Metal–Organic Frameworks-Based Catalysts for Biomass Processing
Abstract
1. Introduction
2. MOFs-Based Catalysts and Their Main Characteristics
3. Rational Design of MOF Catalysts for Biomass Valorization
3.1. Evaluation of Appropriate MOF Structures Relevant to Biomass Valorization
3.2. Choice of MOF Building Blocks
3.3. Organic Linker Choice for MOF Catalyst in Biomass Valorization
3.4. Post-Synthesis Modification of MOF Structures
3.5. Post-Synthesis Modification of Inorganic Nodes
3.6. Post-Synthesis Modification of Organic Linkers
4. MOF Based Hybrid Nanomaterials and MOF-Derived Nanostructures in Biomass Valorization
4.1. MOF-Based Hybrid Nanomaterials
4.2. Embedding Catalytic Active Sites in MOF Host Matrices by Template Synthesis
4.3. Hybrid Materials in the Form of MOF Host Matrices Containing Embedded Metal Nanoparticles
4.4. MOF-Based Composites with Other Matrices
4.5. MOF-Derived Hybrid Materials
4.6. MOF-Derived Carbon Materials
4.7. MOF-Derived Metal Nanostructures
5. Play with Physical Properties: Texture, Geometry and Morphology
6. An Outlook and Perspectives
- Comparison with other “traditional” catalytic systems;
- Energy efficiency;
- Resource and cost economy and optimization;
- Relevance to Green chemistry and sustainable development.
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhanja, P.; Bhaumik, A. Porous nanomaterials as green catalyst for the conversion of biomass to bioenergy. Fuel 2016, 185, 432–441. [Google Scholar] [CrossRef]
- De, S.; Saha, B.; Luque, R. Hydrodeoxygenation processes: Advances on catalytic transformations of biomass-derived platform chemical into hydrocarbon fuels. Bioresour. Technol. 2015, 178, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, X.; Cao, Q.; Dong, L.; Guan, J.; Mu, X. Sustainable Production of Bulk Chemicals; Xian, M., Ed.; Springer Science + Business Media: Dordrecht, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Delidovich, I.; Leonhard, K.; Palkovits, R. Cellulose and hemicellulose valorization: An integrated challenge of catalysis and reaction engineering. Energy Environ. Sci. 2014, 7, 2803–2830. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal–organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, B.; Kailasamb, K.; Sangwana, R.S.; Elumalai, S. Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: A review. Renew. Sustain. Energy Rev. 2018, 82, 2408–2425. [Google Scholar] [CrossRef]
- Sotelo-Boyas, R.; Trejo-Zarraga, F.; Hernandez-Loyo, F.d.J. Hydroconversion of Triglycerides into Green Liquid Fuels. In Hydrogenation; Karam, Y., Ed.; IntechOpen (Open Access), IntechOpen Limited: London, UK, 2012; pp. 188–216, Chapter 8; ISBN 978-953-51-0785-9. [Google Scholar]
- Rodrigues, A.; Bordado, J.C.; Galhano dos Santos, R. Upgrading the glycerol from biodiesel production as a source of energy carriers and chemicals—A technological review for three chemical pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Morales, G.; Paniagua, M. Chemical routes for the conversion of cellulosic platform molecules into high-energy-density biofuels. Chapter 13. In Handbook of Biofuels Production, 2nd ed.; Carol, R.L., Karen, L., Clark, W.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 359–388. ISBN 978-0-08-100455-5. [Google Scholar]
- Sanders, J.P.M.; Clark, J.H.; Harmsen, G.J.; Heeres, H.J.; Heijnen, J.J.; Kersten, S.R.A.; van Swaaij, W.P.M.; Moulijn, J.A. Process intensification in the future production of base chemicals from biomass. Chem. Eng. Process. 2012, 51, 117–136. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.; Janiak, C. MOF catalysts in biomass upgrading towards value-added fine chemicals. CrystEngComm 2017, 19, 4092–4117. [Google Scholar] [CrossRef]
- Hara, M.; Nakajima, K.; Kamata, K. Recent progress in the development of solid catalysts for biomass conversion into high value-added chemicals. Sci. Technol. Adv. Mater. 2015, 16, 034903. [Google Scholar] [CrossRef] [PubMed]
- Fechete, I.; Wang, Y.; Vedrine, J.C. The past, present and future of heterogeneous catalysis. Catal. Today 2012, 189, 2–27. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Smith, R.L., Jr.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energy Combust. Sci. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-organic frameworks: opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, P.; Müller, M.; Corma, A. MOF catalysis in relation to their homogeneous counterparts and conventional solid catalysts. Chem. Sci. 2014, 5, 2979–3007. [Google Scholar] [CrossRef]
- Gascon, J.; Corma, A.; Kapteijn, F.; Llabres, I.; Xamena, F.X. Metal organic framework catalysis: Quo vadis? ACS Catal. 2014, 4, 361–378. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, X.Q.; Jiang, H.L.; Sun, L.B. Metal–organic frameworks for heterogeneous basic catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: synergistic catalysis and tandem reactions. Chem Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal–organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Yaghi, O.M. Metal-organic frameworks: A new class of porous materials. Micropor. Mesopor. Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Pintado-Sierra, M.; Rasero-Almansa, A.M.; Corma, A.; Iglesias, M.; Sẚnchez, F. Bifunctional iridium-(2-aminoterephthalate)–Zr-MOF chemoselective catalyst for the synthesis of secondary amines by one-pot three-step cascade reaction. J. Catal. 2013, 299, 137–145. [Google Scholar] [CrossRef]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal–organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Farha, O.K.; Roberts, J.; Schedt, K.A.; Nguen, S.B.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, V.I.; Kustov, L.M. The application of metal–organic frameworks in catalysis. Petrol. Chem. 2010, 50, 167–180. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.L.; Xu, Q. Metal–organic frameworks as platforms for catalytic applications. Adv. Mater. 2017, 1703663. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Thapa, K.B.; Ju, Q.; Fang, Z.; Huang, W. Heterogeneous catalysts based on mesoporous metal–organic frameworks. Coord. Chem Rev. 2017. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Mixed-metal or mixed-linker metal organic frameworks as heterogeneous catalysts. Catal. Sci. Technol. 2016, 6, 5238–5261. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Tuneable nature of metal organic frameworks as heterogeneous solid catalysts for alcohol oxidation. Chem. Commun. 2017, 53, 10851–10869. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H.; Llabrés i Xamena, F.X. Engineering metal organic frameworks (MOFs) for heterogeneous catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, K.; Li, Y. Greening the processes of metal–organic framework synthesis and their use in sustainable catalysis. ChemSusChem 2017, 10, 3165–3187. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.D.; Zhao, M. Incorporation of molecular catalysts in metal–organic frameworks for highly efficient heterogeneous catalysis. Adv. Mater. 2017, 29, 1605446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Du, Y.; Borgna, A.; Hong, J.; Wang, Y.; Han, J.; Zhang, W.; Xu, R. Post-synthesis modification of a metal–organic framework to construct a bifunctional photocatalyst for hydrogen production. Energy Environ. Sci. 2013, 6, 3229–3234. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Chen, L. Amine-functionalized metal–organic frameworks: Structure, synthesis and applications. RSC Adv. 2016, 6, 32598–32614. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Organic frameworks as versatile hosts of Au nanoparticles in heterogeneous catalysis. ACS Catal. 2017, 7, 2896–2919. [Google Scholar] [CrossRef]
- Falcaro, P.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C.J. Application of metal and metal oxide nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- An, B.; Zeng, L.; Jia, M.; Li, Z.; Lin, Z.; Song, Y.; Zhou, Y.; Cheng, J.; Cheng, W.; Lin, W. Molecular iridium complexes in metal–organic frameworks catalyze CO2 hydrogenation via concerted proton and hydride transfer. J. Am. Chem. Soc. 2017, 139, 17747–17750. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- De Azevedo, D.C.S.; Cardoso, D.M.; Fraga, A.; Pastore, H.O. Zeolites for a sustainable world. Microporous Mesoporous Mater. 2017, 254, 1–2. [Google Scholar] [CrossRef]
- Tafipolsky, M.; Amirjalayer, S.; Schmid, R. Atomistic theoretical models for nanoporous hybrid materials. Microporous Mesoporous Mater. 2010, 129, 304–318. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous catalysis in zeolites, mesoporous silica, and metal–organic frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Hong, D.Y.; Chang, J.S.; Jhung, S.H.; Seo, Y.K.; Kim, J.; Vimont, A.; Daturi, M.; Serre, C.; Férey, G. Amine grafting on coordinatively unsaturated metal centers of MOFs: Consequences for catalysis and metal encapsulation. Angew. Chem. Int. Ed. 2010, 47, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Devaux, A.; Yang, C.H.; De Cola, L.; Fischer, R.A. Highly emissive metal–organic framework composites by host–guest chemistry. Photochem. Photobiol. Sci. 2010, 9, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Arnanz, A.; Pintado-Sierra, M.; Corma, A.; Iglesias, M.; Sanchez, F. Bifunctional metal organic framework catalysts for multistep reactions: MOF-Cu(BTC)-[Pd] catalyst for one-pot heteroannulation of acetylenic compounds. Adv. Synth. Catal. 2012, 354, 1347–1355. [Google Scholar] [CrossRef]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhao, D. Metal–organic frameworks with Lewis acidity: Synthesis, characterization, and catalytic applications. CrystEngComm 2017, 19, 4066–4081. [Google Scholar] [CrossRef]
- Chowdhury, P.; Bikkina, C.; Meister, D.; Dreisbach, F.; Gumma, S. Comparison of adsorption isotherms on Cu-BTC metal organic frameworks synthesized from different routes. Microporous Mesoporous Mater. 2009, 117, 406–413. [Google Scholar] [CrossRef]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane storage in metal–organic frameworks: Current records, surprise findings, and challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mayoral, E.; Cӗjka, J. [Cu3(BTC)2]: A metal–organic framework catalyst for the friedländer reaction. ChemCatChem 2011, 3, 157–159. [Google Scholar] [CrossRef]
- Yepez, R.; Garcıa, S.; Schachat, P.; Saґnchez-Saґnchez, M.; Gonzaґlez-Estefan, J.H.; Gonzaґlez-Zamora, E.; Ibarra, I.A.; Aguilar-Pliego, J. Catalytic activity of HKUST-1 in the oxidation of trans-ferulic acid to vanillin. New J. Chem. 2015, 39, 5112–5115. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.; Vitillo, J.G.; Gianolio, D.; Zavorotynska, O.; Civalleri, B.; Jakobsen, S.; Nilsen, M.H.; Valenzano, L.; Lamberti, C.; Lillerud, K.P.; et al. H2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys. Chem. Chem. Phys. 2012, 14, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- Caratelli, C.; Hajek, J.; Cirujano, F.G.; Francesc, M.W.; Llabrés i Xamena, X.; Van Speybroeck, V. Nature of active sites on UiO-66 and beneficial influence of water in the catalysis of Fischer esterification. J. Catal. 2017, 352, 401–414. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Corma, A.; Llabrés, I.; Xamena, F.X. Zirconium-containing metal organic frameworks as solid acid catalysts for the esterification of free fatty acids: Synthesis of bio diesel and other compounds of interest. Catal. Today 2015, 257, 213–220. [Google Scholar] [CrossRef]
- Phan, A.C.; Doonan, J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Lee, T.; Choi, J.; Yip, A.C.K. On the zeolitic imidazolate framework-8 (ZIF-8) membrane for hydrogen separation from simulated biomass-derived syngas. Microporous Mesoporous Mater. 2016, 233, 70–77. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Gücüyener, C.; van den Bergh, J.; Gascon, J.; Kapteijn, F. Ethane/Ethene separation turned on its head: selective ethane adsorption on the metal−organic framework ZIF-7 through a gate-opening mechanism. J. Am. Chem. Soc. 2010, 132, 17704–17706. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, M.; Usseglio, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P.; Tilset, M. Post-synthetic modification of the metal–organic framework compound UiO-66. J. Mater. Chem. 2010, 20, 9848–9851. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Dutta, S.; Hossain, M.S.A.; Shiddiky, M.J.A.; Tung, K.L.; Shieh, F.K.; Tsung, C.K.; Wu, C.W.; Yamauchi, Y. Strategies for improving the functionality of zeolitic imidazolate frameworks: Tailoring nanoarchitectures for functional applications. Adv. Mater. 2017, 29, 1700213. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, L.; Lu, X. Catalytic conversion of sugars to methyl lactate over Mg-MOF-74 in near-critical methanol solutions. Catal. Commun. 2018, 110, 23–27. [Google Scholar] [CrossRef]
- Holm, M.S.; Pagán-Torres, Y.J.; Saravanamurugan, S.; Riisager, A.; Dumesic, J.A.; Taarning, E. Sn-beta catalysed conversion of hemicellulosic sugars. Green Chem. 2012, 14, 702–706. [Google Scholar] [CrossRef]
- Cabello, C.P.; Gómez-Pozuelo, G.; Opanasenko, M.; Nachtigall, P.; Čejka, J. Metal–organic frameworks M-MOF-74 and M-MIL-100: Comparison of textural, acidic, and catalytic properties. ChemPlusChem 2016, 81, 828–835. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Murillo, B.; Zornoza, B.; Iglesia, O.; Téllez, C.; Coronas, J. Chemocatalysis of sugars to produce lactic acid derivatives on zeolitic imidazolate frameworks. J. Catal. 2016, 334, 60–67. [Google Scholar] [CrossRef]
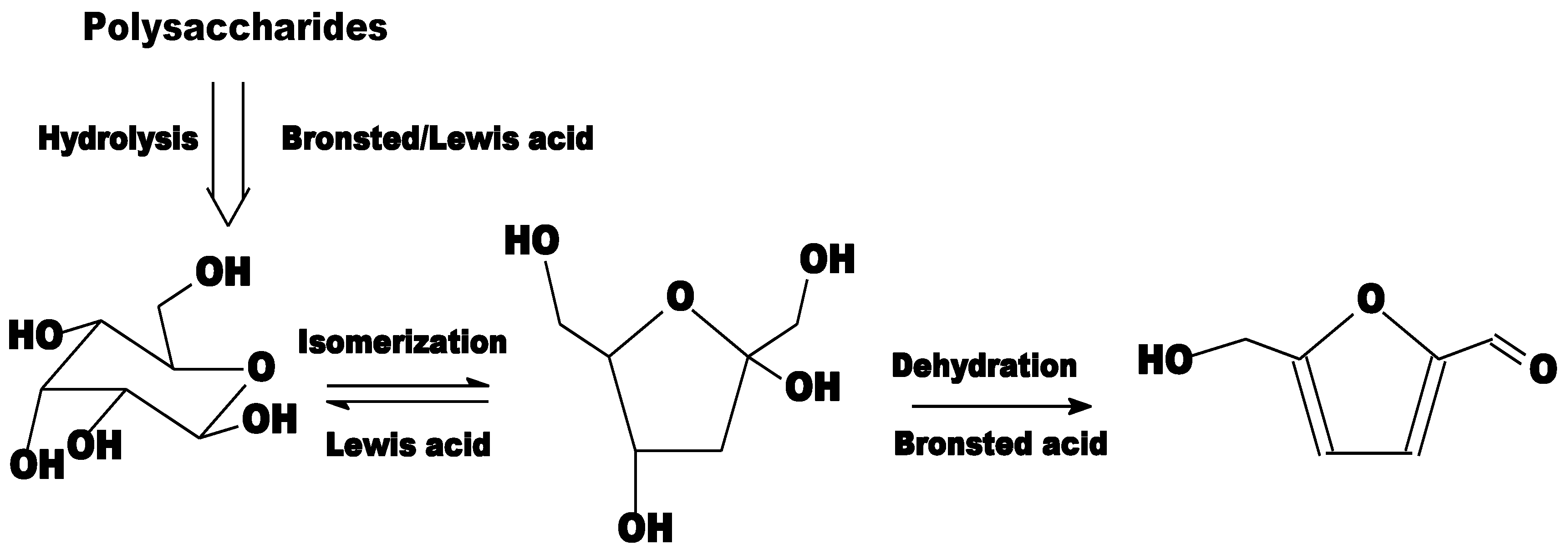
- Kruger, J.S.; Nikolakis, V.; Vlachos, D.G. Carbohydrate dehydration using porous catalysts. Curr. Opin. Chem. Eng. 2012, 1, 312–320. [Google Scholar] [CrossRef]
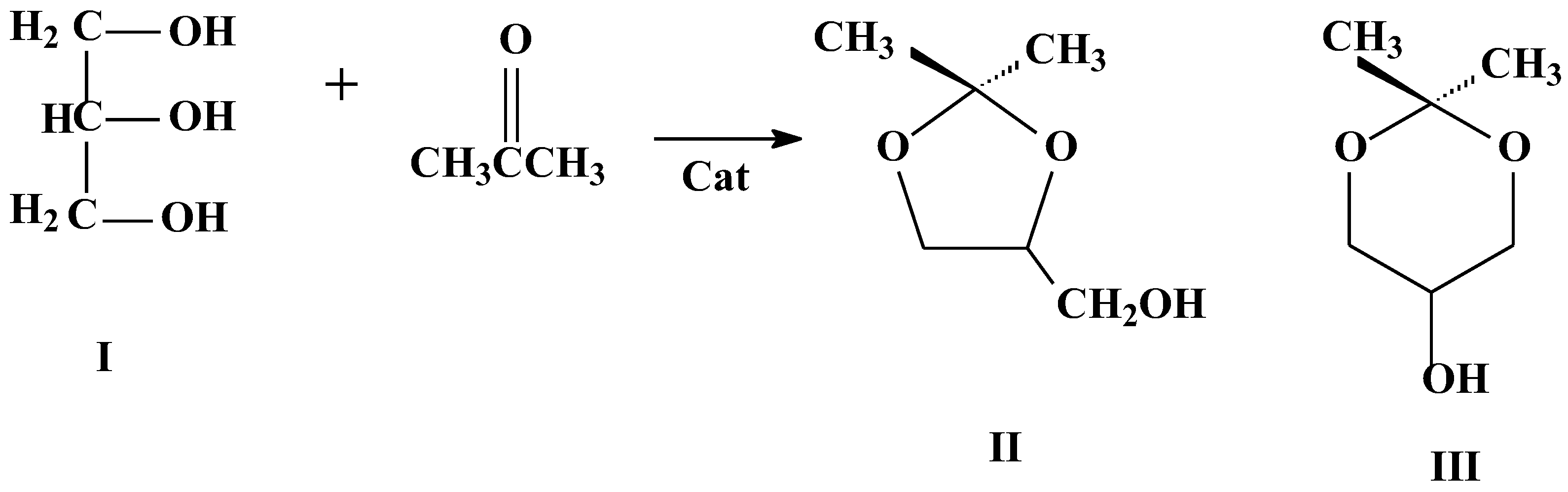
- Timofeeva, M.N.; Panchenko, V.N.; Khan, N.A.; Hasan, Z.; Prosvirin, I.P.; Tsybulya, S.V.; Jhung, S.H. Isostructural metal-carboxylates MIL-100(M) and MIL-53(M.) (M.: V., Al, Fe and Cr) as catalysts for condensation of glycerol with acetone. Appl. Catal. A. Gen. 2017, 529, 167–174. [Google Scholar] [CrossRef]
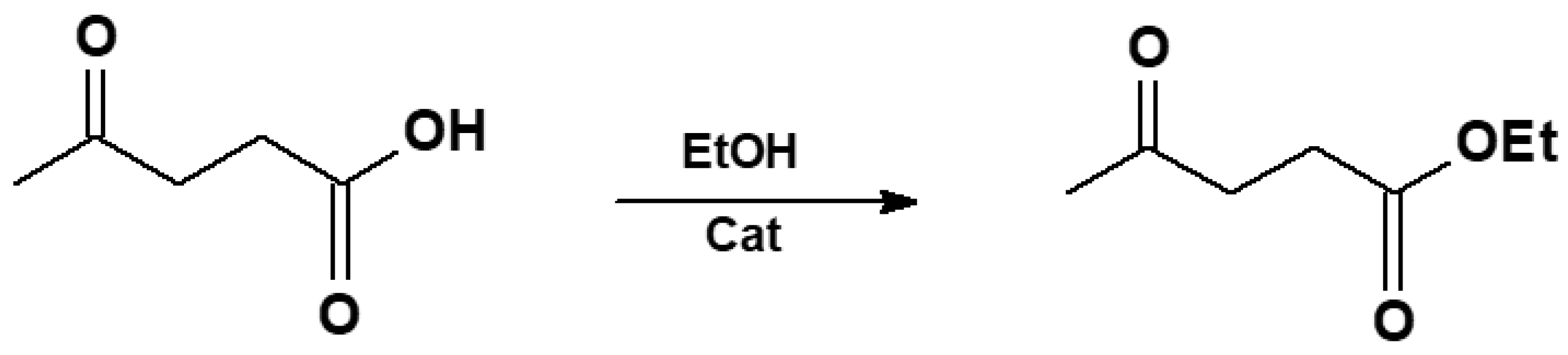
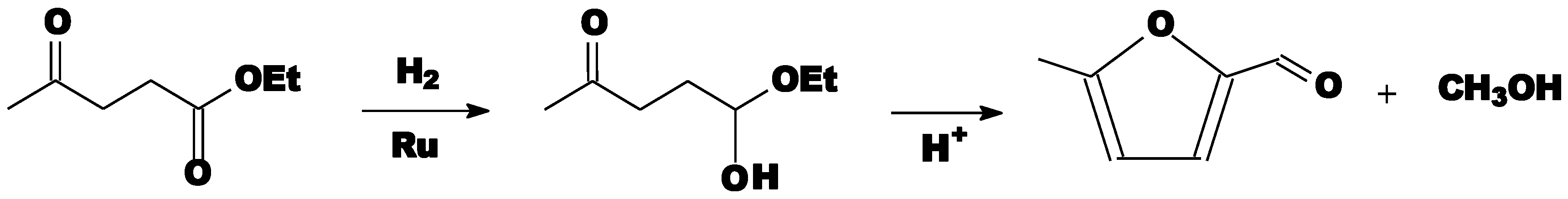
- Zhang, B.; Hao, J.; Sha, Y.; Zhou, H.; Yang, K.; Song, Y.; Ban, Y.; He, R.; Liu, Q. Utilization of lignite derivatives to construct Zr-based catalysts for the conversion of biomass-derived ethyl levulinate. Fuel 2018, 217, 122–130. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Corma, A.; Llabrés, I.; Xamena, F.X. Conversion of levulinic acid into chemicals: Synthesis of biomass derived levulinate esters over Zr-containing MOFs. Chem. Eng. Sci. 2015, 124, 52–60. [Google Scholar] [CrossRef]
- Védrine, J.C. Heterogeneous catalysis on metal oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef]
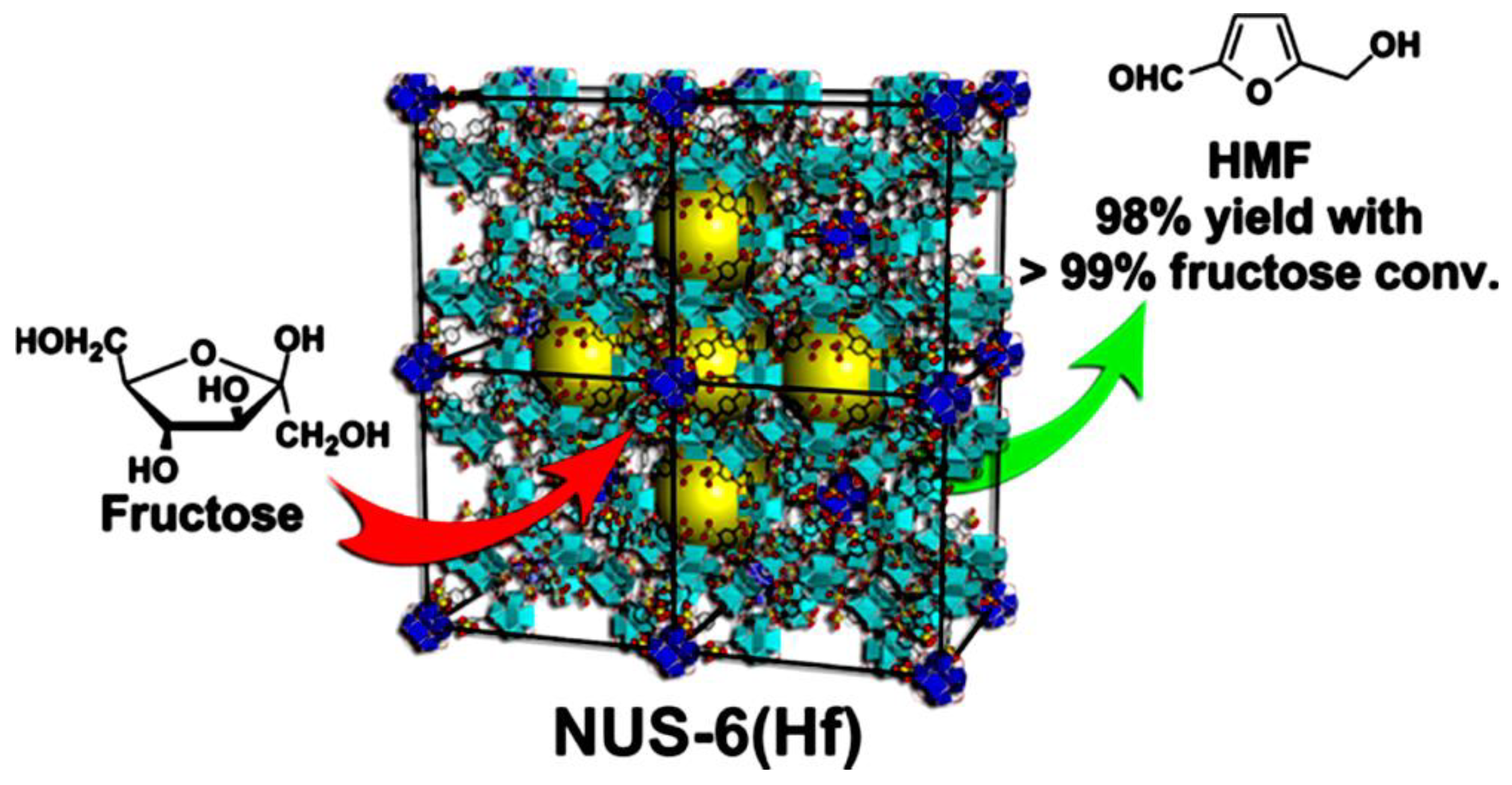
- Hu, Z.; Peng, Y.; Gao, Y.; Qian, Y.; Ying, S.; Yuan, D.; Horike, S.; Ogiwara, N.; Babarao, R.; Wang, Y.; et al. Direct synthesis of hierarchically porous metal−organic frameworks with high stability and strong Brønsted acidity: The decisive role of hafnium in efficient and selective fructose dehydration. Chem. Mater. 2016, 28, 2659–2667. [Google Scholar] [CrossRef]
- Hu, Z.G.; Peng, Y.W.; Tan, K.M.; Zhao, D. Enhanced catalytic activity of a hierarchical porous metal-organic framework CuBTC. CrystEngComm 2015, 17, 7124–7129. [Google Scholar] [CrossRef]
- Wee, L.H.; Lescouet, T.; Fritsch, J.; Bonino, F.; Rose, M.; Sui, Z.; Garrier, E.; Packet, D.; Bordiga, S.; Kaskel, S.; et al. Synthesis of monoglycerides by esterification of oleic acid with glycerol in heterogeneous catalytic process using tin-organic framework catalyst. Catal. Lett. 2013, 143, 356–363. [Google Scholar] [CrossRef]
- Su, Y.; Chang, G.; Zhang, Z.; Xing, H.; Su, B.; Yang, Q.; Ren, Q.; Yang, Y.; Bao, Z. Catalytic dehydration of glucose to 5-hydroxymethylfurfural with a bifunctional metal-organic framework. AIChE J. 2016, 62, 4403–4417. [Google Scholar] [CrossRef]
- Akiyama, G.; Matsuda, R.; Sato, H.; Takata, M.; Kitagawa, S. Cellulose hydrolysis by a new porous coordination polymer decorated with sulfonic acid functional groups. Adv. Mater. 2011, 23, 294–3297. [Google Scholar] [CrossRef] [PubMed]
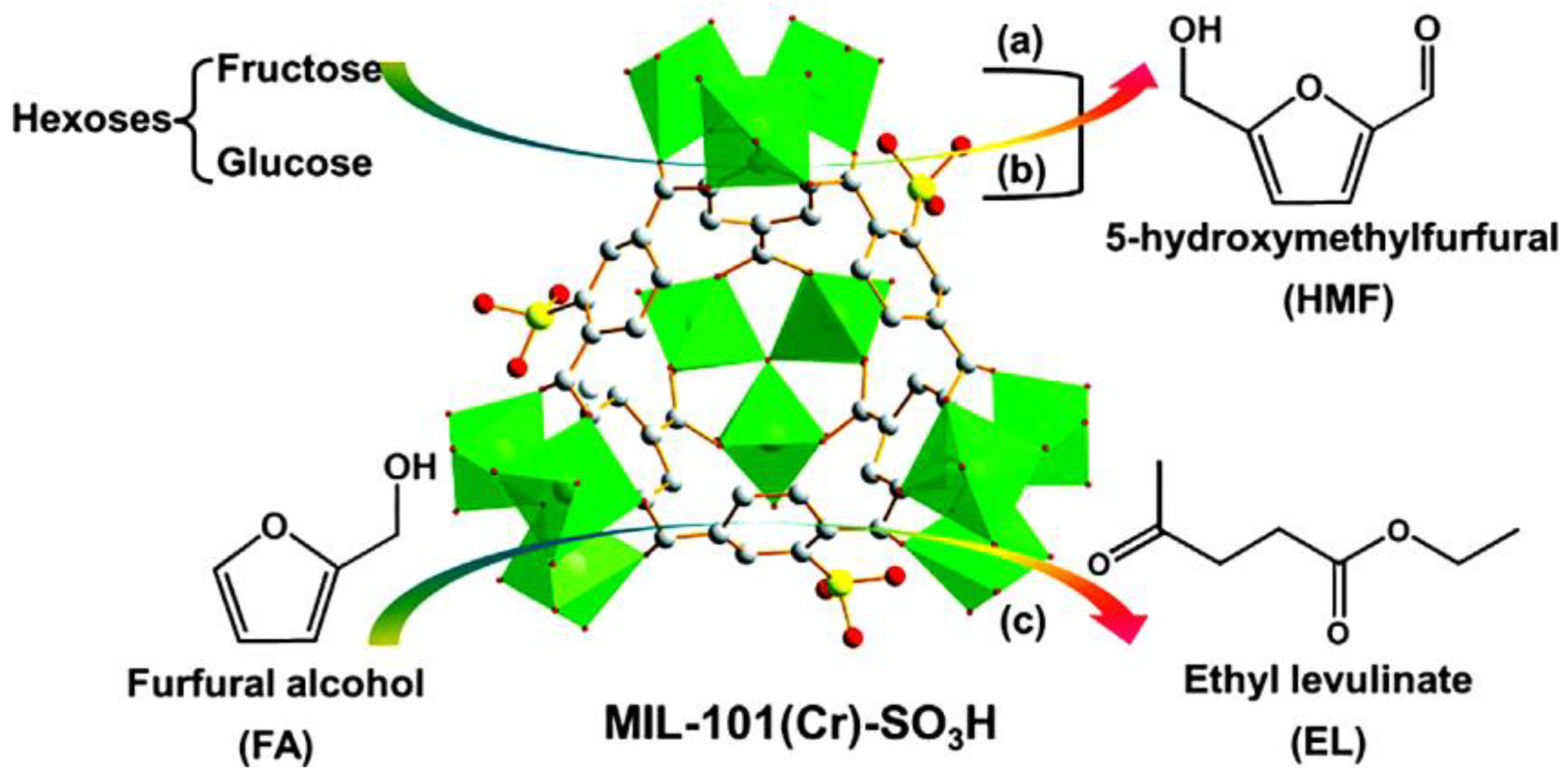
- Herbst, A.; Janiak, C. Selective glucose conversion to 5-hydroxymethylfurfural (5-HMF) instead of levulinic acid with MIL-101Cr MOF-derivatives. New J. Chem. 2016, 40, 7958–7967. [Google Scholar] [CrossRef]
- Liu, X.F.; Li, H.; Zhang, H.; Pan, H.; Huang, S.; Yang, K.L.; Yang, S. Efficient conversion of furfuryl alcohol to ethyl levulinate with sulfonic acid-functionalized MIL-101(Cr). RSC Adv. 2016, 6, 90232–90238. [Google Scholar] [CrossRef]
- Jiang, J.; Yaghi, O.M. Brønsted acidity in metal−organic frameworks. Chem. Rev. 2015, 115, 6966–6997. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Sumby, C.J.; Doonan, C.J. Post-synthetic metalation of metal−organic frameworks. Chem. Soc. Rev. 2014, 43, 5933–5951. [Google Scholar] [CrossRef] [PubMed]
- Juan-Alcanñiz, J.; Gascon, J.; Kapteijn, F. Metal-organic frameworks as scaffolds for the encapsulation of active species: State of the art and future perspectives. J. Mater. Chem. 2012, 22, 10102–10118. [Google Scholar] [CrossRef]
- Chatterjee, A.; Hu, X.; FL-Yuk, L. A dual acidic hydrothermally stable MOF-composite for upgrading xylose to furfural. Appl. Catal. A Gen. 2018. [Google Scholar] [CrossRef]
- Luan, Y.; Zheng, N.; Qi, Y.; Yu, J.; Wang, G. Development of a SO3H-functionalized UiO-66 metal–organic framework by postsynthetic modification and studies of its catalytic activities. Eur. J. Inorg. Chem. 2014, 26, 4268–4272. [Google Scholar] [CrossRef]
- Yabushita, M.; Li, P.; Islamoglu, T.; Kobayashi, H.; Fukuoka, A.; Farha, O.K.; Katz, A. Selective Metal–Organic Framework Catalysis of Glucose to 5-Hydroxymethylfurfural Using Phosphate-Modified NU-1000. Ind. Eng. Chem. Res. 2017, 56, 7141–7148. [Google Scholar] [CrossRef]
- Katz, M.J.; Mondloch, J.E.; Totten, R.K.; Park, J.K.; Nguyen, S.T.; Farha, O.K.; Hupp, J.T. Simple and compelling biomimetic metal-organic framework catalyst for the degradation of nerve agent simulants. Angew. Chem. Int. Ed. 2014, 53, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.G.; Truhlar, D.G. Computational linker design for highly crystalline metal–organic framework NU-1000. Chem. Mater. 2017, 29, 8073–8081. [Google Scholar] [CrossRef]
- Li, P.; Klet, R.C.; Moon, S.Y.; Wang, T.C.; Deria, P.; Peters, A.W.; Klahr, B.M.; Park, H.J.; Al-Juaid, S.S.; Hupp, J.T.; et al. Synthesis of nanocrystals of Zr-based metal–organic frameworks with csq-net: Significant enhancement in the degradation of a nerve agent simulant. Chem. Commun. 2015, 51, 10925–10928. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Hu, X.; Lam, F.L.Y. Towards a recyclable MOF catalyst for efficient production of furfural. Catal. Today 2018, 314, 129–136. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Chen, L.; Liu, R.; Huang, X.; Ye, D. Conversion of fructose into 5-hydroxymethylfurfural catalyzed by recyclable sulfonic acid-functionalized metal–organic frameworks. Green Chem. 2014, 16, 2490–2499. [Google Scholar] [CrossRef]
- Chiappe, C.; Rajamani, S. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate in basic ionic liquids. Pure Appl. Chem. 2012, 84, 755–762. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Frutos, P.D.; Iborra, S.; Noy, M.; Velty, A.; Concepción, P. Chemicals from biomass: Synthesis of GC by transesterification and carbonylation with urea with hydrotalcite catalysts. The role of acid-base pairs. J. Catal. 2010, 269, 140–149. [Google Scholar] [CrossRef]
- Lee, S.D.; Park, G.A.; Kim, D.W.; Park, D.W. Catalytic performance of functionalized IRMOF-3 for the synthesis of glycerol carbonate from glycerol and urea. J. Nanosci. Nanotechnol. 2014, 14, 4551–4556. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhang, Y.; Lin, H.; Liu, C.J. Nanoparticle/metal-organic framework composites for catalytic applications: Current status and perspective. Molecules 2017, 22, 2103. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chao, M.; Yan, B.; Qin, X.; Shen, C.; Xue, H.; Pang, H. Nanoparticle/MOF composites: Preparations and applications. Mater. Horiz. 2017, 4, 557–569. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-derived carbon-based nanomaterials for efficient catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Li, P.; Zeng, H.C. Immobilization of metal–organic framework nanocrystals for advanced design of supported nanocatalysts. Appl. Mater. Interfaces 2016, 43, 29551–29564. [Google Scholar] [CrossRef] [PubMed]
- Casas, N.; Schell, J.; Blom, R.; Maz, M. MOF and UiO-67/MCM-41 adsorbents for pre-combustion CO2 capture by PSA: Breakthrough experiments and process design. Sep. Purif. Technol. 2013, 111, 34–48. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Qiang Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Q.; Jiang, H.L. Metal–organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, W.; Wang, X.; Chen, Z.; Yang, X.; Luque, R.; Li, Y. Catalytically active designer crown-jewel Pd-based nanostructures encapsulated in metal–organic frameworks. Chem. Commun. 2017, 53, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
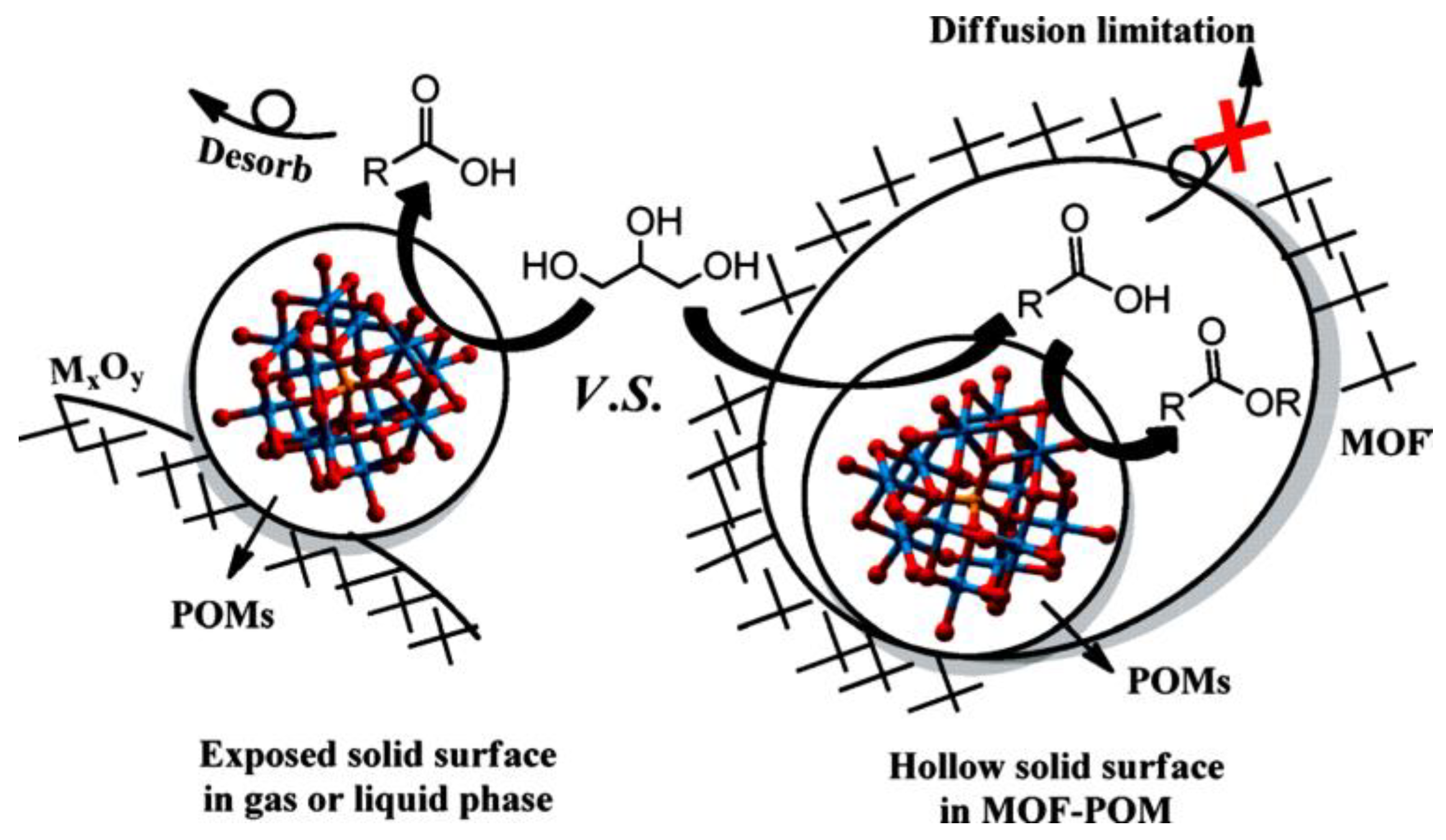
- Juan-Alcanñiz, J.; Ramos-Fernandez, E.V.; Lafont, U.; Gascon, J.; Kapteijn, F. Building MOF bottles around phosphotungstic acid ships: One-pot synthesis of bifunctional polyoxometalate-MIL-101 catalysts. J. Catal. 2010, 269, 229–241. [Google Scholar] [CrossRef]
- Juan-Alcanñiz, J.; Goesten, M.G.; Ramos-Fernandez, E.V.; Gascon, J.; Kapteijn, F. Towards efficient polyoxometalate encapsulationin MIL-100(Cr): Influence of synthesis conditions. New J. Chem. 2012, 36, 977–987. [Google Scholar] [CrossRef]
- Wan, H.; Chen, C.; Wu, Z.; Que, Y.; Feng, Y.; Wang, W.; Liu, X. Encapsulation of Heteropolyanion-Based Ionic Liquid within the Metal−Organic Framework MIL-100(Fe) for Biodiesel Production. ChemCatChem 2015, 7, 441–449. [Google Scholar] [CrossRef]
- Aijaz, A.; Zhu, Q.L.; Tsumori, N.; Akita, T.; Xu, Q. Surfactant-free Pd nanoparticles immobilized to a metal–organic framework with size- and location-dependent catalytic selectivity. Chem. Commun. 2015, 51, 2577–2580. [Google Scholar] [CrossRef] [PubMed]
- Maksimchuk, N.V.; Kholdeeva, O.A.; Kovalenko, K.A.; Fedin, V.P. MIL-101 supported polyoxometalates: Synthesis, characterization, and catalytic applications in selective liquid-phase oxidation. Isr. J. Chem. 2011, 51, 281–289. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, M.; Chen, X.; Yue, B.; He, H. Heterogeneous catalysis of polyoxometalate based organic–inorganic hybrids. Materials 2015, 8, 1545–1567. [Google Scholar] [CrossRef] [PubMed]
- Bohre, A.; Dutta, S.; Saha, B. Upgrading furfurals to drop-in biofuels: An overview. ACS Sustain. Chem. Eng. 2015, 3, 1263–1277. [Google Scholar] [CrossRef]
- Salomon, W.; Yazigi, F.J.; Roch-Marchal, C.; Mialane, P.; Horcajada, P.; Serre, C.; Haouas, M.; Taulelle, F.; Dolbecq, A. Immobilization of Co-containing polyoxometalates in MIL-101(Cr): Structural integrity versus chemical transformation. Dalton Trans. 2014, 43, 12698–12705. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Luo, Z.; Britt, D.K.; Furukawa, H.; Yaghi, O.M.; Hardcastle, K.I.; Hill, C.L. A multiunit catalyst with synergistic stability and reactivity: A polyoxometalate metal organic framework for aerobic decontamination. J. Am. Chem. Soc. 2011, 133, 16839–16846. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Anjali, J.; Yan, Y.; Lee, J.M. Cr-MIL-101-Encapsulated Keggin Phosphomolybdic Acid as a Catalyst for the One-Pot Synthesis of 2,5-Diformylfuran from Fructose. ChemCatChem 2017, 9, 1187–1191. [Google Scholar] [CrossRef]
- Zhang, V.; Degirmenci, Y.; Li, C.; Hensen, E.J.M. Phosphotungstic Acid Encapsulated in Metal–Organic Framework as Catalysts for Carbohydrate Dehydration to 5-Hydroxymethylfurfural. ChemSusChem 2011, 4, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liang, F.; Feng, D.; Xian, M.; Zhang, H.; Liu, H.; Du, F. An efficient route from reproducible glucose to 5-hydroxymethylfurfural catalyzed by porous coordination polymer heterogeneous catalysts. Chem. Eng. J. 2016, 300, 177–184. [Google Scholar] [CrossRef]
- Wee, L.H.; Bajpe, S.R.; anssens, N.; Hermans, I.; Kristof Houthoofd, K.; Kirschhock, C.E.A.; Johan, A.; Martens, J.A. Convenient synthesis of Cu3(BTC)2 encapsulated Keggin heteropolyacid nanomaterial for application in catalysis. Chem. Commun. 2010, 46, 8186–8188. [Google Scholar] [CrossRef] [PubMed]
- Bajpe, S.R.; Kirschhock, C.E.A.; Aerts, A.; Breynaert, E.; Absillis, G.; Parac-Vogt, T.N.; Giebeler, L.; Martens, J. Direct observation of molecular-level template action leading to self-assembly of a porous framework. Chem. Eur. J. 2010, 16, 3926–3932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Q. Conversion of 5-hydroxymethylfurfural into 5-ethoxymethylfurfural and ethyl levulinate catalyzed by MOF-based heteropolyacid materials. Green Chem. 2016, 18, 5884–5889. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, P.; Lu, M. Study on the one-pot oxidative esterification of glycerol with MOF supported polyoxometalates as catalyst. Catal. Sci. Technol. 2015, 5, 3383–3393. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, L.; Kumar, P.; Cybulskis, V.J.; Mkhoyan, M.K.A.; Davis, E.; Tsapatsis, M. A chromium Hydroxide/MIL-101(Cr) MOF composite catalyst and its use for the selective isomerization of glucose to fructose. Angew. Chem. Int. Ed. 2018, 57, 4926–4930. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Y.; Lewis, J.D.; Roman-Leshkov, Y. Lewis acid zeolites for biomass conversion: Perspectives and challenges on reactivity, synthesis, and stability. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 663–692. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Jin, F.Z.; Ma, H.C.; Li, X.B.; Liu, M.Y.; Kan, J.L.; Chen, G.J.; Dong, Y.B. Au@Cu(II)-MOF: Highly efficient bifunctional heterogeneous catalyst for successive oxidation–condensation reactions. Inorg. Chem. 2016, 55, 6685–6691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yuan, K.; Wang, Y.; Li, G.D.; Gu, L.; Hu, W.P.; Zhao, H.J.; Tang, Z.Y. Metal-organic frameworks as selectivity regulators for hydrogenation reactions. Nature 2016, 539, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Liu, H.; Luque, R.; Li, Y. Efficient and selective hydrogenation of biomass-derived furfural to cyclopentanone using Ru catalysts. Green Chem. 2015, 17, 4183–4188. [Google Scholar] [CrossRef]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance material review on cyclopentanone. Food Chem. Toxicol. 2012, 50, S608–S612. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Ren, H.; Li, C.; Liu, J.; Liang, C. Highly selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over MIL-101(Cr)-NH2 supported Pd catalyst at low temperature. Chin. J. Catal. 2018, 39, 319–326. [Google Scholar] [CrossRef]
- Chen, J.; Liu, R.; Guo, Y.; Chen, L.; Gao, H. Selective hydrogenation of biomass-based 5-Hydroxymethylfurfural over catalyst of palladium immobilized on amine-functionalized metal–organic frameworks. ACS Catal. 2015, 5, 722–733. [Google Scholar] [CrossRef]
- Hester, P.; Xu, S.; Liang, W.; Al-Janabi, N.; Vakili, R.; Hill, P.; Muryn, C.A.; Chen, X.; Martin, P.A.; Fan, X. On thermal stability and catalytic reactivity of Zr-based metal–organic framework (UiO-67) encapsulated Pt catalysts. J. Catal. 2016, 340, 85–94. [Google Scholar] [CrossRef]
- Lin, Z.; Cai, X.; Fu, Y.; Zhu, W.; Zhang, F. Cascade catalytic hydrogenation–cyclization of methyl levulinate to form g-valerolactone over Ru nanoparticles supported on a sulfonic acidfunctionalized UiO-66 catalyst. RSC Adv. 2017, 7, 44082–44088. [Google Scholar] [CrossRef]
- Yang, J.; Ma, J.; Yuan, Q.; Zhang, P.; Guan, Y. Selective hydrogenation of furfural on Ru/Al-MIL-53: A comparative study on the effect of aromatic and aliphatic organic linkers. RSC Adv. 2016, 6, 92299–92304. [Google Scholar] [CrossRef]
- Li, H.; Zhao, W.; Fang, Z. Hydrophobic Pd nanocatalysts for one-pot and high-yield production of liquid furanic biofuels at low temperatures. Appl. Catal. B Environ. 2017, 215, 18–27. [Google Scholar] [CrossRef]
- Ning, L.; Liao, S.; Cui, H.; Yu, L.; Tong, X. Selective conversion of renewable furfural with ethanol to produce furan-2-acrolein mediated by Pt@MOF-5. ACS Sustain. Chem. Eng. 2018, 6, 135–142. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhan, D.; van Haandel, L.; Yea, F.; Xue, T.; Hensen, E.J.M.; Guan, Y. Selective liquid phase hydrogenation of furfural to furfuryl alcohol by Ru/Zr-MOFs. J. Mol. Catal. A Chem. 2015, 406, 58–64. [Google Scholar] [CrossRef]
- Li, X.; Tjiptoputro, A.K.; Ding, J.; Xue, J.M.; Zhu, Y. Pd-Ce nanoparticles supported on functional Fe-MIL-101-NH2: An efficient catalyst for selective glycerol oxidation. Catal. Today 2017, 279, 77–83. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Huang, J.; Chen, L.; Ma, L.; Huang, X. Conversion of cellulose and cellobiose into sorbitol catalyzed by ruthenium supported on a polyoxometalate/metal–organic framework hybrid. ChemSusChem 2013, 6, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Hu, X.; Lam, F.L.Y. Catalytic activity of an economically sustainable fly-ash-metal-organic framework composite towards biomass valorization. Catal. Today 2018, 314, 137–146. [Google Scholar] [CrossRef]
- Liu, X.W.; Sun, T.J.; Hu, S.D.; Wang, J.L. Composites of metal–organic frameworks and carbon-based materials: Preparations, functionalities and applications. J. Mater. Chem. A 2016, 4, 3584–3616. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.M.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Zhu, E.; Jin, P.; Wang, K.; Zhao, J.; Li, C.; Yan, Y. Facile synthesis of halloysite nanotubes-supported acidic metal–organic frameworks with tunable acidity for efficient fructose dehydration to 5-Hydroxymethylfurfural. ChemistrySelect 2017, 2, 10413–10419. [Google Scholar] [CrossRef]
- Guimarјes, L.; Enyashin, A.N.; Seifert, G.; Duarte, H.A. Structural, electronic, and mechanical properties of single-walled halloysite nanotube models. J. Phys. Chem. C 2010, 114, 11358–11363. [Google Scholar] [CrossRef]
- Čelič, T.B.; Grilc, M.; Likozar, B.; Tušar, N.N. In-situ generation of Ni nanoparticles from metal–organic framework precursors and their use for biomass hydrodeoxygenation. ChemSusChem 2015, 8, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Insyani, R.; Verma, D.; Kim, S.M.; Kim, J. Direct one-pot conversion of monosaccharides into high-yield 2,5-dimethylfuran over a multifunctional Pd/Zr-based metal–organic framework@sulfonated graphene oxide catalyst. Green Chem. 2017, 19, 2482–2490. [Google Scholar] [CrossRef]
- Yap, M.H.; Fow, K.L.; Chen, G.Z. Synthesis and applications of MOF-derived porous nanostructures. Green Energy Environ. 2017, 2, 218–245. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Li, S.; Gao, L.; Xiao, G. Metal-organic frameworks derived bimetallic Cu-Co catalyst for efficient and selective hydrogenation of biomass-derived furfural to furfuryl alcohol. Mol. Catal. 2017, 436, 128–137. [Google Scholar] [CrossRef]
- Fang, R.; Luque, R.; Li, Y. Selective aerobic oxidation of biomass-derived HMF to 2,5-diformylfuran using a MOF-derived magnetic hollow Fe–Co nanocatalyst. Green Chem. 2016, 18, 3152–3157. [Google Scholar] [CrossRef]
- Fang, R.; Luque, R.; Li, Y. Efficient one-pot fructose to DFF conversion using sulfonated magnetically separable MOF-derived Fe3O4 (111) catalysts. Green Chem. 2017, 19, 647–655. [Google Scholar] [CrossRef]
- Wang, Y.; Sang, S.; Zhu, W.; Gao, L.; Xiao, G. CuNi@C catalysts with high activity derived from metal–organic frameworks precursor for conversion of furfural to cyclopentanone. Chem. Eng. J. 2016, 299, 104–111. [Google Scholar] [CrossRef]
- Lai, H.K.; Chou, Y.Z.; Lee, M.H.; Lin, K.Y.A. Coordination polymer-derived cobalt nanoparticle-embedded carbon nanocomposite as a magnetic multi-functional catalyst for energy generation and biomass conversion. Chem. Eng. J. 2018, 332, 717–726. [Google Scholar] [CrossRef]
- Jin, P.; Zhang, Y.; Chen, Y.; Pan, J.; Dai, X.; Liu, M.; Yan, Y.; Li, C. Facile synthesis of hierarchical porous catalysts for enhanced conversion of fructose to 5-hydroxymethylfurfural. J. Taiwan Inst. Chem. Eng. 2017, 75, 59–69. [Google Scholar] [CrossRef]
- Grilc, M.; Likozar, B.; Levec, J. Hydrodeoxygenation and hydrocracking of solvolysed lignocellulosic biomass by oxide, reduced and sulphide form of NiMo, Ni, Mo and Pd catalysts. Appl. Catal. B 2014, 150–151, 275–287. [Google Scholar] [CrossRef]
- Kong, P.S.; Aroua, M.K.; Daud, W.M.A.W. Conversion of crude and pure glycerol into derivatives: A feasibility evaluation. Renew. Sustain. Energy Rev. 2016, 63, 533–555. [Google Scholar] [CrossRef]
- Massa, M.; Andersson, A.; Finocchio, E.; Busca, G. Gas-phase dehydration of glycerol to acrolein over Al2O3-, SiO2-, and TiO2-supported Nb- and W.-oxide catalysts. J. Catal. 2013, 307, 170–184. [Google Scholar] [CrossRef]
- Huang, L.; Qin, F.; Huang, Z.; Zhuang, Y.; Ma, J.; Xu, H.; Shen, W. Metal–organic framework mediated synthesis of small-sized γ-Alumina as a highly active catalyst for the dehydration of glycerol to acrolein. ChemCatChem 2018, 10, 381–386. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Riches, J.D.; Barry, J.C. γ-Alumina nanofibers prepared from aluminum hydrate with poly(ethylene oxide) surfactant. Chem. Mater. 2002, 14, 2086–2093. [Google Scholar] [CrossRef]
- Shen, S.; Ng, W.K.; Chia, L.S.O.; Dong, R.; Tan, B.H. Morphology controllable synthesis of nanostructured boehmite and γ-Alumina by facile dry gel conversion. Cryst. Growth Des. 2012, 12, 4987–4994. [Google Scholar] [CrossRef]
- Zheng, L.; Li, X.; Du, W.; Shi, D.; Ning, W.; Lu, X.; Hou, Z. Metal-organic framework derived Cu/ZnO catalysts for continuous hydrogenolysis of glycerol. Appl. Catal. B 2017, 203, 146–153. [Google Scholar] [CrossRef]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Férey, G. A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem. Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.U.; Springuel-Huet, M.A.; Coudert, F.X.; Fuchs, A.H.; Boutin, A. Predicting Mixture Coadsorption in Soft Porous Crystals: Experimental and Theoretical Study of CO2/CH4 in MIL-53(Al). Langmuir 2012, 28, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Millange, F.; Serre, C.; Férey, G. Synthesis, structure determination and properties of MIL-53as and MIL-53ht: The first CrIII hybrid inorganic–organic microporous solids: CrIII(OH)·{O2C–C6H4–CO2}·{HO2C–C6H4–CO2H}x. Сhem. Commun. 2002, 822–823. [Google Scholar] [CrossRef]
- Zi, G.; Yan, Z.; Wang, Y.; Chen, Y.; Guo, Y.; Yuan, F.; Gao, W.; Wang, Y.; Wang, J. Catalytic hydrothermal conversion of carboxymethyl cellulose to value-added chemicals over metal–organic framework MIL-53(Al). Carbohydr. Polym. 2015, 115, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramirez, J.; Christensen, K.; Egeblad, C.H.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.H.; Wiktor, C.; Turner, S.; Vanderlinden, W.; Janssens, N.; Bajpe, S.R.; Houthoofd, K.; Van Tendeloo, G.; De Feyter, S.; Kirschhock, C.E.A.; et al. Copper benzene tricarboxylate metal−organic framework with wide permanent mesopores stabilized by keggin polyoxometallate ions. J. Am. Chem. Soc. 2012, 134, 10911–10919. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, Y.; Wang, T.; Wang, X.; Huo, Q.; Liu, Y. Facile Fabricating Hierarchically Porous Metal–Organic Frameworks via a Template-Free Strategy. Cryst. Growth Des. 2016, 16, 504–510. [Google Scholar] [CrossRef]
- Feng, L.; Yuan, S.; Zhang, L.L.; Tan, K.; Li, J.L.; Kirchon, A.; Liu, L.M.; Zhang, P.; Han, Y.; Chabal, Y.J.; Zhou, H.C. Creating hierarchical pores by controlled linker thermolysis in multivariate metal−organic frameworks. Am. Chem. Soc. 2018, 140, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Li, F.; Zhang, H.; Li, J.; Wang, X.; Xi, H. Template synthesis of hierarchical porous metal–organic frameworks with tunable porosity. RSC Adv. 2017, 7, 52245–52251. [Google Scholar] [CrossRef]
- Wee, L.H.; Meledina, M.; Turner, S.; Van Tendeloo, G.; Zhang, K.; Rodriguez-Albelo, L.M.; Masala, A.; Bordiga, S.; Jiang, J.; Navarro, J.A.R.; et al. 1D-2D-3D transformation synthesis of hierarchical metal−organic framework adsorbent for multicomponent alkane separation. J. Am. Chem. Soc. 2017, 139, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.H.; Lescouet, T.; Ethiraj, J.; Bonino, F.; Vidruk, E.; Garrier, E.; Packet, D.; Bordiga, S.; Farrusseng, D.; Herskowitz, M.; et al. Zeolitic Imidazolate Framework-8 Catalyst for Monoglyceride Synthesis. ChemCatChem 2013, 5, 3562–3566. [Google Scholar] [CrossRef]
- Guan, H.Y.; LeBlanc, R.J.; Xie, S.Y.; Yue, Y. Recent progress in the syntheses of mesoporous metal–organic framework materials. Coord. Chem. Rev. 2018, 369, 76–90. [Google Scholar] [CrossRef]
- Junggeburth, S.C.; Schwinghammer, K.; Virdi, K.S.; Scheu, C.; Lotsch, B.V. Towards Mesostructured Zinc Imidazolate Frameworks. Chem. Eur. J. 2012, 18, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wang, Z.U.; Sun, L.B.; Chen, Y.P.; Zhou, H.C. Introduction of functionalized mesopores to metal–organic frameworks via metal–ligand–fragment coassembly. J. Am. Chem. Soc. 2012, 134, 20110–20116. [Google Scholar] [CrossRef] [PubMed]
- McNamara, N.D.; Hicks, J.C. Chelating Agent-Free, Vapor-Assisted Crystallization Method to Synthesize Hierarchical Microporous/Mesoporous MIL-125 (Ti). ACS Appl. Mater. Interfaces 2015, 7, 5338–5346. [Google Scholar] [CrossRef] [PubMed]
- Yabushita, M.; Li, P.; Bernales, V.; Kobayashi, H.; Fukuoka, A.; Gagliardi, L.; Farha, O.K.; Katz, A. Unprecedented selectivity in molecular recognition of carbohydrates by a metal–organic framework. Chem. Commun. 2016, 52, 7094–7097. [Google Scholar] [CrossRef] [PubMed]
- Stavila, V.; Parthasarathi, R.; Davis, R.W.; El Gabaly, F.; Sale, K.L.; Simmons, B.A.; Singh, S.; Allendorf, M.D. MOF-Based Catalysts for Selective Hydrogenolysis of Carbon-Oxygen Ether Bonds. ACS Catal. 2016, 6, 55–59. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Cascade Reactions Catalyzed by Metal Organic Frameworks. ChemSusChem 2014, 7, 2392–2410. [Google Scholar] [CrossRef] [PubMed]
- Nandasiri, M.I.; Jambovane, S.R.; McGrail, B.P.; Schaef, H.T.; Nune, S.K. Adsorption, separation, and catalytic properties of densified metal–organic frameworks. Coord. Chem. Rev. 2016, 311, 38–52. [Google Scholar] [CrossRef]
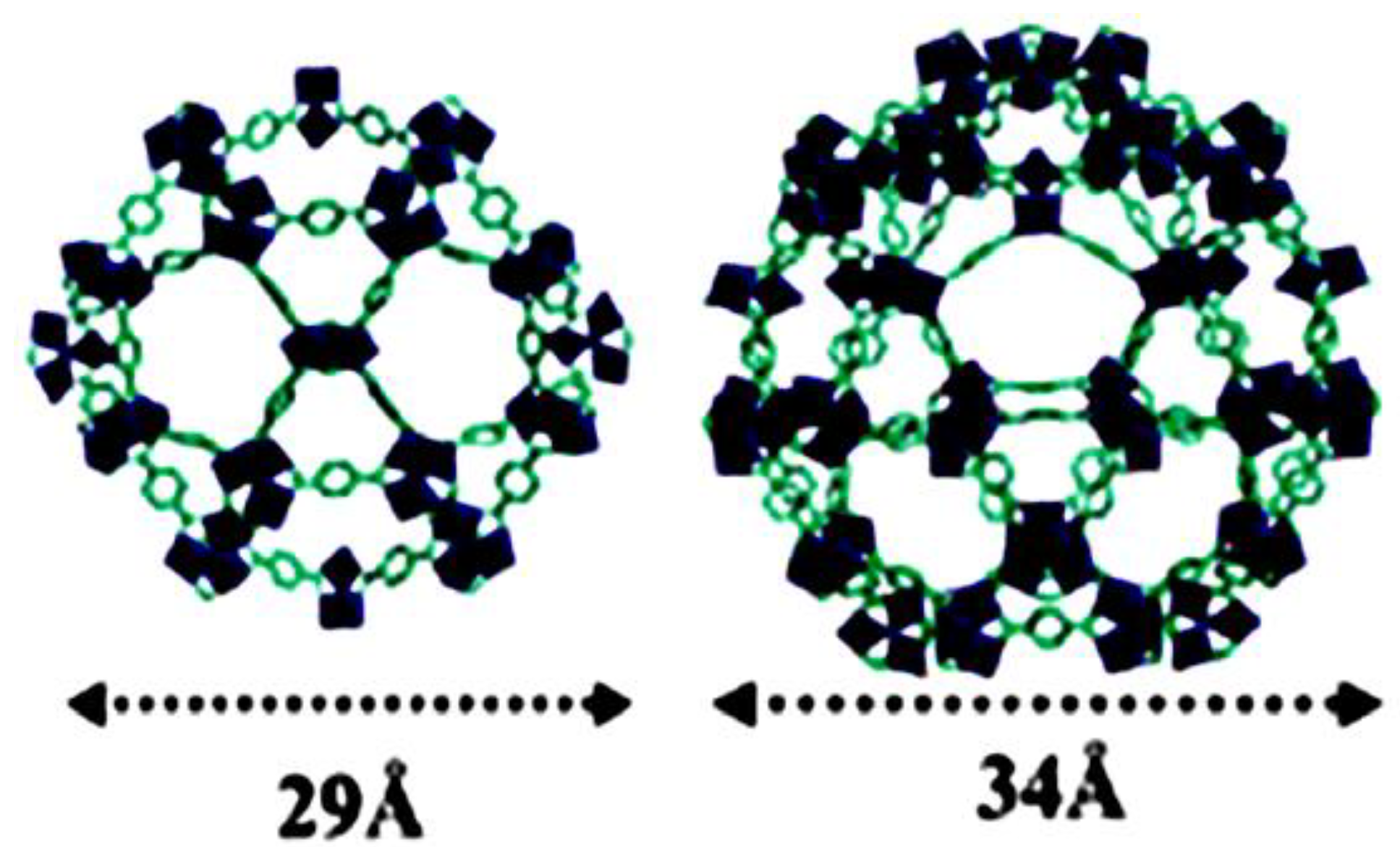
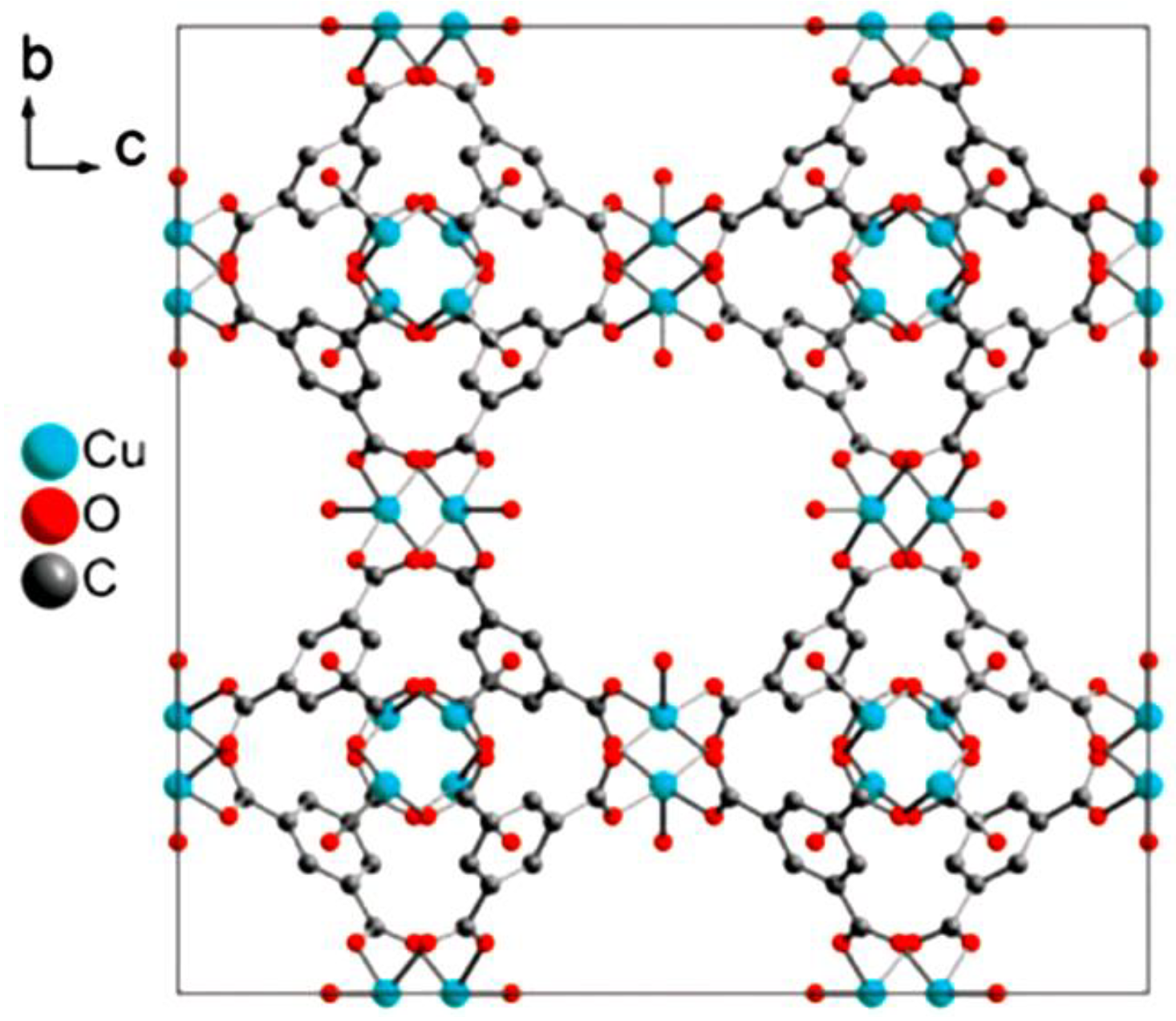

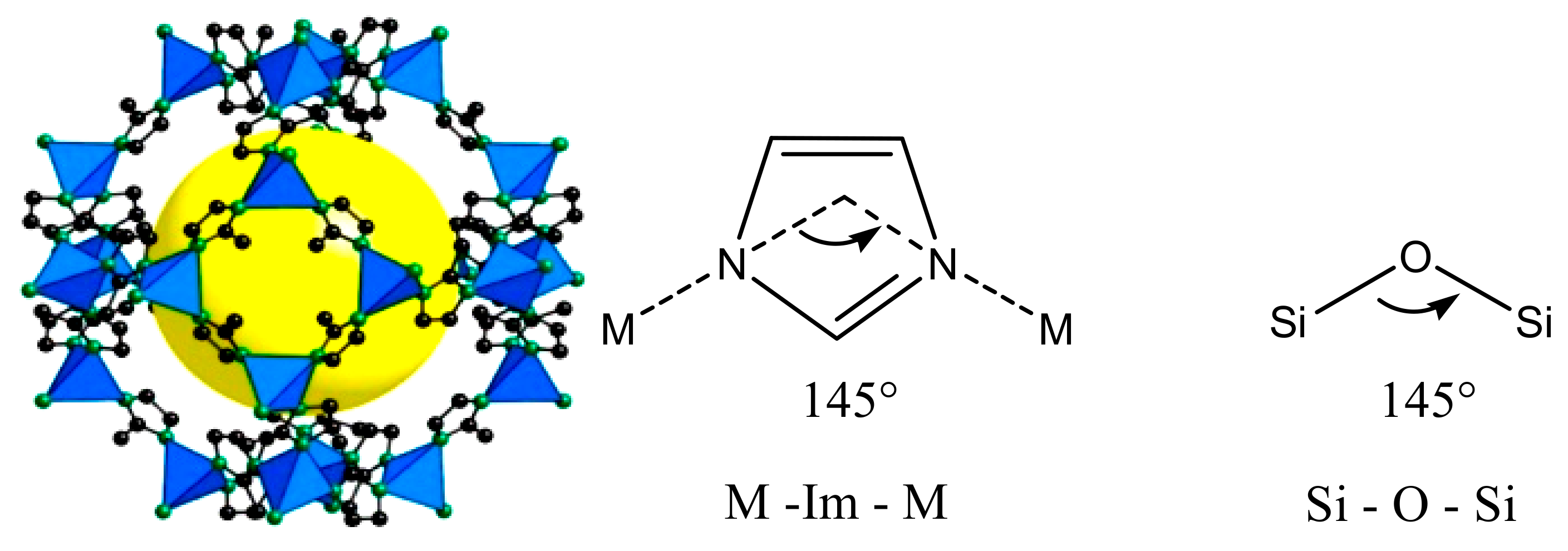
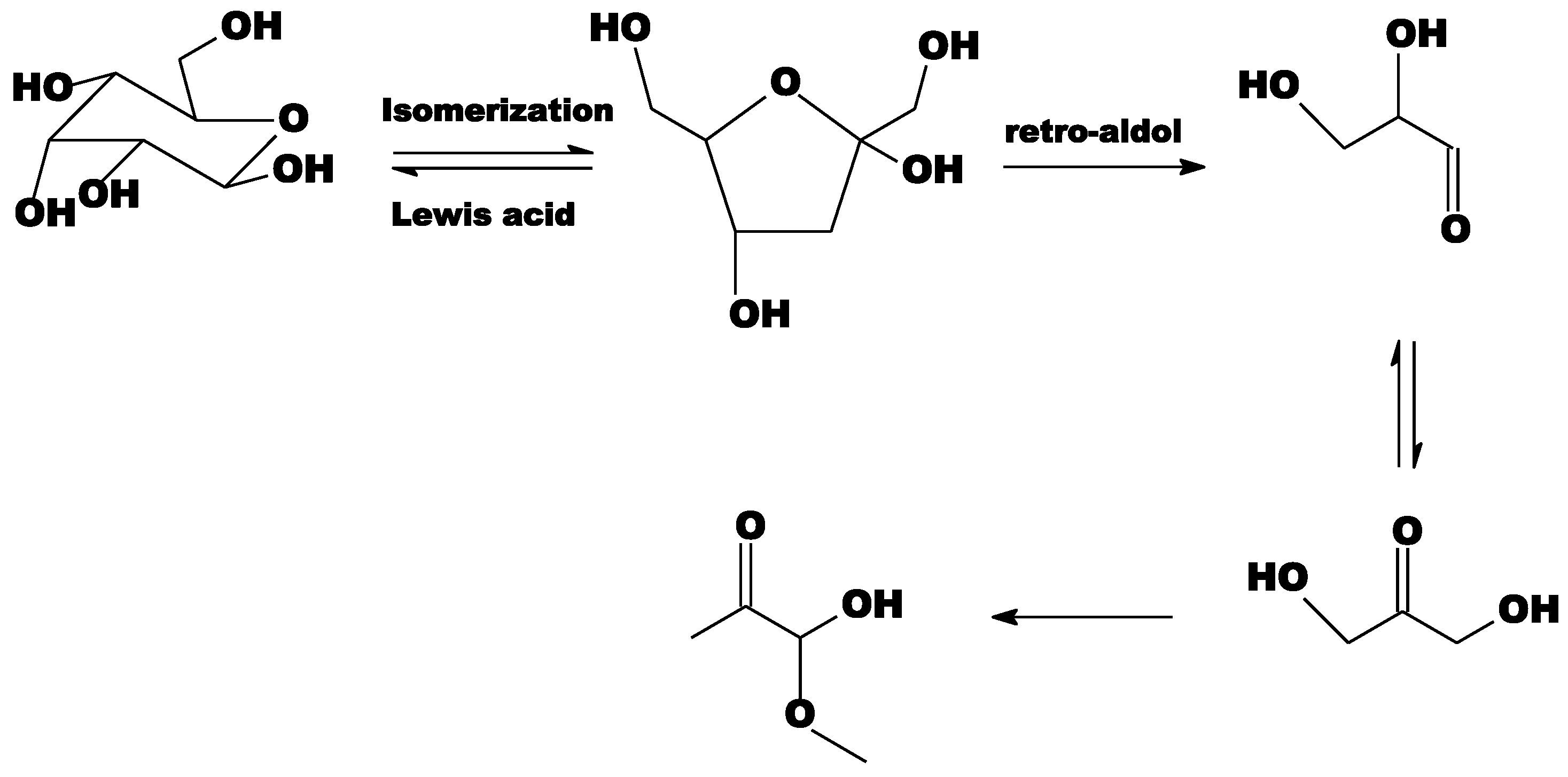
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaeva, V.I.; Nefedov, O.M.; Kustov, L.M. Metal–Organic Frameworks-Based Catalysts for Biomass Processing. Catalysts 2018, 8, 368. https://doi.org/10.3390/catal8090368
Isaeva VI, Nefedov OM, Kustov LM. Metal–Organic Frameworks-Based Catalysts for Biomass Processing. Catalysts. 2018; 8(9):368. https://doi.org/10.3390/catal8090368
Chicago/Turabian StyleIsaeva, Vera I., Oleg M. Nefedov, and Leonid M. Kustov. 2018. "Metal–Organic Frameworks-Based Catalysts for Biomass Processing" Catalysts 8, no. 9: 368. https://doi.org/10.3390/catal8090368
APA StyleIsaeva, V. I., Nefedov, O. M., & Kustov, L. M. (2018). Metal–Organic Frameworks-Based Catalysts for Biomass Processing. Catalysts, 8(9), 368. https://doi.org/10.3390/catal8090368




