Structure and Photocatalytic Activity of PdCrOx Cocatalyst on SrTiO3 for Overall Water Splitting
Abstract
1. Introduction
2. Results and Discussion
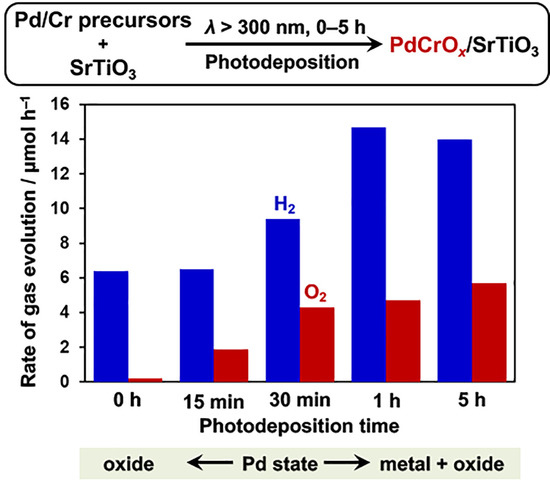
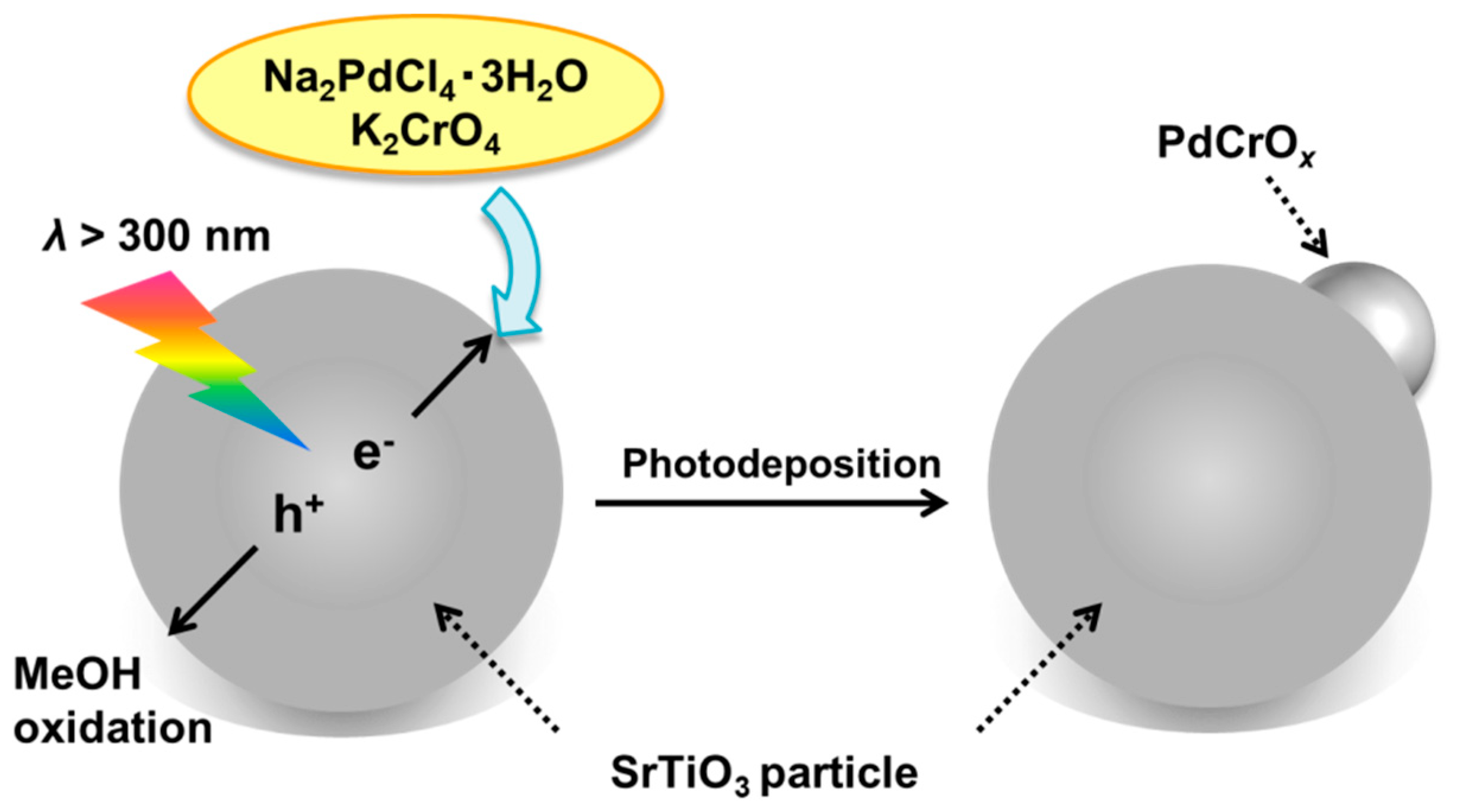
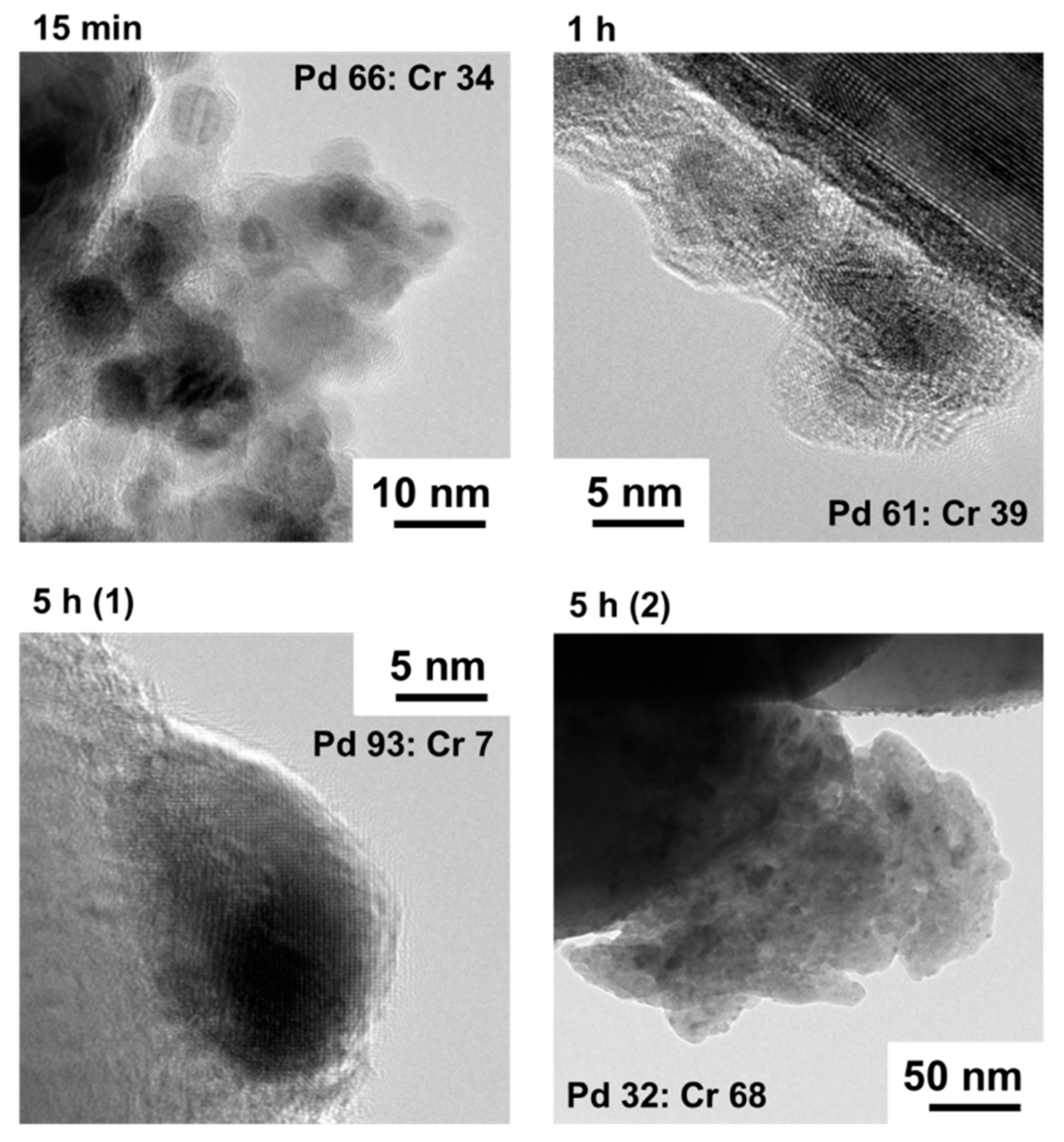
2.1. Deposition of PdCrOx Nanoparticles
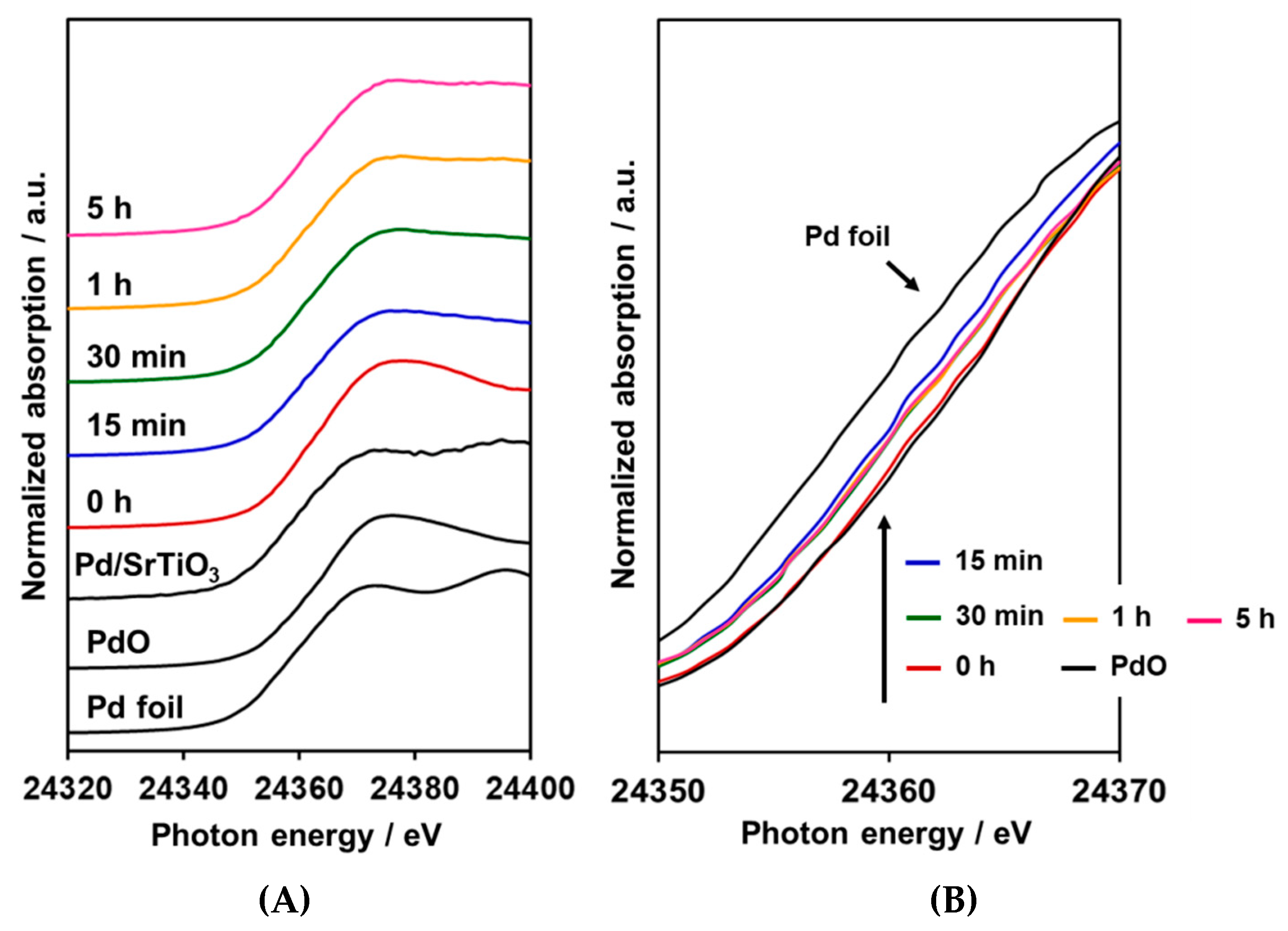
2.2. XANES
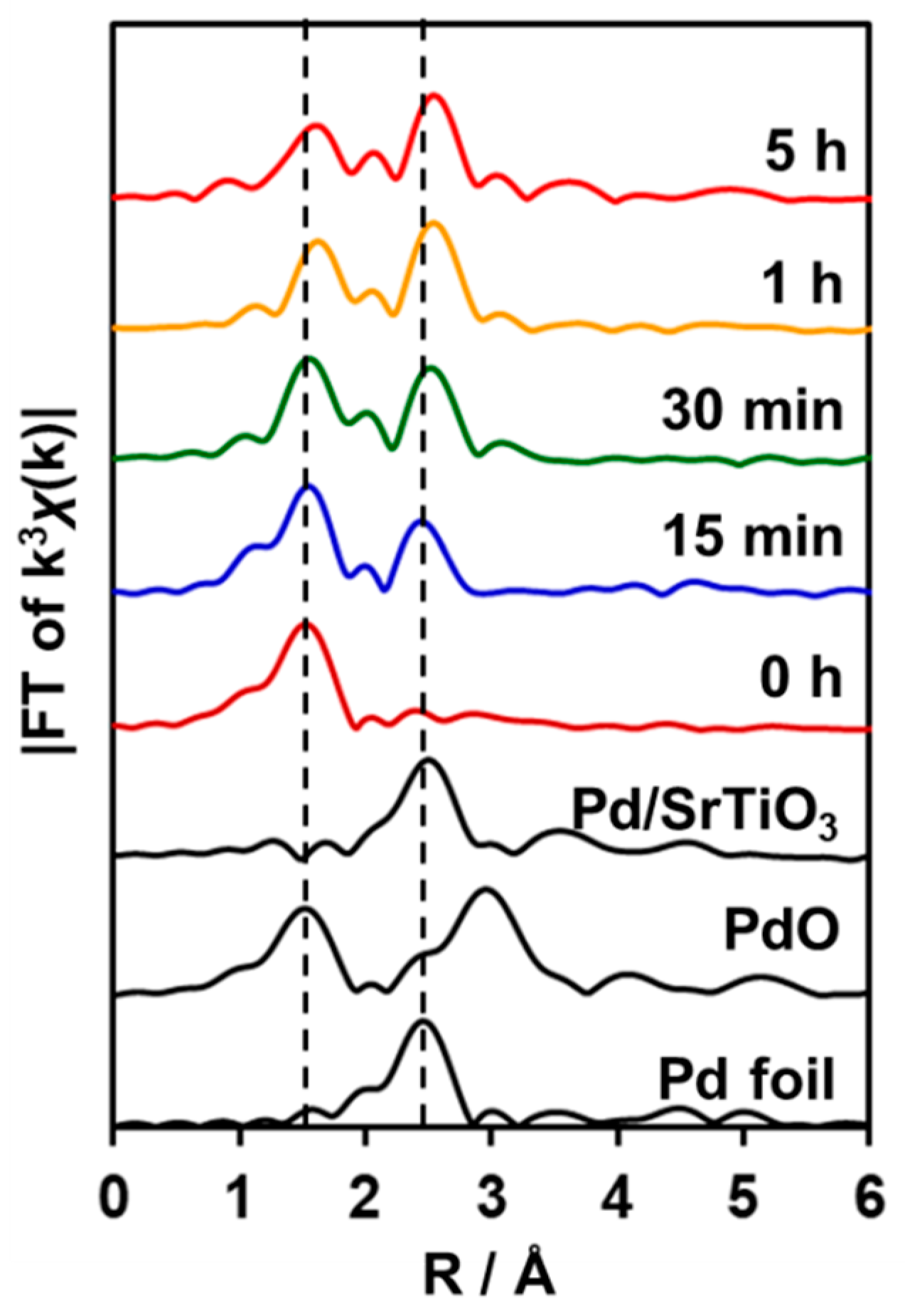
2.3. EXAFS
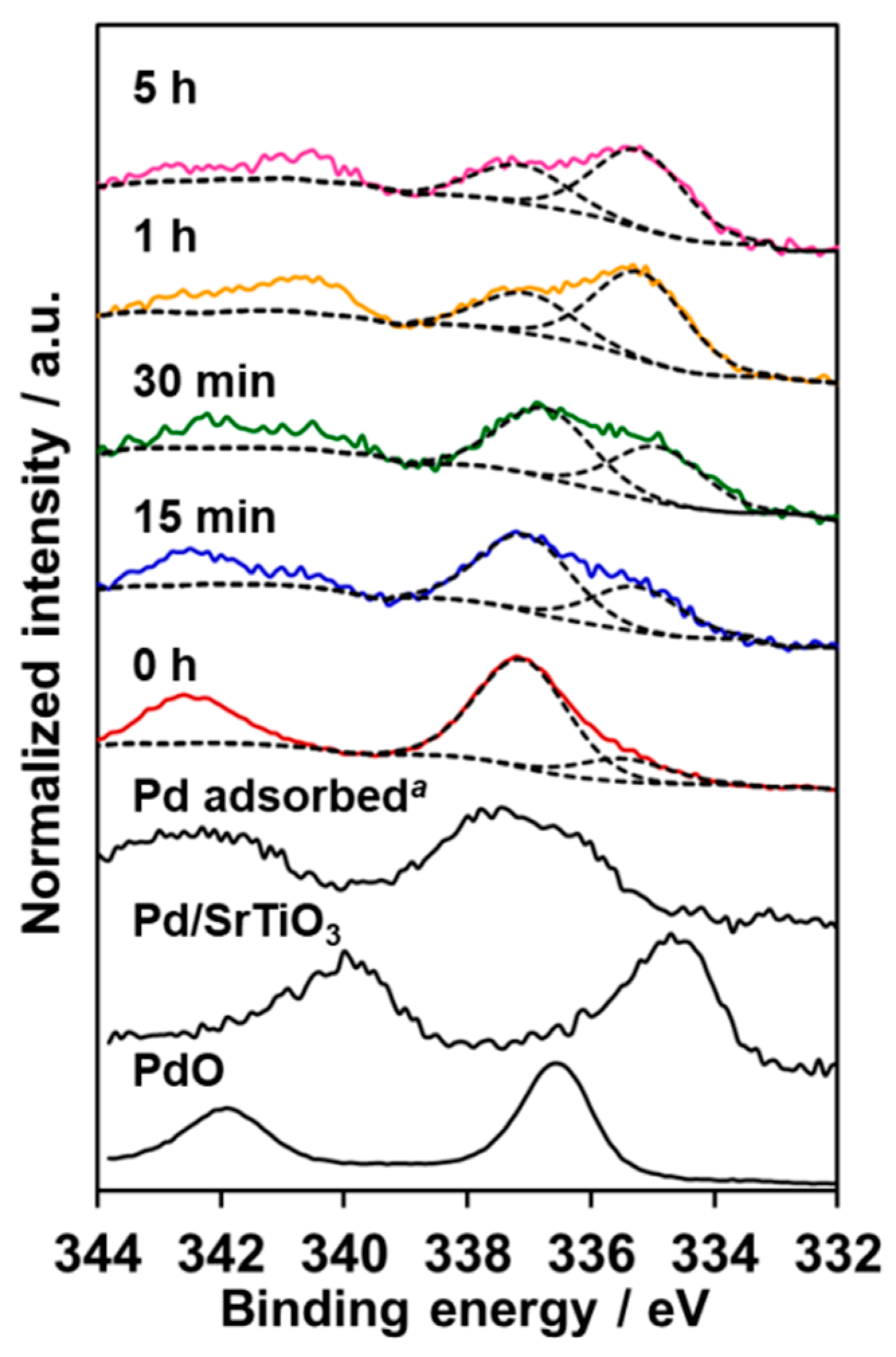
2.4. XPS
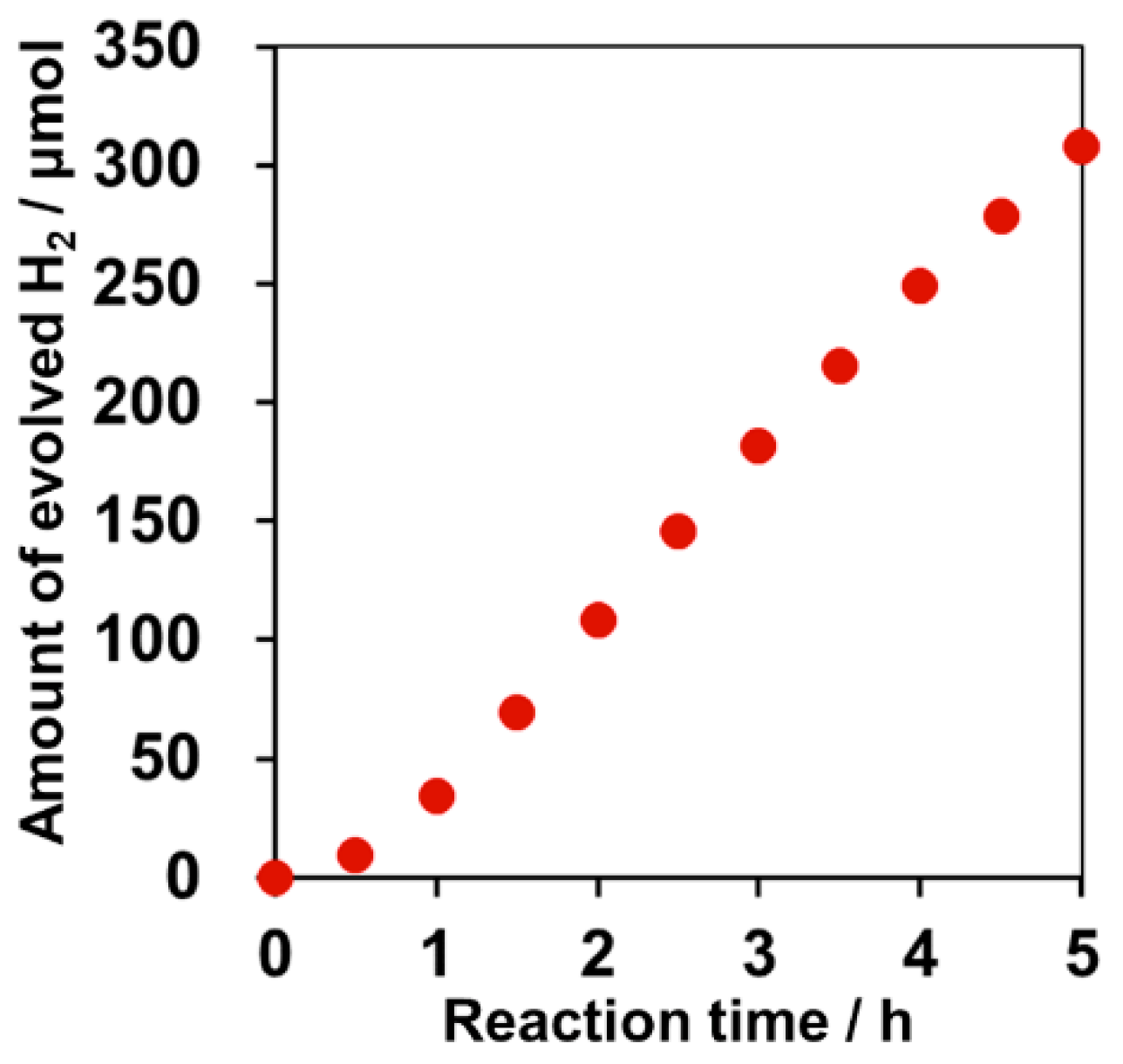
2.5. Effect of PdCrOx Nanoparticles deposited on SrTiO3 upon the Photocatalytic Activity toward Overall Water Splitting
3. Materials and Methods
3.1. Synthesis of SrTiO3
3.2. Cocatalyst Loading
3.3. Characterization
3.4. Photocatalytic Reaction
3.5. Backward Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maeda, K. Photocatalytic Water Splitting Using Semiconductor Particles: History and Recent Developments. J. Photochem. Photobiol. C 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, A.M.; Symes, D.M. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 0003. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Maeda, K.; Mallouk, E.T. Two-Dimensional Metal Oxide Nanosheets as Building Blocks for Artificial Photosynthetic Assemblies. Bull. Chem. Soc. Jpn. 2019, 92, 38–54. [Google Scholar] [CrossRef]
- Ham, Y.; Hisatomi, T.; Goto, Y.; Moriya, Y.; Sakata, Y.; Yamakata, A.; Kubota, J.; Domen, K. Flux-mediated doping of SrTiO3 photocatalysts for efficient overall water splitting. J. Mater. Chem. A 2016, 4, 3027–3033. [Google Scholar] [CrossRef]
- Kato, H.; Kobayashi, M.; Hara, M.; Kakihana, M. Fabrication of SrTiO3 exposing characteristic facets using molten salt flux and improvement of photocatalytic activity for water splitting. Catal. Sci. Technol. 2013, 3, 1733–1738. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Visible-Light-Response and Photocatalytic Activities of TiO2 and SrTiO3 Photocatalysts Codoped with Antimony and Chromium. J. Phys. Chem. B 2002, 106, 5029–5034. [Google Scholar] [CrossRef]
- Kasahara, A.; Nukumizu, K.; Hitoki, G.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. Photoreactions on LaTiO2N under visible light irradiation. J. Phys. Chem. A 2002, 106, 6750–6753. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef]
- Kuriki, R.; Ichibha, T.; Hongo, K.; Lu, D.; Maezono, R.; Kageyama, H.; Ishitani, O.; Oka, K.; Maeda, K. A Stable, Narrow-Gap Oxyfluoride Photocatalyst for Visible-Light Hydrogen Evolution and Carbon Dioxide Reduction. J. Am. Chem. Soc. 2018, 140, 6648–6655. [Google Scholar] [CrossRef]
- Domen, K.; Kudo, A.; Onishi, T. Mechanism of photocatalytic decomposition of water into H2 and O2 over NiO-SrTiO3. J. Catal. 1986, 102, 92–98. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Saito, N.; Inoue, Y.; Domen, K. Improvement of photocatalytic activity of (Ga1−xZnx)(N1−xOx) solid solution for overall water splitting by co-loading Cr and another transition metal. J. Catal. 2006, 243, 303–308. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Saito, N.; Inoue, Y.; Domen, K. Noble-Metal/Cr2O3 Core/Shell Nanoparticles as a Cocatalyst for Photocatalytic Overall Water Splitting. Angew. Chem. Int. Ed. 2006, 45, 7806–7809. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Teramura, K.; Masuda, H.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Efficient Overall Water Splitting under Visible-Light Irradiation on (Ga1−xZnx)(N1−xOx) Dispersed with Rh-Cr Mixed-Oxide Nanoparticles: Effect of Reaction Conditions on Photocatalytic Activity. J. Phys. Chem. B 2006, 110, 13107–13112. [Google Scholar]
- Maeda, K.; Lu, D.; Teramura, K.; Domen, K. Direct deposition of nanoparticulate rhodium–chromium mixed-oxides on a semiconductor powder by band-gap irradiation. J. Mater. Chem. 2008, 18, 3539–3542. [Google Scholar] [CrossRef]
- Kanazawa, T.; Maeda, K. Light-Induced Synthesis of Heterojunctioned Nanoparticles on a Semiconductor as Durable Cocatalysts for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 7165–7172. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Lu, D.; Maeda, K. Photochemical Synthesis of Fe(III)–Cr(III) Mixed Oxide Nanoparticles on Strontium Titanate Powder and Their Application as Water Oxidation Cocatalysts. Chem. Lett. 2016, 45, 967–969. [Google Scholar] [CrossRef]
- Higashi, M.; Domen, K.; Abe, R. Fabrication of an Efficient BaTaO2N Photoanode Harvesting a Wide Range of Visible Light for Water Splitting. J. Am. Chem. Soc. 2013, 135, 10238–10241. [Google Scholar] [CrossRef]
- Yoshida, M.; Takanabe, K.; Maeda, K.; Ishikawa, A.; Kubota, J.; Sakata, Y.; Ikezawa, Y.; Domen, K. Role and function of noble-metal/Cr-layer core/shell structure cocatalysts for photocatalytic overall water splitting studied by model electrodes. J. Phys. Chem. C 2009, 113, 10151–10157. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 2002, 26, 1167–1170. [Google Scholar] [CrossRef]
- Brun, M.; Berthet, A.; Bertolini, C.J. XPS, AES and Auger parameter of Pd and PdO. J. Electron Spectrosc. Relat. Phenom. 1999, 104, 55–60. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
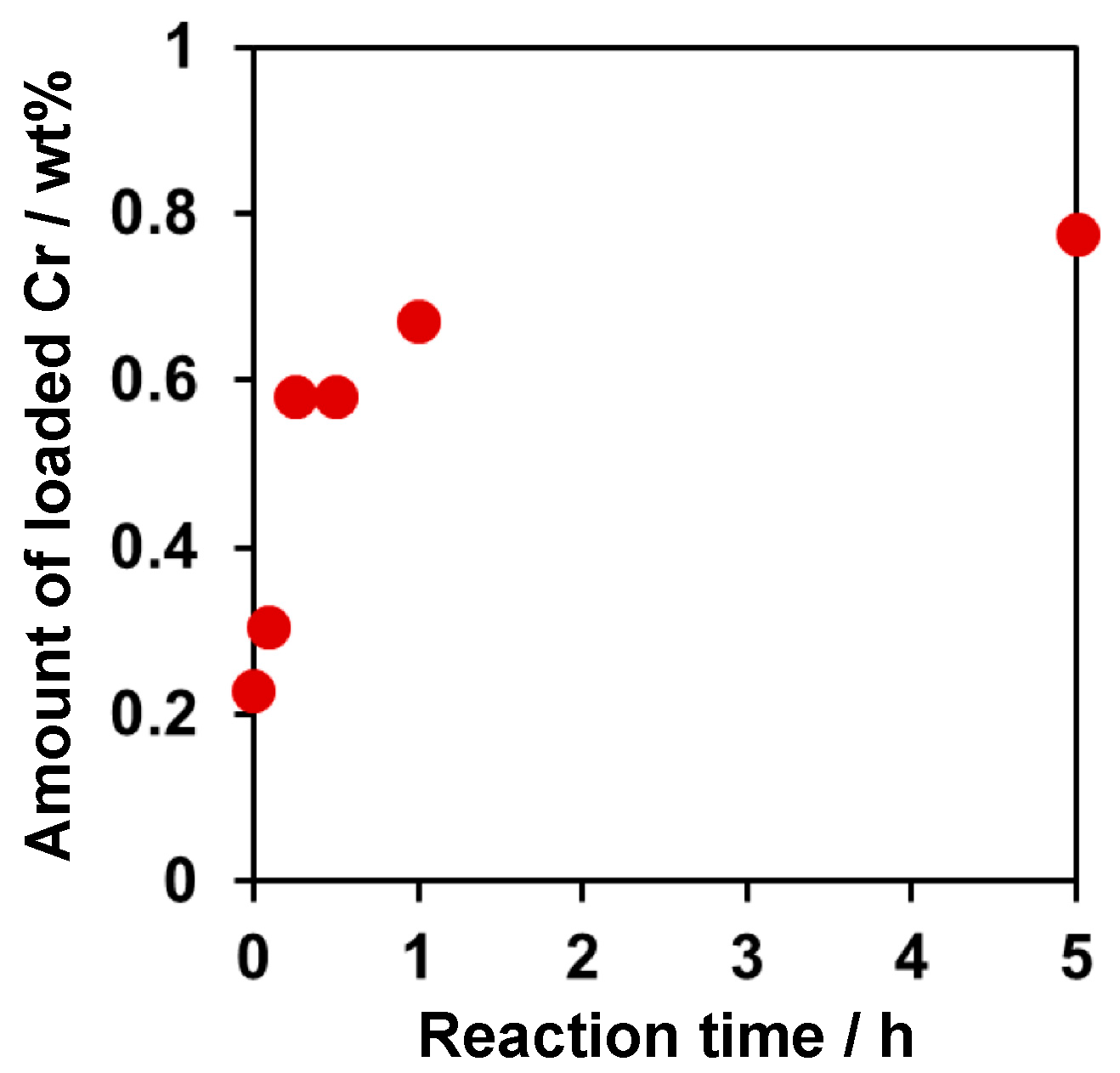
| Entry | Sample | Loaded Cr/wt% | Rate of Gas Evolution/μmol h−1 | |
|---|---|---|---|---|
| H2 | O2 | |||
| 1 | 0 h | 0.2 | 6.4 | 0.2 |
| 2 | 15 min | 0.6 | 6.5 | 1.9 |
| 3 | 30 min | 0.6 | 9.4 | 4.3 |
| 4 | 1 h | 0.7 | 14.7 | 4.7 |
| 5 | 5 h | 0.8 | 14.0 | 5.7 |
| 6 | SrTiO3 | - | 0.1 | N. D. |
| 7 | Pd/SrTiO3 | - | 3.4 | 0.3 |
| 8 | Cr2O3/Pd/SrTiO3 | 0.5 | 5.3 | 1.7 |
| Entry | Sample | Loaded Cr/wt% | Rate of H2 Evolution/μmol h−1 |
|---|---|---|---|
| 1 | 0 h | 0.2 | 12.5 |
| 2 | 15 min | 0.6 | 57.4 |
| 3 | 30 min | 0.6 | 44.2 |
| 4 | 1 h | 0.7 | 50.0 |
| 5 | 5 h | 0.8 | 47.4 |
| 6 | Pd/SrTiO3 | 0 | 43.3 |
| 7 | Cr2O3/Pd/SrTiO3 | 0.5 | 28.2 |
| Entry | Sample | Loaded Cr/wt% | Rate of Diminished Gases/μmol h−1 | |
|---|---|---|---|---|
| H2 | O2 | |||
| 1 | 15 min | 0.6 | 0.1 | trace |
| 2 | 5 h | 0.8 | 2.0 | 1.7 |
| 3 | Pd/SrTiO3 | - | 24.6 | 12.2 |
| Entry | Sample | Loaded Cr/wt% | Atmosphere | Rate/μmol h−1 | |
|---|---|---|---|---|---|
| H2 Evolution | O2 Consumption | ||||
| 1 | 15 min | 0.6 | Ar | 37.5 | - |
| 2 | 15 min | 0.6 | Ar + O2 | 1.1 | 47.7 |
| 3 | 5 h | 0.8 | Ar | 28.1 | - |
| 4 | 5 h | 0.8 | Ar + O2 | 5.5 | 23.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanazawa, T.; Nozawa, S.; Lu, D.; Maeda, K. Structure and Photocatalytic Activity of PdCrOx Cocatalyst on SrTiO3 for Overall Water Splitting. Catalysts 2019, 9, 59. https://doi.org/10.3390/catal9010059
Kanazawa T, Nozawa S, Lu D, Maeda K. Structure and Photocatalytic Activity of PdCrOx Cocatalyst on SrTiO3 for Overall Water Splitting. Catalysts. 2019; 9(1):59. https://doi.org/10.3390/catal9010059
Chicago/Turabian StyleKanazawa, Tomoki, Shunsuke Nozawa, Daling Lu, and Kazuhiko Maeda. 2019. "Structure and Photocatalytic Activity of PdCrOx Cocatalyst on SrTiO3 for Overall Water Splitting" Catalysts 9, no. 1: 59. https://doi.org/10.3390/catal9010059
APA StyleKanazawa, T., Nozawa, S., Lu, D., & Maeda, K. (2019). Structure and Photocatalytic Activity of PdCrOx Cocatalyst on SrTiO3 for Overall Water Splitting. Catalysts, 9(1), 59. https://doi.org/10.3390/catal9010059