ZnO/Ionic Liquid Catalyzed Biodiesel Production from Renewable and Waste Lipids as Feedstocks
Abstract
1. Introduction
2. Results and Discussion
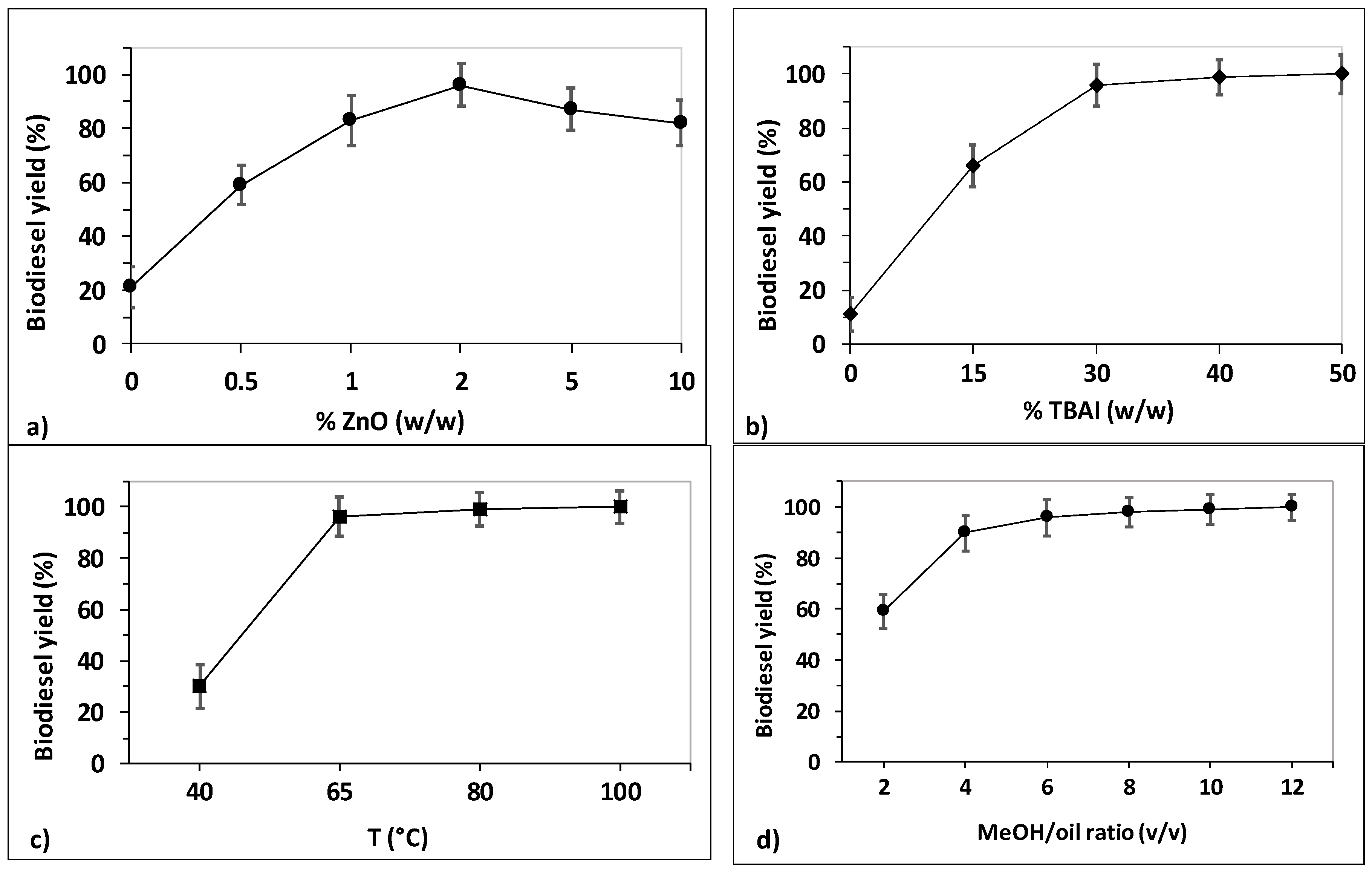
2.1. Optimization of Transesterification Conditions
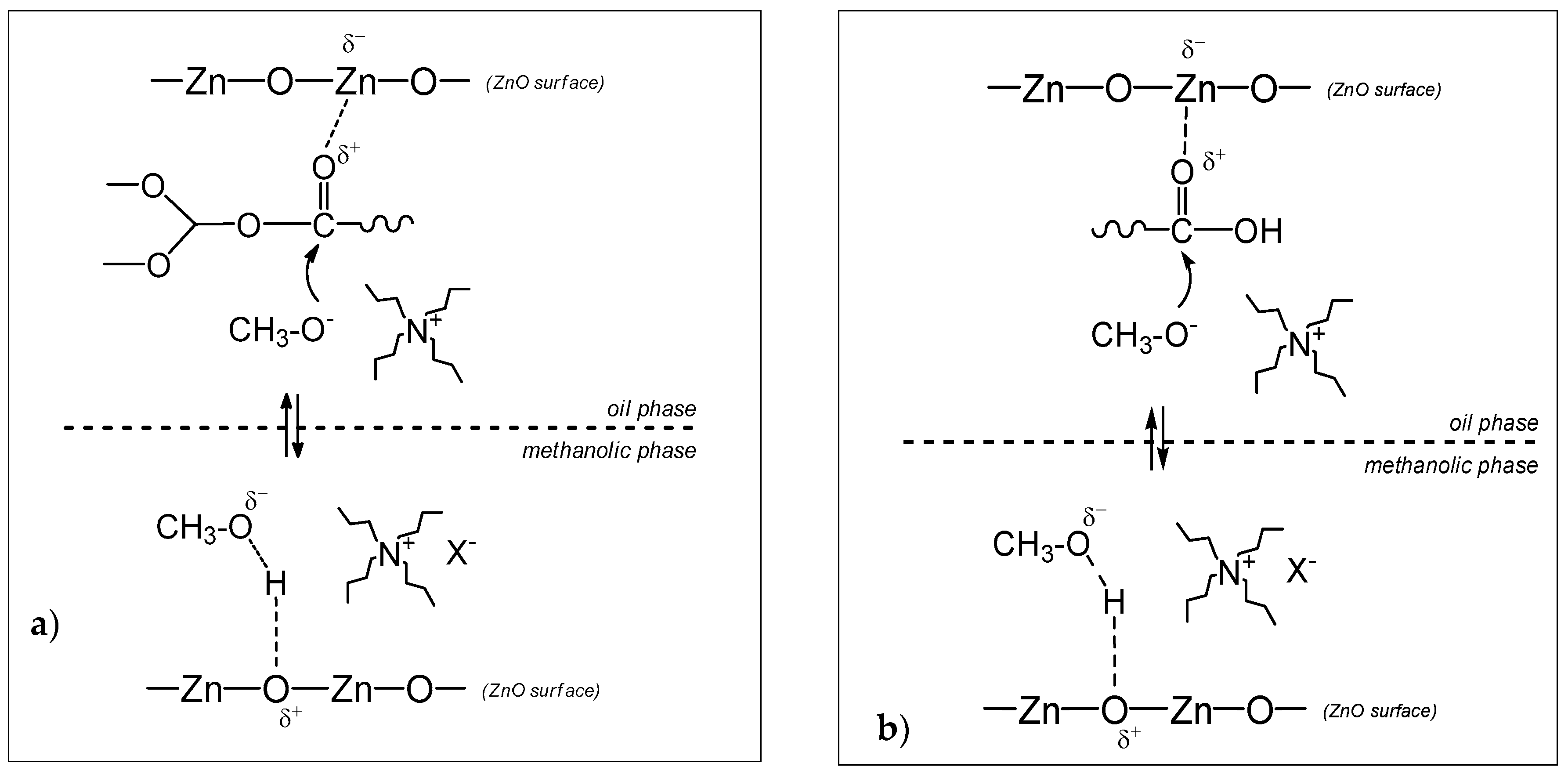
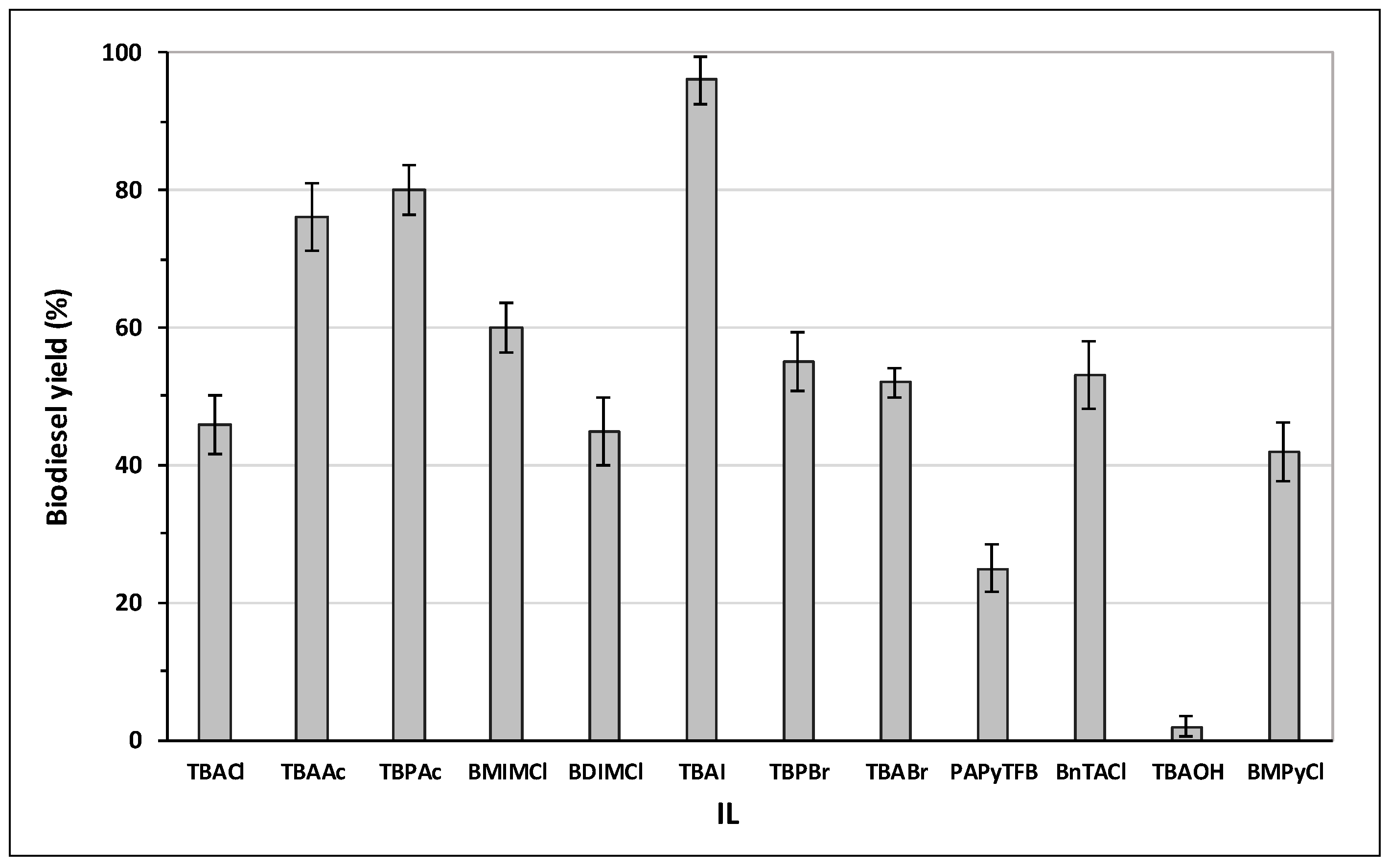
2.2. Influence of the Nature of Ionic Liquid: Mechanistic Insight
2.3. Kinetic and Thermodynamic Parameters
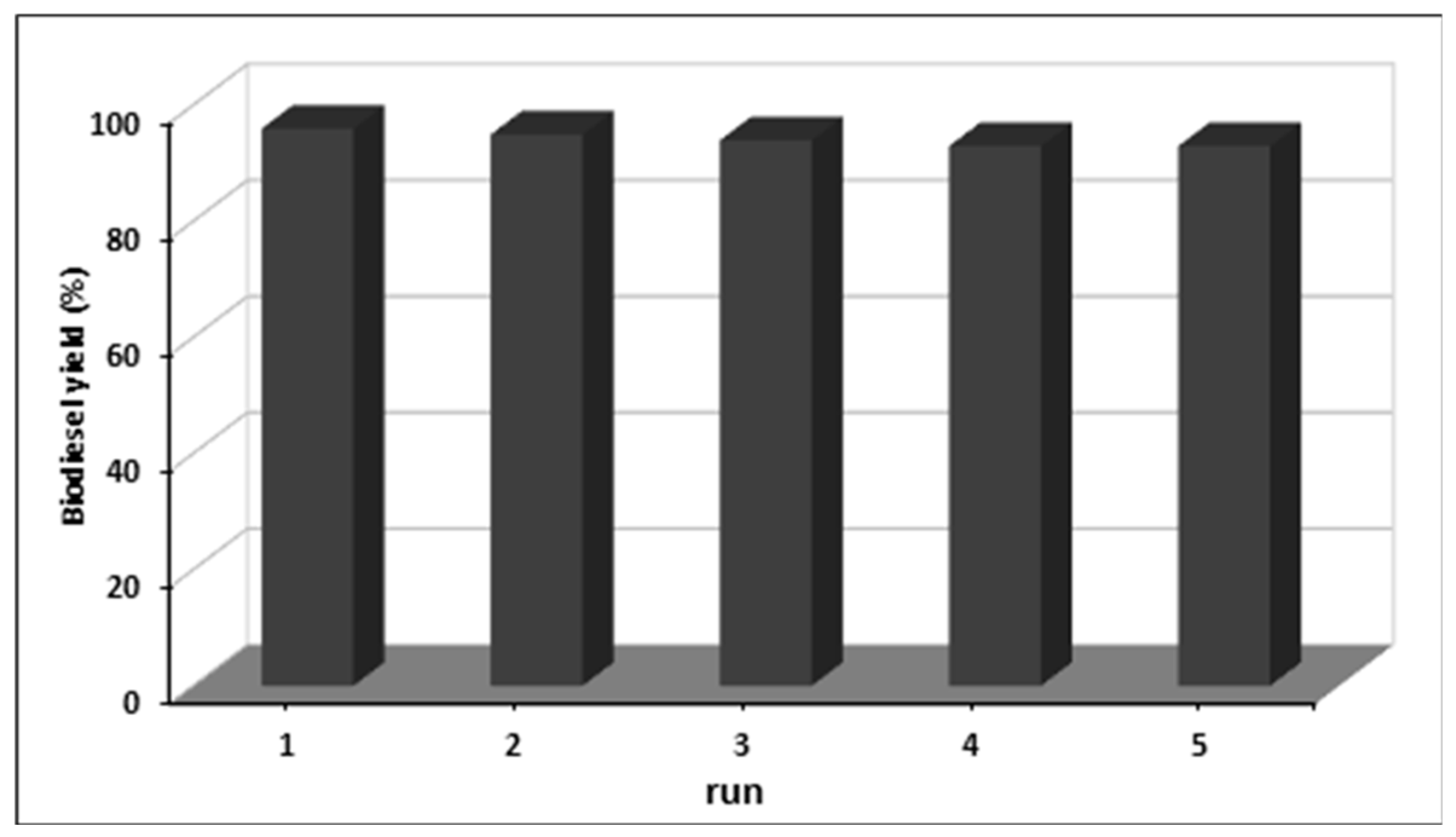
2.4. Recycling Tests
2.5. Transesterification/Esterification of Refined and Wasted Lipid Feedstocks
3. Materials and Methods
3.1. Materials
3.2. Lipids Characterization of Real Sewage Scum
3.3. Transesterification Experiments
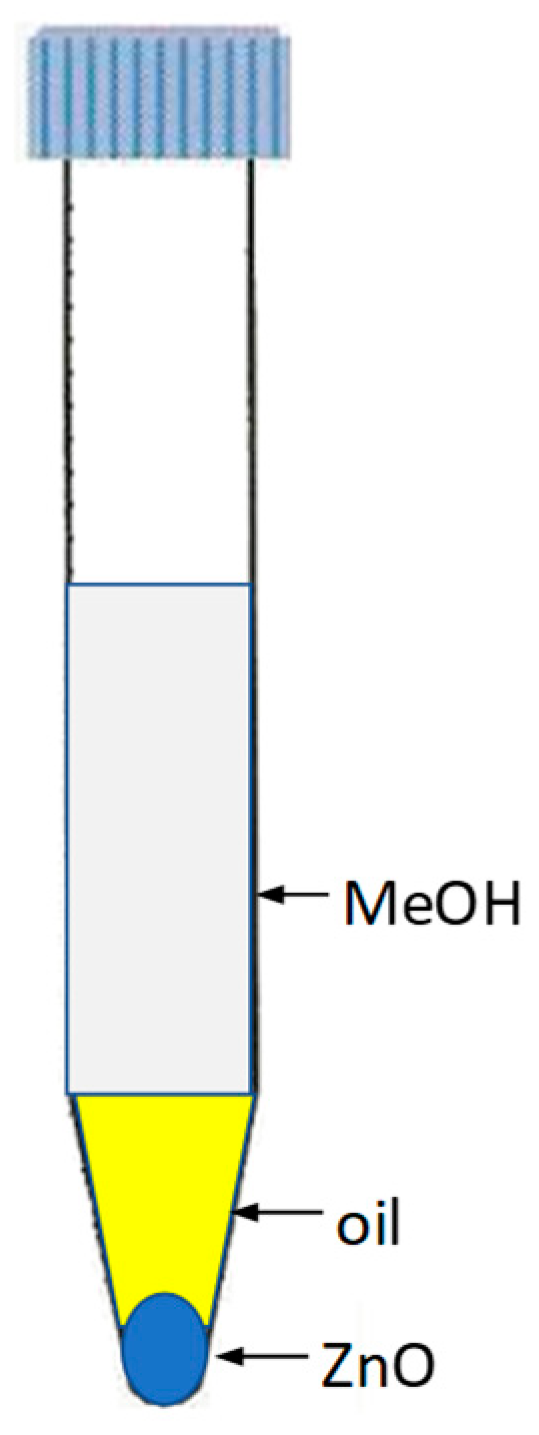
3.4. Procedure for Catalyst Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pradhan, P.; Mahajani, S.M.; Arora, A. Production and utilization of fuel pellets from biomass: A review. Fuel Proc. Technol. 2018, 181, 215–232. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Jahromi, H.; Agblevor, F.A. Hydrodeoxygenation of Aqueous-Phase Catalytic Pyrolysis Oil to Liquid Hydrocarbons Using Multifunctional Nickel Catalyst. Ind. Eng. Chem. Res. 2018, 57, 13257–13268. [Google Scholar] [CrossRef]
- Singh, M.V. Waste and virgin high-density poly(ethylene) into renewable hydrocarbons fuel by pyrolysis-catalytic cracking with a CoCO3 catalyst. J. Anal. Appl. Pyrolysis 2018, 134, 150–161. [Google Scholar] [CrossRef]
- Agblevor, F.A.; Jahromi, H. Aqueous Phase Synthesis of Hydrocarbons from Reactions of Guaiacol and Low Molecular Weight Oxygenates. ChemCatChem 2018, 10, 5201–5214. [Google Scholar] [CrossRef]
- Zecha, K.M.; Dietricha, S.; Reichmuth, M.; Weindorf, W.; Müller-Langer, F. Techno-economic assessment of a renewable bio-jet-fuel production using power-to-gas. Appl. Energy 2018, 231, 997–1006. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Singh, A.; Gaurav, K. Advancement in Catalysts for Transesterification in the Production of Biodiesel: A Review. J. Biochem. Technol. 2018, 7, 1148–1158. [Google Scholar]
- Mishra, V.K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels 2018, 9, 273–289. [Google Scholar] [CrossRef]
- Mele, G.; Annese, C.; De Riccardis, A.; Fusco, C.; Palmisano, L.; Vasapollo, G. D’Accolti, L. Turning lipophilic phthalocyanines/TiO2 composites into efficient photocatalysts for the conversion of CO2 into formic acid under Uv–Vis light irradiation. Appl. Catal. A 2014, 481, 169–172. [Google Scholar] [CrossRef]
- Kim, D.-S.; Hanifzadeh, M.; Kumar, A. Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ. Prog. Sustain. Energy 2018, 37, 7–19. [Google Scholar] [CrossRef]
- Jeevahan, J.; Mageshwaran, G.; Joseph, G.B.; Raj, R.B.D.; Kannan, R.T. Various strategies for reducing Nox emissions of biodiesel fuel used in conventional diesel engines: A review. Chem. Eng. Commun. 2017, 204, 1202–1223. [Google Scholar] [CrossRef]
- Ullah, Z.; Khan, A.S.; Muhammad, N.; Ullah, R.; Alqahtani, A.S.; Shah, S.N.; Ghanem, O.B.; Bustam, M.A.; Man, Z. A review on ionic liquids as perspective catalysts in transesterification of different feedstock oil into biodiesel. J. Mol. Liquids 2018, 266, 673–686. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Gupta, V.K.; Manikanta, A.; Mishra, K.; Singh, S.; Singh, S.; Ramteke, P.W.; Mishra, P.K. Recent development on sustainable biodiesel production using sewage sludge. Biotech 2018, 8, 245. [Google Scholar] [CrossRef]
- Cho, H.U.; Park, J.M. Biodiesel production by various oleaginous microorganisms from organic wastes. Bioresour. Technol. 2018, 256, 502–508. [Google Scholar] [CrossRef]
- Kargbo, D.M. Biodiesel Production from Municipal Sewage Sludges. Energy Fuels 2010, 24, 2791–2794. [Google Scholar] [CrossRef]
- Naylor, R.L.; Higgins, M.M. The rise in global biodiesel production: Implications for food security. Glob. Food Security 2018, 16, 75–84. [Google Scholar] [CrossRef]
- Almeida, E.L.; Andrade, C.M.G.; Andreo Dos Santos, O. Production of Biodiesel via Catalytic Processes: A Brief Review. Int. J. Chem. React. Eng. 2018, 16. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Haj, L.; Al-Muhtaseb, A.H.; Al-Hinai, M.A.; Baawain, M.; Rashid, U.; Ahmad, M.N.M. Current scenario of catalysts for biodiesel production: A critical review. Rev. Chem. Eng. 2018, 34, 267–297. [Google Scholar] [CrossRef]
- Singh Chouhan, A.P.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Pang, D.; Tan, H.; Zhu, R.; Ouyang, F. Producing biodiesel from waste animal oil by modified ZnO. Int. J. Green Energy 2017, 14, 703–711. [Google Scholar] [CrossRef]
- Kwong, T.-L.; Yung, K.-F. One-step production of biodiesel through simultaneous esterification and transesterification from highly acidic unrefined feedstock over efficient and recyclable ZnO nanostar catalyst. Renew. Energy 2016, 90, 450–457. [Google Scholar] [CrossRef]
- Lukić, I.; Kesić, Ž.; Skala, D. Kinetics of Heterogeneous Biodiesel Synthesis Using Supported ZnO as Catalyst. Chem. Eng. Technol. 2014, 37, 1879–1884. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Piraman, S. Biodiesel synthesis by TiO2–ZnO mixed oxide nanocatalyst catalyzed palm oil transesterification process. Bioresour. Technol. 2013, 150, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Fernando, S.D. Transesterification of Soybean Oil Using Heterogeneous Catalysts. Energy Fuels 2008, 22, 2067–2069. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, W. Soybean oil transesterification over zinc oxide modified with alkali earth metals. Fuel Process. Technol. 2007, 88, 631–638. [Google Scholar] [CrossRef]
- Quirino, M.R.; Oliveira, M.J.C.; Keyson, D.; Lucena, G.L.; Oliveira, J.B.L.; Gama, L. Synthesis of zinc oxide by microwave hydrothermal method for application to transesterification of soybean oil (biodiesel). Mater. Chem. Phys. 2017, 185, 24–30. [Google Scholar] [CrossRef]
- Lee, H.V.; Taufiq-Yap, Y.H.; Hussein, M.Z.; Yunus, R. Transesterification of jatropha oil with methanol over Mg–Zn mixed metal oxide catalysts. Energy 2013, 49, 12–18. [Google Scholar] [CrossRef]
- Yan, S.; Salley, S.O.; Simon Ng, K.Y. Simultaneous transesterification and esterification of unrefined or waste oils over ZnO-La2O3 catalysts. Appl. Catal. A: Gen. 2009, 353, 203–212. [Google Scholar] [CrossRef]
- Refaat, A.A. Biodiesel production using solid metal oxide catalysts. Int. J. Environ. Sci. Technol. 2011, 8, 203–221. [Google Scholar] [CrossRef]
- Alba-Rubio, A.C.; Santamaría-González, J.; Mérida-Robles, J.M.; Moreno-Tost, R.; Martin-Alonso, D.; Jiménez-López, A.; Maireles-Torres, P. Heterogeneous transesterification processes by using CaO supported on zinc oxide as basic catalysts. Catal. Today 2010, 149, 281–287. [Google Scholar] [CrossRef]
- Varghese, R.; Henry, J.P.; Irudayaraj, J. Ultrasonication-assisted transesterification for biodiesel production by using heterogeneous ZnO nanocatalyst. Environ. Prog. Sustain. Energy 2018, 37, 1176–1182. [Google Scholar] [CrossRef]
- Nambo, A.; Miralda, C.M.; Jasinski, J.B.; Carreon, M.A. Methanolysis of olive oil for biodiesel synthesis over ZnO nanorods. React. Kinet. Mech. Catal. 2015, 114, 583–595. [Google Scholar] [CrossRef]
- Baskar, G.; Aberna Ebenezer Selvakumari, I.; Aiswarya, R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef]
- Iannone, F.; Casiello, M.; Monopoli, A.; Cotugno, P.; Sportelli, M.C.; Picca, R.A.; Cioffi, N.; Dell’Anna, M.M.; Nacci, A. Ionic liquids/ZnO nanoparticles as recyclable catalyst for polycarbonate depolymerization. J. Mol. Catal. A: Chem. 2017, 426, 107–116. [Google Scholar] [CrossRef]
- Annese, C.; D’Accolti, L.; Filardi, R.; Tommasi, I.; Fusco, C. Oxidative cleavage of lactams in water using dioxiranes: An expedient and environmentally-safe route to ω-nitro acids. Tetrahedron Lett. 2013, 54, 515–517. [Google Scholar] [CrossRef]
- Annese, C.; Abbrescia, D.I.; Catucci, L.; D’Accolti, L.; Denora, N.; Fanizza, I.; Fusco, C.; La Piana, G. Site-dependent biological activity of valinomycin analogs bearing derivatizable hydroxyl sites. J. Pept. Sci. 2013, 19, 751–757. [Google Scholar] [CrossRef]
- Bancquart, S.; Vanhove, C.; Pouilloux, Y.; Barrault, J. Glycerol transesterification with methyl stearate over solid basic catalysts: I. Relationship between activity and basicity. Appl. Catal. A: Gen. 2001, 218, 1–11. [Google Scholar] [CrossRef]
- Gutiérrez-Arnillas, E.; Álvarez, S.M.; Deive, F.J.; Rodríguez, A. Ángeles Sanromán, M. New horizons in the enzymatic production of biodiesel using neoteric solvents. Renew. Energy 2016, 98, 92–100. [Google Scholar] [CrossRef]
- Huang, Y.Q. Synthesis of Biodiesel by Phase Transfer Catalysis. Adv. Energy Sci. Technol. 2013, 291–294, 355–358. [Google Scholar] [CrossRef]
- Long, T.; Deng, Y.; Gan, S.; Chen, J. Application of Choline Chloride·x ZnCl2 Ionic Liquids for Preparation of Biodiesel. Chin. J. Chem. Eng. 2010, 18, 322–327. [Google Scholar] [CrossRef]
- Zimmerman, W.B.; Kokoo, R. Esterification for biodiesel production with a phantom catalyst: Bubble mediated reactive distillation. Appl. Energy 2018, 221, 28–40. [Google Scholar] [CrossRef]
- Monopoli, A.; Cotugno, P.; Iannone, F.; Ciminale, F.; Dell’Anna, M.M.; Mastrorilli, P.; Nacci, A. Ionic-Liquid-Assisted Metal-Free Oxidative Coupling of Amines To Give Imines. Eur. J. Org. Chem. 2014, 5925–5931. [Google Scholar] [CrossRef]
- Martinez, P.A.; Martinez, N.G.; Duran-del-Amor, M.; Media, J.Q. Advances on kinetics and thermodynamics of non-catalytic supercritical methanol transesterification of some vegetable oils to biodiesel. Energy Convers. Manag. 2018, 173, 187–196. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P. Renew. Review of process parameters for biodiesel production from different feedstocks. Sustain. Energy Rev. 2016, 62, 1063–1071. [Google Scholar] [CrossRef]
- Vujicic, D.; Comic, D.; Zarubica, A.; Micic, R.; Boskovic, G. Kinetics of biodiesel synthesis from sunflower oil over CaO heterogeneous catalyst. Fuel 2010, 89, 2054–2061. [Google Scholar] [CrossRef]
- Birla, A.; Singh, B.; Upadhyay, S.N.; Sharma, Y.C. Kinetics studies of synthesis of biodiesel from waste frying oil using a heterogeneous catalyst derived from snail shell. Bioresour. Technol. 2012, 106, 95–100. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sindhanaiselvan, S.; Gandhi, N.N.; Devi, S.S.; Renganathan, S. Optimization and kinetic studies on biodiesel production from underutilized Ceiba Pentandra oil. Fuel 2013, 103, 693–698. [Google Scholar] [CrossRef]
- Farida, M.A.A.; Hassana, M.; Taufiq-Yap, Y.H.; Ibrahime, M.L.; Hasan, M.Y.; Alia, A.A.M.; Othmana, M.R.; Shiraif, Y. Kinetic and thermodynamic of heterogeneously K3PO4/AC-catalysed transesterification via pseudo-first order mechanism and Eyring-Polanyi equation. Fuel 2018, 232, 653–658. [Google Scholar] [CrossRef]
- Razavy, M. Quantum Theory of Tunneling, 1st ed.; World Scientific Publishing Company: Singapore, 2003; ISBN 981-238-018-3. [Google Scholar]
- di Bitonto, L.; Lopez, A.; Mascolo, G.; Mininni, G.; Pastore, C. Efficient solvent-less separation of lipids from municipal wet sewage scum and their sustainable conversion into biodiesel. Renew. Energy 2016, 90, 55–61. [Google Scholar] [CrossRef]
- Pastore, C.; Pagano, M.; Lopez, A.; Mininni, G.; Mascolo, G. Fat, oil and grease waste from municipal wastewater: Characterization, activation and sustainable conversion into biofuel. Water Sci. Technol. 2015, 71, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
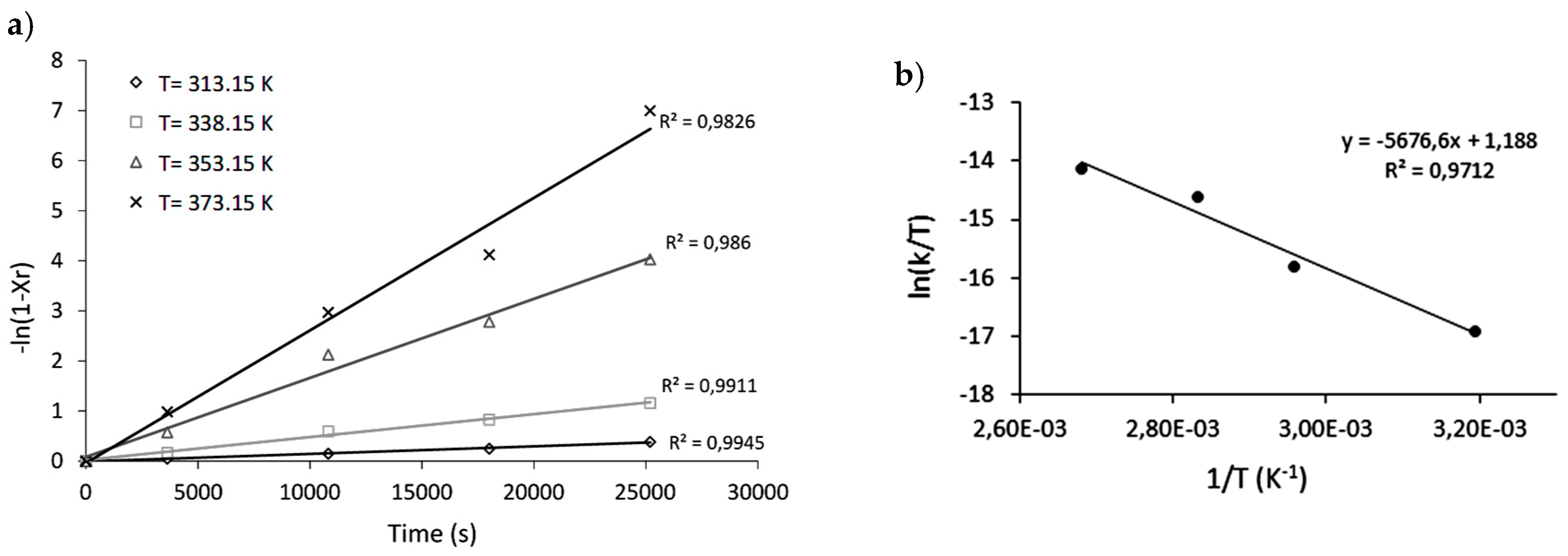
| T (K) | k (s−1) | Ea (KJ/mol) | A (s−1) | ΔG‡ (KJ/mol) | ΔH‡ (KJ/mol) | ΔS‡ (KJ/mol∙K) |
|---|---|---|---|---|---|---|
| 313.15 | 1.4 × 10−5 | 48.46 | 2.4 × 103 | 72.33 2 | 44.90 | −0.081 |
| 338.15 | 4.6 × 10−5 | |||||
| 353.15 | 1.6 × 10−4 | |||||
| 373.15 | 2.7 × 10−4 |
| Feedstock 2 | FFAs (wt.%) | Transesterifiable/Esterifiable Amount (w/w %) | Biodiesel Yield (%) 3 |
|---|---|---|---|
| Soybean oil | 1 | 97 | 96 ± 3 |
| Jatropha oil | <1 | 99 | 95 ± 3 |
| Linseed oil | 2 | 98 | 93 ± 4 |
| Lard (animal fat) | <1 | 94 | 95 ± 4 4 |
| WCO | 1.5 | 96 | 95 ± 3 |
| Fish Oil | <1 | 90 | 92 ± 5 4 |
| Oleic acid | >99 | >99 | 54 ± 4 (99) 5 |
| Olein residue 6 | 76 | 95 | 75 ± 5 |
| Municipal sewage scum 7 | 32 | 60 | 79 ± 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casiello, M.; Catucci, L.; Fracassi, F.; Fusco, C.; Laurenza, A.G.; Di Bitonto, L.; Pastore, C.; D’Accolti, L.; Nacci, A. ZnO/Ionic Liquid Catalyzed Biodiesel Production from Renewable and Waste Lipids as Feedstocks. Catalysts 2019, 9, 71. https://doi.org/10.3390/catal9010071
Casiello M, Catucci L, Fracassi F, Fusco C, Laurenza AG, Di Bitonto L, Pastore C, D’Accolti L, Nacci A. ZnO/Ionic Liquid Catalyzed Biodiesel Production from Renewable and Waste Lipids as Feedstocks. Catalysts. 2019; 9(1):71. https://doi.org/10.3390/catal9010071
Chicago/Turabian StyleCasiello, Michele, Lucia Catucci, Francesco Fracassi, Caterina Fusco, Amelita G. Laurenza, Luigi Di Bitonto, Carlo Pastore, Lucia D’Accolti, and Angelo Nacci. 2019. "ZnO/Ionic Liquid Catalyzed Biodiesel Production from Renewable and Waste Lipids as Feedstocks" Catalysts 9, no. 1: 71. https://doi.org/10.3390/catal9010071
APA StyleCasiello, M., Catucci, L., Fracassi, F., Fusco, C., Laurenza, A. G., Di Bitonto, L., Pastore, C., D’Accolti, L., & Nacci, A. (2019). ZnO/Ionic Liquid Catalyzed Biodiesel Production from Renewable and Waste Lipids as Feedstocks. Catalysts, 9(1), 71. https://doi.org/10.3390/catal9010071