Enhanced Visible Light Photocatalytic Reduction of Cr(VI) over a Novel Square Nanotube Poly(Triazine Imide)/TiO2 Heterojunction
Abstract
:1. Introduction
2. Results and Discussion
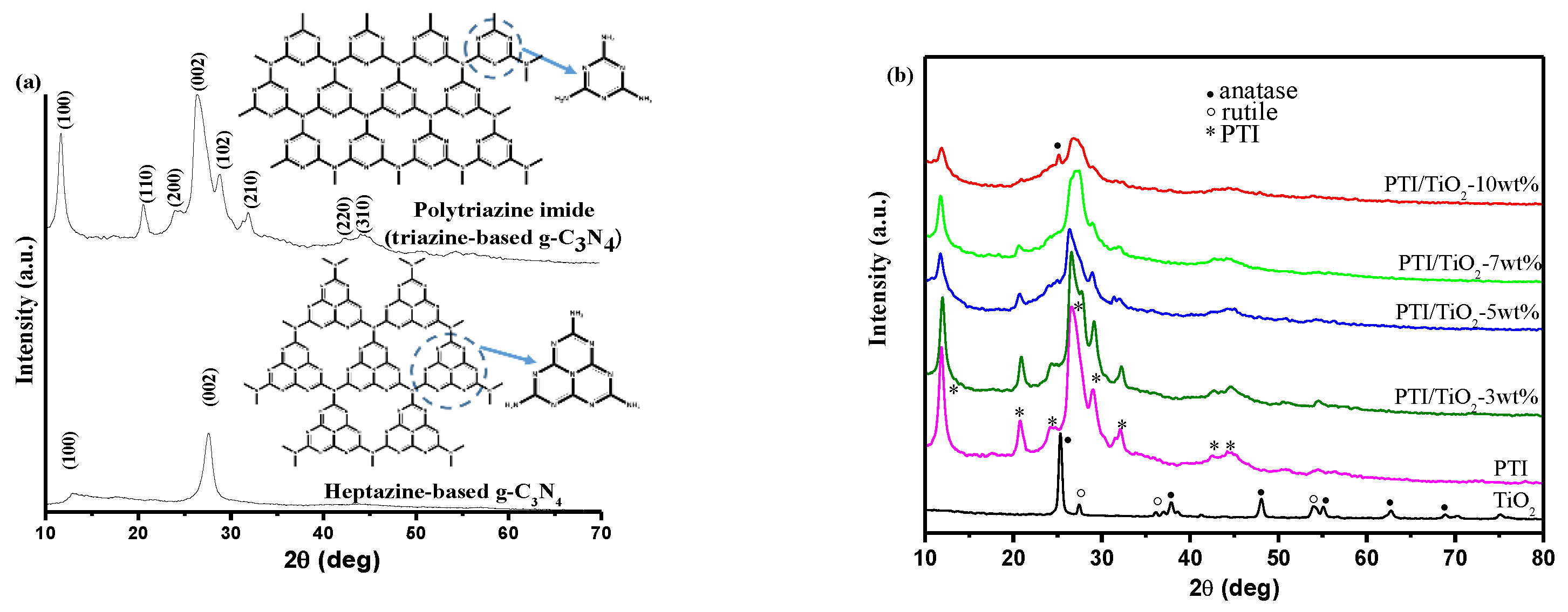
2.1. XRD Analysis
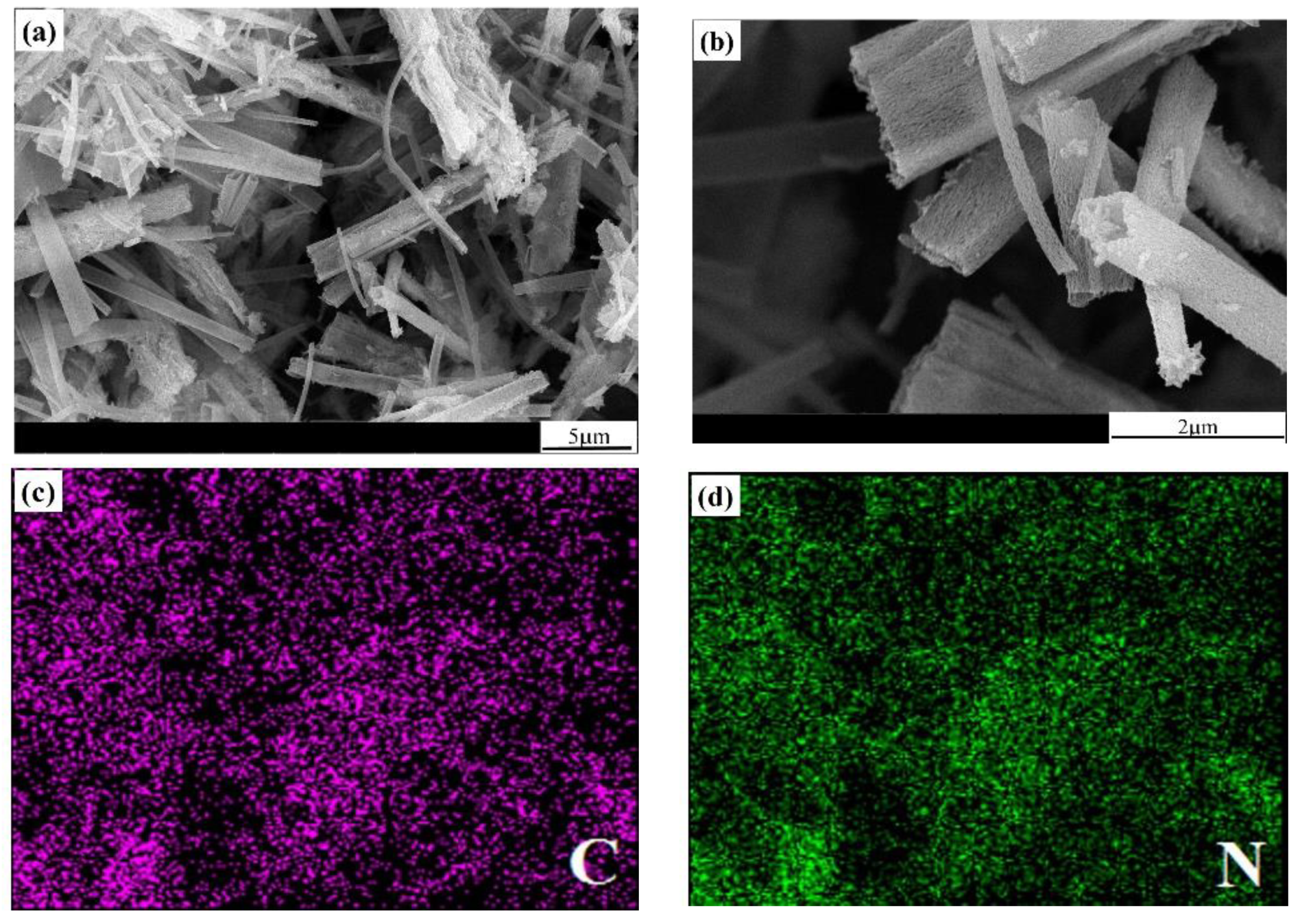
2.2. Morphology Analysis
2.3. UV-Vis DRS Analysis
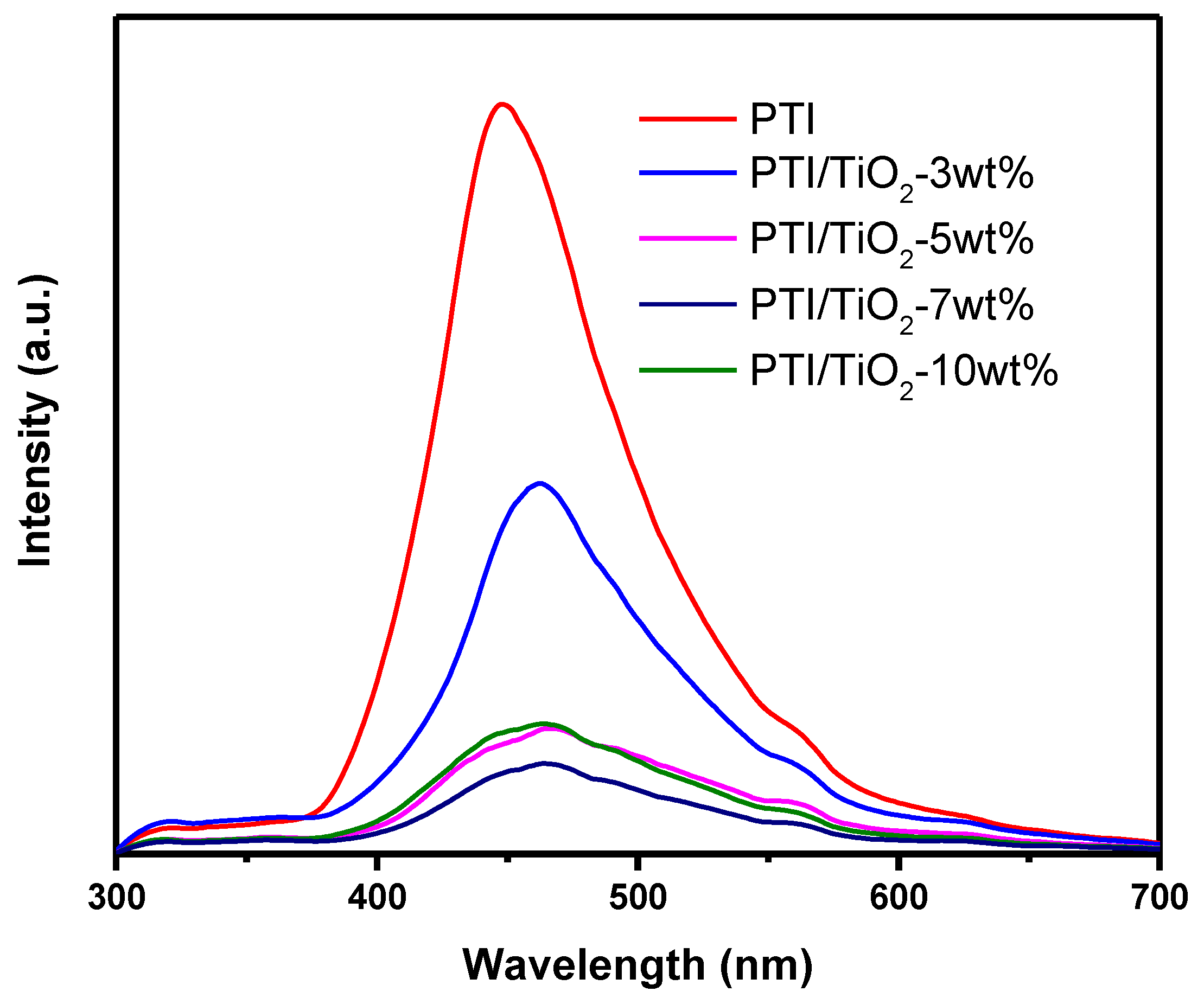
2.4. Photoluminescence Spectroscopy Analysis
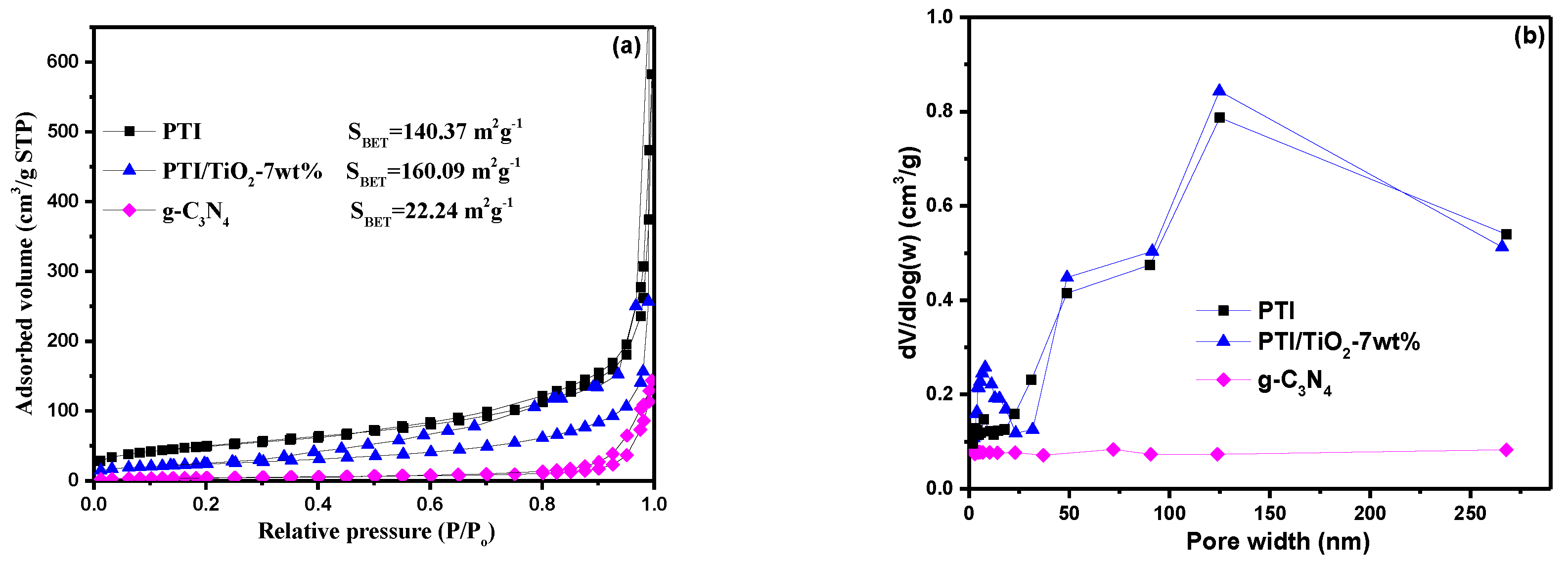
2.5. N2 Adsorption/Desorption Isotherms
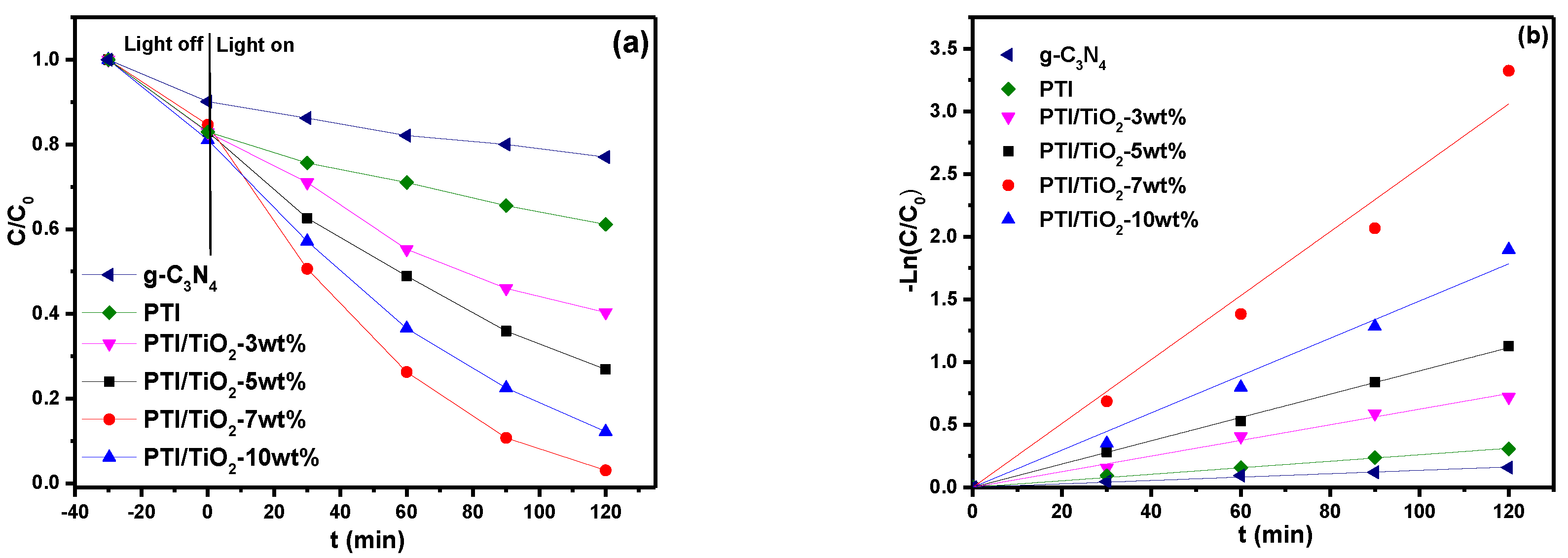
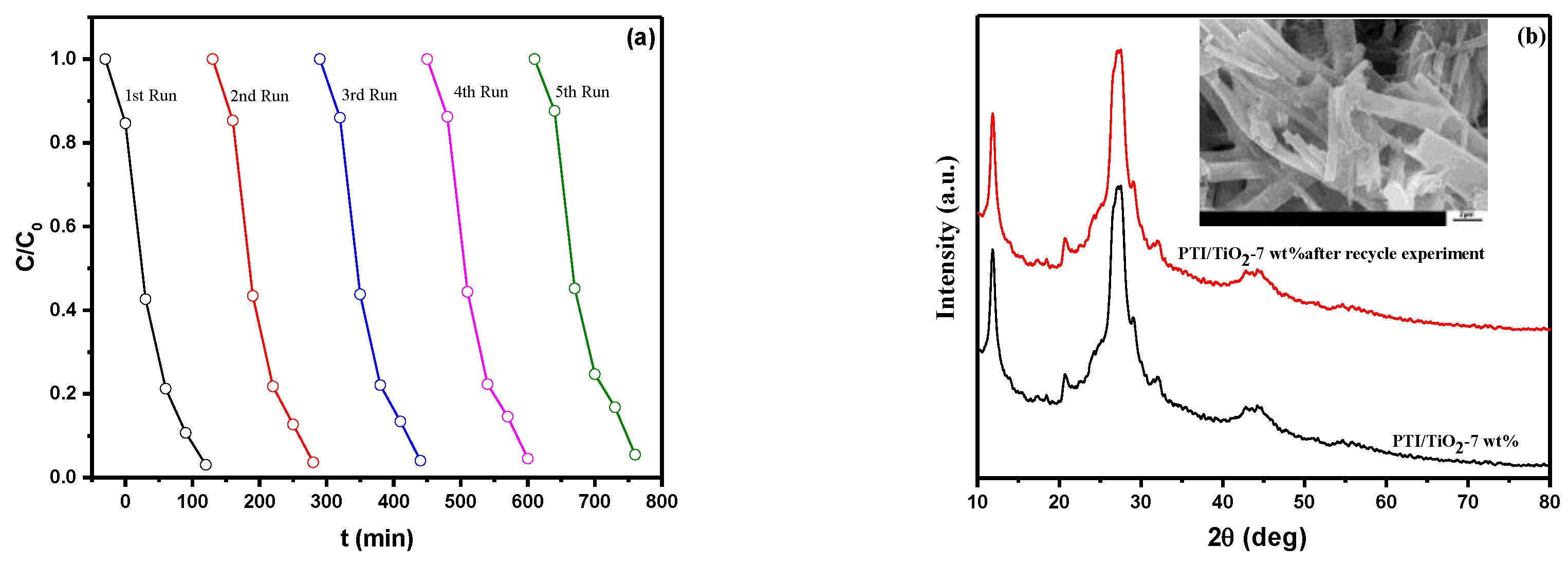
2.6. Photocatalytic Activity Analysis
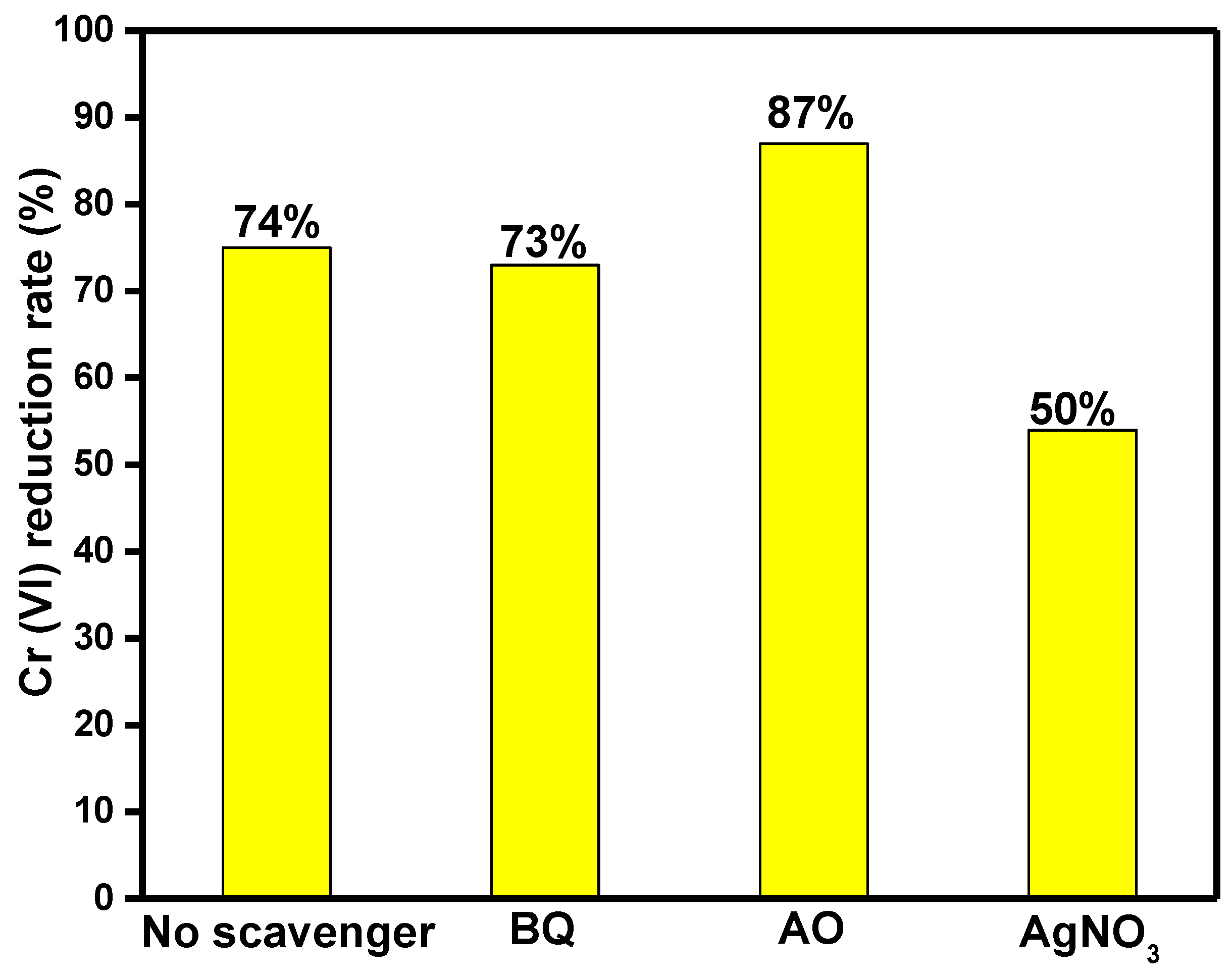
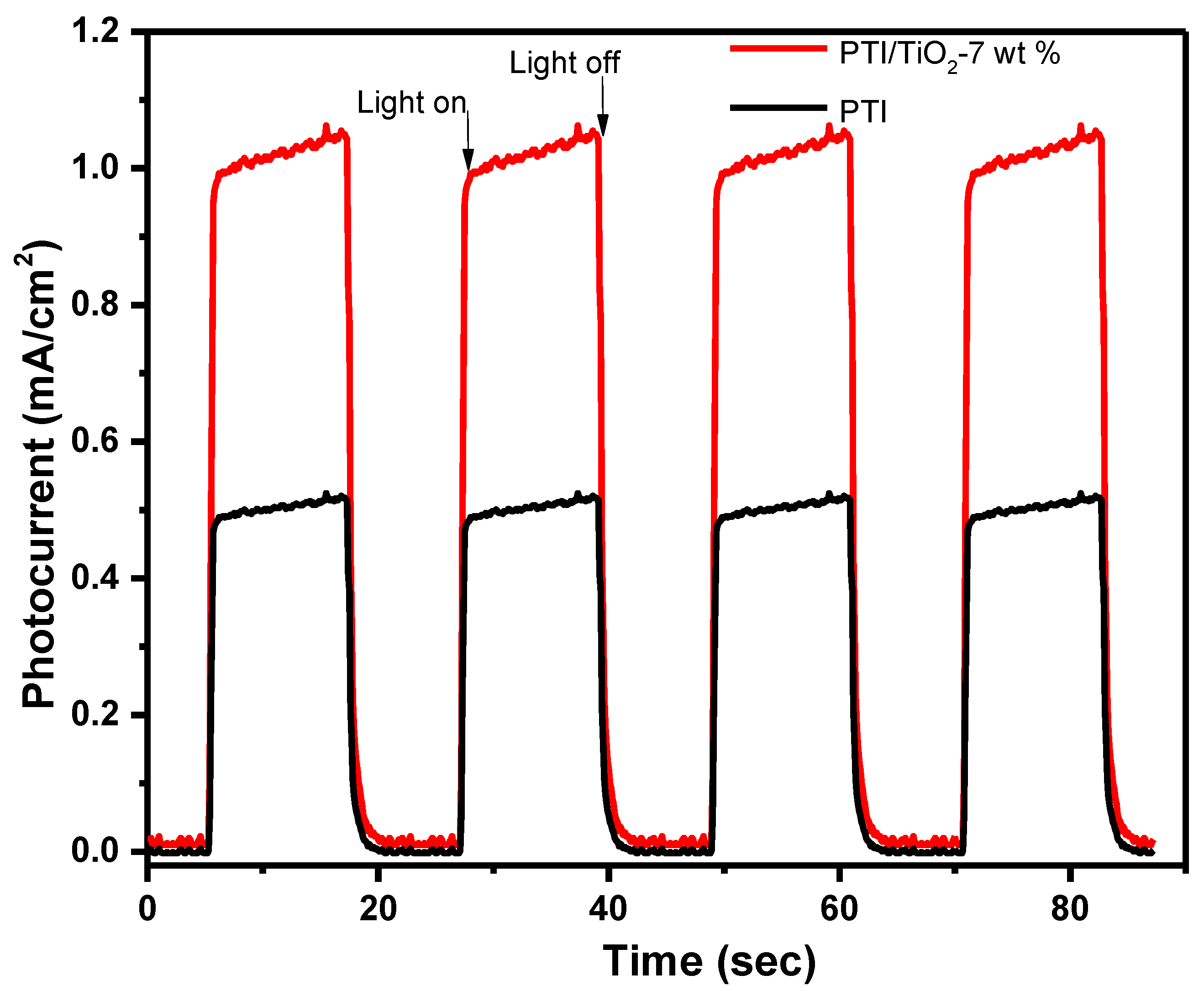
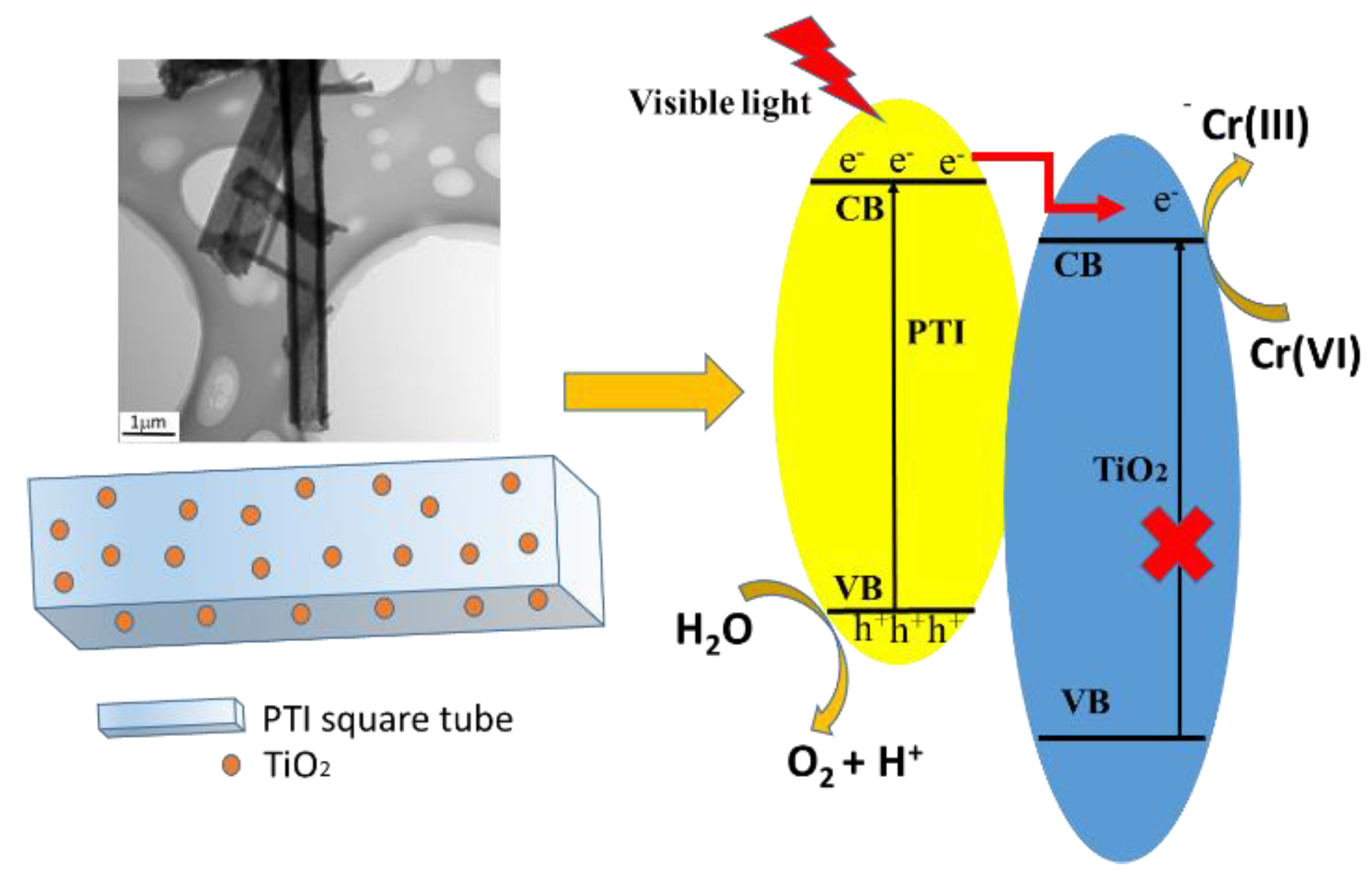
2.7. Photocatalytic Mechanism Analysis
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Photocatalyst
3.3. Characterization of the Prepared Photocatalyst
3.4. Photocatalytic Activity Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kieber, R.J.; Willey, J.D.; Zvalaren, S.D. Chromium speciation in rainwater: Temporal variability and atmospheric deposition. Environ. Sci. Technol. 2002, 36, 5321–5327. [Google Scholar] [CrossRef]
- Dima, J.B.; Sequeiros, C.; Zaritzky, N.E. Hexavalent chromium removal in contaminated water using reticulated chitosan micro/nanoparticles from seafood processing wastes. Chemosphere 2015, 141, 100–111. [Google Scholar] [CrossRef]
- Lu, D.Z.; Zhao, B.; Fang, P.F.; Zhai, S.B.; Li, D.L.; Chen, Z.Q.; Wu, W.H.; Chai, W.Q.; Wu, Y.C.; Qi, N. Facile one-pot fabrication and high photocatalytic performance of vanadium doped TiO2-based nanosheets for visible-light-driven degradation of RhB or Cr(VI). Appl. Surf. Sci. 2015, 359, 435–448. [Google Scholar] [CrossRef]
- Kumar, K.V.A.; Amanchi, S.R.; Sreedhar, B.; Ghosal, P.; Subrahmanyam, C. Phenol and Cr(VI) degradation with Mn ion doped ZnO under visible light photocatalysis. RSC Adv. 2017, 7, 43030–43039. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2 production activity of graphene/C3N4 composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Liang, F.; Wang, J.K.; Li, S.; Zhang, H.J.; Zhang, S.W. Molten salt synthesis of tetragonal carbon nitride hollow tubes and their application for removal of pollutants from wastewater. Appl. Catal. B Environ. 2018, 225, 307–313. [Google Scholar] [CrossRef]
- Bojdys, M.J.; Müller, J.O.; Antonietti, M.A. Ionothermal synthesis of crystalline, condensed, graphitic carbon nitride. Chem. Eur. J. 2008, 14, 8177–8182. [Google Scholar] [CrossRef]
- Schwinghammer, K.; Tuffy, B.; Mesch, M.B.; Wirnhier, C.; Martineau, F.; Taulelle, W.; Lotsch, B.V. Triazine-based carbon nitrides for visible-light-driven hydrogen evolution. Angew. Chem. Int. Ed. 2013, 125, 2495–2499. [Google Scholar] [CrossRef]
- Kroke, E.; Sehwarz, E.M.; Horathbordon, E. Tri-s-triazine derivatives from trichloro-tri-s-triazine to graphitic C3N4 structures. New J. Chem. 2002, 26, 508–512. [Google Scholar] [CrossRef]
- Lee, W.R.; Jun, Y.S.; Park, J.; Stucky, G.D. Crystalline poly(triazine imide) based g-CN as an efficient electrocatalyst for counter electrodes of dye-sensitized solar cells using a triiodide/iodide redox electrolyte. J. Mater. Chem. A 2015, 3, 24232–24236. [Google Scholar] [CrossRef]
- Schwinghammer, K.; Mesch, M.B.; Duppel, V.; Ziegler, C.; Senker, J.; Litsch, B.V. Crystalline carbon nitride nanosheets for improved visible-light hydrogen evolution. J. Am. Chem. Soc. 2014, 136, 1730–1733. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Q.; Yin, N.; Zhang, M.; Liu, X.; Zheng, H.; Wu, G.; Chen, P. Enhanced room-temperature ferromagnetic properties in ultrathin two-dimensional metal-free poly(triazine imide) nanosheets. Carbon 2017, 124, 486–491. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Lu, J.; Cong, Y. Synergistic photoelectrochemical reduction of Cr(VI) and oxidation of organic pollutants by g-C3N4/TiO2-NTs electrodes. Chemosphere 2016, 162, 55–63. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, C.; Chen, S.; Fu, X. Significantly enhanced visible-light photocatalytic activity of g-C3N4 via ZnO modification and the mechanism study. J. Mol. Catal. A Chem. 2013, 368–369, 9–15. [Google Scholar] [CrossRef]
- Peng, W.C.; Li, X.Y. Synthesis of MoS2/g-C3N4 as a solar light-responsive photocatalyst for organic degradation. Catal. Commun. 2014, 49, 63–67. [Google Scholar] [CrossRef]
- Lin, X.H.; Chen, H.; Hu, Z.B.; Hou, Y.D.; Dai, W.X. Enhanced visible light photocatalysis of TiO2 by Co-modification with Eu and Au nanoparticles. Solid State Sci. 2018, 83, 181–187. [Google Scholar] [CrossRef]
- Xiang, Y.G.; Wang, X.P.; Zhang, X.H.; Hou, H.J.; Dai, K.; Huang, Q.Y.; Chen, H. Enhanced visible light photocatalytic activity of TiO2 assisted by organic semiconductors a structure optimization strategy of conjugated polymers. J. Mater. Chem. A 2018, 6, 153–159. [Google Scholar] [CrossRef]
- Ham, Y.; Maeda, K.; Cha, D.; Kazuhiro, T.; Domen, K. Synthesis and photocatalytic activity of poly(triazine imide). Chem. Asian J. 2013, 8, 218–224. [Google Scholar] [CrossRef]
- Wirnhier, E.; Döblinger, M.; Gunzelmann, D.; Senker, J.; Litsch, B.V.; Schnick, W. Poly(triazine imide) with intercalation of lithium and chloride ions [(C3N3)2(NH(x)Li(1-x))3⋅LiCl]: A crystalline 2D carbon nitride network. Chem. Eur. J. 2011, 17, 3213–3221. [Google Scholar] [CrossRef]
- Jin, A.; Jia, Y.; Chen, C.; Liu, X.; Jiang, J.; Chen, X.; Zhang, F. Efficient photocatalytic hydrogen evolution on band structure tuned polytriazine/heptazine based carbon nitride heterojunctions with ordered needle-like morphology achieved by an in situ molten salt method. J. Phys. Chem. C 2017, 121, 21497–21509. [Google Scholar] [CrossRef]
- Liu, H.; Chen, D.; Wang, Z.; Jing, H.; Zhang, R. Microwave-assisted molten-salt rapid synthesis of isotype triazine/heptazine based g-C3N4, heterojunctions with highly enhanced photocatalytic hydrogen evolution performance. Appl. Catal B Environ. 2017, 203, 300–313. [Google Scholar] [CrossRef]
- Gao, H.L.; Yan, S.C.; Wang, J.J.; Huang, Y.A.; Wang, P.; Li, Z.S. Towards efficient solar hydrogen production by intercalated carbon nitride photocatalyst. Phys. Chem. Chem. Phys. 2013, 15, 18077–18084. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science. 2002, 297, 2243–2245. [Google Scholar] [CrossRef]
- Chen, X.F.; Wei, J.; Hou, R.J.; Liang, Y.; Xie, Z.L.; Zhu, Y.G.; Zhang, X.W.; Wang, H.T. Growth of g-C3N4 on mesoporous TiO2 spheres with high photocatalytic activity under visible light irradiation. Appl. Catal. B 2016, 188, 342–350. [Google Scholar] [CrossRef]
- Ye, B.Y.; Han, X.X.; Yan, M.D.; Zhang, H.H.; Xi, F.; Dong, X.P.; Liu, J.Y. Fabrication of metal-free two dimensional/two dimensional homojunction photocatalyst using various carbon nitride nanosheets as building blocks. J. Colloid Interface Sci. 2017, 507, 209–216. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.C.; Sun, D.D.; Ng, W.J. Graphene oxide–CdS composite with high photocatalytic degradation and disinfection activities under visible light irradiation. J. Hazard. Mater. 2013, 250–251, 412–420. [Google Scholar] [CrossRef]
- Singh, P.; Mondal, K.; Sharma, A. Reusable electrospun mesoporous ZnO nanofiber mats for photocatalytic degradation of polycyclic aromatic hydrocarbon dyes in wastewater. J. Colloid Interface Sci. 2013, 394, 208–215. [Google Scholar] [CrossRef]
- Wei, H.T.; Zhang, Q.; Zhang, Y.C.; Yang, Z.J.; Zhu, A.P.; Dionysiou, D. Enhancement of the Cr(VI) adsorption and photocatalytic reduction activity of g-C3N4 by hydrothermal treatment in HNO3 aqueous solution. Appl. Catal. A Gen. 2016, 521, 9–18. [Google Scholar] [CrossRef]
- Wang, X.; Hong, M.Z.; Zhang, F.W.; Zhuang, Z.Y.; Yu, Y. Recyclable nanoscale zero valent iron doped g-C3N4/MoS2 for efficient photocatalysis of RhB and Cr(VI) driven by visible light. ACS Sustain. Chem. Eng. 2016, 7, 4055–4063. [Google Scholar] [CrossRef]
- Hu, X.F.; Ji, H.H.; Chang, F.; Luo, Y.M. Simultaneous photocatalytic Cr(VI) reduction and 2,4,6-TCP oxidation over g-C3N4 under visible light irradiation. Catal. Today 2014, 224, 34–40. [Google Scholar] [CrossRef]
- Huang, W.Y.; Liu, N.; Zhang, X.D.; Wu, M.H.; Tang, L. Metal organic framework g-C3N4 /MIL-53(Fe) heterojunctions with enhanced photocatalytic activity for Cr(VI) reduction under visible light. Appl. Surf. Sci. 2017, 425, 107–116. [Google Scholar] [CrossRef]
- Sun, M.; Fang, Y.; Kong, Y.; Sun, S.; Yu, Z. Graphitic carbon nitride (g-C3N4) coated titanium oxide nanotube arrays with enhanced photo-electrochemical performance. Dalton Trans. 2016, 45, 2702–12709. [Google Scholar] [CrossRef]
- Liu, F.; Yu, J.; Tu, G.; Qu, L.; Xiao, J.; Liu, Y.; Wang, L.; Lei, J.; Zhang, J. Carbon nitride coupled Ti-SBA15 catalyst for visible-light-driven photocatalytic reduction of Cr (VI) and the synergistic oxidation of phenol. Appl. Catal. B Environ. 2017, 201, 1–11. [Google Scholar] [CrossRef]
- Ding, M.; Wang, W.; Zhou, Y.; Lu, C.; Ni, Y.; Xu, Z. Facile in situ synthesis of 2D porous g-C3N4 and g-C3N4/P25(N) heterojunction with enhanced quantum effect for efficient photocatalytic application. J. Alloys Compd. 2015, 635, 34–40. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Ma, C.; Guan, Q.; Lu, Z.; Huo, P. Melamine modified P25 with heating method and enhanced the photocatalytic activity on degradation of ciprofloxacin. Appl. Surf. Sci. 2015, 329, 17–22. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Ning, G.; Zhao, P. Enhanced Visible Light Photocatalytic Reduction of Cr(VI) over a Novel Square Nanotube Poly(Triazine Imide)/TiO2 Heterojunction. Catalysts 2019, 9, 55. https://doi.org/10.3390/catal9010055
Yan X, Ning G, Zhao P. Enhanced Visible Light Photocatalytic Reduction of Cr(VI) over a Novel Square Nanotube Poly(Triazine Imide)/TiO2 Heterojunction. Catalysts. 2019; 9(1):55. https://doi.org/10.3390/catal9010055
Chicago/Turabian StyleYan, Xin, Guotao Ning, and Peng Zhao. 2019. "Enhanced Visible Light Photocatalytic Reduction of Cr(VI) over a Novel Square Nanotube Poly(Triazine Imide)/TiO2 Heterojunction" Catalysts 9, no. 1: 55. https://doi.org/10.3390/catal9010055
APA StyleYan, X., Ning, G., & Zhao, P. (2019). Enhanced Visible Light Photocatalytic Reduction of Cr(VI) over a Novel Square Nanotube Poly(Triazine Imide)/TiO2 Heterojunction. Catalysts, 9(1), 55. https://doi.org/10.3390/catal9010055



