Rice Husk Derived Porous Silica as Support for Pd and CeO2 for Low Temperature Catalytic Methane Combustion
Abstract
:1. Introduction
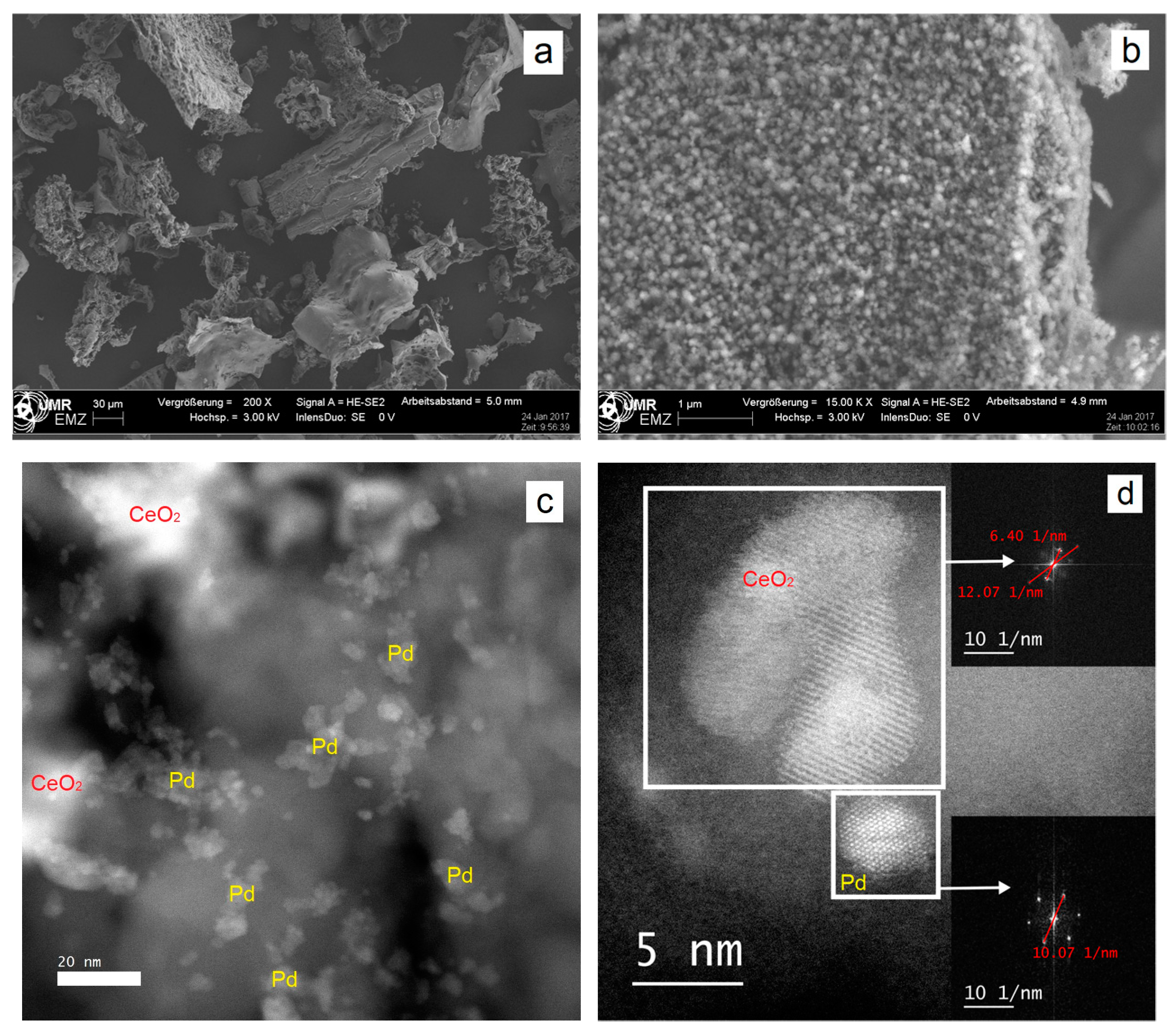
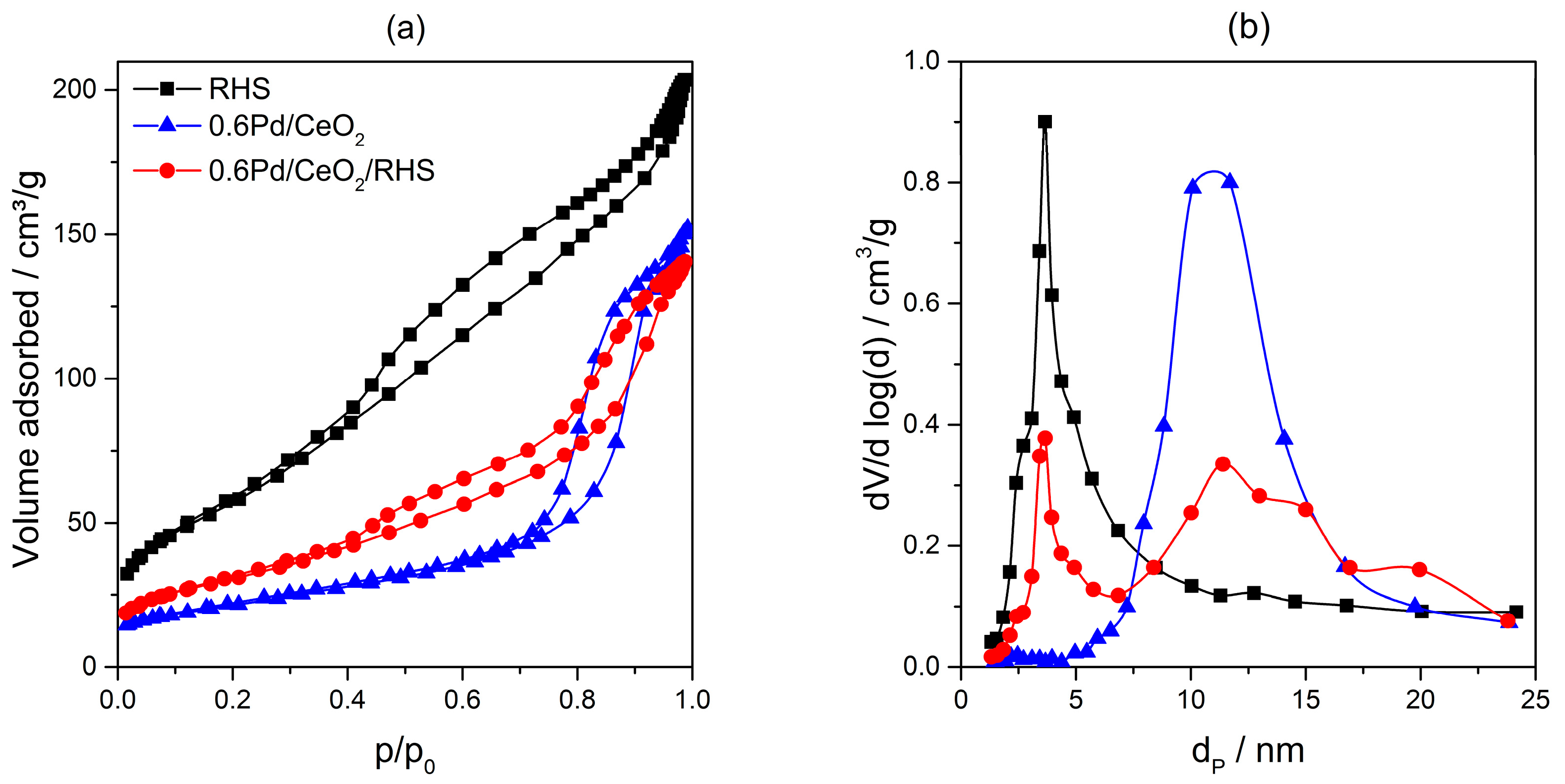
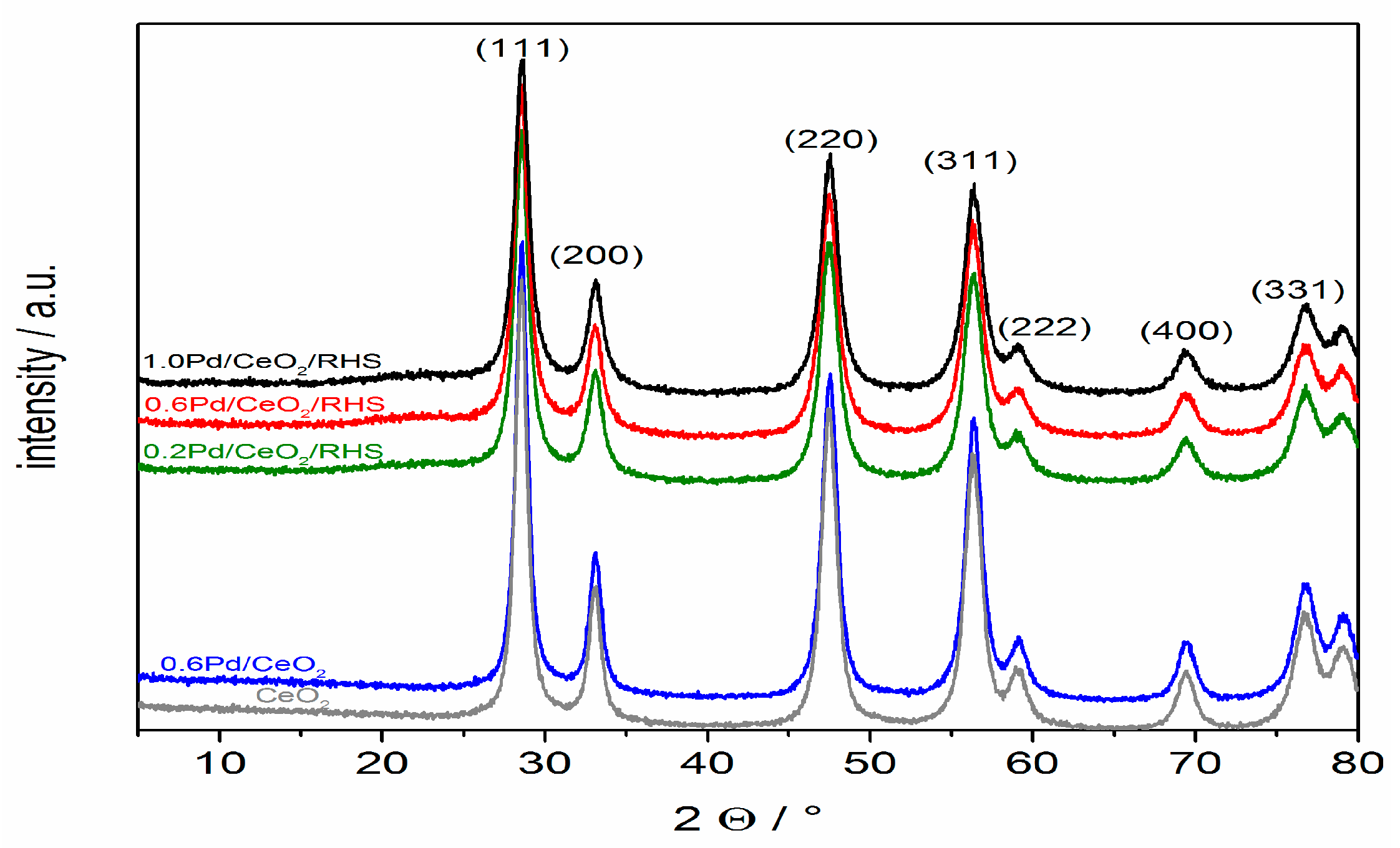
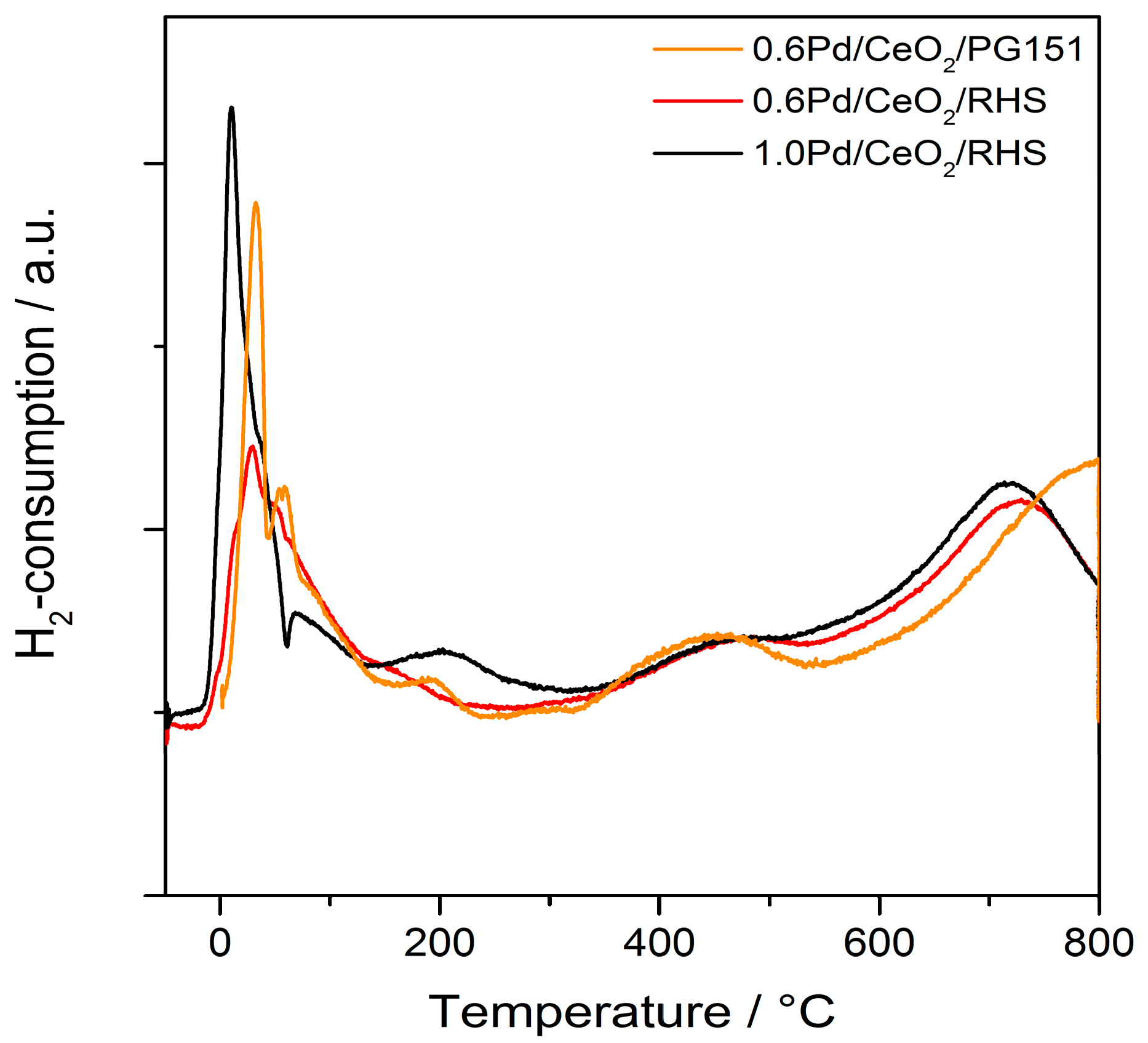
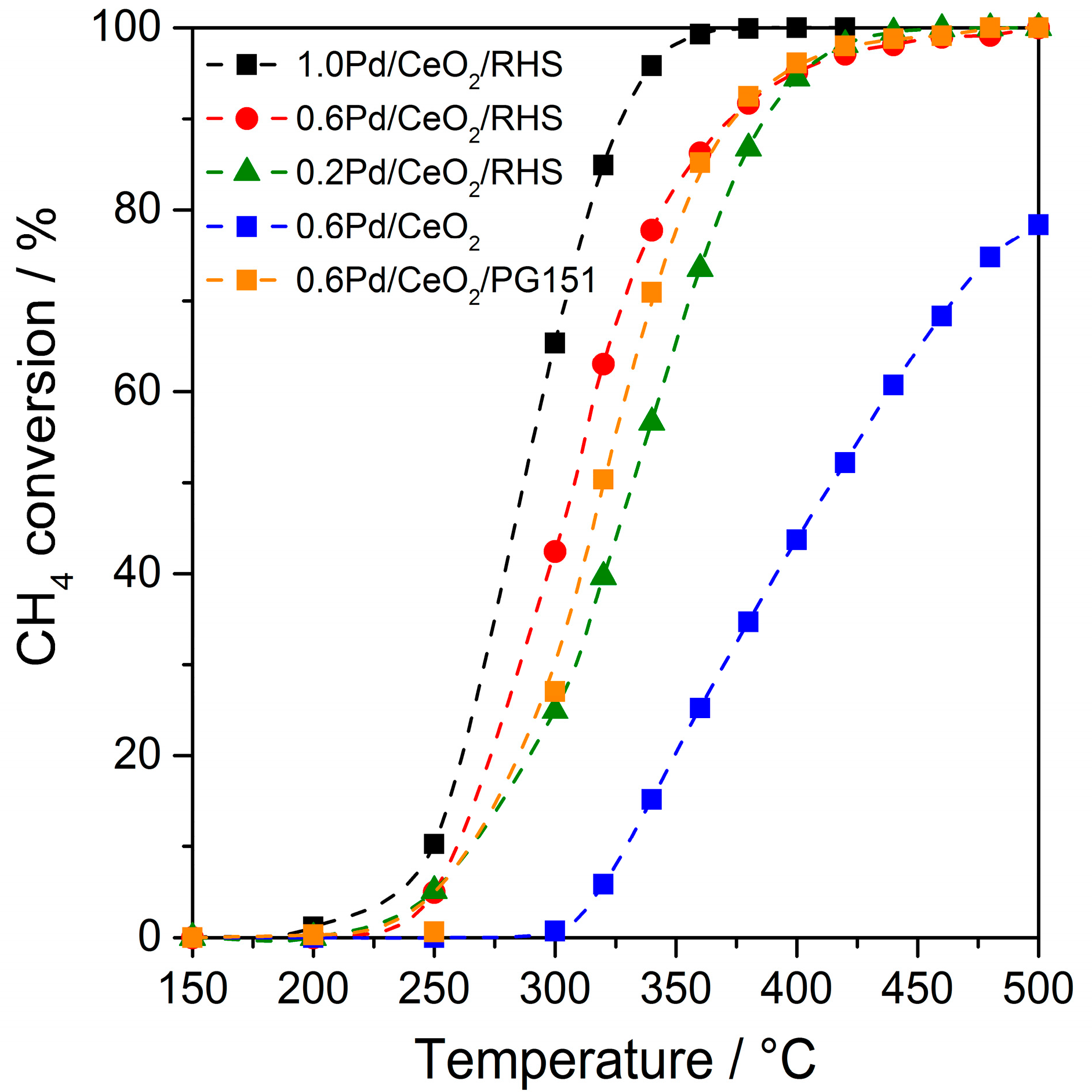
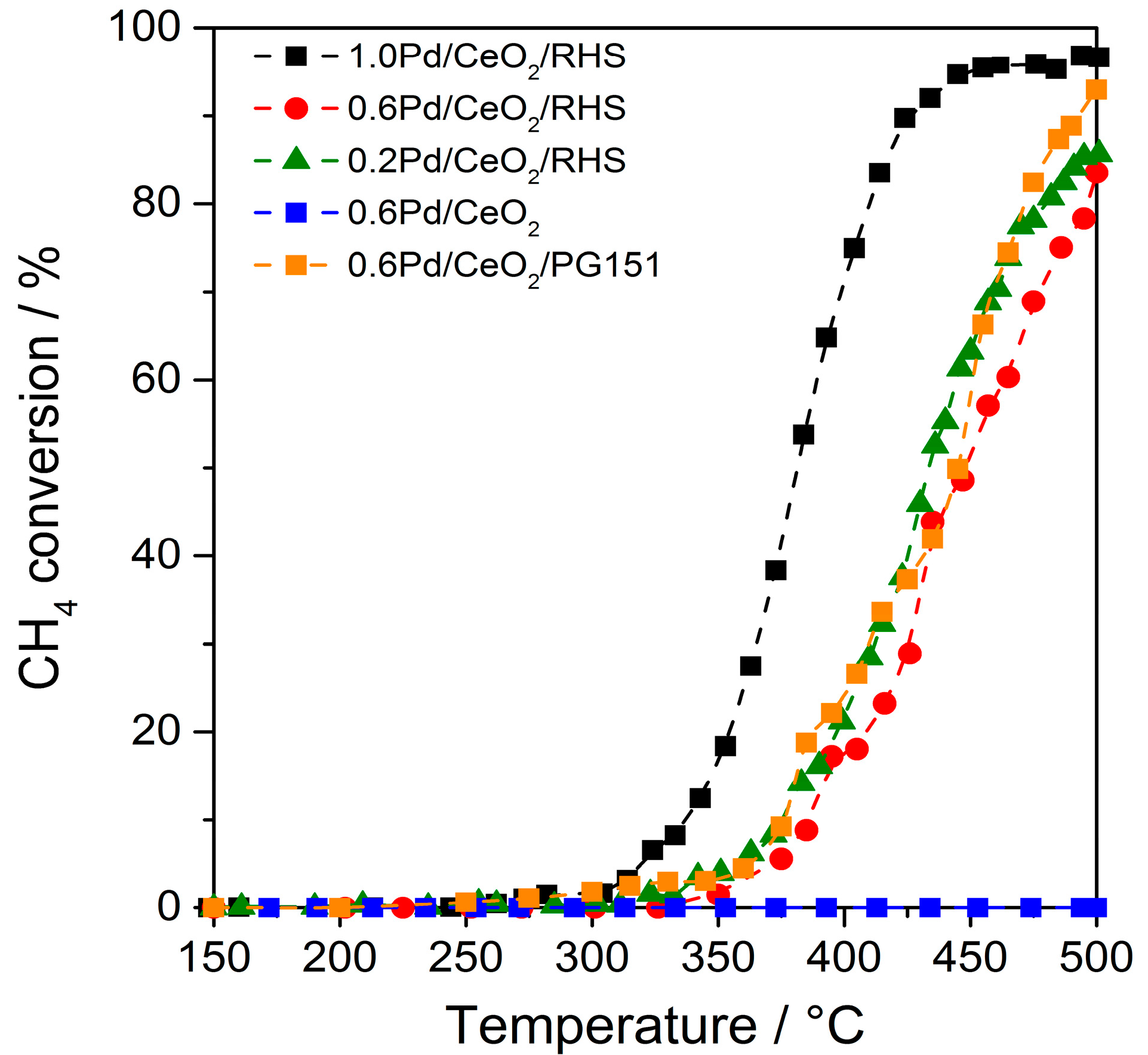
2. Results and Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Testing of Methane Combustion Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malyan, S.K.; Bhatia, A.; Kumar, A.; Gupta, D.K.; Singh, R.; Kumar, S.S.; Tomer, R.; Kumar, O.; Jain, N. Methane production, oxidation and mitigation: A mechanistic understanding and comprehensive evaluation of influencing factors. Sci. Total Environ. 2016, 572, 874–896. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 2009, 1, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Seiler, W.; Holzapfel-Pschorn, A.; Conrad, R.; Scharffe, D. Methane emission from rice paddies. J. Atmos. Chem. 1983, 1, 241–268. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Calheiro, D.; Kieling, A.G.; Moraes, C.A.M.; Rocha, T.L.A.C.; Brehm, F.A.; Modolo, R.C.E. Characterization of rice husk ash produced using different biomass combustion techniques for energy. Fuel 2016, 165, 351–359. [Google Scholar] [CrossRef]
- Moraes, C.A.M.; Fernandes, I.J.; Calheiro, D.; Kieling, A.G.; Brehm, F.A.; Rigon, M.R.; Berwanger Filho, J.A.; Schneider, I.A.H.; Osorio, E. Review of the rice production cycle: By-products and the main applications focusing on rice husk combustion and ash recycling. Waste Manag. Res. 2014, 32, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Pode, R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain. Energy Rev. 2016, 53, 1468–1485. [Google Scholar] [CrossRef]
- Kalapathy, U.; Proctor, A.; Shultz, J. An improved method for production of silica from rice hull ash. Bioresour. Technol. 2002, 85, 285–289. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, B.; Gao, W.; Qu, Y.; Wang, L.; Wang, Z.; Zhu, Y. A recyclable method for production of pure silica from rice hull ash. Powder Technol. 2012, 217, 497–501. [Google Scholar] [CrossRef]
- Alyosef, H.A.; Eilert, A.; Welscher, J.; Ibrahim, S.S.; Denecke, R.; Schwieger, W.; Enke, D. Characterization of Biogenic Silica Generated by Thermo Chemical Treatment of Rice Husk. Part. Sci. Technol. 2013, 31, 524–532. [Google Scholar] [CrossRef]
- Schneider, D.; Wassersleben, S.; Weiß, M.; Denecke, R.; Stark, A.; Enke, D. A Generalized procedure for the production of high-grade, porous biogenic silica. Waste Biomass Valoriz. 2018. [Google Scholar] [CrossRef]
- Alyosef, H.; Roggendorf, H.; Schneider, D.; Inayat, A.; Welscher, J.; Schwieger, W.; Münster, T.; Kloess, G.; Ibrahim, S.; Enke, D. Comparative study between direct and pseudomorphic transformation of rice husk ash into MFI-type zeolite. Molecules 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Alyosef, H.; Uhlig, H.; Münster, T.; Kloess, G.; Einicke, W.-D.; Gläser, R.; Enke, D. Biogenic silica from rice husk ash–Sustainable sources for the synthesis of value added silica. Chem. Eng. 2014, 37, 667–672. [Google Scholar]
- Adam, F.; Appaturi, J.N.; Iqbal, A. The utilization of rice husk silica as a catalyst: Review and recent progress. Catal. Today 2012, 190, 2–14. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kreft, S.; Georgi, G.; Fulda, G.; Pohl, M.-M.; Seeburg, D.; Berger-Karin, C.; Kondratenko, E.V.; Wohlrab, S. Improved catalytic methane combustion of Pd/CeO2 catalysts via porous glass integration. Appl. Catal. B Environ. 2015, 179, 313–320. [Google Scholar] [CrossRef]
- Enke, D.; Janowski, F.; Schwieger, W. Porous glasses in the 21st century––A short review. Microporous Mesoporous Mater. 2003, 60, 19–30. [Google Scholar] [CrossRef]
- Ishihara, T.; Shigematsu, H.; Abe, Y.; Takita, Y. Effects of additives on the Activity of palladium catalysts for methane combustion. Chem. Lett. 1993, 22, 407–410. [Google Scholar] [CrossRef]
- Li, Z.; Hoflund, G.B. Catalytic oxidation of methane over Pd/Co3O4. React. Kinet. Catal. Lett. 1999, 66, 367–374. [Google Scholar] [CrossRef]
- Eguchi, K.; Arai, H. Low temperature oxidation of methane over Pd-based catalysts—effect of support oxide on the combustion activity. Appl. Catal. A Gen. 2001, 222, 359–367. [Google Scholar] [CrossRef]
- Ciuparu, D.; Lyubovsky, M.R.; Altman, E.; Pfefferle, L.D.; Datye, A. Catalytic combustion of methane over palladium-based catalysts. Catal. Rev. 2002, 44, 593–649. [Google Scholar] [CrossRef]
- Li, Z.; Hoflund, G.B. A review on complete oxidation of methane at low temperatures. J. Nat. Gas Chem. 2003, 12, 153–160. [Google Scholar]
- Xiao, L.-H.; Sun, K.-P.; Xu, X.-L.; Li, X.-N. Low-temperature catalytic combustion of methane over Pd/CeO2 prepared by deposition-precipitation method. Catal. Commun. 2005, 6, 796–801. [Google Scholar] [CrossRef]
- Ercolino, G.; Grzybek, G.; Stelmachowski, P.; Specchia, S.; Kotarba, A.; Specchia, V. Pd/Co3O4-based catalysts prepared by solution combustion synthesis for residual methane oxidation in lean conditions. Catal. Today 2015, 257, 66–71. [Google Scholar] [CrossRef]
- Ercolino, G.; Stelmachowski, P.; Specchia, S. Catalytic performance of Pd/Co3O4 on SiC and ZrO2 open cell foams for process intensification of methane combustion in lean conditions. Ind. Eng. Chem. Res. 2017, 56, 6625–6636. [Google Scholar] [CrossRef]
- Seeburg, D.; Liu, D.; Radnik, J.; Atia, H.; Pohl, M.-M.; Schneider, M.; Martin, A.; Wohlrab, S. Structural changes of highly active Pd/MeOx (Me = Fe, Co, Ni) during catalytic methane combustion. Catalysts 2018, 8, 42. [Google Scholar] [CrossRef]
- Vassalini, I.; Alessandri, I. Switchable stimuli-responsive heterogeneous catalysis. Catalysts 2018, 8, 569. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Pech-Canul, M.I.; González, L.A. Review on the physicochemical treatments of rice husk for production of advanced materials. Chem. Eng. J. 2015, 264, 899–935. [Google Scholar] [CrossRef]
- Schliermann, T.; Hartmann, I.; Beidaghy Dizaji, H.; Zeng, T.; Schneider, D.; Wassersleben, S.; Enke, D.; Jobst, T.; Lange, A.; Roelofs, F.; et al. High quality biogenic silica from combined energetic and material utilization of agricultural residues. In Proceedings of the 7th International Symposium on Energy from Biomass and Waste (VENICE2018), Venice, Italy, 15–18 October 2018. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Muto, K.-I.; Katada, N.; Niwa, M. Complete oxidation of methane on supported palladium catalyst: Support effect. Appl. Catal. A Gen. 1996, 134, 203–215. [Google Scholar] [CrossRef]
- Leitenburg, C.D.; Trovarelli, A.; Kašpar, J. A temperature-programmed and transient kinetic study of CO2 activation and methanation over CeO2 supported noble metals. J. Catal. 1997, 166, 98–107. [Google Scholar] [CrossRef]
- Sasikala, R.; Gupta, N.M.; Kulshreshtha, S.K. Temperature-programmed reduction and CO oxidation studies over Ce–Sn mixed oxides. Catal. Lett. 2001, 71, 69–73. [Google Scholar] [CrossRef]
- Ferrer, V.; Moronta, A.; Sánchez, J.; Solano, R.; Bernal, S.; Finol, D. Effect of the reduction temperature on the catalytic activity of Pd-supported catalysts. Catal. Today 2005, 107–108, 487–492. [Google Scholar] [CrossRef]
- Gremminger, A.T.; Pereira de Carvalho, H.W.; Popescu, R.; Grunwaldt, J.-D.; Deutschmann, O. Influence of gas composition on activity and durability of bimetallic Pd-Pt/Al2O3 catalysts for total oxidation of methane. Catal. Today 2015, 258, 470–480. [Google Scholar] [CrossRef]
| Samples | Nitrogen Sorption | ICP-OES | |||
|---|---|---|---|---|---|
| SBET (m2/g) | Vt (mL/g) | Pd % | Ce % | Si % | |
| RHS | 217 | 0.34 | - | - | - |
| 0.6Pd/CeO2 | 76 | 0.24 | 0.7 | 76.9 | - |
| 0.6Pd/CeO2/RHS | 111 | 0.22 | 0.7 | 32.0 | 18.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Seeburg, D.; Kreft, S.; Bindig, R.; Hartmann, I.; Schneider, D.; Enke, D.; Wohlrab, S. Rice Husk Derived Porous Silica as Support for Pd and CeO2 for Low Temperature Catalytic Methane Combustion. Catalysts 2019, 9, 26. https://doi.org/10.3390/catal9010026
Liu D, Seeburg D, Kreft S, Bindig R, Hartmann I, Schneider D, Enke D, Wohlrab S. Rice Husk Derived Porous Silica as Support for Pd and CeO2 for Low Temperature Catalytic Methane Combustion. Catalysts. 2019; 9(1):26. https://doi.org/10.3390/catal9010026
Chicago/Turabian StyleLiu, Dongjing, Dominik Seeburg, Stefanie Kreft, René Bindig, Ingo Hartmann, Denise Schneider, Dirk Enke, and Sebastian Wohlrab. 2019. "Rice Husk Derived Porous Silica as Support for Pd and CeO2 for Low Temperature Catalytic Methane Combustion" Catalysts 9, no. 1: 26. https://doi.org/10.3390/catal9010026
APA StyleLiu, D., Seeburg, D., Kreft, S., Bindig, R., Hartmann, I., Schneider, D., Enke, D., & Wohlrab, S. (2019). Rice Husk Derived Porous Silica as Support for Pd and CeO2 for Low Temperature Catalytic Methane Combustion. Catalysts, 9(1), 26. https://doi.org/10.3390/catal9010026








