The Reduction Temperature Effect of Fe–Co/MgO Catalyst on Characteristics of Multi-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Results & Discussion
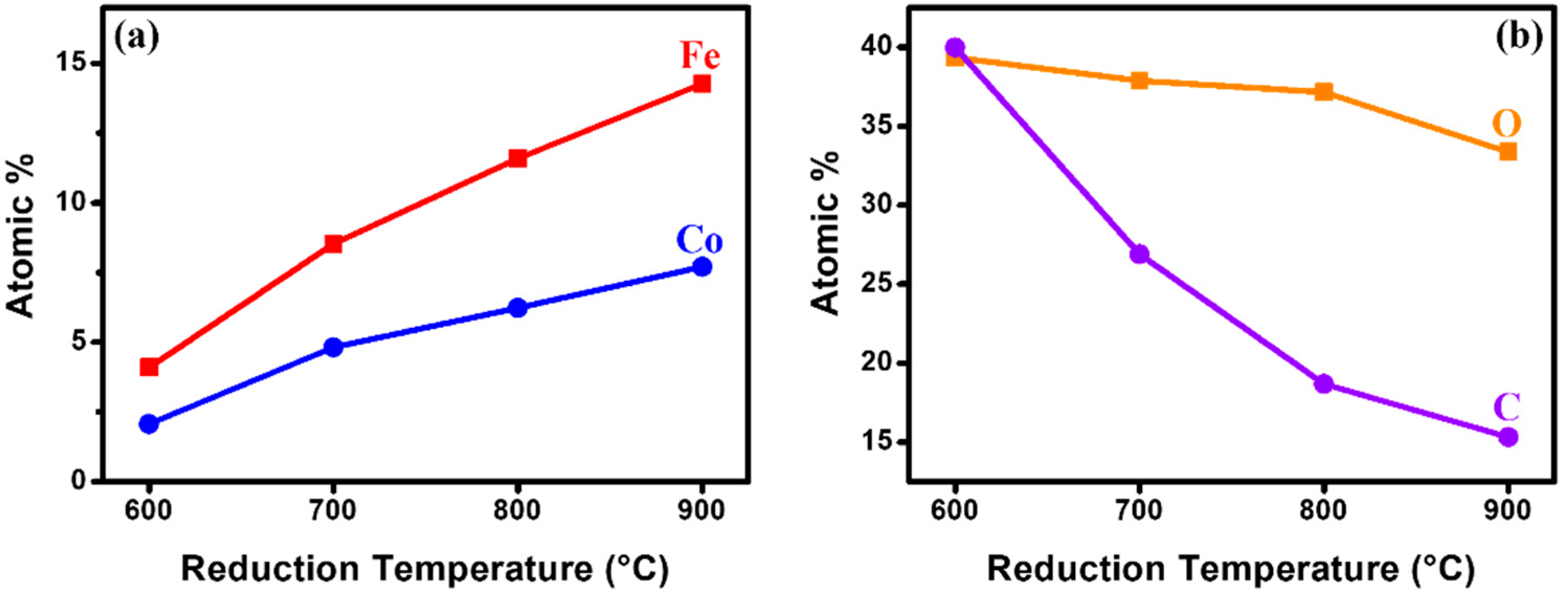
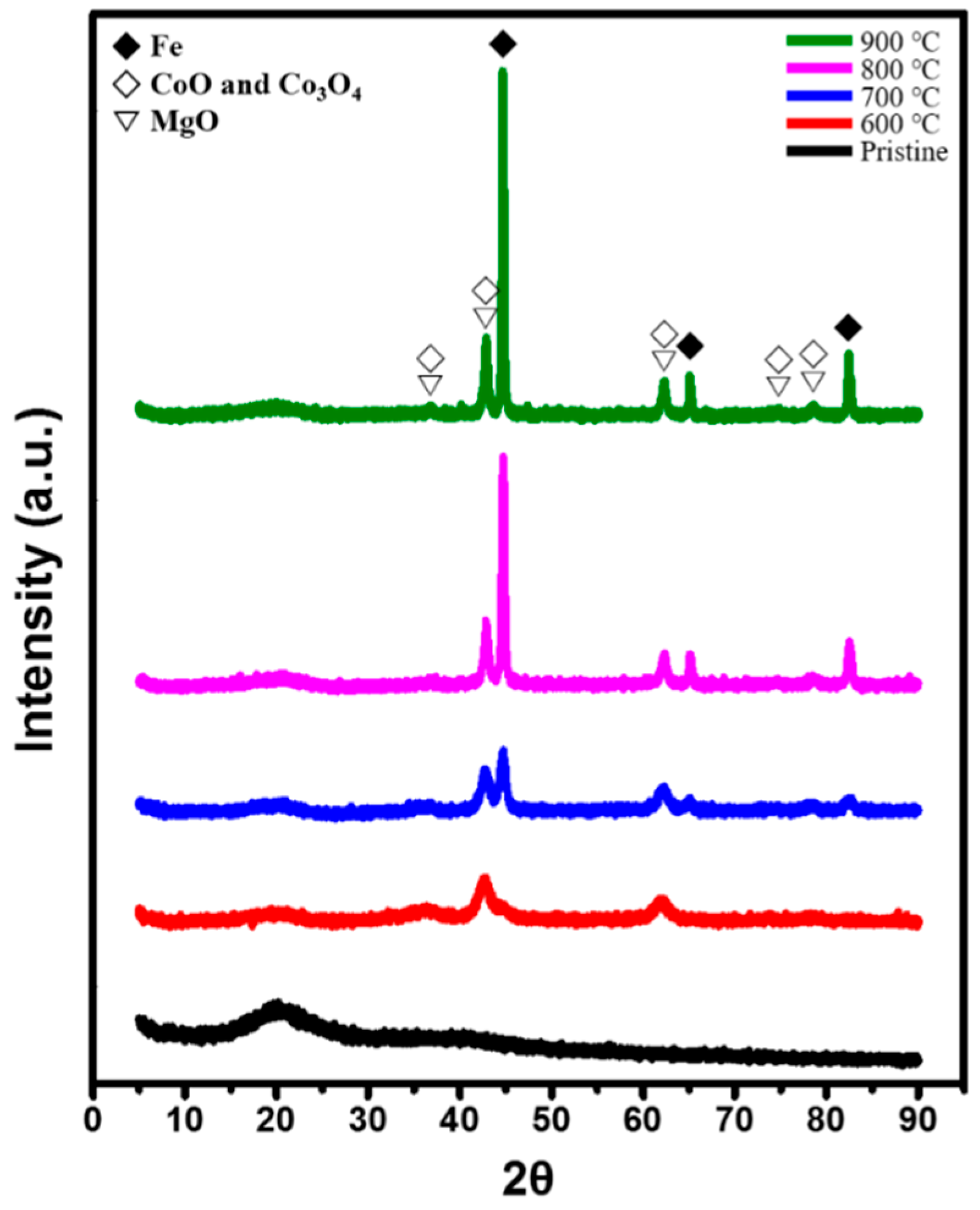
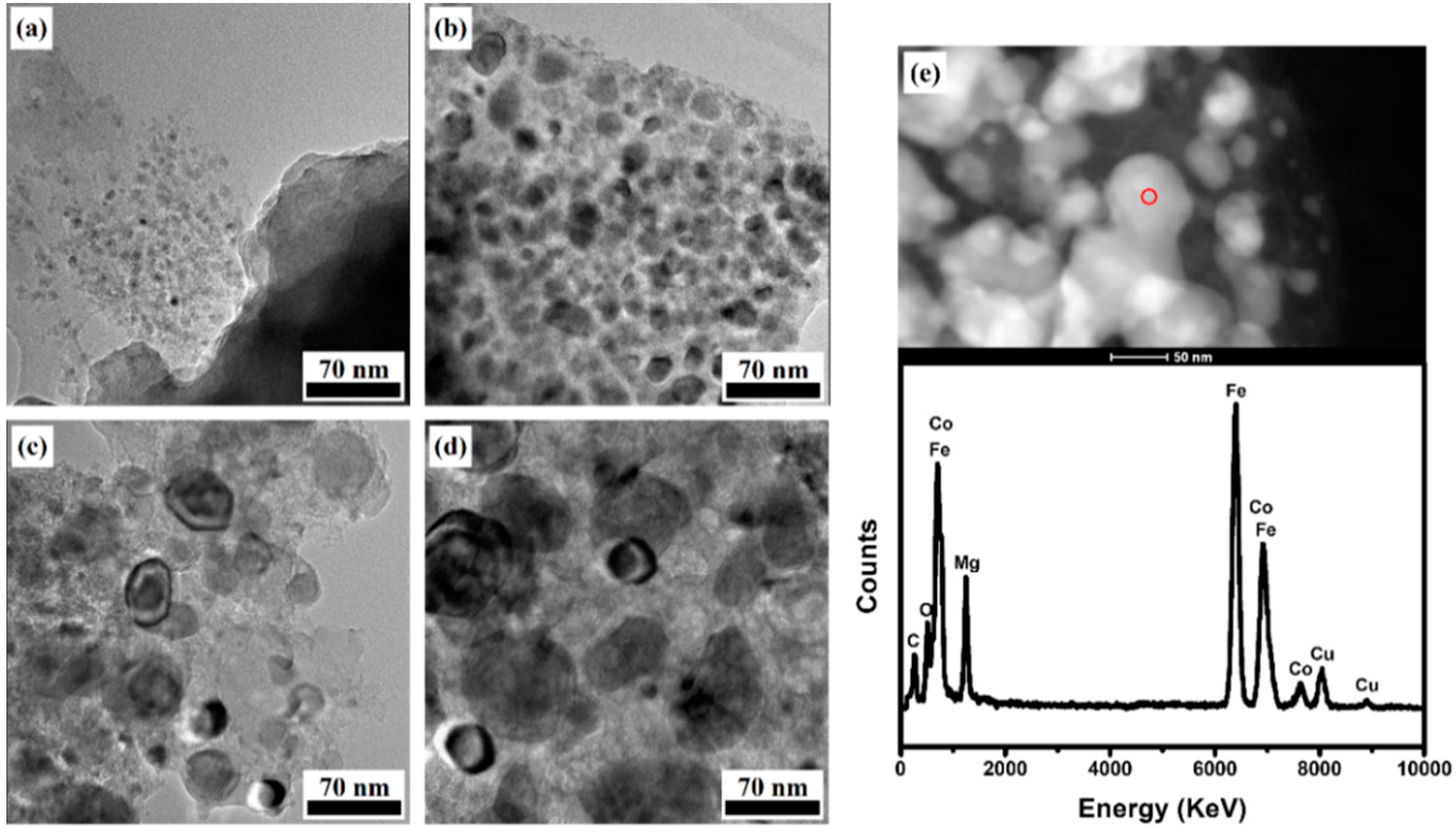
2.1. Characterization of Fe–Co/MgO Catalyst after Reduction
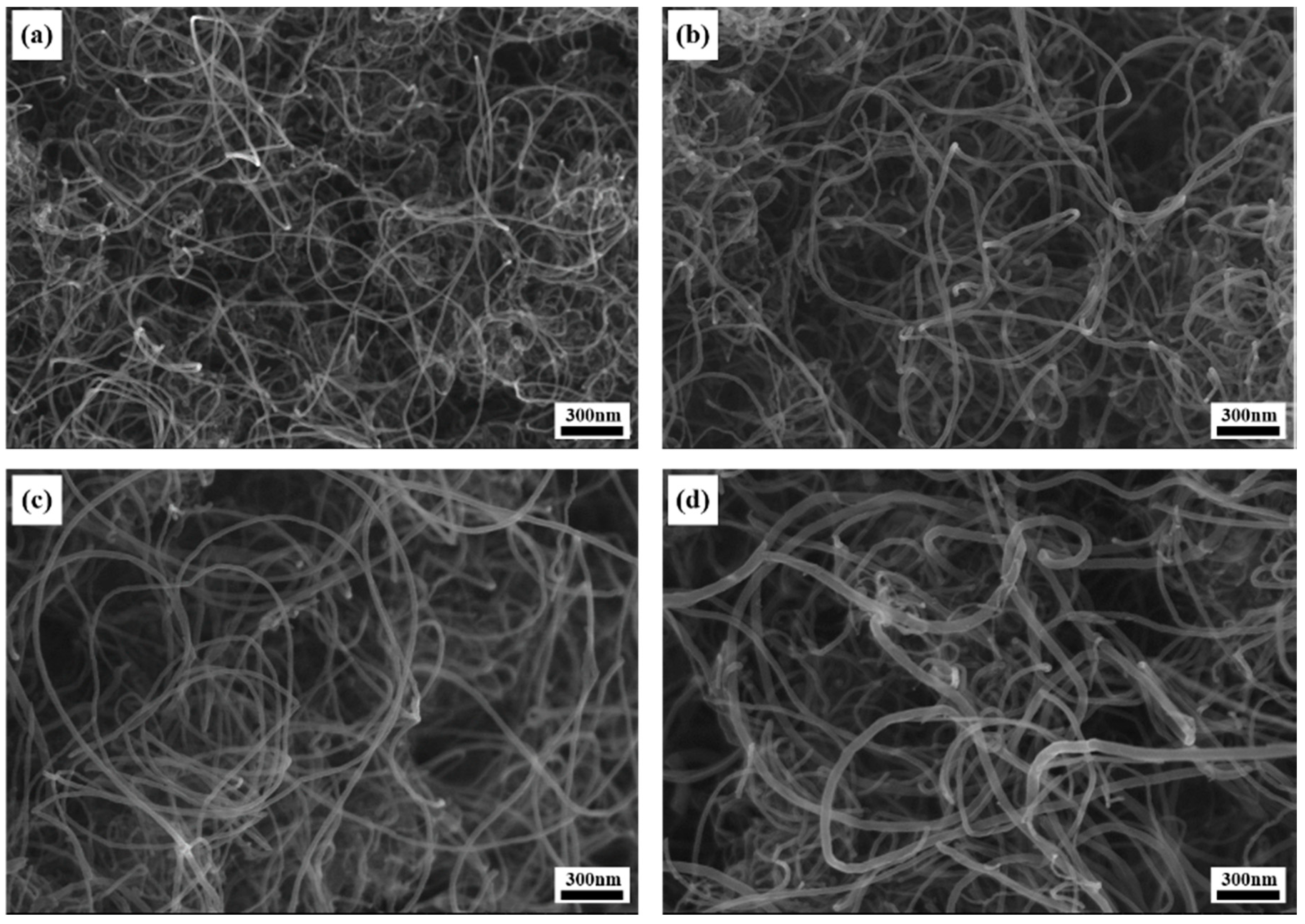
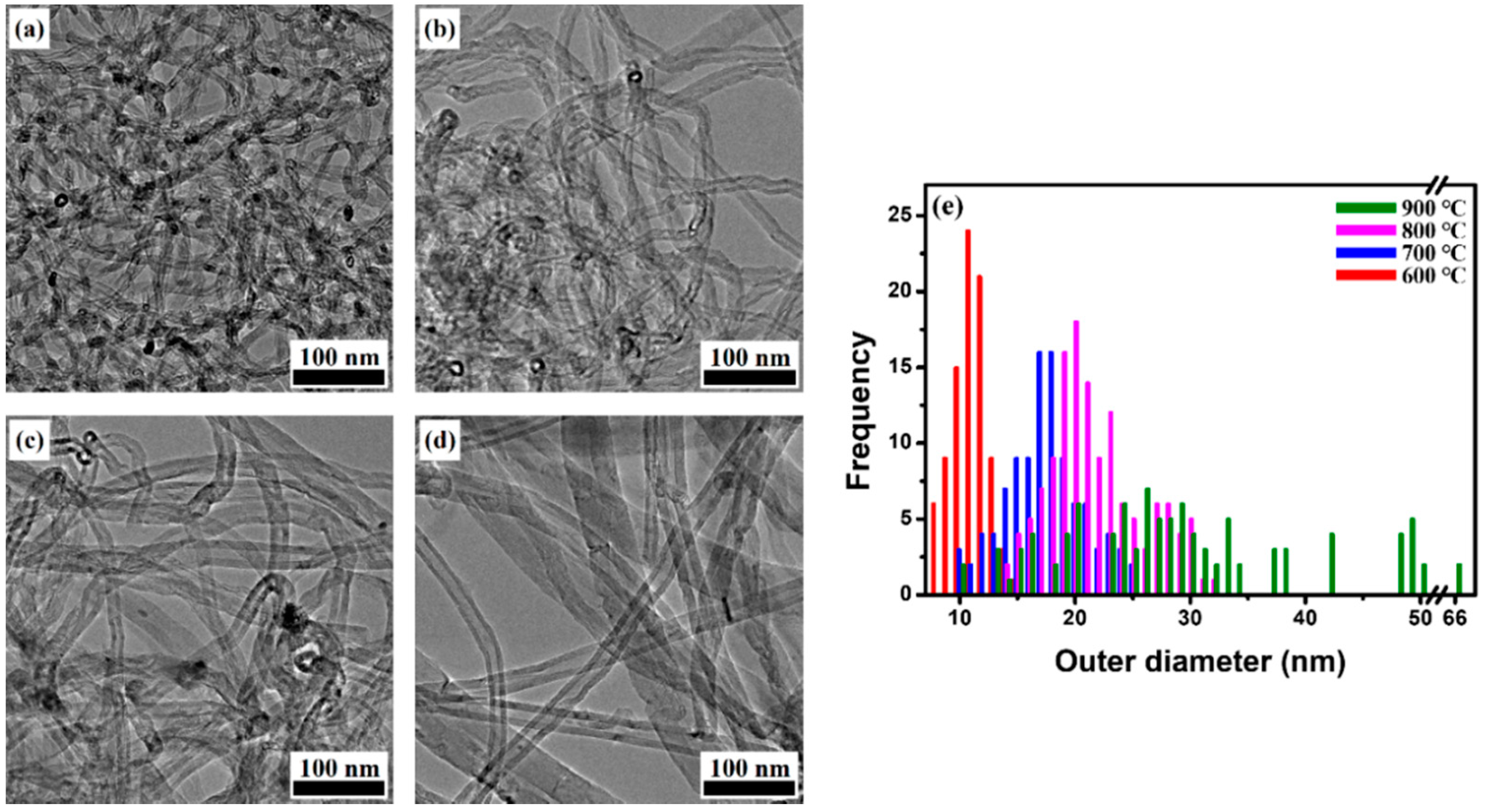
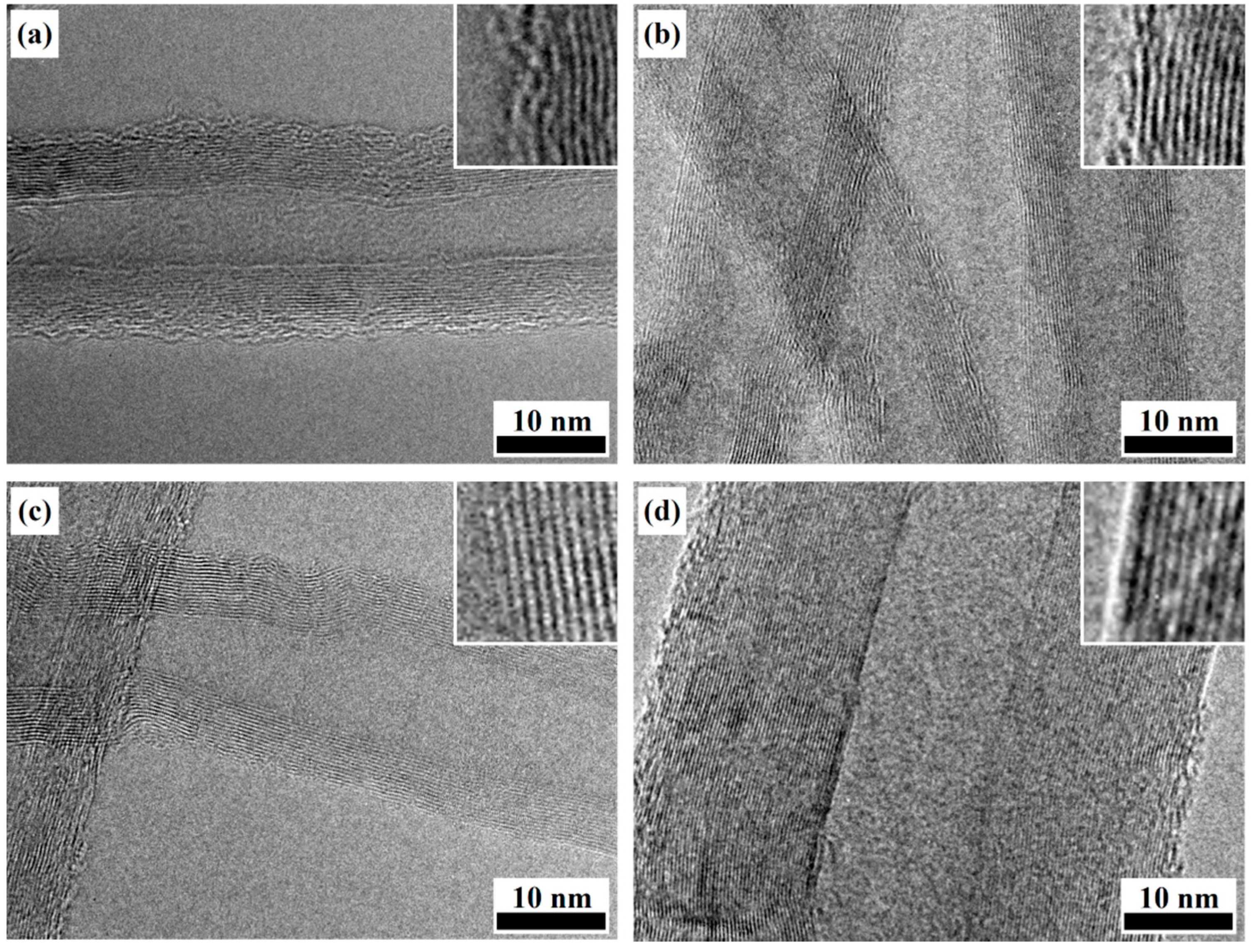
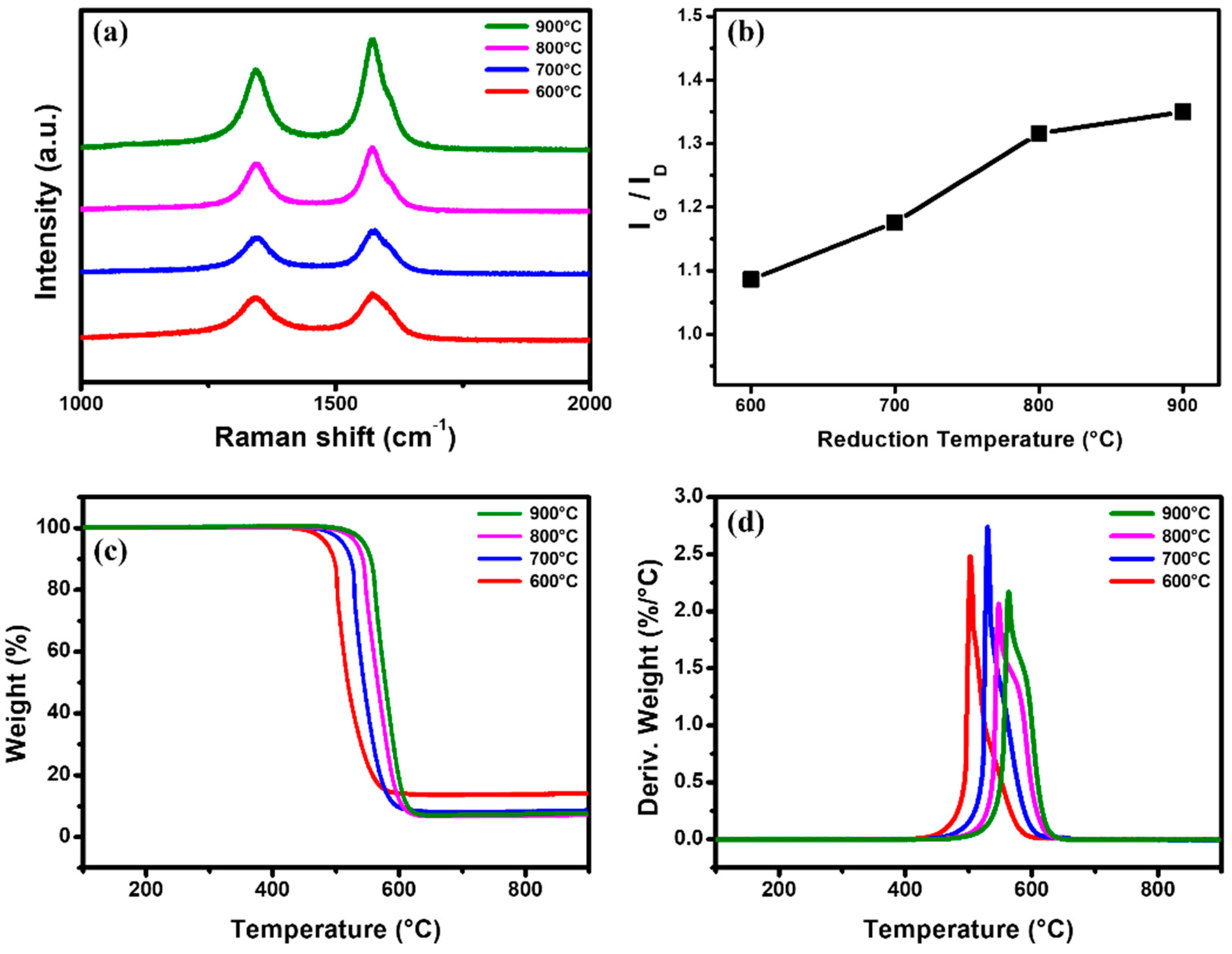
2.2. Analysis of Synthesized MWCNTs Using Catalyst Reduced at Different Temperature
3. Experimental Section
3.1. Fabrication of Catalyst and the Growth of MWCNTs
3.2. Characterization of Catalyst and MWCNTs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Yun, K.N.; Jeon, S.G.; Kim, J.I.; Saito, Y.; Milne, W.I.; Lee, C.J. High performance field emission of carbon nanotube film emitters with a triangular shape. Carbon 2015, 89, 404–410. [Google Scholar] [CrossRef]
- Cao, Q.; Rogers, J.A. Ultrathin films of single-walled carbon nanotubes for electronics and sensors: A review of fundamental and applied aspects. Adv. Mater. 2009, 21, 29–53. [Google Scholar] [CrossRef]
- Mirri, F.; Ma, A.W.K.; Hsu, T.T.; Behabtu, N.; Eichmann, S.L.; Young, C.C.; Tsentalovich, D.E.; Pasquali, M. High-performance carbon nanotube transparent conductive films by scalable dip coating. ACS Nano 2012, 6, 9737–9744. [Google Scholar] [CrossRef] [PubMed]
- Lota, G.; Fic, K.; Frackowiak, E. Carbon nanotubes and their composites in electrochemical applications. Energ. Environ. Sci. 2011, 4, 1592–1605. [Google Scholar] [CrossRef]
- Odom, T.W.; Huang, J.L.; Kim, P.; Lieber, C.M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 1998, 391, 62–64. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Sharma, N.N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Maser, W.K.; Munoz, E.; Benito, A.M.; Martinez, M.T.; de la Fuente, G.F.; Maniette, Y.; Anglaret, E.; Sauvajol, J.L. Production of high-density single-walled nanotube material by a simple laser-ablation method. Chem. Phys. Lett. 1998, 292, 587–593. [Google Scholar] [CrossRef]
- Lyu, S.C.; Liu, B.C.; Lee, S.H.; Park, C.Y.; Kang, H.K.; Yang, C.W.; Lee, C.J. Large-scale synthesis of high-quality single-walled carbon nanotubes by catalytic decomposition of ethylene. J. Phys. Chem. B 2004, 108, 1613–1616. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed]
- Magrez, A.; Seo, J.W.; Smajda, R.; Mionic, M.; Forro, L. Catalytic cvd synthesis of carbon nanotubes: Towards high yield and low temperature growth. Materials 2010, 3, 4871–4891. [Google Scholar] [CrossRef] [PubMed]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis-review. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Lee, C.J.; Lyu, S.C.; Cho, Y.R.; Lee, J.H.; Cho, K.I. Diameter-controlled growth of carbon nanotubes using thermal chemical vapor deposition. Chem. Phys. Lett. 2001, 341, 245–249. [Google Scholar] [CrossRef]
- Rummeli, M.H.; Kramberger, C.; Schaffel, F.; Borowiak-Palen, E.; Gemming, T.; Rellinghaus, B.; Jost, O.; Loffler, M.; Ayala, P.; Pichler, T.; et al. Catalyst size dependencies for carbon nanotube synthesis. Phys. Status Solidi B 2007, 244, 3911–3915. [Google Scholar] [CrossRef]
- Huang, Y.L.; Vlachos, D.G.; Chen, J.G.G. Synthesis of rigid and stable large-inner-diameter multiwalled carbon nanotubes. RSC Adv. 2012, 2, 2685–2687. [Google Scholar] [CrossRef]
- Yeoh, W.M.; Lee, K.Y.; Chai, S.P.; Lee, K.T.; Mohamed, A.R. Effective synthesis of carbon nanotubes via catalytic decomposition of methane: Influence of calcination temperature on metal-support interaction of co-mo/mgo catalyst. J. Phys. Chem. Solids 2013, 74, 1553–1559. [Google Scholar] [CrossRef]
- Tran, K.Y.; Heinrichs, B.; Colomer, J.F.; Pirard, J.P.; Lambert, S. Carbon nanotubes synthesis by the ethylene chemical catalytic vapour deposition (CCVD) process on Fe, Co, and Fe–Co/Al2O3 sol-gel catalysts. Appl. Catal. A Gen. 2007, 318, 63–69. [Google Scholar] [CrossRef]
- Pelech, I.; Narkiewicz, U.; Kaczmarek, A.; Jedrzejewska, A. Preparation and characterization of multi-walled carbon nanotubes grown on transition metal catalysts. Pol. J. Chem. Technol. 2014, 16, 117–122. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Wang, Y.Q.; Wang, Z.L. Preparation of monodispersed Fe–Mo nanoparticles as the catalyst for cvd synthesis of carbon nanotubes. Chem. Mater. 2001, 13, 1008–1014. [Google Scholar] [CrossRef]
- Datta, S.S.; Strachan, D.R.; Khamis, S.M.; Johnson, A.T.C. Crystallographic etching of few-layer graphene. Nano Lett. 2008, 8, 1912–1915. [Google Scholar] [CrossRef] [PubMed]
- Ci, L.J.; Song, L.; Jariwala, D.; Elias, A.L.; Gao, W.; Terrones, M.; Ajayan, P.M. Graphene shape control by multistage cutting and transfer. Adv. Mater. 2009, 21, 4487–4491. [Google Scholar] [CrossRef]
- Garces, L.J.; Hincapie, B.; Zerger, R.; Suib, S.L. The effect of temperature and support on the reduction of cobalt oxide: An in situ x-ray diffraction study. J. Phys. Chem. C 2015, 119, 5484–5490. [Google Scholar] [CrossRef]
- Hassan, N.A.; El-Molla, S.A.; Mohamed, G.M.; Fagal, G.A. Effect of ZrO2-doping of nanosized Fe2O3/MgO system on its structural, surface and catalytic properties. Mater. Res. Bull. 2012, 47, 2655–2661. [Google Scholar] [CrossRef]
- Mao, Z.; Wu, Q.Z.; Wang, M.; Yang, Y.S.; Long, J.; Chen, X.H. Tunable synthesis of SiO2-encapsulated zero-valent iron nanoparticles for degradation of organic dyes. Nanoscale Res. Lett. 2014, 9, 501. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Li, X.Q.; Cao, J.S.; Zhang, W.X.; Wang, H.P. Characterization of zero-valent iron nanoparticles. Adv. Colloid Interface Sci. 2006, 120, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Maurin, G.; Stepanek, I.; Bernier, P.; Colomer, J.F.; Nagy, J.B.; Henn, F. Segmented and opened multi-walled carbon nanotubes. Carbon 2001, 39, 1273–1278. [Google Scholar] [CrossRef]
- Jang, J.W.; Lee, K.W.; Oh, I.H.; Lee, E.M.; Kim, I.M.; Lee, C.E.; Lee, C.J. Magnetic Fe catalyst particles in the vapor-phase-grown multi-walled carbon nanotubes. Solid State Commun. 2008, 145, 561–564. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A. Unusual properties and structure of carbonnanotubes. Annu. Rev. Mater. Res. 2004, 34, 247–278. [Google Scholar] [CrossRef]
- Irurzun, V.M.; Tan, Y.Q.; Resasco, D.E. Sol-gel synthesis and characterization of Co–Mo/silica catalysts for single-walled carbon nanotube production. Chem. Mater. 2009, 21, 2238–2246. [Google Scholar] [CrossRef]
- Dubey, P.; Choi, S.K.; Kim, B.; Lee, C.J. Synthesis of thin-multiwalled carbon nanotubes by Fe–Mo/MgO catalyst using sol-gel method. Carbon Lett. 2012, 13, 99–108. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, P.; Lee, C.J. The Reduction Temperature Effect of Fe–Co/MgO Catalyst on Characteristics of Multi-Walled Carbon Nanotubes. Catalysts 2018, 8, 361. https://doi.org/10.3390/catal8090361
Kim P, Lee CJ. The Reduction Temperature Effect of Fe–Co/MgO Catalyst on Characteristics of Multi-Walled Carbon Nanotubes. Catalysts. 2018; 8(9):361. https://doi.org/10.3390/catal8090361
Chicago/Turabian StyleKim, Paul, and Cheol Jin Lee. 2018. "The Reduction Temperature Effect of Fe–Co/MgO Catalyst on Characteristics of Multi-Walled Carbon Nanotubes" Catalysts 8, no. 9: 361. https://doi.org/10.3390/catal8090361
APA StyleKim, P., & Lee, C. J. (2018). The Reduction Temperature Effect of Fe–Co/MgO Catalyst on Characteristics of Multi-Walled Carbon Nanotubes. Catalysts, 8(9), 361. https://doi.org/10.3390/catal8090361



