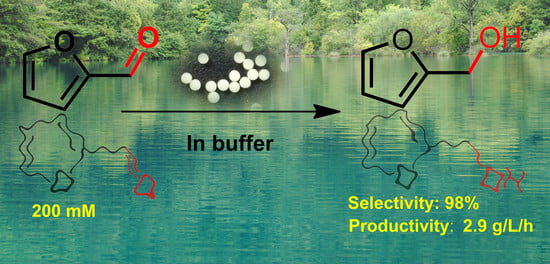
Selective Synthesis of Furfuryl Alcohol from Biomass-Derived Furfural Using Immobilized Yeast Cells
Abstract
:1. Introduction
2. Results and Discussion
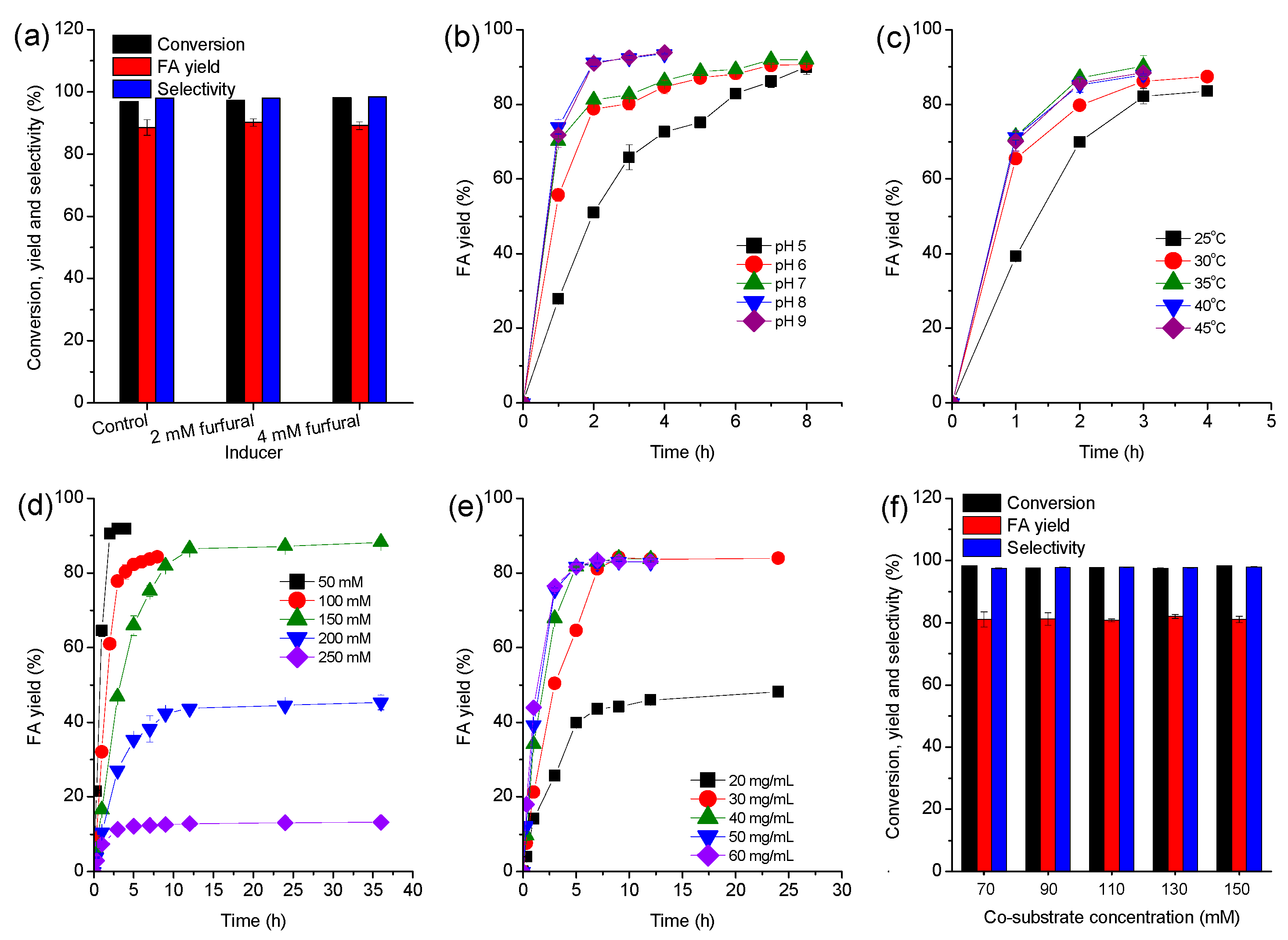
2.1. Process Optimization
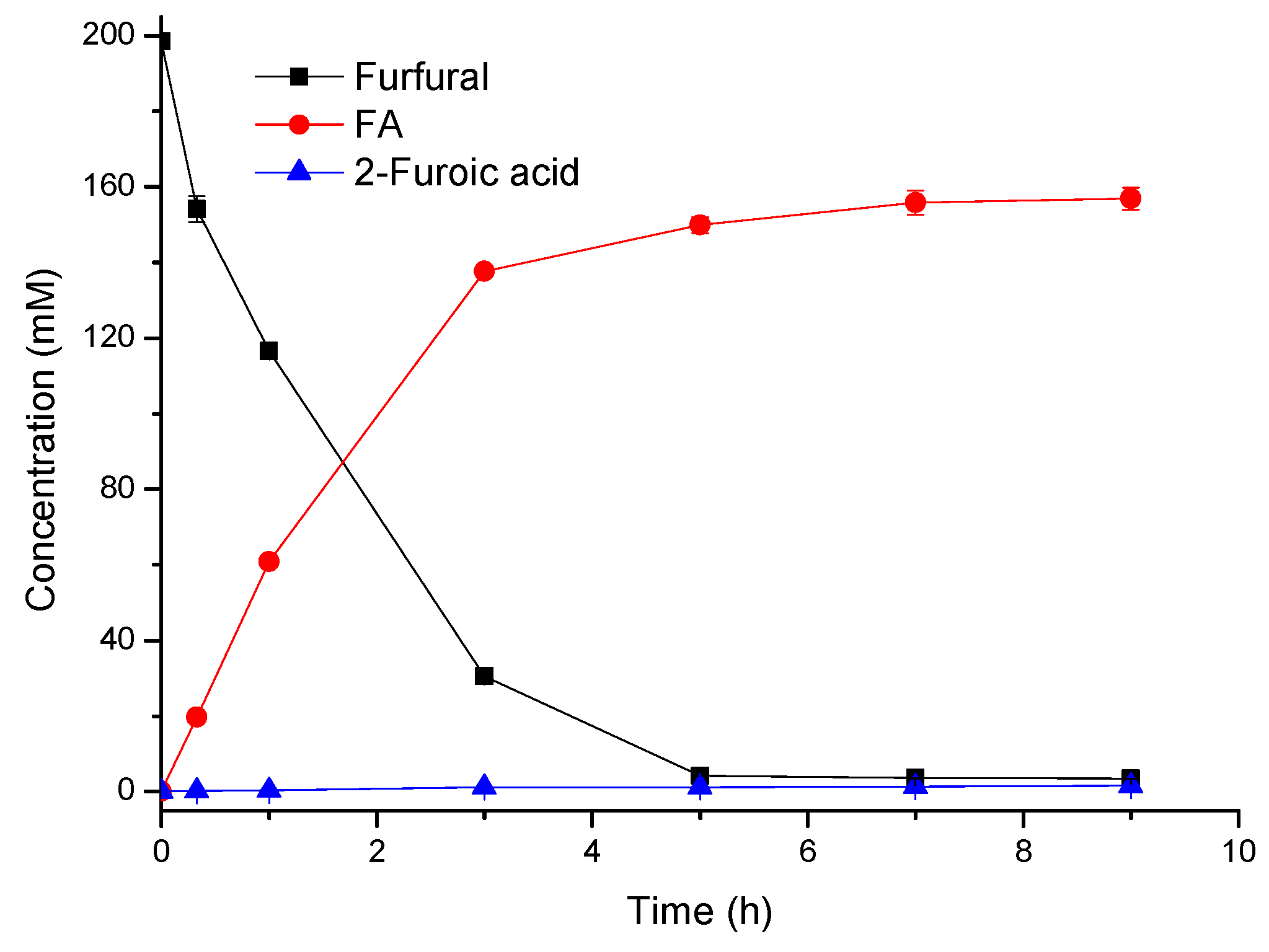
2.2. Improved Synthesis of FA
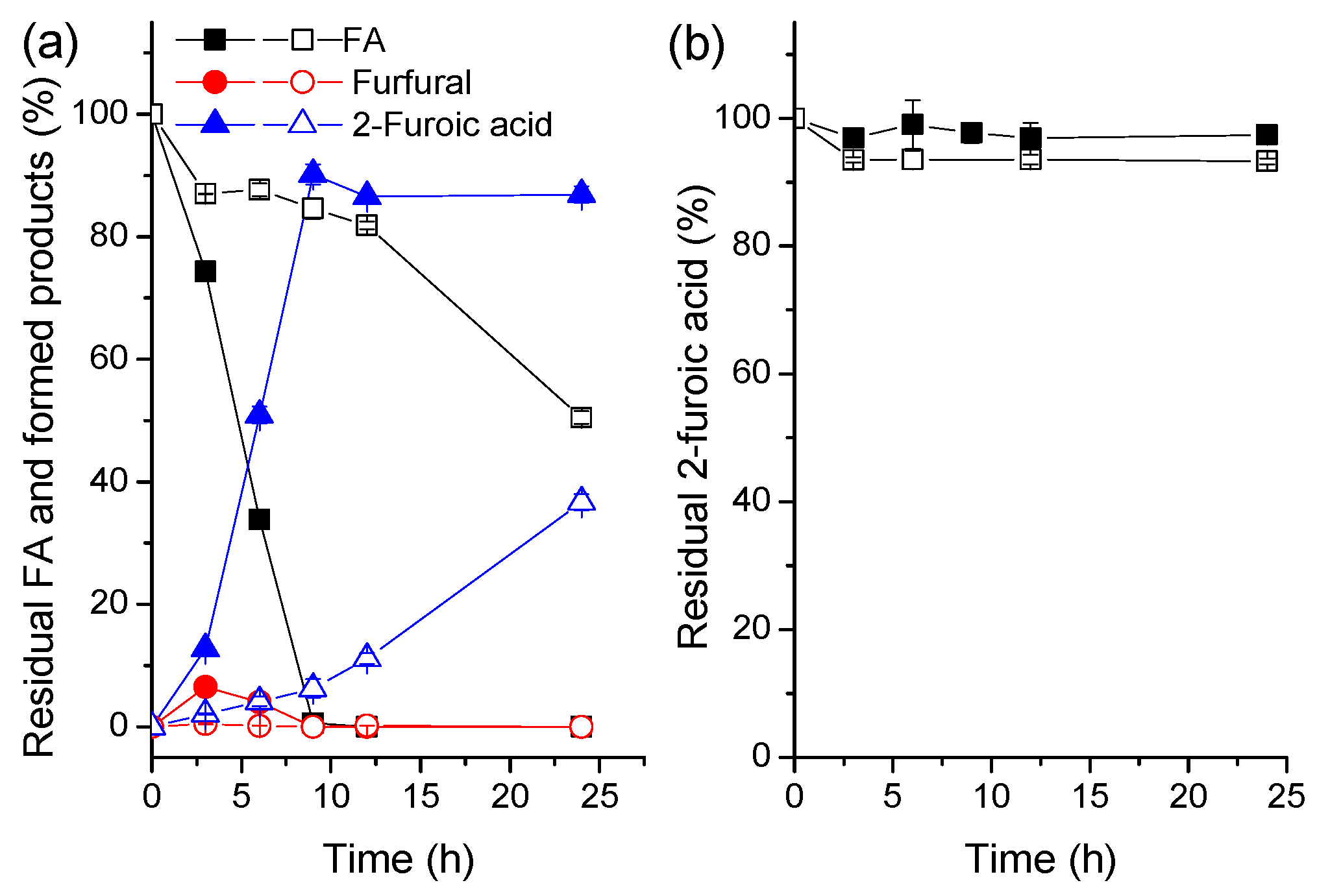
2.3. Scale-Up Synthesis and Separation of FA
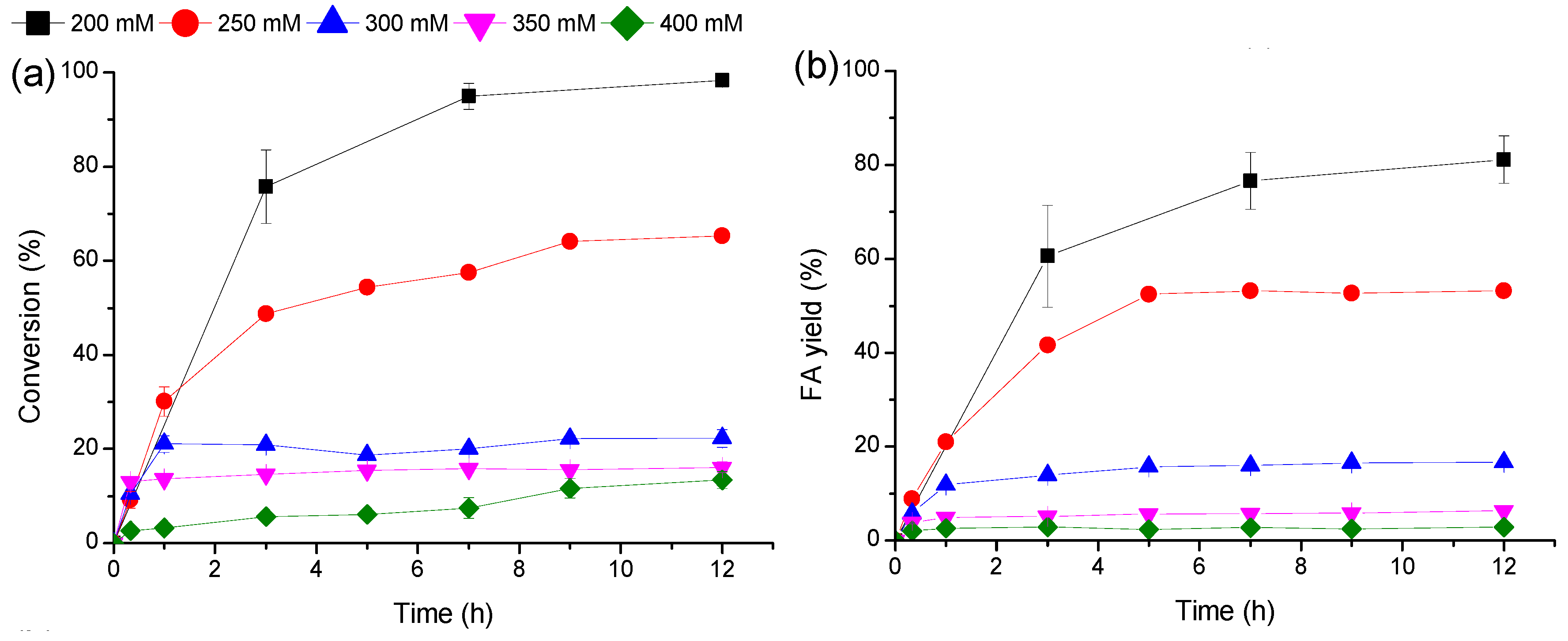
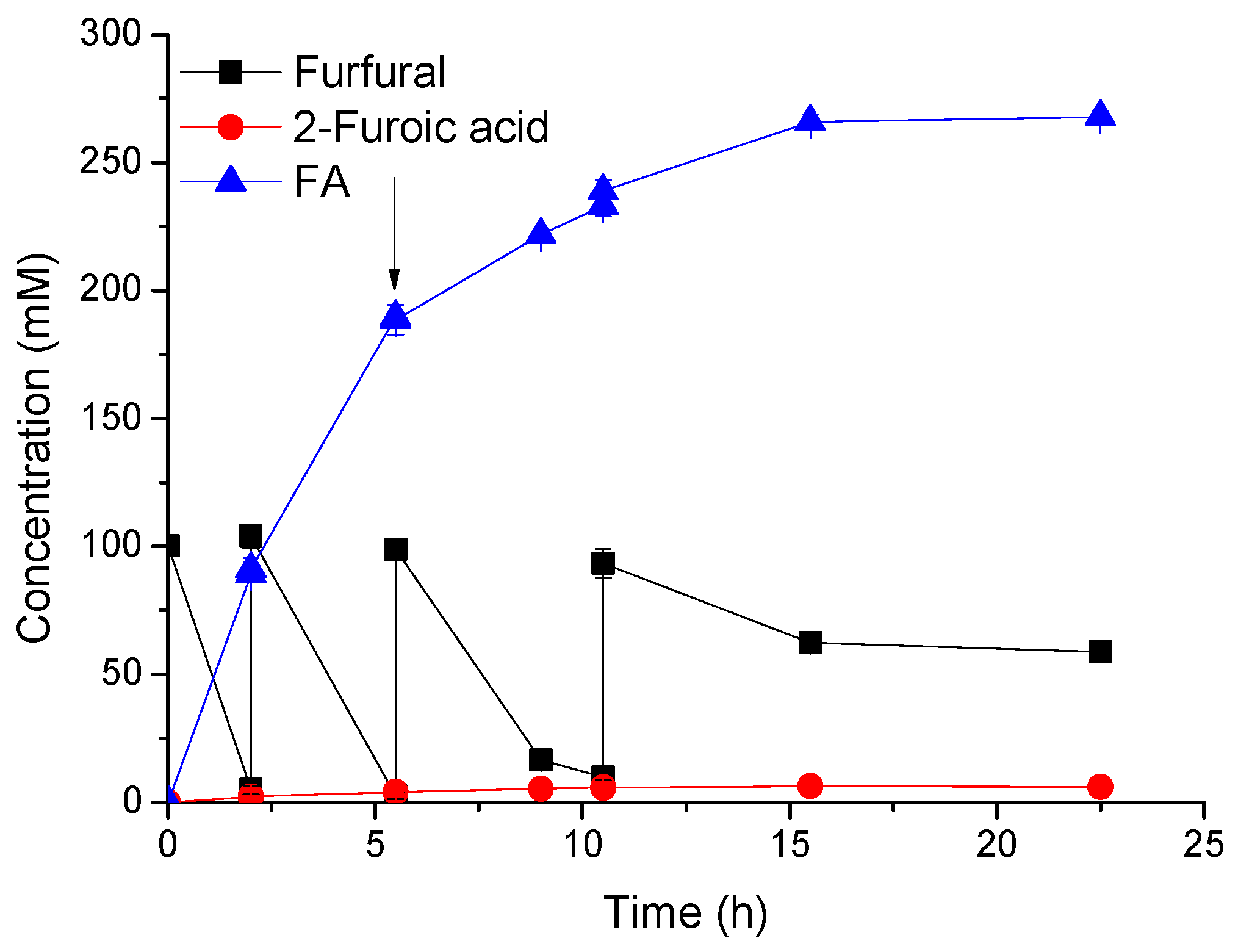
2.4. FA Synthesis by a Fed-Batch Strategy
3. Materials and Methods
3.1. Materials
3.2. Cultivation and Immobilization of Microbial Cells
3.3. General Procedure for the Synthesis of FA
3.4. Scale-Up Production and Extraction of FA
3.5. FA Synthesis via Fed-Batch Feeding of Substrate
3.6. HPLC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates-the US department of energy’s “top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sadaba, I.; Lopez Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Sharma, R.V.; Das, U.; Sammynaiken, R.; Dalai, A.K. Liquid phase chemo-selective catalytic hydrogenation of furfural to furfuryl alcohol. Appl. Catal. A Gen. 2013, 454, 127–136. [Google Scholar] [CrossRef]
- Liu, D.; Zemlyanov, D.; Wu, T.; Lobo-Lapidus, R.J.; Dumesic, J.A.; Miller, J.T.; Marshall, C.L. Deactivation mechanistic studies of copper chromite catalyst for selective hydrogenation of 2-furfuraldehyde. J. Catal. 2013, 299, 336–345. [Google Scholar] [CrossRef]
- Gupta, K.; Rai, R.K.; Singh, S.K. Metal catalysts for the efficient transformation of biomass-derived HMF and furfural to value added chemicals. ChemCatChem 2018, 10, 2326–2349. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Pereira, P.C. Biocatalysis engineering: The big picture. Chem. Soc. Rev. 2017, 46, 2678–2691. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Guajardo, N. Biocatalytic valorization of furans: Opportunities for inherently unstable substrates. ChemSusChem 2017, 10, 4123–4134. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Liu, Z.L.; Slininger, P.J.; Dien, B.S.; Berhow, M.A.; Kurtzman, C.P.; Gorsich, S.W. Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. J. Ind. Microbiol. Biotechnol. 2004, 31, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Slininger, P.J.; Gorsich, S.W. Enhanced biotransformation of furfural and hydroxymethylfurfural by newly developed ethanologenic yeast strains. Appl. Biochem. Biotechnol. 2005, 121, 451–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, B.; Ezeji, T.C. Biotransformation of furfural and 5-hydroxymethyl furfural (HMF) by Clostridium acetobutylicum ATCC 824 during butanol fermentation. New Biotechnol. 2012, 29, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Boopathy, R.; Bokang, H.; Daniels, L. Biotransformation of furfural and 5-hydroxymethyl furfural by enteric bacteria. J. Ind. Microbiol. 1993, 11, 147–150. [Google Scholar] [CrossRef]
- Liu, Z.L.; Moon, J.; Andersh, B.J.; Slininger, P.J.; Weber, S. Multiple gene-mediated NAD(P)H-dependent aldehyde reduction is a mechanism of in situ detoxification of furfural and 5-hydroxymethylfurfural by saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2008, 81, 743–753. [Google Scholar] [PubMed]
- Wang, X.; Yomano, L.P.; Lee, J.Y.; York, S.W.; Zheng, H.; Mullinnix, M.T.; Shanmugam, K.T.; Ingram, L.O. Engineering furfural tolerance in Escherichia coli improves the fermentation of lignocellulosic sugars into renewable chemicals. Proc. Natl. Acad. Sci. USA 2013, 110, 4021–4026. [Google Scholar] [CrossRef]
- Boopathy, R. Anaerobic biotransformation of furfural to furfuryl alcohol by a methanogenic archaebacterium. Int. Biodeterior. Biodegrad. 2009, 63, 1070–1072. [Google Scholar] [CrossRef]
- Zhang, D.; Ong, Y.L.; Li, Z.; Wu, J.C. Biological detoxification of furfural and 5-hydroxyl methyl furfural in hydrolysate of oil palm empty fruit bunch by Enterobacter sp. FDS8. Biochem. Eng. J. 2013, 72, 77–82. [Google Scholar] [CrossRef]
- Li, Y.M.; Zhang, X.Y.; Li, N.; Xu, P.; Lou, W.Y.; Zong, M.H. Biocatalytic reduction of HMF to 2,5-bis(hydroxymethyl)furan by HMF-tolerant whole cells. ChemSusChem 2017, 10, 372–378. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-C.; Jiang, C.-X.; Jiang, J.-W.; Di, J.-H.; Liu, F.; Ding, Y.; Qing, Q.; Ma, C.-L. One-pot chemo-enzymatic synthesis of furfuralcohol from xylose. Bioresour. Technol. 2017, 238, 698–705. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ding, Y.; Ma, C.; Di, J.; Jiang, C.; Li, A. One-pot conversion of biomass-derived xylose to furfuralcohol by a chemo-enzymatic sequential acid-catalyzed dehydration and bioreduction. Green Chem. 2017, 19, 3844–3850. [Google Scholar] [CrossRef]
- Yan, Y.; Bu, C.; He, Q.; Zheng, Z.; Ouyang, J. Efficient bioconversion of furfural to furfuryl alcohol by Bacillus coagulans NL01. RSC Adv. 2018, 8, 26720–26727. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zong, M.H.; Li, N. Whole-cell biocatalytic selective oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid. Green Chem. 2017, 19, 4544–4551. [Google Scholar] [CrossRef]
- Xu, Z.H.; Cheng, A.D.; Xing, X.P.; Zong, M.H.; Bai, Y.P.; Li, N. Improved synthesis of 2,5-bis(hydroxymethyl)furan from 5-hydroxymethylfurfural using acclimatized whole cells entrapped in calcium alginate. Bioresour. Technol. 2018, 262, 177–183. [Google Scholar] [CrossRef]
- Almeida, J.R.M.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Meynial-Salles, I.; Dorotyn, S.; Soucaille, P. A new process for the continuous production of succinic acid from glucose at high yield, titer, and productivity. Biotechnol. Bioeng. 2008, 99, 129–135. [Google Scholar] [CrossRef]
- Villa, G.P.; Bartroli, R.; López, R.; Guerra, M.; Enrique, M.; Peñas, M.; Rodríquez, E.; Redondo, D.; Jglesias, I.; Díaz, M. Microbial transformation of furfural to furfuryl alcohol by Saccharomyces cerevisiae. Acta Biotechnol. 1992, 12, 509–512. [Google Scholar] [CrossRef]
- Vassileva, A.; Burhan, N.; Beschkov, V.; Spasova, D.; Radoevska, S.; Ivanova, V.; Tonkova, A. Cyclodextrin glucanotransferase production by free and agar gel immobilized cells of Bacillus circulans ATCC 21783. Process Biochem. 2003, 38, 1585–1591. [Google Scholar] [CrossRef]
- He, Y.-C.; Xu, J.-H.; Su, J.-H.; Zhou, L. Bioproduction of glycolic acid from glycolonitrile with a new bacterial isolate of Alcaligenes sp. ECU0401. Appl. Biochem. Biotechnol. 2010, 160, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Ikeda, K.; Ijima, H.; Kawakami, K. Fabrication of poly(vinyl alcohol) hydrogel beads crosslinked using sodium sulfate for microorganism immobilization. Process Biochem. 2011, 46, 566–571. [Google Scholar] [CrossRef]
- Aparecida de Assis, S.; Ferreira, B.S.; Fernandes, P.; Guaglianoni, D.G.; Cabral, J.M.S.; Oliveira, O.M.M.F. Gelatin-immobilized pectinmethylesterase for production of low methoxyl pectin. Food Chem. 2004, 86, 333–337. [Google Scholar] [CrossRef]

| Polymers (w/v) | Polymer Amount (mg) | Losses (%) | ||
|---|---|---|---|---|
| Furfural | FA | 2-Furoic Acid | ||
| Control | none | 4 ± 2 | 3 ± 1 | n.d. 1 |
| Calcium alginate (2.5%) | 150 | 7 ± 2 | 10 ± 1 | 2 ± 1 |
| 350 | 14 ± 1 | 15 ± 3 | 3 ± 3 | |
| Agar (2%) | 150 | 10 ± 1 | 10 ± 0 | 9 ± 1 |
| 350 | 16 ± 1 | 15 ± 1 | 14 ± 0 | |
| Gelatin (15%) | 350 | 26 ± 1 | 14 ± 0 | 9 ± 2 |
| Carrageenan (3%) | 350 | 10 ± 3 | 11 ± 0 | 15 ± 0 |
| Poly(vinyl alcohol) (10%) | 350 | 21 ± 1 | 18 ± 2 | 9 ± 2 |
| Biocatalyst | Reaction Conditions | Substrate Concentration (mM) | Time (h) | C/Y 1 (%) | Selectivity (%) | Productivity 2 (g/L/h) | Ref. |
|---|---|---|---|---|---|---|---|
| Enterobacter sp. FDS8 | 4.6 mg (dry weight)/mL cells, pH 7, 30 °C, in lignocellulosic hydrolysate | 19 | 3 | n.a. 3 | n.a. | 0.5 4 | [20] |
| Clostridium acetobutylicum ATCC 824 | 1% of the inoculate culture, pH 5.6, 30 °C, in a sugar cane molasses medium | 17 | 12 | 100 (C) | 100 | 0.1 | [15] |
| S. cerevisiae 307-12-F40 | 1% of the inoculate culture, 30 °C, in a synthetic complete medium containing 20 mM glucose | 30 | 30 | 70 (Y) | n.a. | <0.1 4 | [14] |
| S. cerevisiae 354 | Cell concentration is not available, pH 6.7, 35 °C, in a P2 medium containing approximately 333 mM glucose | 62.5 | 6 | 100 (Y) | 100 | 1.0 | [29] |
| B. coagulans NL01 | Molglucose:Molfurfural = 5:2, 9 mg (dry weight)/mL cells, pH 7, 50 °C, in phosphate buffer | 42 | 3 | 96 (C) | 87 | 1.3 4 | [24] |
| E. coli CCZU-A13 | Molglucose:Molfurfural = 1:1, 100 mg (wet weight)/mL cells, pH 6.5, 30 °C, in KH2PO4-K2HPO4 buffer | 200 | 12 | 94 (Y) | n.a. | 1.5 | [23] |
| 300 | 12 | 74 (Y) | n.a. | 1.8 | [23] | ||
| E. coli CCZU-K14 | Molglucose:Molfurfural = 3:2, 400 mM xylose, 100 mg (wet weight)/mL cells, pH 6.5, 30 °C, in KH2PO4-K2HPO4 buffer | 200 | 24 | 100 (Y) | 100 | 0.8 | [22] |
| M. guilliermondii SC1103 | Molglucose:Molfurfural = 7:15, 50 mg (wet weight)/mL cells, pH 8, 35 °C, in Tris-HCl buffer | 200 | 7 | 79 (Y) | 99 | 2.2/2.9 (5 h) | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-Y.; Xu, Z.-H.; Zong, M.-H.; Wang, C.-F.; Li, N. Selective Synthesis of Furfuryl Alcohol from Biomass-Derived Furfural Using Immobilized Yeast Cells. Catalysts 2019, 9, 70. https://doi.org/10.3390/catal9010070
Zhang X-Y, Xu Z-H, Zong M-H, Wang C-F, Li N. Selective Synthesis of Furfuryl Alcohol from Biomass-Derived Furfural Using Immobilized Yeast Cells. Catalysts. 2019; 9(1):70. https://doi.org/10.3390/catal9010070
Chicago/Turabian StyleZhang, Xue-Ying, Zhong-Hua Xu, Min-Hua Zong, Chuan-Fu Wang, and Ning Li. 2019. "Selective Synthesis of Furfuryl Alcohol from Biomass-Derived Furfural Using Immobilized Yeast Cells" Catalysts 9, no. 1: 70. https://doi.org/10.3390/catal9010070
APA StyleZhang, X.-Y., Xu, Z.-H., Zong, M.-H., Wang, C.-F., & Li, N. (2019). Selective Synthesis of Furfuryl Alcohol from Biomass-Derived Furfural Using Immobilized Yeast Cells. Catalysts, 9(1), 70. https://doi.org/10.3390/catal9010070