bpy)3]2+ ⇌ M-[Co(III)(bpy)3]3+ + e−
M-[Co(II)(bpy)3]2+ + e− ⇌ M-[Co(I)(bpy)3]+ (M = BKC micelles)
2.2. cmc and Solubilization Constant Evaluation of BKC by CV using [Co(II)(bpy)3]2+/[Co(III)(bpy)3]3+ Redox Couple
In Figure 2, the peak current initially shows constant from 0.01 mM up to 0.48 mM, then there is a sudden drop in current when the concentration of BKC (cBKC) is increased to 10 mM. A similar trend is followed at all scan rates studied. This sudden variation in ipA1 with cBKC may identify the aggregation of monomer amphiphiles forming higher oligomers of BKC, which in turn predicts the cmc values for BKC. The break point value ca. 0.48 mM is near to the cmc of BKC observed in water from surface tension measurements, viz. 0.35 mM [33], and from light scattering measurements, viz. 0.4 mM [34]. The light scattering study also reports a value 0.02 mM for the cmc of BKC in 0.04 M NaCl [34]. The present study almost correlates with cmc of the light scattering measurements.
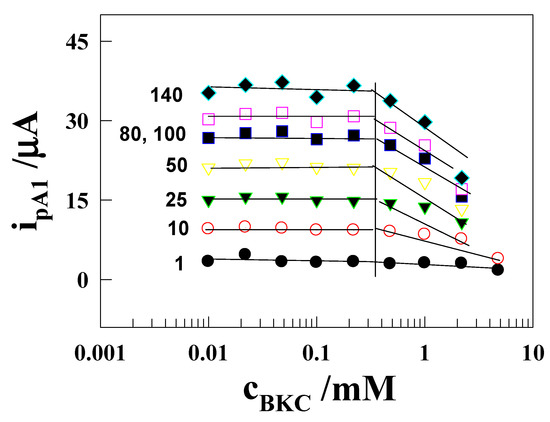
Figure 2.
Anodic peak (A1) current as a function of BKC concentration for the oxidation of 1 mM [Co(II)(bpy)3]2+ on a GCE in 0.1 M Na2SO4 solution at various scan rates.
According to Figure 3, there is almost no change in E1/2 (difference in A1 and C1 peak potential) values with increasing BKC concentration up to 0.48 mM, but there is a positive E1/2 value shift in subsequent concentrations from 1 to 4.8 mM of BKC (Figure 3 shows a definite E1/2 value of 2.2 mM). The shifts in potential can be correlated directly with the relative extent of the solubilization of the redox components within the micelles [26,27,30,31] using Equation (3):
E1/2,M-Wr = EW0’ + (RT/nF)ln(KR/KO)
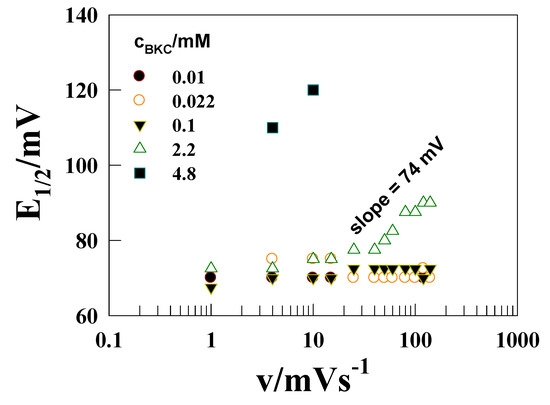
Figure 3.
E1/2 versus log(v) plots for the oxidation of 1 mM [Co(II)(bpy)3]2+ on a GCE in 0.1 M Na2SO4 at various concentrations of BKC.
The experimentally measured E1/2M-W in the presence of BKC and E1/2W without BKC difference is 25 mV (E1/2M-W = 95 mV and Ew0’ = 70 mV), which is giving rise to the partition coefficient ratio KCo(II)/KCo(III) for Co(III)/Co(II) redox couple to be 2.9. This implies a preferential partitioning of [Co(II)(bpy)3]2+, than [Co(III)(bpy)3]3+, into the BKC micellar phase. The larger KCo(II) value for the association of complex than for its oxidized counterpart can be attributed to greater hydrophobic nature of the divalent complex, supported by the reduced charge on the cobalt center, which provides electrostatic advantage of the 2+ charge, since its electrostatic repulsion with the cationic micellar entity is smaller. The predominance of hydrophobic interactions has also been observed for [Co(III)(phen)3]3+/[Co(II)(phen)3]2+ couple by Davies and Hussam [30] in SDS micelles, which collectively tells that BKC solubilize also the cationic counterpart into its micelle.
2.3. Partition Coefficient and Association Constant by Diffusion Coefficient of the Co(II)/Co(III) Redox Couple
Figure 4 shows the variation of ipA1 with v1/2 for various cBKC. The linearity is decreased with increasing the BKC concentration, but at low scan rate, the peak current value obey linearity with passes through the origin. The condition predicted by the Randle–Sevcik equation for a diffusion controlled electron transfer process, namely, peak current passing through the origin and also varying linearly with v1/2 is strictly followed only in the absence of BKC, and at low concentrations of BKC, confirms that BKC allows C(III)/Co(II) via diffusion controlled electron transfer process. The diffusion coefficient of the micelle-solubilized Co(II) complex, DM-W-2/3¸ was calculated from ipa1 vs. v1/2 slope using Equation (4), which is valid for an electrode process controlled by diffusion [32]:
where n is the number of electrons of the redox couple (n = 1 here); Ageo is the electrode area, and cM-W-Co(II) is the bulk concentration of [Co(II)(bpy)3]2+ in the presence of defined BKC concentration. DM-W2/3 values slightly decreased with BKC concentration up to 1 mM, as predicted by the linear interaction theory [17], then sharply decreased in additional BKC concentration (Figure 5).
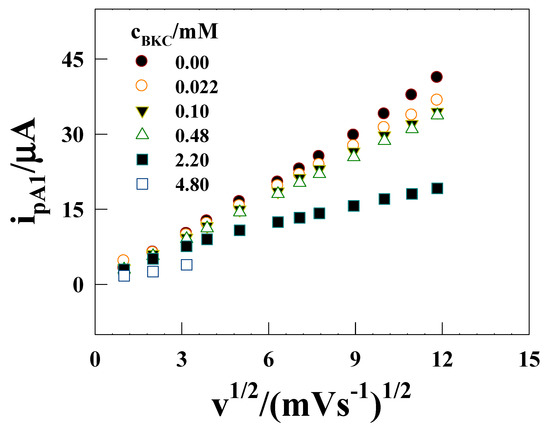
Figure 4.
ipA1 versus v1/2 plots for the oxidation of 1 mM [Co(II)(bpy)3]2+ on a GCE in 0.1 M Na2SO4 solution in presence of various concentrations of BKC (mM mentioned in the figure).
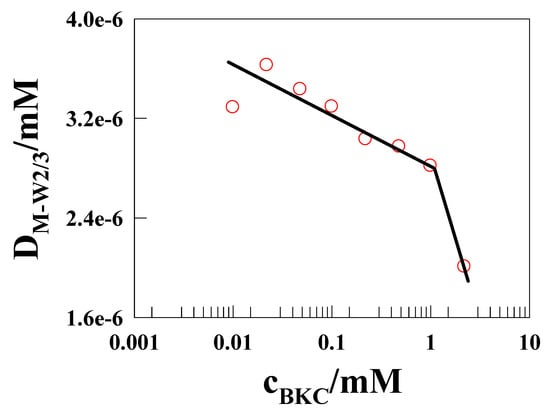
Figure 5.
Diffusion coefficient of surfactant bound-[Co(II)(bpy)3]2+ from CV as a function of BKC concentration (mM).
A decrease in diffusion coefficient value when the increasing BKC concentration associates with the larger micelle aggregates formation, which means solubilization of the Co(II)/Co(III) within the micellar region. In the present case of the preferably polar head-confined Co(II) complex-bound BKC micelles, their effective size could also increase due to the additional electrostatic repulsion-induced Co(II) among the adjacent surface sites acting along the surface periphery at the micelle head/solution boundary. These larger BKC micelles would allow the slower diffusion of Co(II) probe-solubilized aggregates with smaller diffusion coefficients. In addition, the diffusion coefficient value change with BKC concentration can be used to identify the cmc of BKC.
The measured diffusion coefficient of the micelle-bound Co(II) complex in the aqueous/micellar mixture, DM-W2/3, is contributed by free complex and the bound complex. Thus, considering the partitioning of the complex between the free and the micelle bound forms, DM-W2/3 is described by a concentration (mole fraction) weighted average of the two forms [35,36,37,38] for a partitioning of [Co(II)(bpy)3]2+ between micelle bound (Sn.R) and free (RW) form in the BKC micellar medium (Sn), which can be written as
DM-W2/3 = Ff⋅DWR + Fb⋅DBKC
Here, DWR is the diffusion coefficient of free [Co(II)(bpy)3]2+ measured in a 0.1 M Na2SO4 aqueous solution without surfactant. DBKC is the diffusion coefficient of BKC micelle.
Since Ff + Fb=1, Equation (6) can be expressed in terms of diffusion parameters as
Ff = (DM-W2/3 − DBKC)/(DWR − DBKC)
Fb = (DM-W2/3 − DWR)/(DBKC − DWR)
KR = [Sn⋅R]/[RW] = Fb/Ff = (DM-W2/3 − DWR)/ (DBKC − DM-W2/3)
Thus, Equations (9)–(11) are a method to calculate Ff, Fb, and KR, respectively, from diffusion coefficient values. Mandal and Nair [39] have adopted this method to calculate the partition parameters and the mole fractions in the micellar phase for three redox forms of [Co(en)3](ClO4)3 in CTAB and CPC (Cetylpyridinium chloride) micellar solution. By substituting experimentally measured diffusion coefficient values in Equations (7)–(9), the micellar solubilization constant, KCo(II), fractions of solubilized and free probe molecules are calculated to be 3.8 × 104 M−1 using the values of DWR = 3.42 × 10−6 cm2 s−1 and DBKC = 0.39 × 10−6 cm2 s−1 [40]. The calculated thermodynamic parameters are presented in Table 1. The micelle solubilized fraction of Co(II) species is negligible up to 0.1 mM of BKC, and gradually increases up to 2.2 mM, where Co(II) is partitioned equally into the micellar phase. The partition coefficient, KCo(II), increases with cBKC and shows linear segment with slope (Figure 4) {KCo(II)/ [BKC]} = 3.8 × 102 M−1. The corresponding association constant is, therefore, 3.9 × 104 M−1 (KCo(II) × n, n = 102 for BKC), which confirms the BKC allows the probe molecule (Co(II)) equally distributed in the micellar system.

Table 1.
Experimentally measured diffusion coefficient of [Co(II)(bpy)3]2+ complex in micellar solutions by CV (DM-W2/3), mole fraction of micellar bound Co(II) complex (Fb), mole fraction of unbound Co(II) complex (Ff), and partition coefficient of Co(II) complex (KCo(II)) in 0.1 M Na2SO4 containing different concentrations of BKC.
2.4. BKC Effect on Catalysis by Product Analysis
As shown in Figure 1, curve b, the C2 and A2 redox couple in the presence of 0.1 M BKC was adopted to check the catalytic reduction of allyl chloride (a model VOC solution). The CV of [Co(II)(bpy)3]2+ in different surfactant with various substrates proved its mediated catalytic reduction [19,23,24,25]. The product identification may lead to the effectiveness of BKC in catalytic reaction on allyl chloride. Figure 6 shows the current response during the bulk electrolysis of allyl chloride (5 mM), [Co(II)(bpy)3]2+ (1 mM, the 5:1 ratio of allylchloride to cobalt enables to follow pseudo first order condition), and BKC (100 mM) in 0.1 M Na2SO4 solution. The current sharply increased and reached to 4 mA in 2 h and started to decrease a little, which indicates the reduction reaction was enabled without blocking and the reaction was completed in 2 h. Figure 7A’,B’ presents GC/MS data of the reduction products collected from a 3-h electrolyzed solution of allyl chloride. They show three GC peaks at three different rt (3.59, 4.28, and 6.12) (Figure 7A’) corresponding to the fragment ions at m/z = 82, 138, and 136 in their respective mass spectrum (Figure 7B’). The three compounds predicted by these fragment ions at the above m/z are likely to be 1,5-hexadiene (3) (m/z = 82), 1,4,8-nonatriene (4) (m/z = 138), and 1,8-nonadiene (5) (m/z = 136). The absence of an allyl chloride substrate GC peak (rt = 3.22) in the GC spectrum for the electrolyzed solution confirms the complete degradation of allyl chloride by the BKC micelle solubilized Co(I) mediation process. Scheme 1 presents the possible allyl chloride reduction mechanism based on the results of both CV and bulk electrolysis, accounting for these three reduction products.
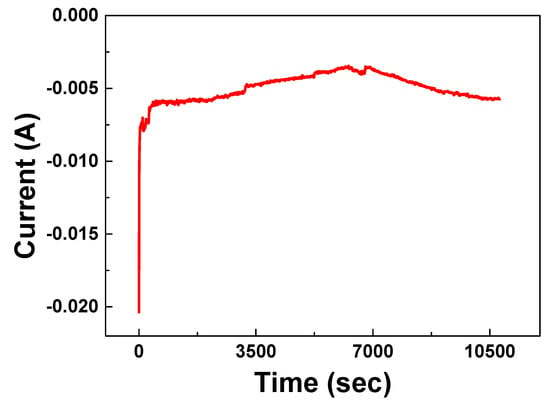
Figure 6.
Chronoamperometric curve during mediated electrocatalytic reduction of allyl chloride. Conditions: working solution (250 mL) = 5 mM allyl chloride+ 1 mM [Co(II)(bpy)3]2++ 100 mM BKC in 0.1 M Na2SO4 solution; Electrodes = Carbon cathode and Pt anode; Applied potential = −1300 mV.
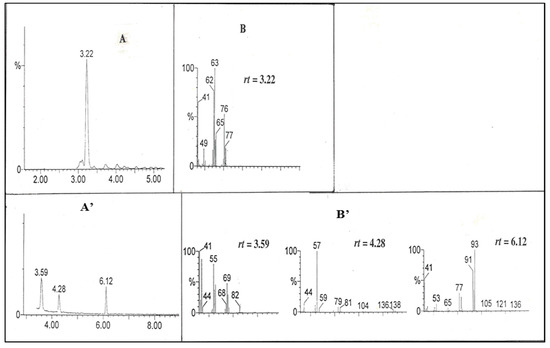
Figure 7.
(A,A’) Gas chromatograms and (B,B’) mass spectra of different systems: (A,B) pure AC (allyl chloride); (A’,B’) AC degradation products from constant-potential bulk electrolysis using a carbon plate cathode of 5 mM ally chloride + 1 mM [Co(II)(bpy)3]2+ + 100 mM BKC in a 0.1 M Na2SO4 aqueous solution. Potential applied = −1300 mV (SCE). Mass spectra are given for different retention times (rt) indicated in the figure.
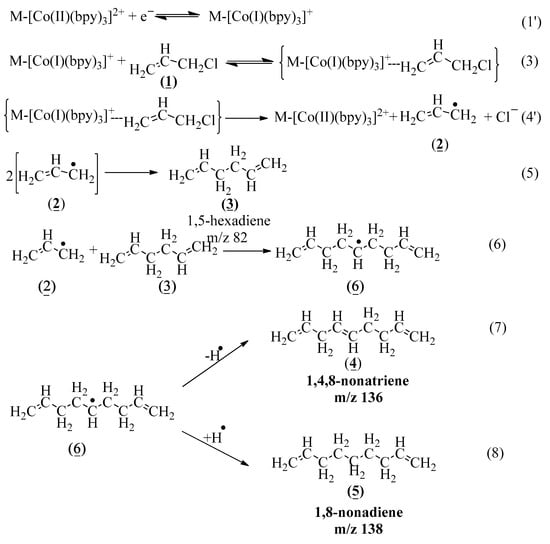
Scheme 1.
Mechanism of electrocatalytic ally chloride reduction mediated by M-[Co(I)(bpy)3]1+ in the presence of BKC surfactant.
As shown in Scheme 1, the mechanism for the formation of degradation products (3), (4), and (5) begins with heterogeneous electron transfer to convert the BKC micelle bound-Co(II)(bipyridine) precursor to the BKC-Co(I) complex catalyst in Reaction (1’), which is followed by a reaction with the substrate allyl chloride to form a {M-[Co(I)(bpy)3]+∙∙∙ally chloride} complex according to Reaction (3). The Co(I) within the mediator–substrate complex could transfer an electron electrocatalytically to the adjacent allyl chloride to give the allyl radical (2) and a Cl− ion and regenerate the precursor Co(II) species in a slow rate-determining step (Reaction (4’)). The radical (2) formed could dimerize immediately to form the GC/MS identified 1,5-hexadiene (3) (m/z = 82) (Reaction (5)). Alternatively, the radical (2) could react with the product, i.e., 1,5-hexadiene (3) in Reaction (6), to give an intermediate radical (6), which upon eliminating a hydrogen radical (Reaction (7)) forms a product, i.e., 1,4,8-nonatriene (4) (m/z = 136). The same intermediate radical (6), upon abstracting a hydrogen radical (reaction 8), affords the other GC/MS identified product, 1,8-nonadiene (5) (m/z = 138). Note that TDTAB (tetradecyltrimethlyammonium bromide) surfactant solubilized b-methyl allyl chloride found a chlorinated product [24] and TS-1 (Titanium silicate-1) in ethanol with H2O2 produced 99% epichlorohydrin [41], but the unusual and complete non-chlorinated products formation could be due the stabilization by the BKC micellar solution, evidenced by the high partition coefficient and diffusion coefficient, into its hydrophobic region. Also, the stabilization of chemicals in BKC is evidenced by slow drug delivery in antibacterial drugs [42]. The above ally chloride reduction mechanism satisfactorily accounts for the GC/MS-identified degradation products, and for the stoichiometry coefficient σ = 1, because one mole of mediator is needed to reduce 1 mole of the substrate. On the other hand, the source for OH∙ radical (in Reaction (8)) could not be accounted for, and the reaction intermediates have not been identified or characterized independently.
3. Material and methods
3.1. Chemicals
Benzalkonium chloride was purified by repeated washing with anhydrous ether [43]. Allyl chloride was distilled twice (BP = 46 °C) prior to use. The Co(II)-tris-bipyridine perchlorate complex was prepared using the procedure reported elsewhere [44]. All other reagents and solvents were of AR grade and used as received.
3.2. Electrochemical Studies
The CV experiments were carried out using a Wenking potentiostat (ST 72, Gerhard Bank Elektroniks, Göttingen, Germany), a Wenking signal generator (VSG 83, Gerhard Bank Elektroniks, Göttingen, Germany) and a X-Y-t recorder (WX 2300, Graphtec, Tokyo, Japan) in combination. A conventional gas-tight three-electrode electrolysis cell was used with a glassy carbon disk, a Pt plate, and a dip type saturated calomel electrode (SCE) as the working, counter, and reference electrodes, respectively. Before each experiment, the GCE surface was polished using a fine mesh alumina on a velvet cloth followed by ultrasonic cleaning in double distilled water and washing with methanol. Charcoal-treated double distilled water was used to prepare all experimental solutions. Dry nitrogen was used to degas the solutions and the temperature was maintained at 25 ± 0.2 °C for all experiments.
Bulk electrolysis for the de-chlorination products was carried out using a large-sized carbon plate cathode in a divided cell containing 250 mL of a stirred electrolyte solution under a N2 atmosphere with Pt as the anode at a constant potential that was 100 mV negative of the reduction peak of the catalyst (−1300 mV) in the presence of the substrate. After approximately 3-h electrolysis, the products from the catholyte were extracted into ether and analyzed using a Perkin-Elmer Auto System XL gas chromatograph with a Turbo Mass Spectrometer (EI.70 eV, Perkin-Elmer, Waltham, MA, USA). A 30 m × 0.25 mm × 1 μm capillary column (Perkin-Elmer Elite series PE-5, Perkin-Elmer, Waltham, MA, USA) with helium as the carrier gas at a flow rate of 1.0 mL min−1 and an oven temperature programmed between 80 and 250 °C at a rate of 10 °C min−1.
4. Conclusions
The paper reported that the benzyl headgroup-bearing BKC micelles exerted significantly different effects on the oxidation of [Co(II)(bpy)3]2+ to [Co(III)(bpy)3]3+ as well as on the effect of electrocatalysis by the BKC bound electrogenerated Co(I)(bpy) complex. The CV analysis with different BKC concentrations demonstrates the identification of cmc and diffusion coefficient of BKC surfactant. The presence of bulkier hydrophobic benzyl headgroups in BKC led to the preferred solubilization of the Co(II) complex (KCo(II)/KCo(III) = 2.9), a more positive onset potential for Co(II) oxidation to Co(III), and considerably larger micellar aggregates with decreasing DM-W2/3 values with concentration. These studies clearly correlated the behavioral differences of the BKC surfactant on the different redox couples of the same mediator. The highly stable micelle-solubilized Co(I) mediator and the total de-chlorination of allyl chloride accompanied by three non-chloro organics as the only de-chlorination products in the BKC solution highlight the efficiency of the hydrophobic benzyl headgroup-bearing surfactant towards the de-chlorination process. This study also highlights the power of the CV technique as the diagnostic electrochemical tool in characterizing the micellar systems as well as in in electrocatalytic systems.
Author Contributions
Investigation, G.M.; Project administration, I.-S.M.; Supervision, K.C.P.; Writing—original draft, G.M.; Writing—review & editing, I.-S.M.
Funding
This study was supported by the National Research Foundation of Korea (NRF) funded through the Ministry of Engineering Science and Technology (MEST) (Grant No. 2017R1A2A1A05001484) from Korean Government and the University Grants Commission, Government of India. K.C.P wishes to thank the Korea Federation of Science and Technology Societies (KOFST, Republic of Korea) for the offer of Invited Scientist through the ‘Brain Pool Program’. The authors also wish to thank A. K. Mohanakrishnan of Organic Chemistry Department, University of Madras for his help in interpreting the GC/MS results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mc Cay, P.H.; Ocampo-Sosa, A.A.; Fleming, G.T.A. Effect of subinhibitory concentrations of benzalkonium chloride on the competitiveness of Pseudomonas aeruginosa grown in continuous culture. Microbiology 2010, 156, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Thorrold, C.A.; Letsoalo, M.E.; Dusé, A.G.; Marais, E. Efflux pump activity in fluoroquinolone and tetracycline resistant Salmonella and E. coli implicated in reduced susceptibility to household antimicrobial cleaning agents. Int. J. Food Microbiol. 2007, 113, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhu, S.; Zhang, S. Experimental and theoretical studies of benzalkonium chloride as an inhibitor for carbon steel corrosion in sulfuric acid. J. Ind. Eng. Chem. 2015, 24, 174–180. [Google Scholar] [CrossRef]
- Tamborini, L.H.; Casco, M.E.; Militello, M.P.; Silvestre-Albero, J.; Barbero, C.A.; Acevedo, D.F. Successful application of a commercial cationic surfactant mixture (benzalkonium chloride) as porosity stabilizer in porous carbons fabrication. Colloids Surf. A 2016, 509, 449–456. [Google Scholar] [CrossRef]
- Ohta, K.; Kawamoto, M.; Mizuno, T.; Lowy, D.A. Electrochemical reduction of carbon dioxide in methanol at ambient temperature and pressure. J. Appl. Electrochem. 1998, 28, 717–724. [Google Scholar] [CrossRef]
- Naitoh, A.; Ohta, K.; Mizuno, T.; Yoshida, H.; Sakai, M.; Noda, H. Electrochemical reduction of carbon dioxide in methanol at low temperature. Electrochim. Acta 1993, 38, 2177–2179. [Google Scholar] [CrossRef]
- Parr, A.C.S.; Smith, M.J.; Beveridge, C.M.; Kerr, A.; Cowling, M.J.; Hodgkiess, T. Optical assessment of a fouling-resistant surface (PHEMA/ benzalkonium chloride) after exposure to a marine environment. Adv. Mater. Opt. Electr. 1998, 8, 187–193. [Google Scholar] [CrossRef]
- Cowling, M.J. Marine Sciences and Technologies; Weydert, M., Ed.; European Commission: Luxembourg, 1995; Volume 2, pp. 1045–1058. [Google Scholar]
- Smith, T.J.; Wang, C.; Abbott, N.L. Influence of Self-Assembling Redox Mediators on Charge Transfer at Hydrophobic Electrodes. Langmuir 2015, 31, 10638–10648. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, G.W.; Sandoval, M.; Ciringh, Y.; Xue, G.; Jaeger, D.; Kompanik, K.; Jiao, J.; Gelotte, K.M. Determination of benzalkonium chloride partition in micelle solutions using ultrafiltration method. AAPS PharmSciTech 2009, 10, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Rippie, E.G. Micellar Distribution Equilibria: Ultracentrifugal Study of Apparent Partition Coefficients. J. Pharm. Sci. 1977, 66, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1989. [Google Scholar]
- Rusling, J.F. Electroanalytical Chemistry; Bard, A.J., Ed.; Marcel Dekker: New York, NY, USA, 1994; Volume 19, pp. 1–88. [Google Scholar]
- Valencia, D.P.; González, F.J. Estimation of diffusion coefficients by using a linear correlation between the diffusion coefficient and molecular weight. J. Electroanal. Chem. 2012, 681, 121–126. [Google Scholar] [CrossRef]
- Macpherson, J.V.; Unwin, P.R. Determination of the Diffusion Coefficient of Hydrogen in Aqueous Solution Using Single and Double Potential Step Chronoamperometry at a Disk Ultramicroelectrode. Anal. Chem. 1997, 69, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Gao, X. Steady-State Voltammetry with Stationary Disk Millielectrodes in Magnetic Fields: Nonlinear Dependence of the Mass-Transfer Limited Current on the Electron Balance of the Faradaic Process. J. Phys. Chem. B 1999, 103, 5832–5840. [Google Scholar] [CrossRef]
- Ferreira, T.L.; Sato, B.M.; El Seoud, O.A.; Bertotti, M. Application of Microelectrode Voltammetry to Study the Properties of Surfactant Solutions: Alkyltrimethylammonium Bromides. J. Phys. Chem. B 2010, 114, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Rusling, J.F.; Shi, C.-N.; Gosser, D.K.; Shukla, S.S. Electrocatalytic reactions in organized assemblies: Part I. Reduction of 4-bromobiphenyl in cationic and non-ionic micelles. J. Electroanal. Chem. 1988, 240, 201–216. [Google Scholar] [CrossRef]
- Kamau, G.N.; Rusling, J.F. Electrocatalytic reactions in organized assemblies: Part III. Reduction of allyl halides by bipyridyl derivatives of cobalt in anionic and cationic micelles. J. Electroanal. Chem. 1988, 240, 217–226. [Google Scholar] [CrossRef]
- Shi, C.; Rusling, J.F.; Wang, Z.; Willis, W.S.; Winiecki, A.M.; Suib, S.L. Electrocatalytic reactions in organized assemblies. 6. Electrochemical and spectroscopic studies of catalytic clay micelle electrodes. Langmuir 1989, 5, 650–660. [Google Scholar] [CrossRef]
- Iwunze, M.O.; Rusling, J.F. Aqueous lamellar surfactant system for mediated electrolytic dechlorination of polychlorinated biphenyls. J. Electroanal. Chem. 1989, 266, 197–201. [Google Scholar] [CrossRef]
- He, W.Y.; Fontmorin, J.M.; Hapiot, P.; Soutrel, I.; Floner, D.; Fourcade, F.; Amrane, A.; Geneste, F. A new bipyridyl cobalt complex for reductive dechlorination of pesticides. Electrochim. Acta 2016, 207, 313–320. [Google Scholar] [CrossRef]
- Muthuraman, G.; Chandrasekara Pillai, K. Surfactant effects on mediated electrocatalytic dechlorination of allylchloride. J. Mol. Catal. A Chem. 2001, 169, 137–146. [Google Scholar] [CrossRef]
- Muthuraman, G.; Chandrasekara Pillai, K. Dechlorination of β-methylallyl chloride by electrogenerated [Co(I)(bipyridine)3]+: An electrochemical study in the presence of cationic surfactants. J. Colloid Interface Sci. 2006, 297, 687–695. [Google Scholar] [CrossRef]
- Pillai, K.C.; Muthuraman, G.; Moon, I.-S. Surfactant structural effects on mediated electrocatalytic dechlorination: Links between the micellar enhancement of dechlorination reactions and micellar properties. J. Colloid Interface Sci. 2018, 512, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Georges, J.; Desmettre, S. Electrochemistry of ferrocene in anionic, cationic and nonionic micellar solutions. Effet of the micelle solubilization of the half-wave potentials. Electrochim. Acta 1984, 29, 521–525. [Google Scholar] [CrossRef]
- Kaifer, A.E.; Bard, A.J. Micellar effects on the reductive electrochemistry of methylviologen. J. Phys. Chem. 1985, 89, 4876–4880. [Google Scholar] [CrossRef]
- Zana, R.; Mackay, R.A. Polargographic measurement of micellar diffusion coefficients. Langmuir 1986, 2, 109–113. [Google Scholar] [CrossRef]
- Kamau, G.N.; Leipert, T.; Shukla, S.S.; Rusling, J.F. Electrochemistry of bipyridyl derivatives of cobalt in solutions of anionic and cationic micelles. J. Electroanal. Chem. 1987, 233, 173–187. [Google Scholar] [CrossRef]
- Davies, K.; Hussam, A. Electrochemical studies of metal complexes in sodium dodecyl sulfate micellar solution. Langmuir 1993, 9, 3270–3276. [Google Scholar] [CrossRef]
- Myers, S.A.; Mackay, R.A.; Brajter-Toth, A. Solution microstructure and electrochemical reactivity. Effect of probe partitioning on electrochemical formal potentials in microheterogeneous solutions. Anal. Chem. 1993, 65, 3447–3453. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications; Wiley-Interscience: New York, NY, USA, 1980. [Google Scholar]
- Corrin, M.L.; Harkins, W.D. Determination of the Critical Concentration for Micelle Formation in Solutions of Colloidal Electrolytes by the Spectral Change of a Dye1. J. Am. Chem. Soc. 1947, 69, 679–683. [Google Scholar] [CrossRef]
- Okano, L.T.; Quina, F.H.; El Seoud, O.A. Fluorescence and Light-Scattering Studies of the Aggregation of Cationic Surfactants in Aqueous Solution: Effects of Headgroup Structure. Langmuir 2000, 16, 3119–3123. [Google Scholar] [CrossRef]
- Rusling, J.F.; Shi, C.N.; Kumosinski, T.F. Diffusion of micelle-bound molecules to electrodes in solutions of ionic surfactants. Anal. Chem. 1988, 60, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.P.; Scott, K. Interaction of cetyltrimethylammonium chloride micelles under static and hydrodynamic conditions. J. Chem. Soc. Faraday Trans. 1996, 92, 4541–4545. [Google Scholar] [CrossRef]
- Evans, D.H. Multicomponent diffusion with chemical reactions and its effect in voltammetry. J. Electroanal. Chem. 1989, 258, 451–456. [Google Scholar] [CrossRef]
- McIntire, G.L.; Chiappardi, D.M.; Casselberry, R.L.; Blount, H.N. Electrochemistry in ordered systems. 2. Electrochemical and spectroscopic examination of the interactions between nitrobenzene and anionic, cationic, and nonionic micelles. J. Phys. Chem. 1982, 86, 2632–2640. [Google Scholar] [CrossRef]
- Mandal, A.B.; Nair, B.U. Cyclic voltammetric technique for the determination of the critical micelle concentration of surfactants, self-diffusion coefficient of micelles, and partition coefficient of an electrochemical probe. J. Phys. Chem. 1991, 95, 9008–9013. [Google Scholar] [CrossRef]
- Smith, M.J.; Flowers, T.H.; Cowling, M.J.; Duncan, H.J. Method for the measurement of the diffusion coefficient of benzalkonium chloride. Water Res. 2002, 36, 1423–1428. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Mao, G.; Zheng, X. Synthesis of TS-1 from an Inorganic Reactant System and Its Catalytic Properties for Allyl Chloride Epoxidation. Ind. Eng. Chem. Res. 2012, 51, 12730–12738. [Google Scholar] [CrossRef]
- Rios-Castillo, A.G.; Umana, F.F.; Rodriguez-Jerez, J.J. Long-term antibacterial efficacy of disinfectants based on benzalkonium chloride and sodium hypochlorite tested on surfaces against resistant gram-positive bacteria. Food Control 2018, 93, 219–225. [Google Scholar] [CrossRef]
- Ledbetter, J.W.; Bowen, J.R. Integral spectrophotometric titrations using eosin for the determination of the critical micelle concentration of alkyldimethylbenzylammonium chlorides. Anal. Chem. 1971, 43, 773–774. [Google Scholar] [CrossRef]
- Burstall, F.H.; Nyholm, R.S. Studies in co-ordination chemistry. Part XIII. Magnetic moments and bond types of transition-metal complexes. J. Chem. Soc. 1952, 681, 3570–3579. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).