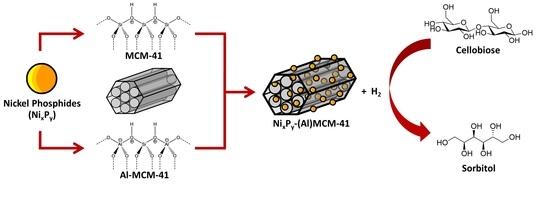
One-Pot Catalytic Conversion of Cellobiose to Sorbitol over Nickel Phosphides Supported on MCM-41 and Al-MCM-41
Abstract
:1. Introduction
2. Results and Discussion
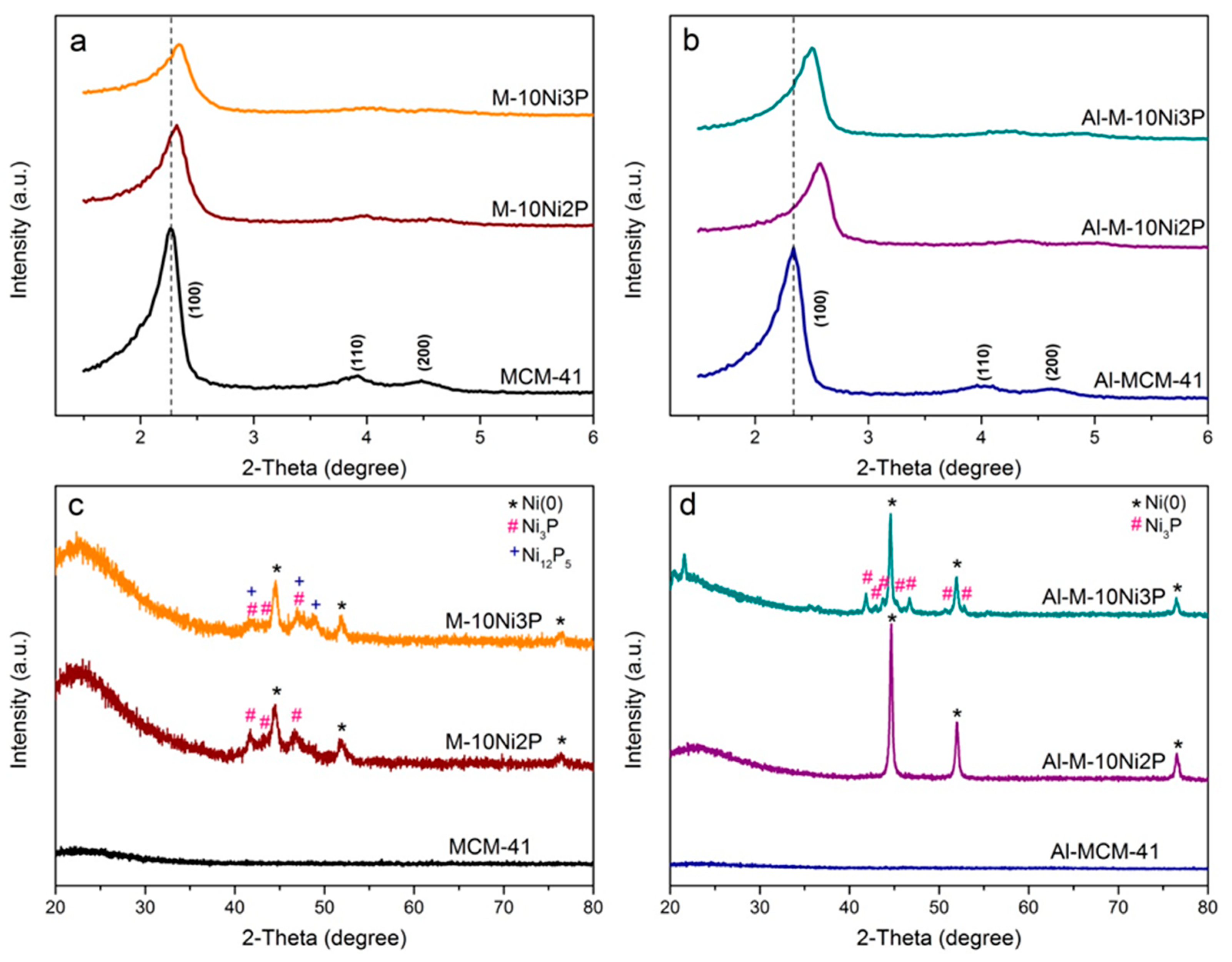
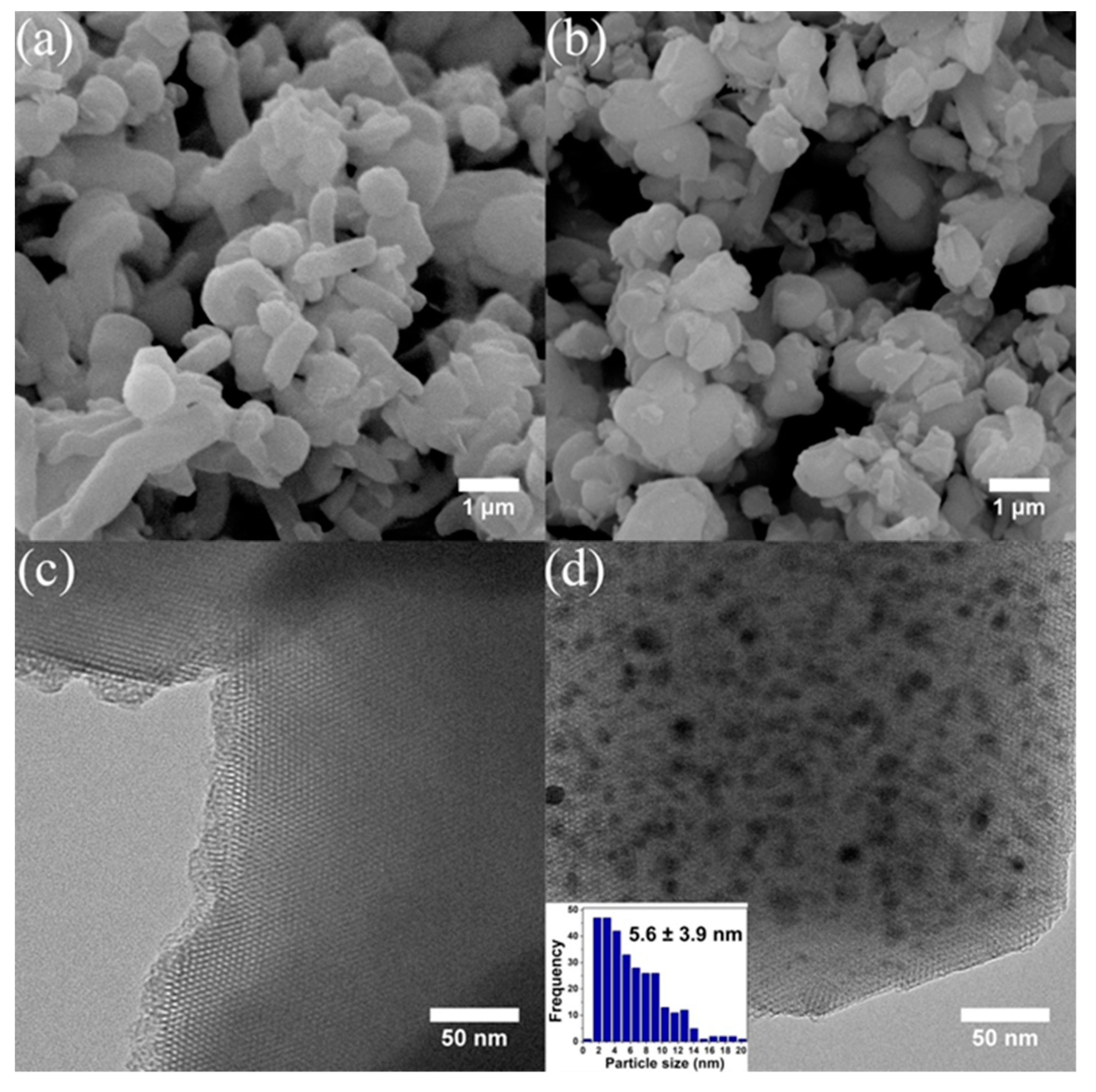
2.1. Characterization of the Catalysts
2.2. Catalytic Conversion of Cellobiose to Sorbitol
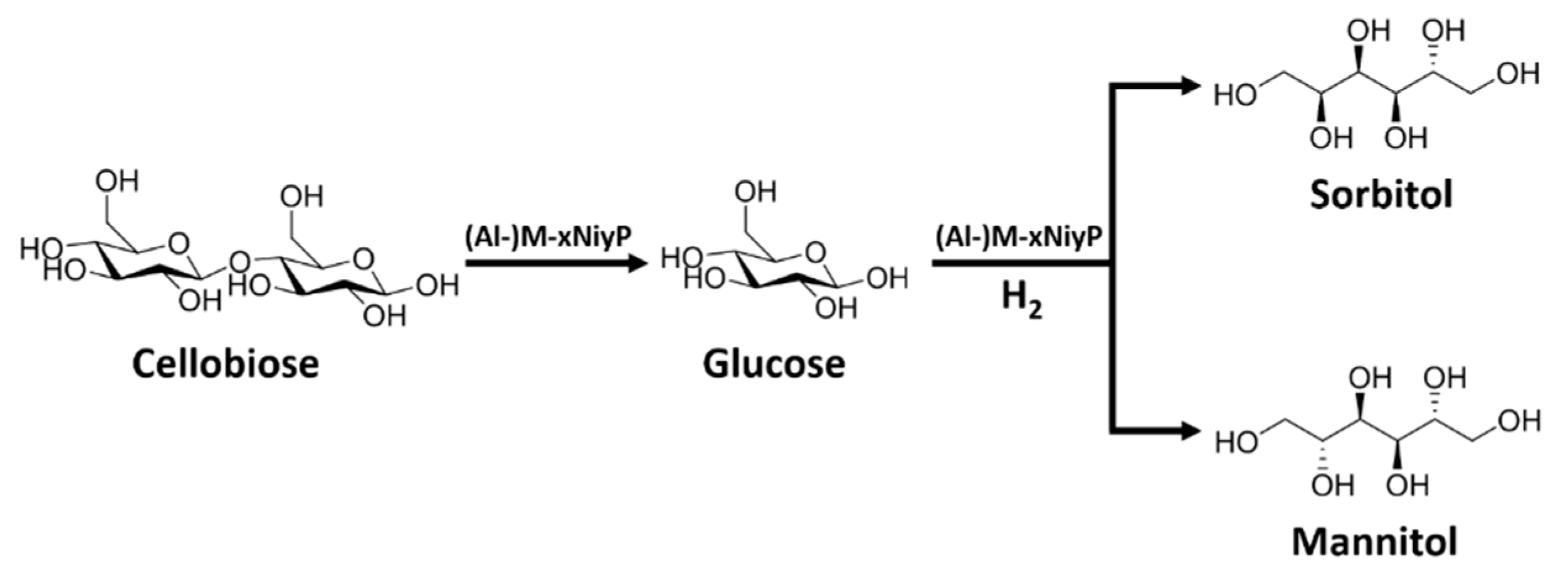
2.3. Catalytic Mechanism
2.4. Comparison of Catalytic Performance of Various Metal-Based Catalysts
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Nickel Phosphide Nanoparticles Supported on MCM-41 and Al-MCM-41
3.3. Materials Characterization
3.4. Catalytic Cellobiose Conversion and Product Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yabushita, M.; Kobayashi, H.; Fukuoka, A. Catalytic transformation of cellulose into platform chemicals. Appl. Catal. B 2014, 145, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Fukuoka, A. Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem. 2013, 15, 1740–1763. [Google Scholar] [CrossRef]
- Dhepe, P.L.; Fukuoka, A. Cellulose conversion under heterogeneous catalysis. ChemSusChem 2008, 1, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Palkovits, R.; Schüth, F. Depolymerization of cellulose using solid catalysts in ionic liquids. Angew. Chem. Int. Ed. 2008, 47, 8047–8050. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Cheng, K.; Zhang, H.; Deng, W.; Zhang, Q.; Wang, Y. Mesoporous h-zsm-5 as an efficient catalyst for conversions of cellulose and cellobiose into methyl glucosides in methanol. Catal. Today 2016, 274, 60–66. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Lee, Y.-Y.; Peng, W.-H.; Wu, K.C.W. Cellulosic conversion in ionic liquids (ils): Effects of h2o/cellulose molar ratios, temperatures, times, and different ils on the production of monosaccharides and 5-hydroxymethylfurfural (hmf). Catal. Today 2011, 174, 65–69. [Google Scholar] [CrossRef]
- An, D.; Ye, A.; Deng, W.; Zhang, Q.; Wang, Y. Selective conversion of cellobiose and cellulose into gluconic acid in water in the presence of oxygen, catalyzed by polyoxometalate-supported gold nanoparticles. Chem. Eur. J. 2012, 18, 2938–2947. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, A.; Zhang, T. A new 3d mesoporous carbon replicated from commercial silica as a catalyst support for direct conversion of cellulose into ethylene glycol. Chem. Commun. 2010, 46, 862–864. [Google Scholar] [CrossRef]
- Deng, W.; Liu, M.; Tan, X.; Zhang, Q.; Wang, Y. Conversion of cellobiose into sorbitol in neutral water medium over carbon nanotube-supported ruthenium catalysts. J. Catal. 2010, 271, 22–32. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.-B.; Wu, S.-B.; Liu, Y. Advances in the catalytic production and utilization of sorbitol. Ind. Eng. Chem. Res. 2013, 52, 11799–11815. [Google Scholar] [CrossRef]
- Fukuoka, A.; Dhepe, P.L. Catalytic conversion of cellulose into sugar alcohols. Angew. Chem. Int. Ed. Engl. 2006, 45, 5161–5163. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Zhao, C.; Luo, C.; Dyson, P.J.; Liu, H.; Kou, Y. One-step conversion of cellobiose to c6-alcohols using a ruthenium nanocluster catalyst. J. Am. Chem. Soc. 2006, 128, 8714–8715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, S.; Li, B.; Zhang, H. Direct conversion of cellobiose into sorbitol and catalyst deactivation mechanism. Catal. Commun. 2012, 29, 180–184. [Google Scholar] [CrossRef]
- Almeida, J.M.A.R.; Da Vià, L.; Demma Carà, P.; Carvalho, Y.; Romano, P.N.; Peña, J.A.O.; Smith, L.; Sousa-Aguiar, E.F.; Lopez-Sanchez, J.A. Screening of mono- and bi-functional catalysts for the one-pot conversion of cellobiose into sorbitol. Catal. Today 2017, 279, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Geboers, J.; Van de Vyver, S.; Carpentier, K.; Jacobs, P.; Sels, B. Efficient hydrolytic hydrogenation of cellulose in the presence of ru-loaded zeolites and trace amounts of mineral acid. Chem. Commun. (Camb.) 2011, 47, 5590–5592. [Google Scholar] [CrossRef]
- Kusserow, B.; Schimpf, S.; Claus, P. Hydrogenation of glucose to sorbitol over nickel and ruthenium catalysts. Adv. Synth. Catal. 2003, 345, 289–299. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Wu, Q.; Zhang, C.; Yu, Y.; Lan, M.; Wei, X.; Ying, Z.; Liu, T.; Liang, G.; et al. Synthesis of ni/mesoporous zsm-5 for direct catalytic conversion of cellulose to hexitols: Modulating the pore structure and acidic sites via a nanocrystalline cellulose template. Green Chem. 2016, 18, 3315–3323. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Liu, Y.; Li, B. Hydrogenation of glucose over reduced ni/cu/al hydrotalcite precursors. Catal. Commun. 2013, 35, 23–26. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Shi, H. Deoxygenation of methyl laurate as a model compound to hydrocarbons on ni2p/sio2, ni2p/mcm-41, and ni2p/sba-15 catalysts with different dispersions. Energy Fuels 2013, 27, 3400–3409. [Google Scholar] [CrossRef]
- Koranyi, T.; Vit, Z.; Poduval, D.; Ryoo, R.; Kim, H.; Hensen, E. Sba-15-supported nickel phosphide hydrotreating catalysts. J. Catal. 2008, 253, 119–131. [Google Scholar] [CrossRef]
- Oyama, S.; Lee, Y. The active site of nickel phosphide catalysts for the hydrodesulfurization of 4,6-dmdbt. J. Catal. 2008, 258, 393–400. [Google Scholar] [CrossRef]
- Alexander, A.M.; Hargreaves, J.S. Alternative catalytic materials: Carbides, nitrides, phosphides and amorphous boron alloys. Chem. Soc. Rev. 2010, 39, 4388–4401. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Kobayashi, H.; Hara, K.; Fukuoka, A. Phase change of nickel phosphide catalysts in the conversion of cellulose into sorbitol. ChemSusChem 2012, 5, 920–926. [Google Scholar] [CrossRef]
- Mathew, A.; Parambadath, S.; Kim, S.Y.; Ha, H.M.; Ha, C.-S. Diffusion mediated selective adsorption of zn2+ from artificial seawater by mcm-41. Microporous Mesoporous Mater. 2016, 229, 124–133. [Google Scholar] [CrossRef]
- Song, H.; Wang, J.; Wang, Z.; Song, H.; Li, F.; Jin, Z. Effect of titanium content on dibenzothiophene HDS performance over Ni2P/Ti-MCM-41 catalyst. J. Catal. 2014, 311, 257–265. [Google Scholar] [CrossRef]
- Wang, R.; Smith, K.J. The effect of preparation conditions on the properties of high-surface area ni2p catalysts. Appl. Catal. A 2010, 380, 149–164. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (technical report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Kadi, M.W.; Hameed, A.; Mohamed, R.M.; Ismail, I.M.I.; Alangari, Y.; Cheng, H.-M. The effect of pt nanoparticles distribution on the removal of cyanide by tio2 coated al-mcm-41 in blue light exposure. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710. [Google Scholar] [CrossRef]
- Gokulakrishnan, N.; Pandurangan, A.; Somanathan, T.; Sinha, P.K. Uptake of decontaminating agent from aqueous solution: A study on adsorption behaviour of oxalic acid over al-mcm-41 adsorbents. J. Porous Mater. 2010, 17, 763–771. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Zhang, Y.; Zhao, Y.; Liew, K.; Hong, J. Ru catalysts supported on al–sba-15 with high aluminum content and their bifunctional catalytic performance in fischer–tropsch synthesis. Catal. Sci. Technol. 2014, 4, 1005–1011. [Google Scholar] [CrossRef]
- Mokaya, R. Post-synthesis grafting of al onto mcm-41. Chem. Commun. 1997, 22, 2185–2186. [Google Scholar] [CrossRef]
- Yang, Y.; Ochoa-Hernández, C.; Pizarro, P.; de la Peña O’Shea, V.A.; Coronado, J.M.; Serrano, D.P. Influence of the ni/p ratio and metal loading on the performance of nixpy/sba-15 catalysts for the hydrodeoxygenation of methyl oleate. Fuel 2015, 144, 60–70. [Google Scholar] [CrossRef]
- Wu, S.-K.; Lai, P.-C.; Lin, Y.-C. Atmospheric hydrodeoxygenation of guaiacol over nickel phosphide catalysts: Effect of phosphorus composition. Catal. Lett. 2014, 144, 878–889. [Google Scholar] [CrossRef]
- Prins, R. Hydrogen spillover. Facts and fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.N.; Wang, A.Q.; Zheng, M.Y.; Zhang, T. Selective transformation of cellulose into sorbitol by using a bifunctional nickel phosphide catalyst. ChemSusChem 2010, 3, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, A.; Sankaranarayanan, T.M.; Gómez, G.; Moreno, I.; Coronado, J.M.; Pizarro, P.; Serrano, D.P. Evaluation of transition metal phosphides supported on ordered mesoporous materials as catalysts for phenol hydrodeoxygenation. Green Chem. 2016, 18, 1938–1951. [Google Scholar] [CrossRef]
- Negahdar, L.; Oltmanns, J.U.; Palkovits, S.; Palkovits, R. Kinetic investigation of the catalytic conversion of cellobioseto sorbitol. Appl. Catal. B 2014, 147, 677–683. [Google Scholar] [CrossRef]
- Liu, M.; Deng, W.; Zhang, Q.; Wang, Y.; Wang, Y. Polyoxometalate-supported ruthenium nanoparticles as bifunctional heterogeneous catalysts for the conversions of cellobiose and cellulose into sorbitol under mild conditions. Chem. Commun. (Camb.) 2011, 47, 9717–9719. [Google Scholar] [CrossRef]
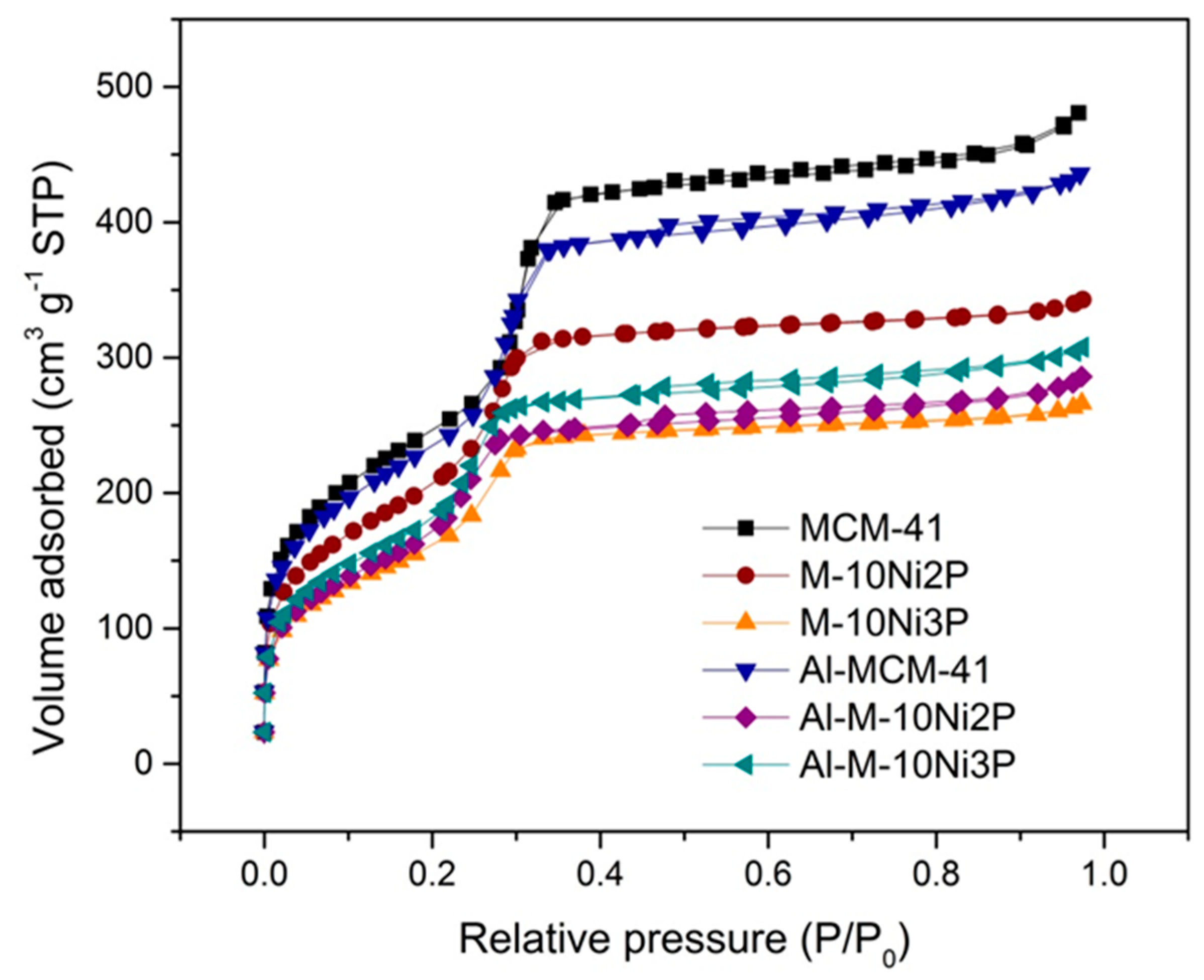




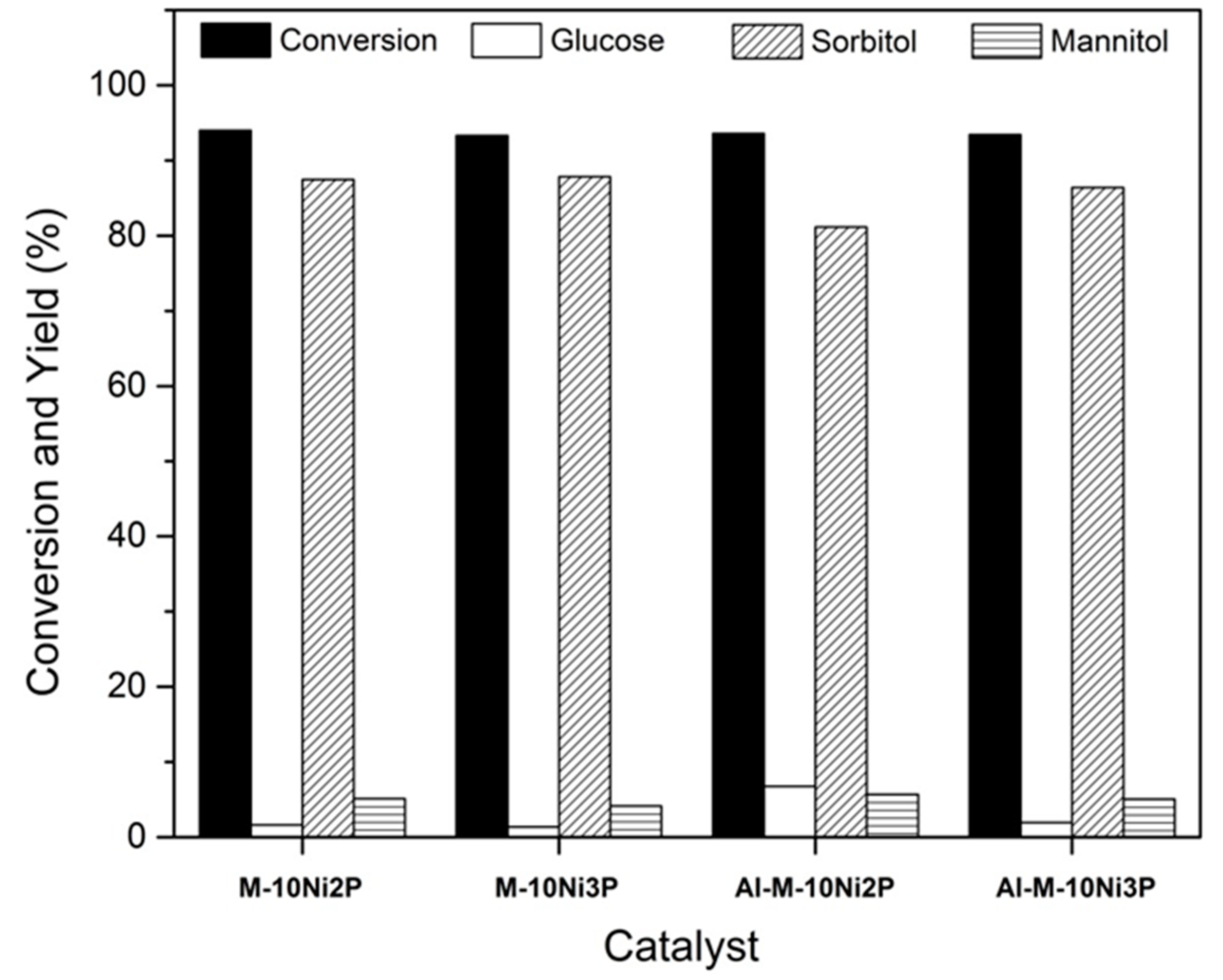

| Materials | BET Surface Area a (m2 g−1) | Internal Surface Area b (m2 g−1) | External Surface Area b (m2 g−1) | Pore Volume c (cm3 g−1) | Pore Diameter c (nm) |
|---|---|---|---|---|---|
| MCM-41 | 900 | 865 | 71 | 0.68 | 2.43 |
| M-10Ni2P | 771 | 747 | 27 | 0.43 | 2.43 |
| M-10Ni3P | 577 | 552 | 23 | 0.33 | 2.43 |
| Al-MCM-41 | 843 | 767 | 84 | 0.59 | 2.43 |
| Al-M-10Ni2P | 622 | 570 | 48 | 0.35 | 2.43 |
| Al-M-10Ni3P | 643 | 616 | 62 | 0.39 | 2.43 |
| Materials | Ni (wt.%) a | P (wt.%) a | Total Acidity (μmol g−1) b |
|---|---|---|---|
| MCM-41 | - | - | 2 |
| M-10Ni2P | 9.45 | 1.80 | 85 |
| M-10Ni3P | 9.62 | 2.58 | 30 |
| Al-MCM-41 | - | - | 6 |
| Al-M-10Ni2P | 9.59 | 1.83 | 125 |
| Al-M-10Ni3P | 9.85 | 2.62 | 53 |
| Catalyst | Time (h) | Temp. (°C) | Pressure of H2 (MPa) | Cellobiose Conversion (%) | Sorbitol Yield (%) | Reference |
|---|---|---|---|---|---|---|
| M-10Ni3P | 3 | 150 | 4 | 95.0 | 43.5 | This work |
| M-10Ni3P | 3 | 180 | 4 | 93.3 | 87.8 | This work |
| Pd with pH 2 | 12 | 120 | 4 | 100 | 0 | [13] |
| Pt with pH 2 | 12 | 120 | 4 | 100 | 18.5 | [13] |
| Ru with pH 2 | 12 | 120 | 4 | 100 | 100 | [13] |
| Ru/C | 12 | 120 | 4 | 100 | <1 | [13] |
| Ru/Cs3PW12O40 | 6 | 140 | 2 | 100 | 86 | [40] |
| Ru/Cs2HPW12O40 | 6 | 140 | 2 | 100 | 93 | [40] |
| Ru/C + 0.05 wt.% H3PO4 | 1 | 185 | 3 | n.r. a | 87.1 | [14] |
| 3%RuNPs/Amberlyst 15 | 5 | 150 | 4 | 100 | 81.6 | [15] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anutrasakda, W.; Eiamsantipaisarn, K.; Jiraroj, D.; Phasuk, A.; Tuntulani, T.; Liu, H.; Tungasmita, D.N. One-Pot Catalytic Conversion of Cellobiose to Sorbitol over Nickel Phosphides Supported on MCM-41 and Al-MCM-41. Catalysts 2019, 9, 92. https://doi.org/10.3390/catal9010092
Anutrasakda W, Eiamsantipaisarn K, Jiraroj D, Phasuk A, Tuntulani T, Liu H, Tungasmita DN. One-Pot Catalytic Conversion of Cellobiose to Sorbitol over Nickel Phosphides Supported on MCM-41 and Al-MCM-41. Catalysts. 2019; 9(1):92. https://doi.org/10.3390/catal9010092
Chicago/Turabian StyleAnutrasakda, Wipark, Kanyanok Eiamsantipaisarn, Duangkamon Jiraroj, Apakorn Phasuk, Thawatchai Tuntulani, Haichao Liu, and Duangamol Nuntasri Tungasmita. 2019. "One-Pot Catalytic Conversion of Cellobiose to Sorbitol over Nickel Phosphides Supported on MCM-41 and Al-MCM-41" Catalysts 9, no. 1: 92. https://doi.org/10.3390/catal9010092
APA StyleAnutrasakda, W., Eiamsantipaisarn, K., Jiraroj, D., Phasuk, A., Tuntulani, T., Liu, H., & Tungasmita, D. N. (2019). One-Pot Catalytic Conversion of Cellobiose to Sorbitol over Nickel Phosphides Supported on MCM-41 and Al-MCM-41. Catalysts, 9(1), 92. https://doi.org/10.3390/catal9010092