Theoretical Study on the Hydrogenation Mechanisms of Model Compounds of Heavy Oil in a Plasma-Driven Catalytic System
Abstract
1. Introduction
2. Results
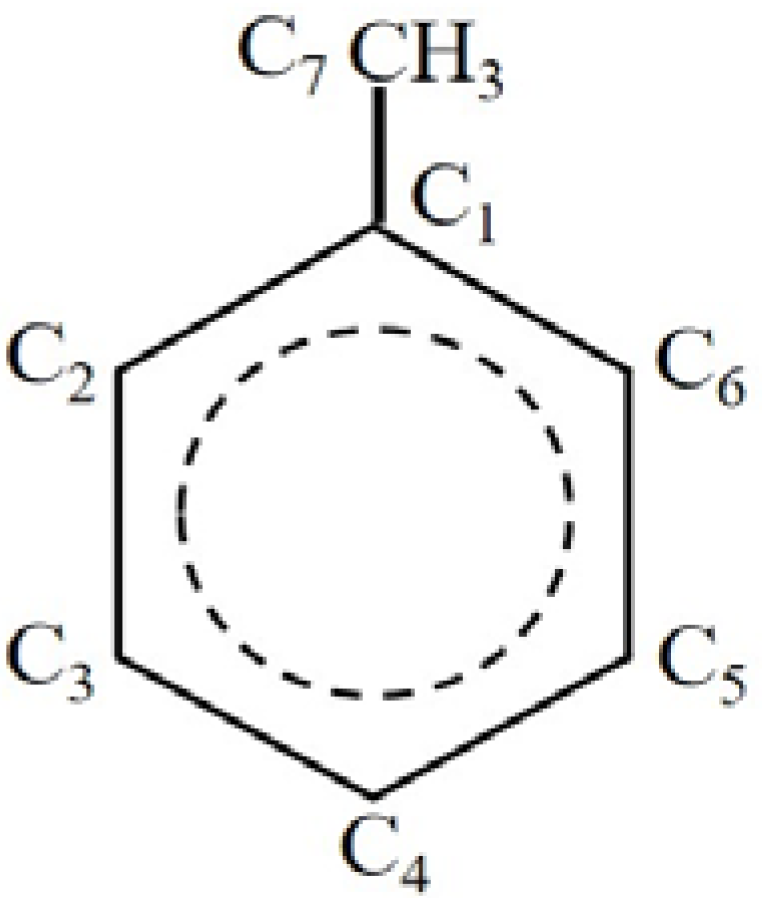
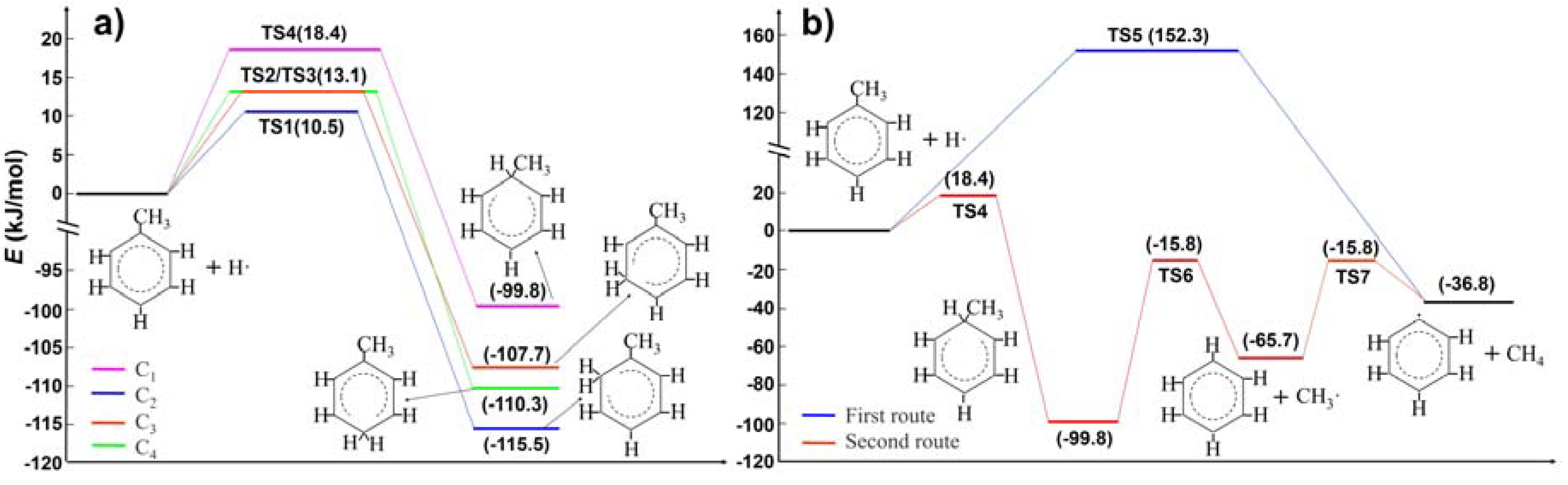
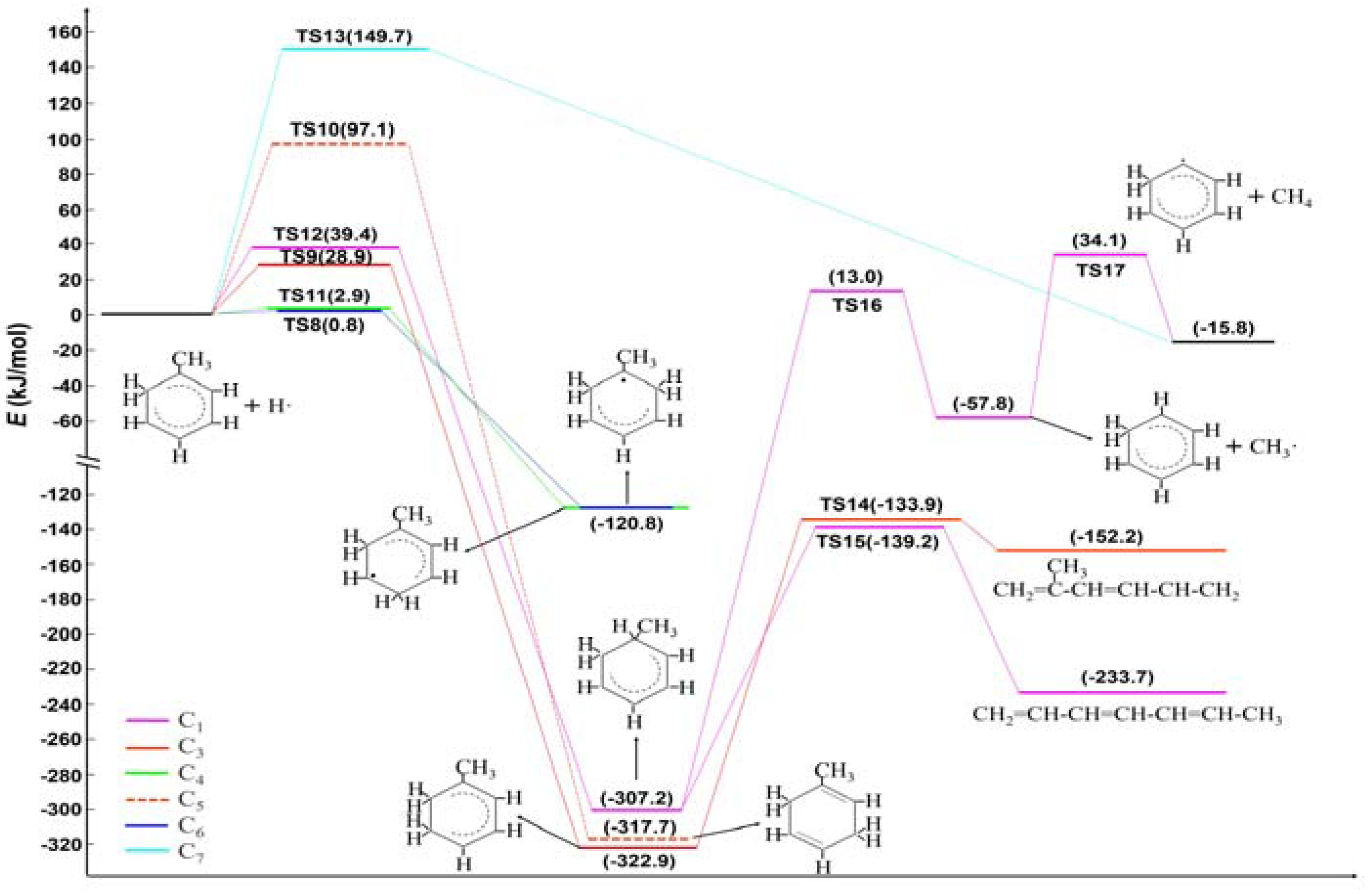
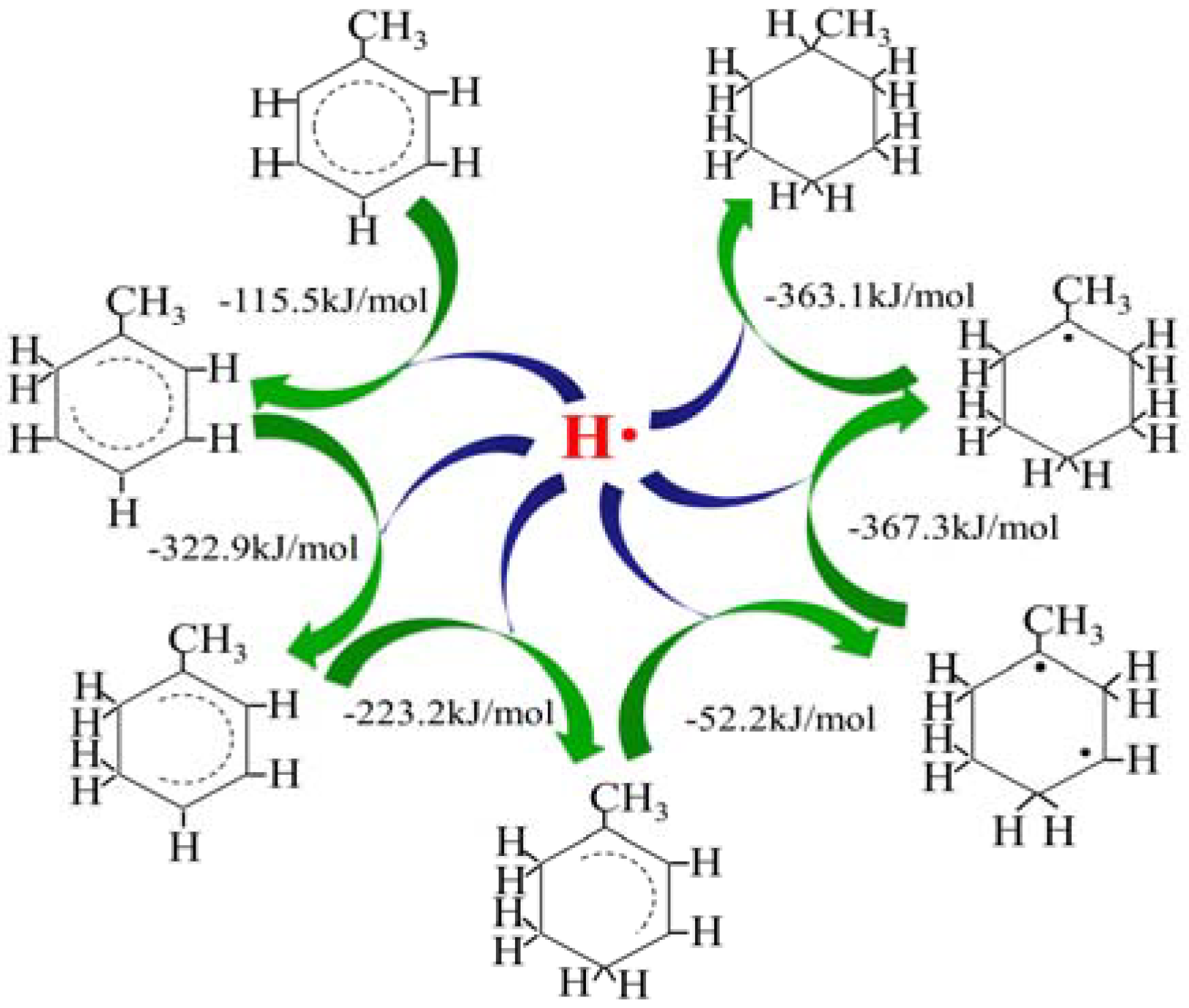
2.1. Hydrogen Atom Reacted with Toluene
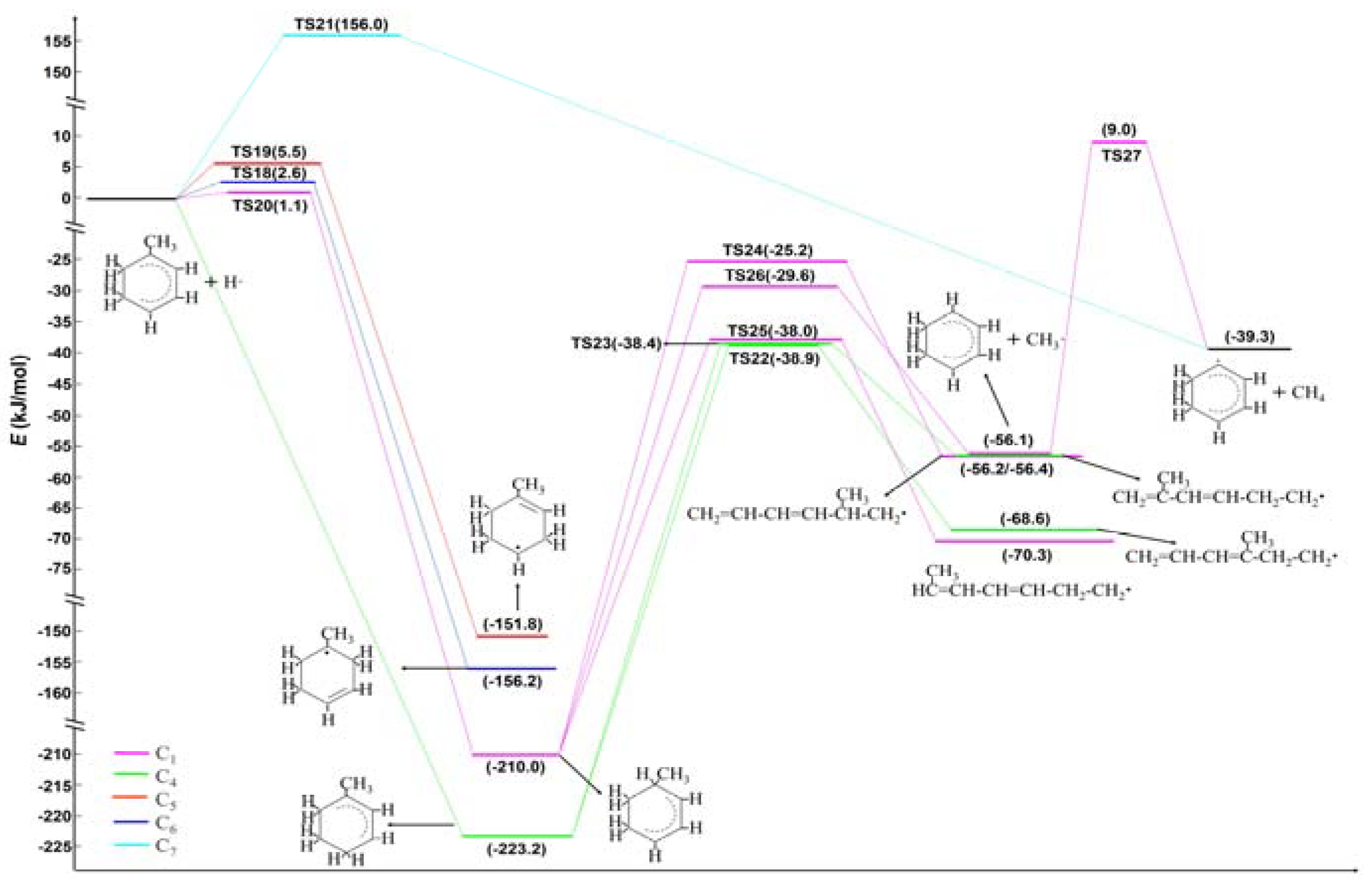
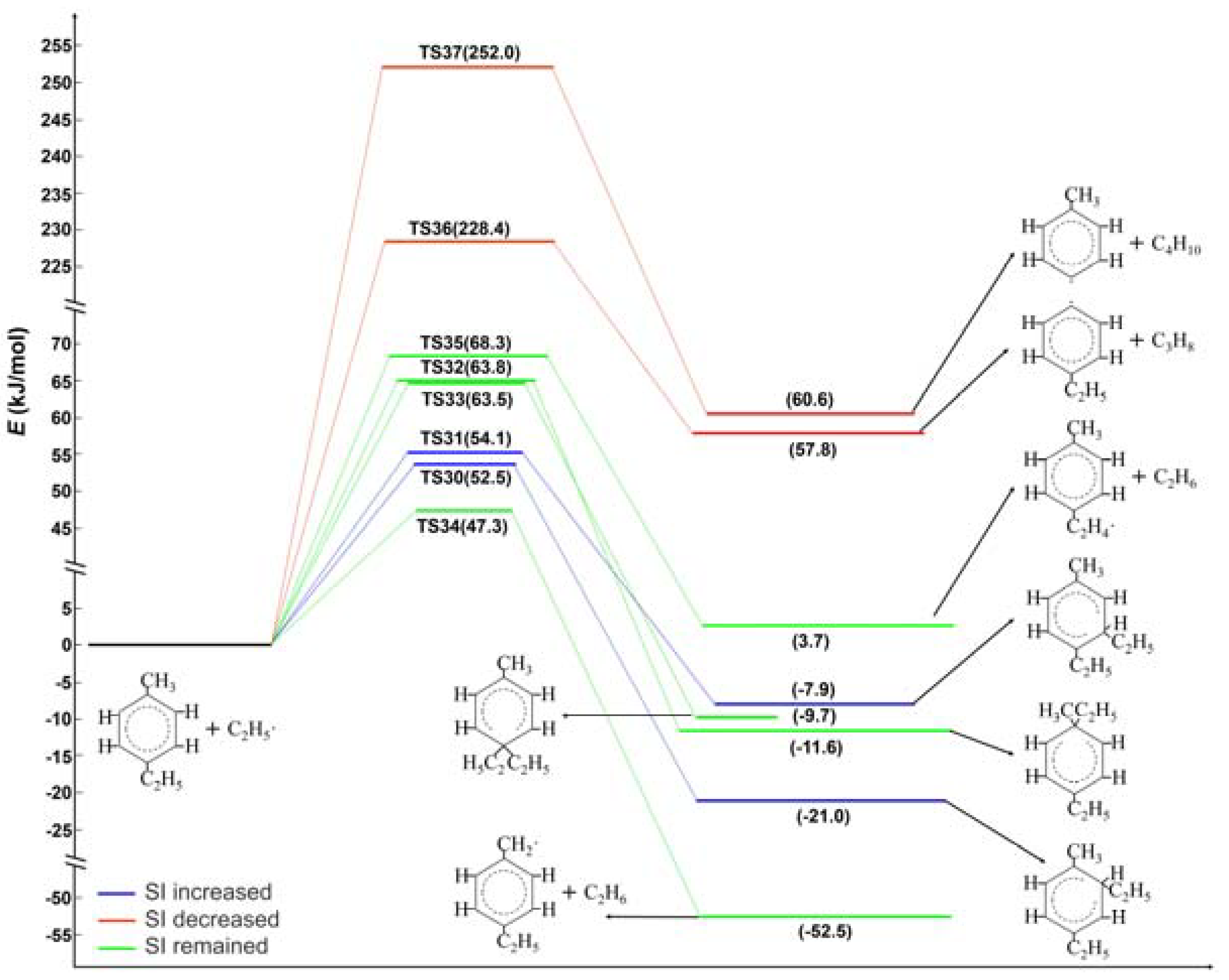
2.2. Ethyl Radical Reacted with 4-Ethyltoluene
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rana, M.S.; Samano, V.; Ancheyta, J.; Diaz, J.A.I. A review of recent advances on process technologies for upgrading of heavy oils and residua. Fuel 2007, 86, 1216–1231. [Google Scholar] [CrossRef]
- Speight, J.G. New approaches to hydroprocessing. Catal. Today 2004, 98, 55–60. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, D.; Deng, W.; Que, G. A review of slurry-phase hydrocracking heavy oil technology. Energy Fuels 2007, 21, 3057–3062. [Google Scholar] [CrossRef]
- Furimsky, E. Selection of catalysts and reactors for hydroprocessing. Appl. Catal. A 1998, 171, 177–206. [Google Scholar] [CrossRef]
- Zhang, X.; Chodakowski, M.; Shaw, J.M. Impact of multiphase behavior on coke deposition in a commercial hydrotreating catalyst under sedimentation conditions. Energy Fuels 2005, 19, 1405–1411. [Google Scholar] [CrossRef]
- Yang, M.G.; Nakamura, I.; Fujimoto, K. Hydro-thermal cracking of heavy oils and its model compound. Catal. Today 1998, 43, 273–280. [Google Scholar] [CrossRef]
- Hao, H.G.; Wu, B.S.; Yang, J.L.; Guo, Q.; Yang, Y.; Li, Y.W. Non-thermal plasma enhanced heavy oil upgrading. Fuel 2015, 149, 162–173. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Leys, C.; Van Langenhove, H. Combining non-thermal plasma with heterogeneous catalysis in waste gas treatment: A review. Appl. Catal. B 2008, 78, 324–333. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Chirokov, A.; Gutsol, A.; Fridman, A. Atmospheric pressure plasma of dielectric barrier discharges. Pure Appl. Chem. 2005, 77, 487–495. [Google Scholar] [CrossRef]
- Rollier, J.D.; Gonzalez-Aguilar, J.; Petitpas, G.; Darmon, A.; Fulcheri, L.; Metkemeijer, R. Experimental study on gasoline reforming assisted by nonthermal arc discharge. Energy Fuels 2008, 22, 556–560. [Google Scholar] [CrossRef]
- Eliasson, B.; Liu, C.J.; Kogelschatz, U. Direct conversion of methane and carbon dioxide to higher hydrocarbons using catalytic dielectric-barrier discharges with zeolites. Ind. Eng. Chem. Res. 2000, 39, 1221–1227. [Google Scholar] [CrossRef]
- Li, M.W.; Tian, Y.L.; Xu, G.H. Characteristics of carbon dioxide reforming of methane via alternating current (AC) corona plasma reactions. Energy Fuels 2007, 21, 2335–2339. [Google Scholar] [CrossRef]
- Matsui, Y.; Kawakami, S.; Takashima, K.; Katsura, S.; Mizuno, A. Liquid-phase fuel reforming at room temperature using non-thermal plasma. Energy Fuels 2005, 19, 1561–1565. [Google Scholar] [CrossRef]
- Veerapandian, S.K.; Leys, C.; De Geyter, N.; Morent, R. Abatement of VOCs using packed bed non-thermal plasma reactors: A review. Catalysts 2017, 7, 113. [Google Scholar] [CrossRef]
- Yap, D.; Tatibouët, J.M.; Batiot-Dupeyrat, C. Catalyst assisted by non-thermal plasma in dry reforming of methane at low temperature. Catal. Today 2018, 299, 263–271. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, Y.; Yang, J.; Zhang, S.; Zhang, J.; Zheng, C. Research progress of pollutants removal from coal-fired flue gas using non-thermal plasma. Renew. Sustain. Energy Rev. 2017, 67, 791–810. [Google Scholar] [CrossRef]
- Pan, Y.X.; Han, Y.; Liu, C.J. Pathways for steam reforming of dimethyl ether under cold plasma conditions: A DFT study. Fuel 2007, 86, 2300–2307. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, C.; Cao, Y. DFT study on pathways of steam reforming of ethanol under cold plasma conditions for hydrogen generation. Int. J. Hydrogen Energy 2010, 35, 1951–1956. [Google Scholar] [CrossRef]
- Mistry, H.; Choi, Y.W.; Bagger, A.; Scholten, F.; Bonifacio, C.; Sinev, I.; Stach, E.A. Enhanced carbon dioxide electroreduction to carbon monoxide over defect rich plasma-activated silver catalysts. Angew. Chem. 2017, 129, 11552–11556. [Google Scholar] [CrossRef]
- Shirazi, M.; Neyts, E.C.; Bogaerts, A. DFT study of Ni-catalyzed plasma dry reforming of methane. Appl. Catal. B 2017, 205, 605–614. [Google Scholar] [CrossRef]
- Somers, W.; Bogaerts, A.; van Duin, A.C.T.; Neyts, E.C. Plasma species interacting with nickel surfaces: Toward an atomic scale understanding of plasma-catalysis. J. Phys. Chem. C 2012, 116, 20958–20965. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Gusarov, S.; Kuznicki, S.M.; Kovalenko, A. Theoretical modeling of zeolite nanoparticle surface acidity for heavy oil upgrading. J. Phys. Chem. C 2008, 112, 6794–6810. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, S.; Zhou, Y.; Wei, Q.; Zhou, W. Oxygen effects on the structure and hydrogenation activity of the MoS2 active site: A mechanism study by DFT calculation. Fuel 2017, 194, 63–74. [Google Scholar] [CrossRef]
- Hou, P.; Zhou, Y.; Guo, W.; Ren, P.; Guo, Q.; Xiang, H.; Yang, Y. Rational design of hydrogen-donor solvents for direct coal liquefaction. Energy Fuels 2018, 32, 4715–4723. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, A. 02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, H.; Lian, P.; Gong, J.; Gao, R. Theoretical Study on the Hydrogenation Mechanisms of Model Compounds of Heavy Oil in a Plasma-Driven Catalytic System. Catalysts 2018, 8, 381. https://doi.org/10.3390/catal8090381
Hao H, Lian P, Gong J, Gao R. Theoretical Study on the Hydrogenation Mechanisms of Model Compounds of Heavy Oil in a Plasma-Driven Catalytic System. Catalysts. 2018; 8(9):381. https://doi.org/10.3390/catal8090381
Chicago/Turabian StyleHao, Haigang, Pengfei Lian, Juhui Gong, and Rui Gao. 2018. "Theoretical Study on the Hydrogenation Mechanisms of Model Compounds of Heavy Oil in a Plasma-Driven Catalytic System" Catalysts 8, no. 9: 381. https://doi.org/10.3390/catal8090381
APA StyleHao, H., Lian, P., Gong, J., & Gao, R. (2018). Theoretical Study on the Hydrogenation Mechanisms of Model Compounds of Heavy Oil in a Plasma-Driven Catalytic System. Catalysts, 8(9), 381. https://doi.org/10.3390/catal8090381



