Efficient Catalytic Dehydration of High-Concentration 1-Butanol with Zn-Mn-Co Modified γ-Al2O3 in Jet Fuel Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
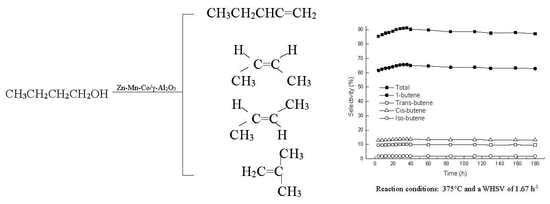
2.2. Catalytic Activity of γ-Al2O3 and Zn-Mn-Co/γ-Al2O3
2.3. Optimization of Temperature and WHSV for Butanol Dehydration
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Experimental Design
i = 1, 2, …, k; j = 1, 2, …, k; i ≠ j,
3.4. 1-Butanol Dehydration Experiments
3.5. Analytical Methods
3.6. Catalyst Characterization
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Tacconi, D. Sustainable bio kerosene: Process routes and industrial demonstration activities in aviation biofuels. Appl. Energy 2014, 136, 767–774. [Google Scholar] [CrossRef]
- Danilo, S.B.; Adriano, P.M. Jet fuel production in eucalyptus pulp mills: Economics and carbon footprint of ethanol vs. butanol pathway. Bioresour. Technol. 2018, 268, 9–19. [Google Scholar]
- Zhang, W.L.; Liu, Z.Y.; Liu, Z.; Li, F.L. Butanol production from corncob residue using Clostridium beijerinckii NCIMB 8052. Lett. Appl. Microbiol. 2012, 55, 240–246. [Google Scholar] [CrossRef]
- Ujor, V.; Agu, C.V.; Gopalan, V.; Ezeji, T.C. Allopurinol-mediated lignocellulose-derived microbial inhibitor tolerance by Clostridium beijerinckii during acetone-butanol-ethanol (ABE) fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 3729–3740. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.N.; Liu, H.J.; Yan, X.; Zhou, Y.J.; Cheng, K.K.; Zhang, J.A. High-efficiency acetone-butanol-ethanol production and recovery in non-strict anaerobic gas-stripping fed-batch fermentation. Appl. Microbiol. Biotechnol. 2017, 101, 8029–8039. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Zhao, J.B.; Liu, F.F.; Lu, C.C.; Yang, S.T.; Bai, F.W. Two-stage in situ gas stripping for enhanced butanol fermentation and energy-saving product recovery. Bioresour. Technol. 2013, 135, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Du, G.Q.; Sun, J.X.; Chen, L.J.; Gao, S.S.; Yu, M.L.; Yang, S.T.; Bai, F.W. Characterization of gas stripping and its integration with acetone-butanol-ethanol fermentation for high-efficient butanol production and recovery. Biochem. Eng. J. 2014, 83, 55–61. [Google Scholar] [CrossRef]
- West, R.M.; Braden, D.J.; Dumesic, J.A. Production of alkanes from biomass derived carbohydrates on bi-functional catalysts employing niobium-based supports. J. Catal. 2009, 10, 1743–1746. [Google Scholar] [CrossRef]
- Sun, H.; Blass, S.; Michor, E.; Schmidt, L. Autothermal reforming of butanol to butenes in a staged millisecond reactor: Effect of catalysts and isomers. Appl. Catal. A Gen. 2012, 445–446, 35–41. [Google Scholar] [CrossRef]
- Sedjame, H.J.; Lafaye, G.; Barbier, J., Jr. N-butanol removal over alumina supported platinum catalysts. Appl. Catal. B Environ. 2013, 132–133, 132–141. [Google Scholar] [CrossRef]
- Zhang, D.; Alhajri, R.; Barri, S.A.; Chadwick, D. One-step dehydration and isomerisation of n-butanol to iso-butene over zeolite catalysts. Chem. Commun. 2010, 46, 4088–4090. [Google Scholar] [CrossRef] [PubMed]
- Macho, V.; Jurecekova, E.; Hudec, J. Dehydration of C4 alkanols conjugated with a positional and skeletal isomerisation of the formed C4 alkenes. Appl. Catal. A Gen. 2001, 214, 251–257. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.Z. Increased fermentability of enzymatically hydrolyzed steam-exploded corn stover for butanol production by removal of fermentation inhibitors. Process Biochem. 2001, 46, 604–607. [Google Scholar] [CrossRef]
- Nahreen, S.; Gupta, R.B. Conversion of the acetone-butanol-ethanol (ABE) mixture to hydrocarbons by catalytic dehydration. Eenergy Fuel 2013, 27, 2116–2125. [Google Scholar] [CrossRef]
- Lopez-Pedrajas, S.; Estevez, R.; Schnee, J.; Schnee, J.; Gaigneaux, E.M.; Luna, D.; Bautista, F.M. Study of the gas-phase glycerol oxidehydration on systems based on transition metals (Co, Fe, V) and aluminium phosphate. Mol. Catal. 2018, 455, 68–77. [Google Scholar] [CrossRef]
- Popa, A.; Sasca, V.; Verdes, O.; Oszko, A. Preparation and catalytic properties of cobalt salts of Keggin type heteropolyacids supported on mesoporous silica. Catal. Today 2018, 306, 233–242. [Google Scholar] [CrossRef]
- Armenta, M.A.; Valdez, R.; Silva-Rodrigo, R.; Olivas, A. Diisopropyl ether production via 2-propanol dehydration using supported iron oxides catalysts. Fuel 2019, 236, 934–941. [Google Scholar] [CrossRef]
- Li, F.Y.; Liu, Z.Z.; Shen, D.J.; Zhang, M.H. Catalytic dehydration of ethanol to ethylene over nickel modified HZSM-5 catalysts. Mod. Chem. Ind. 2013, 33, 54–57. [Google Scholar]
- Wang, W.; Cheng, K.K.; Xue, J.W.; Zhang, J.A. Optimization of ethylene production from ethanol dehydration using Zn-Mn-Co/HZSM-5 by response surface methodology. Chin. J. Biotechnol. 2011, 27, 412–418. [Google Scholar]
- Osman, A.I.; Abu-Dahrieh, J.K.; Rooney, D.W.; Thompson, J.; Halawy, S.A.; Mohamed, M.A. Surface hydrophobicity and acidity effect on alumina catalyst in catalytic methanol dehydration reaction. J. Chem. Technol. Biotechnol. 2017, 92, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Said, E.A.A.; El-Wahab, M.M.M.A.; Abdelhak, M.M. The role of Brønsted acid site strength on the catalytic performance of phosphotungstic acid supported on nano γ-alumina catalysts for the dehydration of ethanol to diethyl ether. React. Kinet. Mech. Catal. 2017, 122, 1–17. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, M.H.; Ye, G. Influence of different metal ions on the rehydration properties of aluminas. Acta Sini Pet Process Sect. 1999, 15, 25–27. [Google Scholar]
- Fei, J.H.; Yang, M.X.; Hou, Z.Y.; Zheng, X.M. Effect of the addition of manganese and zinc on the properties of copper-based catalyst for the synthesis of syngas to dimethyl ether. Eenergy Fuel 2004, 18, 1584–1587. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Phung, T.K.; Lagazzo, A.; Crespo, M.Á.R.; Escribano, V.S.; Busca, G.A. Study of commercial transition aluminas and of their catalytic activity in the dehydration of ethanol. J. Catal. 2014, 311, 102–113. [Google Scholar] [CrossRef]
- Abattista, F.; Delmastro, S.; Gozzelino, G.; Mazza, D.; Vallino, M.; Busca, G.; Lorenzelli, V.; Ramis, G. Surface characterization of amorphous alumina and its crystallization products. J. Catal. 1989, 117, 42–51. [Google Scholar] [CrossRef]
- Morterra, C.; Magnacca, G. A case study: Surface chemistry and surface structure of catalytic aluminas, as studied by vibrational spectroscopy of adsorbed species. Catal. Today 1996, 27, 497–532. [Google Scholar] [CrossRef]
- Khabtou, S.; Chevreau, T.; Lavalley, J.C. Quantitative infrared study of the distinct acidic hydroxyl groups contained in modified Y zeolites. Microporous Mater. 1994, 3, 133–148. [Google Scholar] [CrossRef]
- Zheng, H.D.; Lin, Y.; Wang, M.C.; Liu, J.; Wu, D.; Chen, J.J.; Yin, G.H.; Oyama, S.T.; Zhao, S.Y. The influence of solvent polarity on the dehydrogenation of isoborneol over a Cu/ZnO/Al2O3 catalyst. Catal. Today 2019, 323, 44–53. [Google Scholar] [CrossRef]
- Ashour, S.S. Factors Affecting the Activity and Selectivity of Alumina Catalysts in the Dehydration of 1-Butanol. Adsorpt. Sci. Technol. 2004, 22, 475–483. [Google Scholar] [CrossRef]
- El-Molla, S.A. Surface and catalytic properties of Cr2O3/MgO system doped with manganese and cobalt oxides. Appl. Catal. A Gen. 2005, 280, 189–197. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Tang, Z.; Liu, C.G. Effects of rehydration of alumina on its structural properties, surface acidity, and HDN activity of quinoline. Appl. Catal. A Gen. 2012, 437–438, 79–89. [Google Scholar] [CrossRef]








| Catalyst | SBET (m2/g) | Vad (cm3/g) | r (nm) |
|---|---|---|---|
| γ-Al2O3 | 264.46 | 0.67 | 10.14 |
| Zn-Mn-Co/γ-Al2O3 | 211.70 | 0.57 | 10.72 |
| Catalyst | Weak LAS (μmol/g) | Strong LAS (μmol/g) |
|---|---|---|
| γ-Al2O3 | 240.8 | 153.8 |
| Zn-Mn-Co/γ-Al2O3 | 266.2 | 170.2 |
| Temperature (°C) | Conversion of 1-Butanol (%) | Selectivity of Butenes (%) | Selectivity of DBE (%) | Other Hydrocarbons (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 1- | Trans- | Cis- | Iso- | Total | ||||
| 300 | 71.21 | 65.72 | 3.87 | 6.96 | 0.77 | 77.32 | 21.88 | - |
| 350 | 82.63 | 63.18 | 7.32 | 9.61 | 1.76 | 81.87 | 16.76 | 0.43 |
| 400 | 98.56 | 56.97 | 17.13 | 12.29 | 2.61 | 89.00 | 6.74 | 2.16 |
| 450 | 100 | 47.15 | 20.06 | 17.54 | 2.94 | 87.69 | - | 10.43 |
| Temperature (°C) | Conversion of 1-Butanol (%) | Selectivity of Butenes (%) | Selectivity of DBE (%) | Other Hydrocarbons (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 1- | Trans- | Cis- | Iso- | Total | ||||
| 300 | 80.34 | 65.11 | 5.70 | 9.77 | 0.82 | 81.39 | 17.21 | - |
| 350 | 95.25 | 64.67 | 9.59 | 12.98 | 1.25 | 88.49 | 9.35 | 0.51 |
| 400 | 100 | 57.79 | 20.45 | 15.13 | 2.87 | 96.24 | - | 2.59 |
| 450 | 100 | 33.62 | 25.05 | 24.29 | 3.77 | 86.73 | - | 11.77 |
| WHSV (h−1) | Conversion of 1-Butanol (%) | Selectivity of Butenes (%) | Selectivity of DBE (%) | Other Hydrocarbons (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 1- | Trans- | Cis- | Iso- | Total | ||||
| 1 | 98.01 | 55.58 | 11.21 | 11.05 | 2.48 | 80.32 | 17.89 | 0.37 |
| 2 | 82.63 | 63.18 | 7.31 | 9.62 | 1.76 | 81.87 | 16.76 | 0.43 |
| 3 | 68.56 | 68.69 | 4.82 | 6.70 | 0.83 | 81.04 | 17.22 | 0.39 |
| 4 | 51.04 | 70.75 | 4.28 | 4.53 | 0.13 | 79.69 | 18.52 | 0.32 |
| WHSV (h−1) | Conversion of 1-Butanol (%) | Selectivity of Butenes (%) | Selectivity of DBE (%) | Other Hydrocarbons (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 1- | Trans- | Cis- | Iso- | Total | ||||
| 1 | 100 | 60.60 | 11.58 | 15.29 | 2.90 | 90.37 | 8.05 | 0.48 |
| 2 | 95.25 | 64.67 | 9.59 | 12.98 | 1.25 | 88.49 | 9.35 | 0.51 |
| 3 | 79.45 | 72.65 | 7.16 | 9.69 | 0.74 | 90.24 | 8.87 | 0.41 |
| 4 | 65.49 | 74.97 | 5.74 | 8.10 | 0.36 | 89.17 | 9.01 | 0.48 |
| Run | Temperature (°C) | WHSV (h−1) | Butenes Yield (%) |
|---|---|---|---|
| 1 | 400 | 2.5 | 90.15 |
| 2 | 400 | 2.5 | 90.54 |
| 3 | 350 | 4.0 | 58.80 |
| 4 | 450 | 4.0 | 77.64 |
| 5 | 400 | 2.5 | 89.79 |
| 6 | 450 | 1.0 | 89.23 |
| 7 | 350 | 1.0 | 90.57 |
| 8 | 400 | 2.5 | 89.96 |
| 9 | 400 | 4.0 | 77.21 |
| 10 | 350 | 2.5 | 76.31 |
| 11 | 400 | 1.0 | 99.33 |
| 12 | 400 | 2.5 | 89.82 |
| 13 | 450 | 2.5 | 84.43 |
| 14 | 400 | 2.5 | 88.26 |
| Source | Sum of Squares | F Value | p-Value Prob > F |
|---|---|---|---|
| Model | 1240.91 | 642.51 | <0.0001 |
| X1 (Temperature) | 108.98 | 282.14 | <0.0001 |
| X2 (WHSV) | 715.32 | 1851.86 | <0.0001 |
| X1X2 | 101.65 | 263.15 | <0.0001 |
| X1X1 | 239.26 | 619.40 | <0.0001 |
| X2X2 | 6.15 | 15.93 | <0.0052 |
| Residual | 2.70 | ||
| Lack of Fit | 0.58 | 0.36 | 0.7855 |
| Pure Error | 2.13 | ||
| Cor Total | 1268.49 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Liu, H.-J.; Yan, X.; Zhou, Y.-J.; Lin, Z.-N.; Mi, S.; Cheng, K.-K.; Zhang, J.-A. Efficient Catalytic Dehydration of High-Concentration 1-Butanol with Zn-Mn-Co Modified γ-Al2O3 in Jet Fuel Production. Catalysts 2019, 9, 93. https://doi.org/10.3390/catal9010093
Wu J, Liu H-J, Yan X, Zhou Y-J, Lin Z-N, Mi S, Cheng K-K, Zhang J-A. Efficient Catalytic Dehydration of High-Concentration 1-Butanol with Zn-Mn-Co Modified γ-Al2O3 in Jet Fuel Production. Catalysts. 2019; 9(1):93. https://doi.org/10.3390/catal9010093
Chicago/Turabian StyleWu, Jing, Hong-Juan Liu, Xiang Yan, Yu-Jie Zhou, Zhang-Nan Lin, Shuo Mi, Ke-Ke Cheng, and Jian-An Zhang. 2019. "Efficient Catalytic Dehydration of High-Concentration 1-Butanol with Zn-Mn-Co Modified γ-Al2O3 in Jet Fuel Production" Catalysts 9, no. 1: 93. https://doi.org/10.3390/catal9010093
APA StyleWu, J., Liu, H.-J., Yan, X., Zhou, Y.-J., Lin, Z.-N., Mi, S., Cheng, K.-K., & Zhang, J.-A. (2019). Efficient Catalytic Dehydration of High-Concentration 1-Butanol with Zn-Mn-Co Modified γ-Al2O3 in Jet Fuel Production. Catalysts, 9(1), 93. https://doi.org/10.3390/catal9010093