Co-Aromatization of n-Butane and Methanol over PtSnK-Mo/ZSM-5 Zeolite Catalysts: The Promotion Effect of Ball-Milling
Abstract
:1. Introduction
2. Results and Discussion
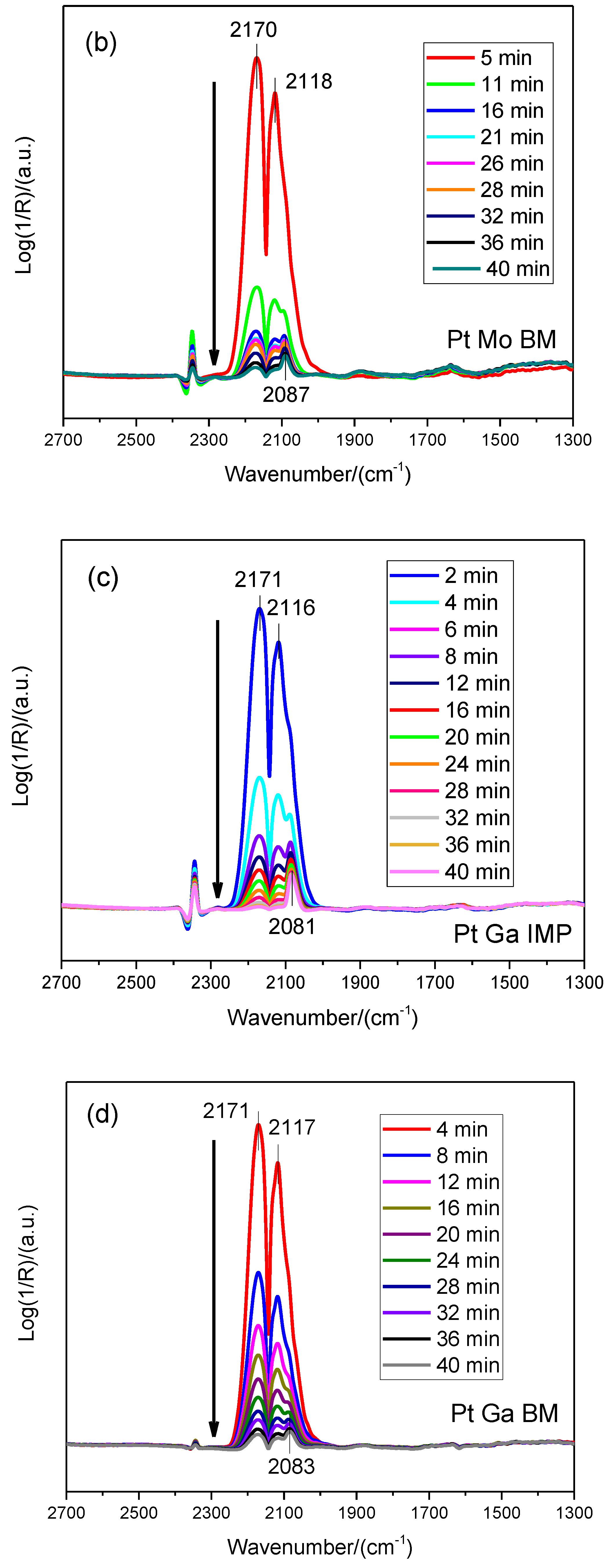
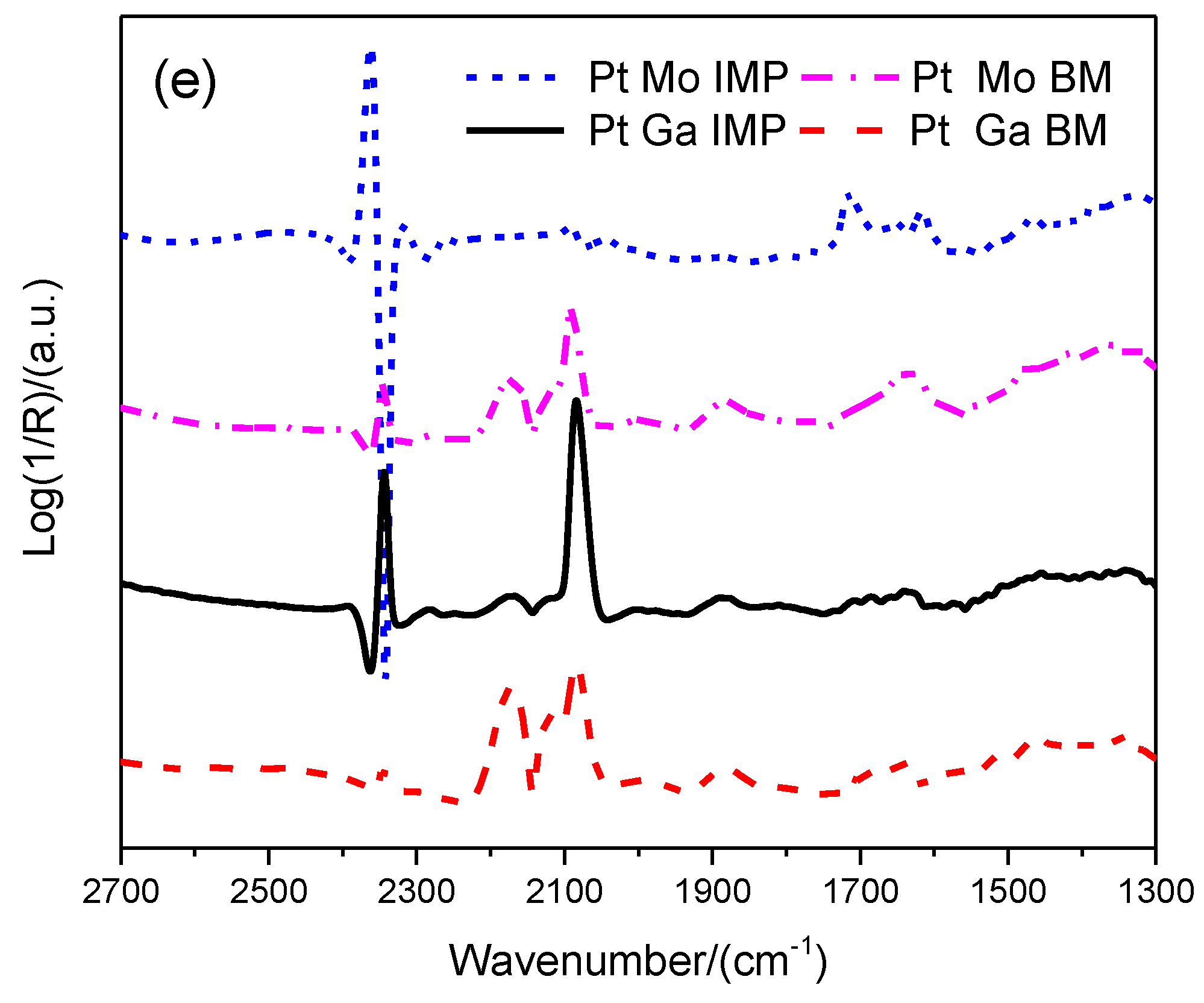
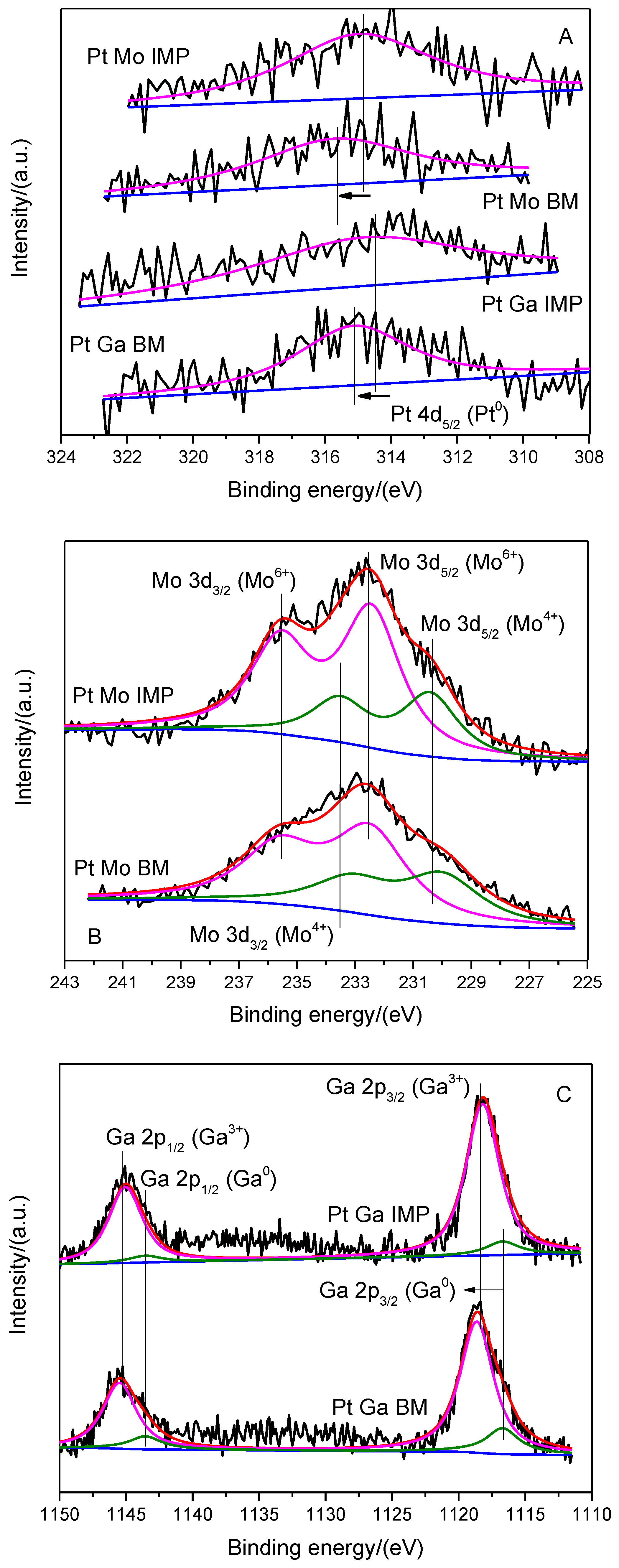
2.1. Catalyst Characterization
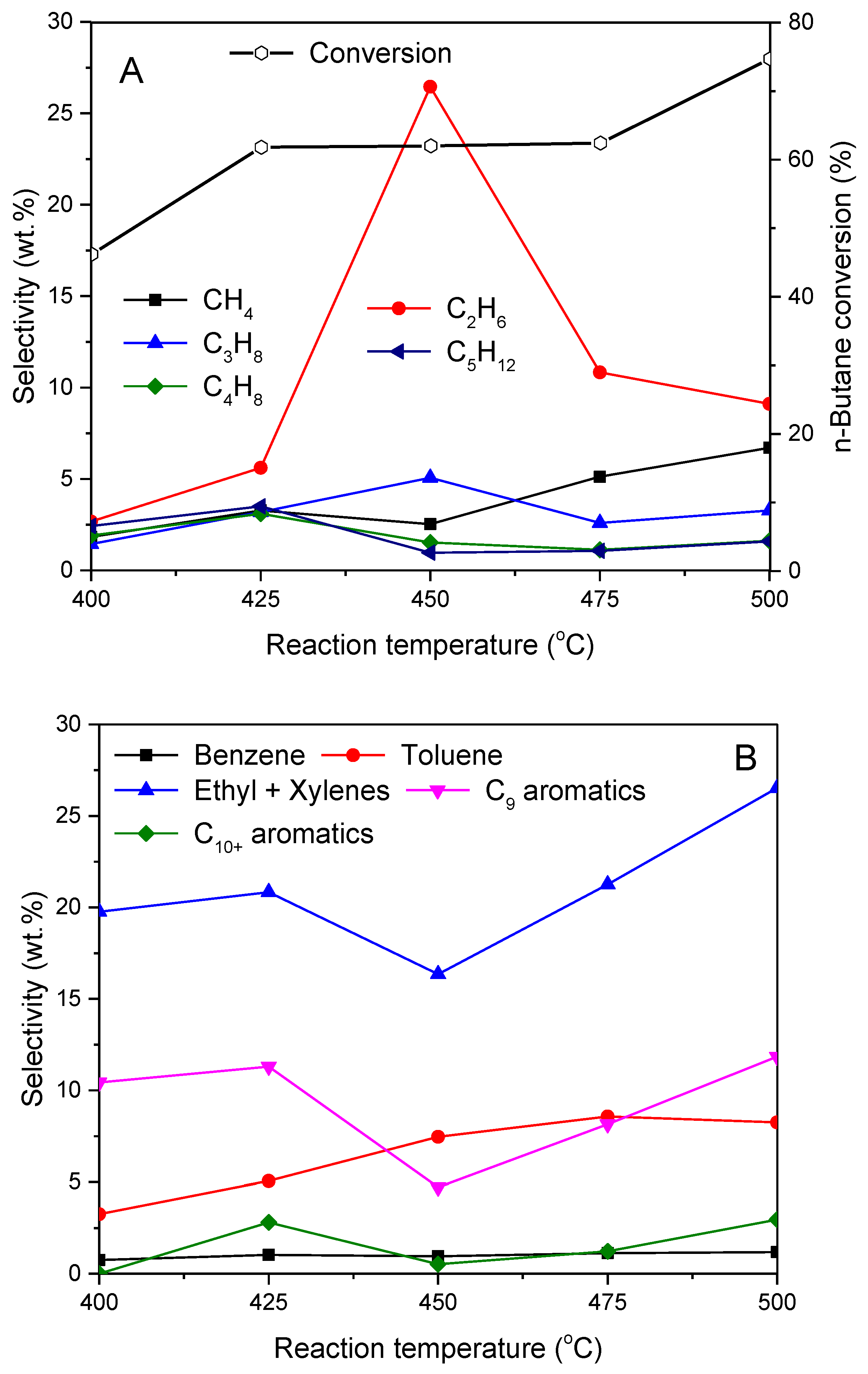
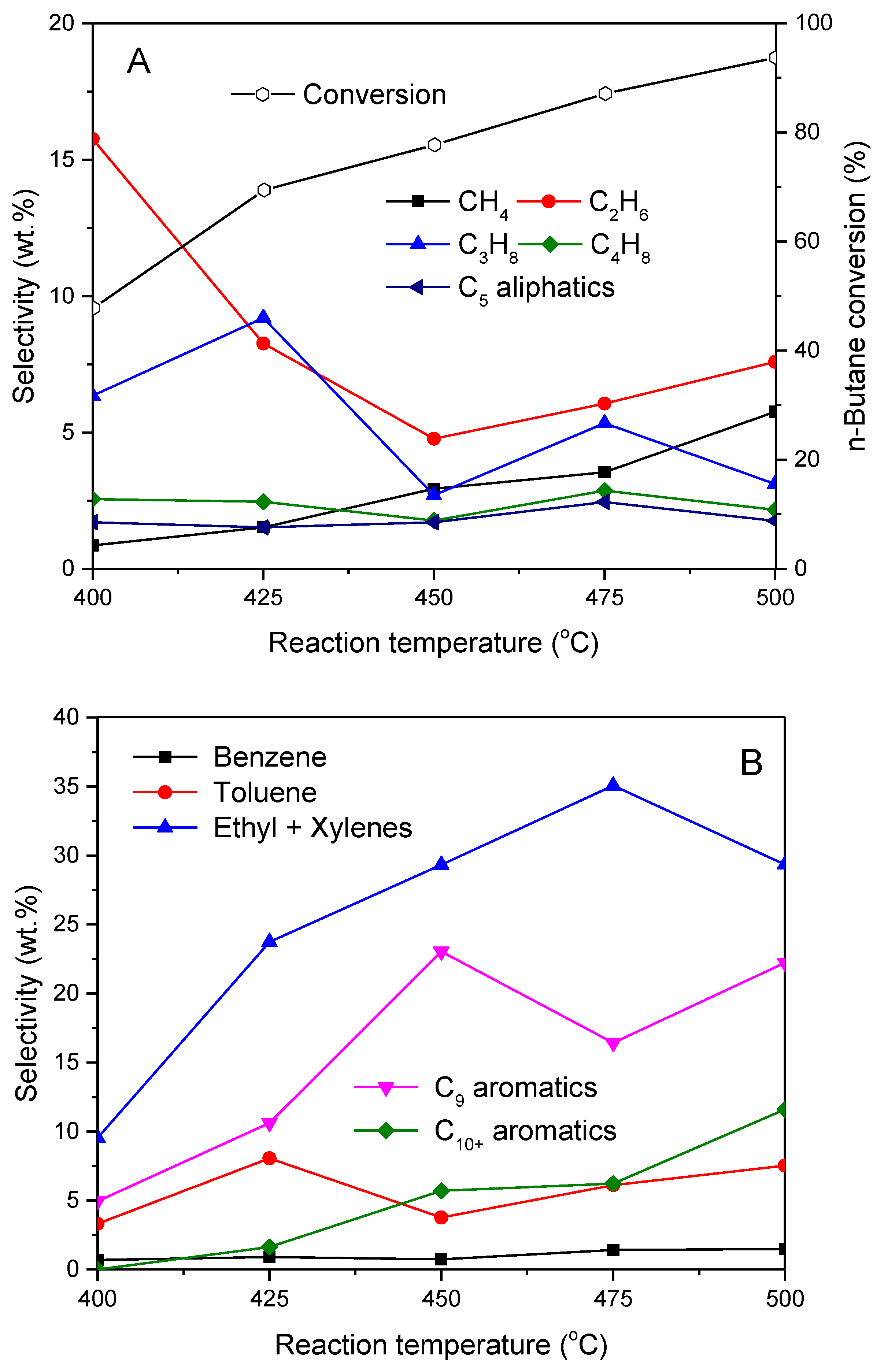
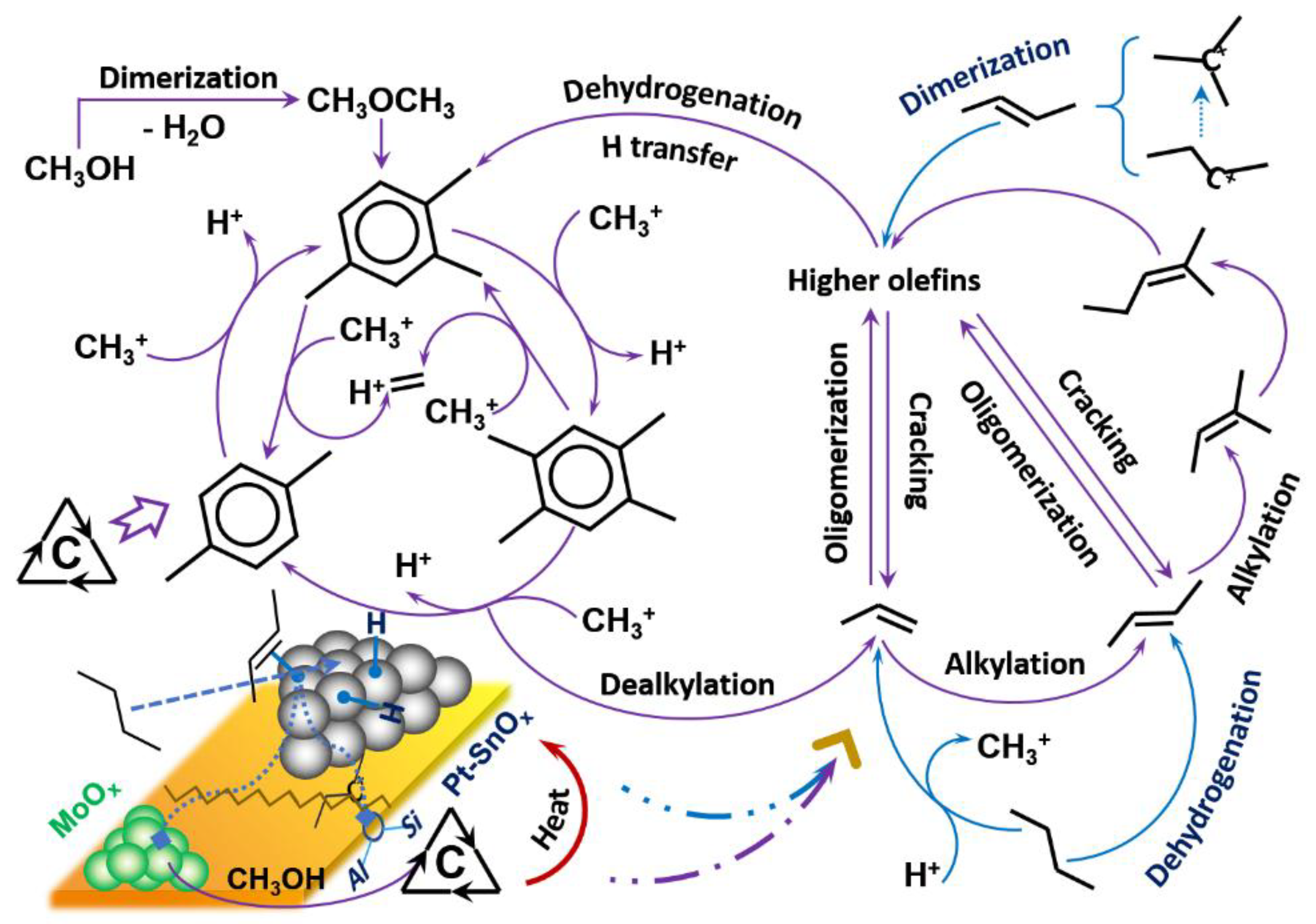
2.2. Influence of Preparation Method on the Aromatization of Cofeeding n-Butane with Methanol
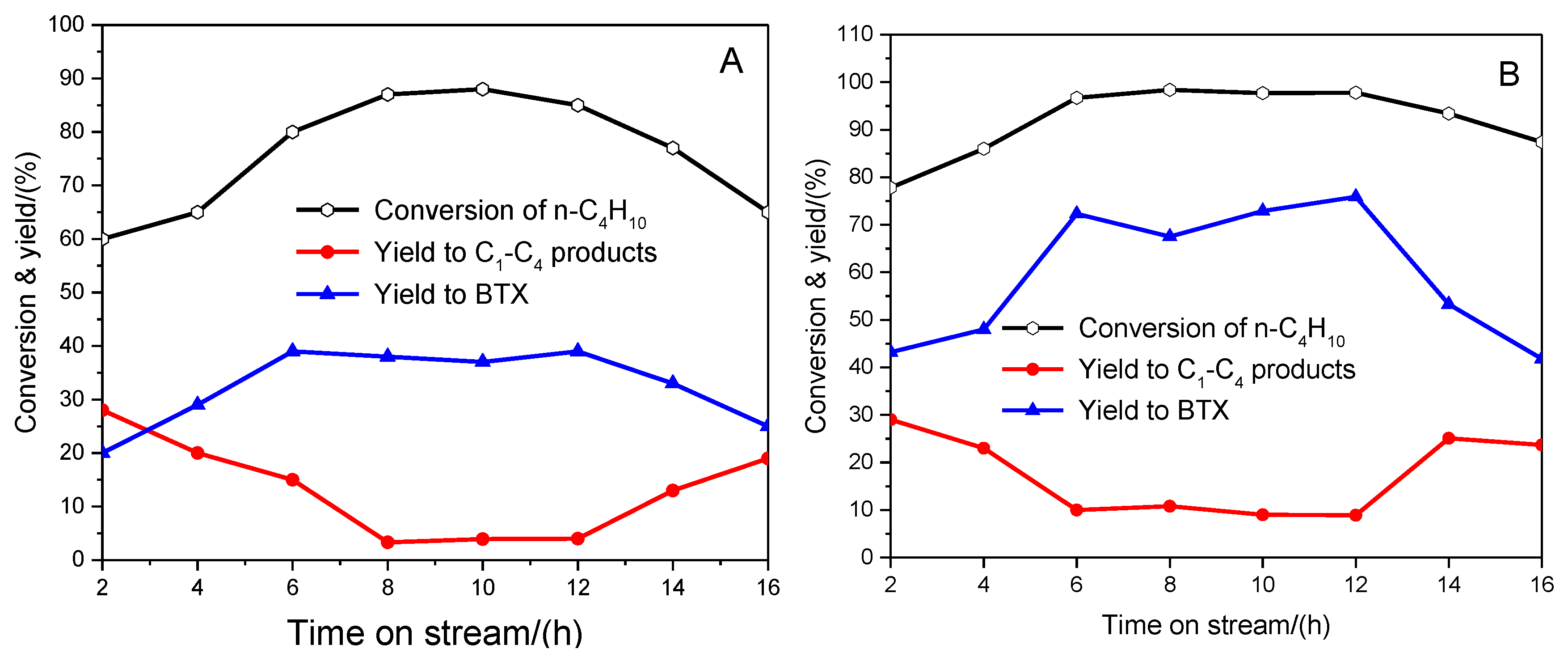
2.3. PtSnK–Mo/ZSM-5 Catalyst Stability for Co-Aromatization
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mi, D. Analysis of domestic and foreign aromatics production and market supply and demand pricing (Chinese version). Chem. Ind. 2017, 35, 59–64. [Google Scholar]
- Dai, W.; Yang, L.; Wang, C.; Wang, X.; Wu, G.; Guan, N.; Obenaus, U.; Hunger, M.; Li, L. Effect of n-Butanol Cofeeding on the Methanol to Aromatics Conversion over Ga-Modified Nano H-ZSM-5 and Its Mechanistic Interpretation. ACS Catal. 2018, 8, 1352–1362. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, J.; Zhang, K.; Feng, W.; Liu, S.; Ding, C.; Liu, P. Nanocrystallite self-assembled hierarchical ZSM-5 zeolite microsphere for methanol to aromatics. Microporous Mesoporous Mater. 2017, 247, 103–115. [Google Scholar] [CrossRef]
- Conte, M.; Lopez-Sanchez, J.A.; He, Q.; Morgan, D.J.; Ryabenkova, Y.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Kiely, C.J.; Khalid, K.; et al. Modified zeolite ZSM-5 for the methanol to aromatics reaction. Catal. Sci. Technol. 2012, 2, 105–112. [Google Scholar] [CrossRef]
- Lai, P.C.; Chen, C.H.; Hsu, H.Y.; Lee, C.H.; Lin, Y.C. Methanol aromatization over Ga-doped desilicated HZSM-5. RSC Adv. 2016, 6, 67361–67371. [Google Scholar] [CrossRef]
- Xin, Y.; Qi, P.; Duan, X.; Lin, H.; Yuan, Y. Enhanced Performance of Zn–Sn/HZSM-5 Catalyst for the Conversion of Methanol to Aromatics. Catal. Lett. 2013, 143, 798–806. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, W.; Kong, C.; Wei, F. Increasing para-Xylene Selectivity in Making Aromatics from Methanol with a Surface-Modified Zn/P/ZSM-5 Catalyst. ACS Catal. 2015, 5, 2982–2988. [Google Scholar] [CrossRef]
- Mier, D.; Aguayo, A.T.; Gayubo, A.G.; Olazar, M.; Bilbao, J. Synergies in the production of olefins by combined cracking of n-butane and methanol on a HZSM-5 zeolite catalyst. Chem. Eng. J. 2010, 160, 760–769. [Google Scholar] [CrossRef]
- Song, C.; Liu, S.; Li, X.; Xie, S.; Liu, Z.; Xu, L. Influence of reaction conditions on the aromatization of cofeeding n-butane with methanol over the Zn loaded ZSM-5/ZSM-11 zeolite catalyst. Fuel Process. Technol. 2014, 126, 60–65. [Google Scholar] [CrossRef]
- Song, C.; Liu, K.; Zhang, D.; Liu, S.; Li, X.; Xie, S.; Xu, L. Effect of cofeeding n-butane with methanol on aromatization performance and coke formation over a Zn loaded ZSM-5/ZSM-11 zeolite. Appl. Catal. A 2014, 470, 15–23. [Google Scholar] [CrossRef]
- Fila, V.; Bernauer, M.; Bernauer, B.; Sobalik, Z. Effect of addition of a second metal in Mo/ZSM-5 catalyst for methane aromatization reaction under elevated pressures. Catal. Today 2015, 256, 269–275. [Google Scholar] [CrossRef]
- Tshabalala, T.E.; Scurrell, M.S. Aromatization of n-hexane over Ga, Mo and Zn modified H-ZSM-5 zeolite catalysts. Catal. Commun. 2015, 72, 49–52. [Google Scholar] [CrossRef]
- Barthos, R.; Solymosi, F. Aromatization of n-heptane on Mo2C-containing catalysts. J. Catal. 2005, 235, 60–68. [Google Scholar] [CrossRef]
- Solymosi, F.; Széchenyi, A. Aromatization of n-butane and 1-butene over supported Mo2C catalyst. J. Catal. 2004, 223, 221–231. [Google Scholar] [CrossRef]
- Barthos, R.; Bánsági, T.; Zakar, T.S.; Solymosi, F. Aromatization of methanol and methylation of benzene over Mo2C/ZSM-5 catalysts. J. Catal. 2007, 247, 368–378. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2011, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Gu, B.; Liu, X.; Kang, J.; Zhang, Q.; Wang, Y. Direct and Highly Selective Conversion of Synthesis Gas into Lower Olefins: Design of a Bifunctional Catalyst Combining Methanol Synthesis and Carbon–Carbon Coupling. Angew. Chem. Int. Ed. 2016, 128, 4803–4806. [Google Scholar] [CrossRef]
- Lin, Q.; Guo, Z.; Pan, L.; Xiang, X. Zn-Cr double metal cyanide catalysts synthesized by ball milling for the copolymerization of CO2/propylene oxide, phthalic anhydride/propylene oxide, and CO2/propylene oxide/phthalic anhydride. Catal. Commun. 2015, 64, 114–118. [Google Scholar]
- Pineda, A.; Balu, A.M.; Campelo, J.M.; Romero, A.A.; Carmona, D.; Balas, F.; Santamaria, J.; Luque, R. A dry milling approach for the synthesis of highly active nanoparticles supported on porous materials. ChemSusChem 2011, 4, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Caeiro, G.; Carvalho, R.H.; Wang, X.; Lemos, M.A.N.D.A.; Lemos, F.; Guisnet, M.; Ribeiro, F.R. Activation of C2–C4 alkanes over acid and bifunctional zeolite catalysts. J. Mol. Catal. A 2006, 255, 131–158. [Google Scholar] [CrossRef]
- Sattler, J.J.; Ruizmartinez, J.; Santillanjimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, S.; Song, Y.; Xu, L. Butene Catalytic Cracking to Propene and Ethene over Potassium Modified ZSM-5 Catalysts. Catal. Lett. 2005, 103, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Epelde, E.; Santos, J.I.; Florian, P.; Aguayo, A.T.; Gayubo, A.G.; Bilbao, J.; Castaño, P. Controlling coke deactivation and cracking selectivity of MFI zeolite by H3PO4 or KOH modification. Appl. Catal. A 2015, 505, 105–115. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, X.; Xie, S.; Wang, Q.; Xu, L. The Effect of Acidity on Olefin Aromatization over Potassium Modified ZSM-5 Catalysts. Catal. Lett. 2004, 97, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, S.; Wu, G.; Gong, J. Synthesis of stable Ni-CeO2 catalysts via ball-milling for ethanol steam reforming. Catal. Today 2014, 233, 53–60. [Google Scholar] [CrossRef]
- Wan, Z.; Wu, W.; Li, G.; Wang, C.; Yang, H.; Zhang, D. Effect of SiO2/Al2O3 ratio on the performance of nanocrystal ZSM-5 zeolite catalysts in methanol to gasoline conversion. Appl. Catal. A 2016, 523, 312–320. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Shi, J.; Zhou, S.; Sheng, X.; Zhang, Z.; Xiang, S. Comparative study of bimetallic Pt-Sn catalysts supported on different supports for propane dehydrogenation. J. Mol. Catal. A 2014, 381, 138–147. [Google Scholar] [CrossRef]
- Morán, C.; González, E.; Sánchez, J.; Solano, R.; Carruyo, G.; Moronta, A. Dehydrogenation of ethylbenzene to styrene using Pt, Mo, and Pt-Mo catalysts supported on clay nanocomposites. J. Colloid Interface Sci. 2007, 315, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Tshabalala, T.E.; Coville, N.J.; Scurrell, M.S. Dehydroaromatization of methane over doped Pt/Mo/H-ZSM-5 zeolite catalysts: The promotional effect of tin. Appl. Catal. A 2014, 485, 238–244. [Google Scholar] [CrossRef]
- Meriaudeau, P.; Albano, K.; Naccache, C. Promotion of platinum-based catalysts for methanol synthesis from syngas. J. Chem. Soc. Faraday Trans. L 1987, 83, 2113–2118. [Google Scholar] [CrossRef]
- Silva, M.A.P.D.; Mellovieira, R.A.; Schmal, M. Interaction between Pt and MoO3 dispersed over alumina. Appl. Catal. A 2000, 190, 177–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Yang, K.; Li, Y.; Wang, Y.; Xu, Y.; Wu, P. Effect of hydrothermal treatment on catalytic properties of PtSnNa/ZSM-5 catalyst for propane dehydrogenation. Microporous Mesoporous Mater. 2006, 96, 245–254. [Google Scholar] [CrossRef]
- Kolobova, E.; Pestryakov, A.; Mamontov, G.; Kotolevich, Y.; Bogdanchikova, N.; Farias, M.; Vosmerikov, A.; Vosmerikova, L.; Corberan, V.C. Low-temperature CO oxidation on Ag/ZSM-5 catalysts: Influence of Si/Al ratio and redox pretreatments on formation of silver active sites. Fuel 2017, 188, 121–131. [Google Scholar] [CrossRef]
- Muddada, N.B.; Olsbye, U.; Fuglerud, T.; Vidotto, S.; Marsella, A.; Bordiga, S.; Gianolio, D.; Leofanti, G.; Lamberti, C. The role of chlorine and additives on the density and strength of Lewis and Brønsted acidic sites of γ-Al2O3 support used in oxychlorination catalysis: A FTIR study. J. Catal. 2011, 284, 236–246. [Google Scholar] [CrossRef]
- Ro, I.B.; Aragao, Z.J.; Brentzel, Y.; Liu, K.R.; Rivera-Dones, M.R.; Ball, D.; Zanchet, G.W.; Huber, J.A. Dumesic, Intrinsic Activity of Interfacial Sites for Pt-Fe and Pt-Mo Catalysts in the Hydrogenation of Carbonyl Groups. Appl. Catal. B 2018, 231, 182–190. [Google Scholar] [CrossRef]
- Matsuda, T.; Ohno, T.; Hiramatsu, Y.; Li, Z.; Sakagami, H.; Takahashi, N. Effects of the amount of MoO3 on the catalytic properties of H2-reduced Pt/MoO3–SiO2 for heptane isomerization. Appl. Catal. A 2009, 362, 40–46. [Google Scholar] [CrossRef]
- Pidko, E.A.; Hensen, E.J.M.; Santen, R.A.V. Dehydrogenation of Light Alkanes over Isolated Gallyl Ions in Ga/ZSM-5 Zeolites. J. Phys. Chem. C 2007, 111, 13068–13075. [Google Scholar] [CrossRef]
- Yoshida, H.; Narisawa, S.; Fujita, S.; Ruixia, L.; Arai, M. In situ FTIR study on the formation and adsorption of CO on alumina-supported noble metal catalysts from H2 and CO2 in the presence of water vapor at high pressures. Phys. Chem. Chem. Phys. 2012, 14, 4724–4733. [Google Scholar] [CrossRef] [PubMed]
- Brandt, R.K.; Sorbello, R.S.; Greenler, R.G. Site-specific, coupled-harmonic-oscillator model of carbon monoxide adsorbed on extended, single-crystal surfaces and on small crystals of platinum. Surf. Sci. 1992, 271, 605–615. [Google Scholar] [CrossRef]
- Ding, K.; Gulec, A.; Johnson, A.M.; Schweitzer, N.M.; Stucky, G.D.; Marks, L.D.; Stair, P.C. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 2015, 350, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Pattamakomsan, K.; Ehret, E.; Morfin, F.; Gélin, P.; Jugnet, Y.; Prakash, S.; Bertolini, J.C.; Panpranot, J.; Aires, F.J.C.S. Selective hydrogenation of 1,3-butadiene over Pd and Pd–Sn catalysts supported on different phases of alumina. Catal. Today 2011, 164, 28–33. [Google Scholar] [CrossRef]
- Busca, G. Spectroscopic characterization of the acid properties of metal oxide catalysts. Catal. Today 1998, 41, 191–206. [Google Scholar] [CrossRef]
- Daturi, M.; Appel, L.G. Infrared Spectroscopic Studies of Surface Properties of Mo/SnO2 Catalyst. J. Catal. 2002, 209, 427–432. [Google Scholar] [CrossRef]
- Seo, H.; Lee, J.K.; Hong, U.G.; Park, G.; Yoo, Y.; Lee, J.; Chang, H.; Song, I.K. Direct dehydrogenation of n-butane over Pt/Sn/M/γ-Al2O3 catalysts: Effect of third metal (M) addition. Catal. Commun. 2014, 47, 22–27. [Google Scholar] [CrossRef]
- Jaroszewska, K.; Masalska, A.; Marek, D.; Grzechowiak, J.R.; Zemska, A. Effect of support composition on the activity of Pt and PtMo catalysts in the conversion of n-hexadecane. Catal. Today 2014, 223, 76–86. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Huber, G.W.; Sánchez-Castillo, M.A.; Dumesic, J.A.; Rodríguez-Reinoso, F.; Sepúlveda-Escribano, A. Effect of Sn addition to Pt/CeO2–Al2O3 and Pt/Al2O3 catalysts: An XPS, 119Sn Mössbauer and microcalorimetry study. J. Catal. 2006, 241, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Gao, L.; Zheng, S. Investigation of the dispersion of MoO3 onto the support of mesoporous silica MCM-41. Appl. Catal. A 2002, 236, 163–171. [Google Scholar] [CrossRef]
- Choi, J.G.; Thompson, L.T. XPS study of as-prepared and reduced molybdenum oxides. Appl. Surf. Sci. 1996, 93, 143–149. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J.; King, R.C., Jr. Handbook of X-ray photoelectron spectroscopy: A reference book of standard spectra for identification and interpretation of XPS data. Chem. Phys. Lett. 1992, 220, 7–10. [Google Scholar]
- Nowak, J. Quartararo, Effect of H2-O2 pre-treatments on the state of gallium in Ga/H-ZSM-5 propane aromatisation catalysts. Appl. Catal. A 2003, 251, 107–120. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, D.; He, X.; Li, Y.; Zhu, X. Methylation of benzene with methanol over HZSM-11 and HZSM-5: A density functional theory study. J. Mol. Catal. A 2016, 424, 351–357. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Arzumanov, S.S.; Freude, D.; Stepanov, A.G. Propane Aromatization on Zn-Modified Zeolite BEA Studied by Solid-State NMR In Situ. J. Phys. Chem. C 2010, 114, 12681–12688. [Google Scholar] [CrossRef]
- Karp, E.M.; Silbaugh, T.L.; Campbell, C.T. Energetics of Adsorbed CH3 and CH on Pt(111) by Calorimetry: Dissociative Adsorption of CH3I. J. Phys. Chem. C 2013, 117, 6325–6336. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Al-Yassir, N.; Al-Khattaf, S.; Čejka, J. Aromatization of alkanes over Pt promoted conventional and mesoporous gallosilicates of MEL zeolite. Catal. Today 2012, 179, 61–72. [Google Scholar] [CrossRef]
- Martinez-Espin, J.S.; de Wispelaere, K.; Erichsen, M.W.; Svelle, S.; Janssens, T.V.W.; van Speybroeck, V.; Beato, P.; Olsbye, U. Benzene co-reaction with methanol and dimethyl ether over zeolite and zeotype catalysts: Evidence of parallel reaction paths to toluene and diphenylmethane. J. Catal. 2017, 349, 136–148. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, L.; Cao, Q.; Wen, Z.; Zhou, Z.; Xu, Y.; Zhu, X. Steamed Zn/ZSM-5 catalysts for improved methanol aromatization with high stability. Fuel Process. Technol. 2017, 162, 66–77. [Google Scholar] [CrossRef]
- Song, C.; Li, X.; Zhu, X.; Liu, S.; Chen, F.; Liu, F.; Xu, L. Influence of the state of Zn species over Zn-ZSM-5/ZSM-11 on the coupling effects of cofeeding n-butane with methanol. Appl. Catal. A 2016, 519, 48–55. [Google Scholar] [CrossRef]
- Baldovino-Medrano, V.G.; Giraldo, S.A.; Centeno, A. The functionalities of Pt–Mo catalysts in hydrotreatment reactions. Fuel 2010, 89, 1012–1018. [Google Scholar] [CrossRef]
- Yang, K.; Liu, C. Hydrogenation of isoprene over gold supported on CeO2, ZrO2, SiO2 and N–SiO2. J. Ind. Eng. Chem. 2015, 28, 161–170. [Google Scholar] [CrossRef]
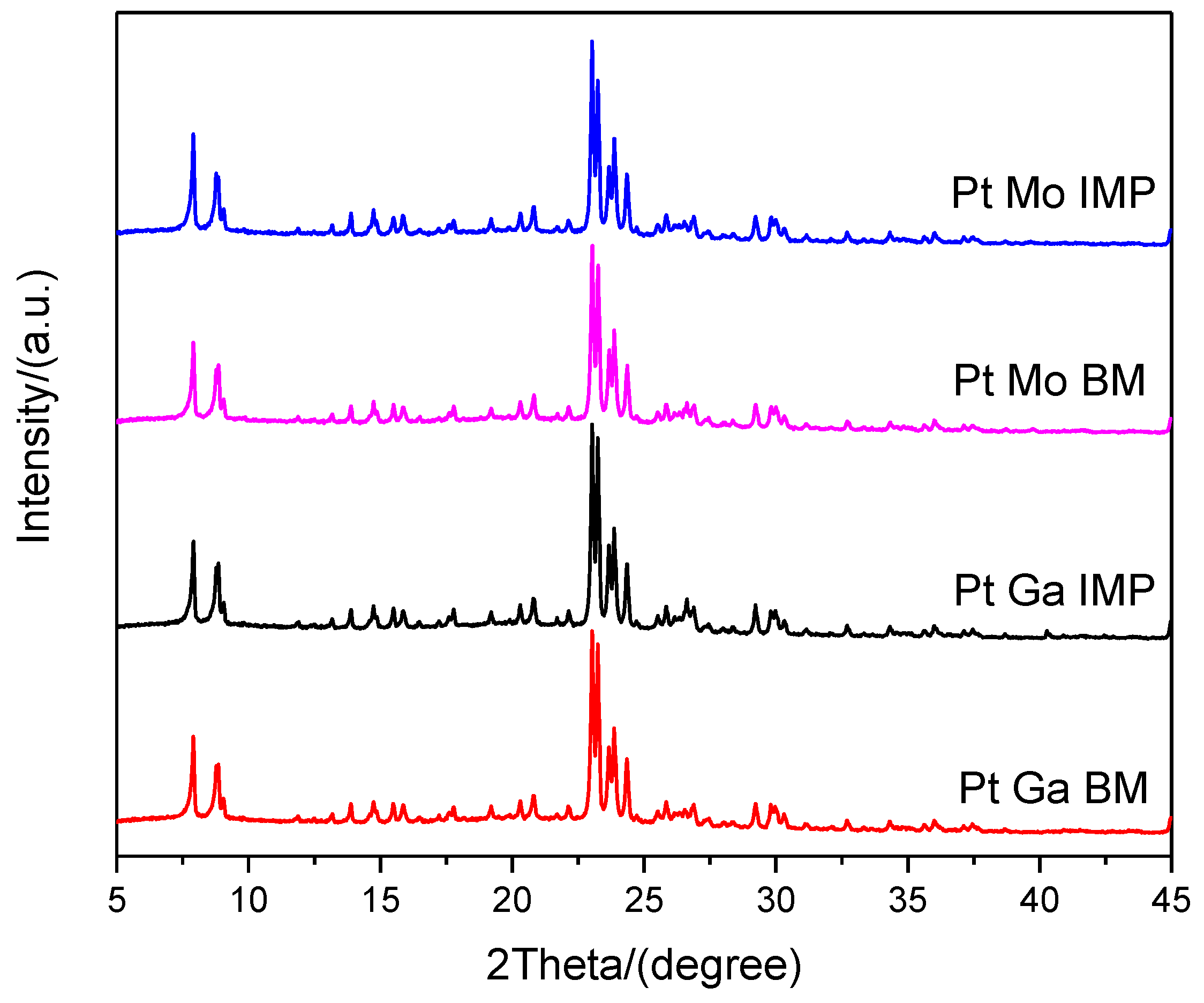
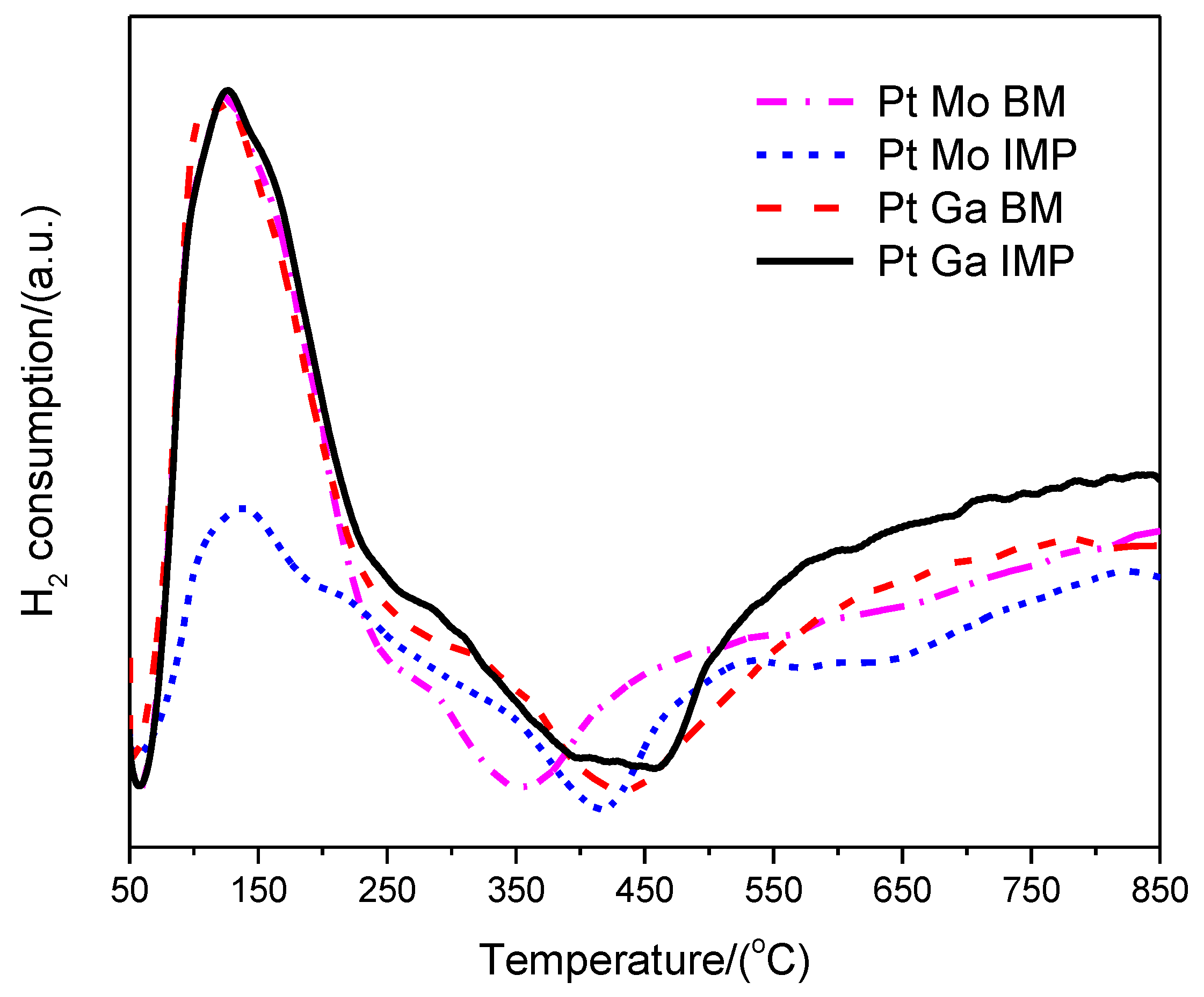
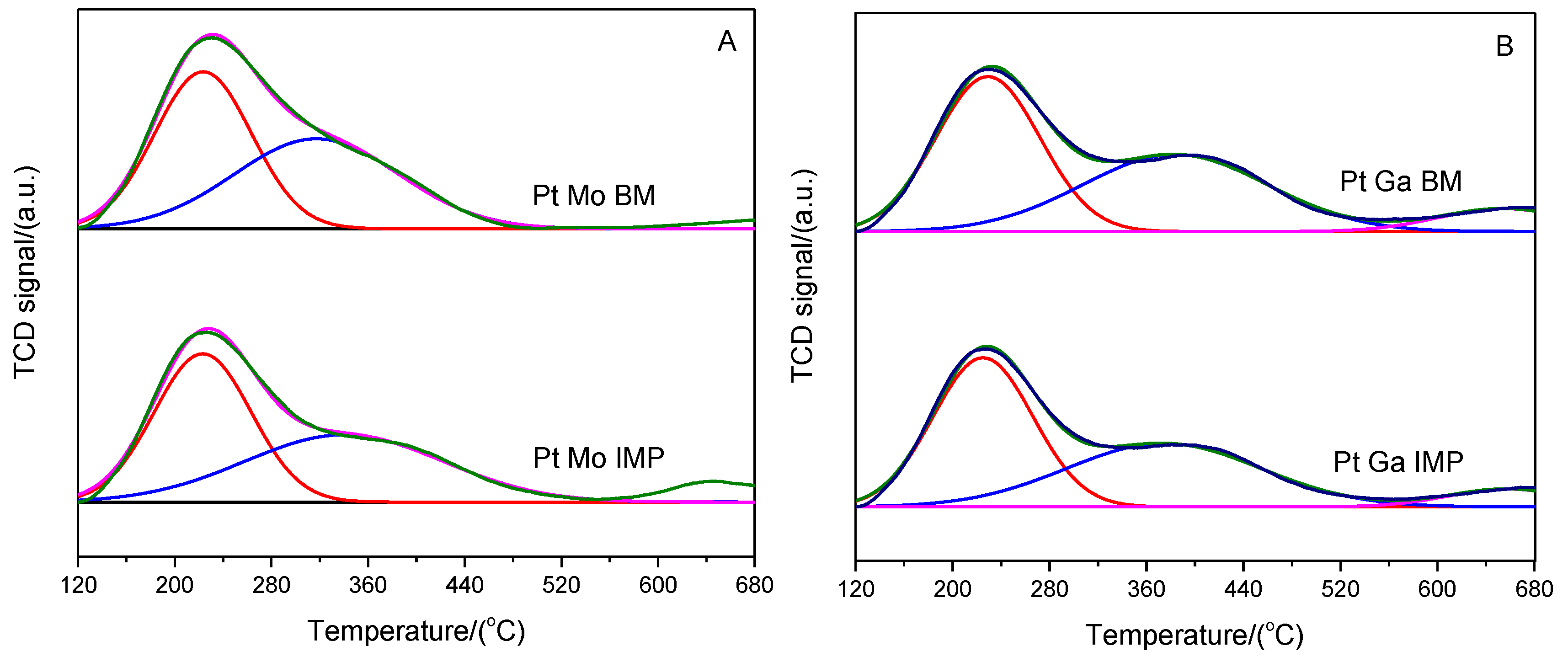
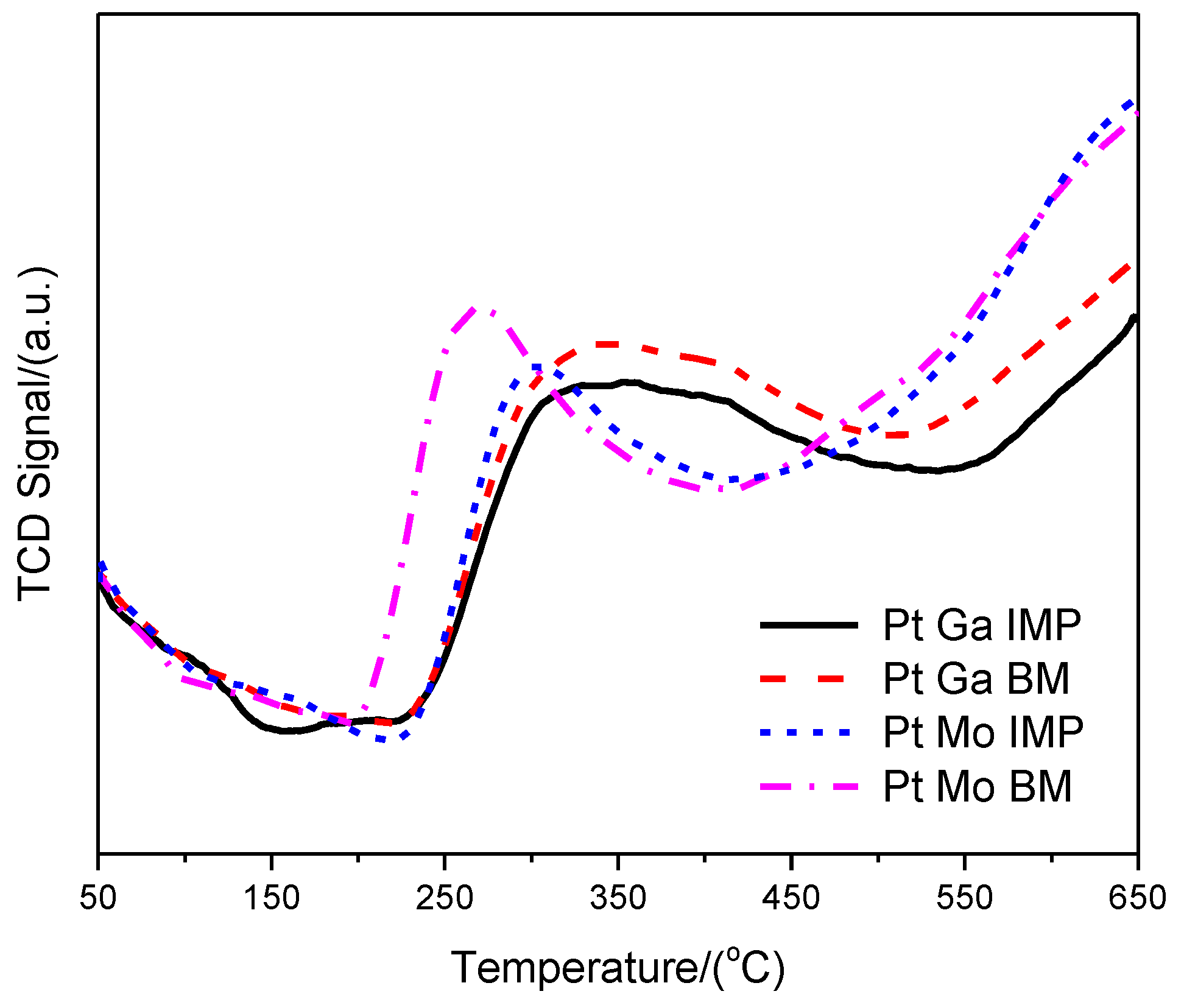

| Sample | XRF Analysis (wt.%) | SBET (m2·g−1) | t-Plot | ||||
|---|---|---|---|---|---|---|---|
| Pt | Sn | Mo/Ga | Smicro (m2·g−1) | Vmicro (cm3·g−1) | D (nm) | ||
| Pt Mo IMP | 0.45 | 0.93 | 1.44 | 333 | 246 | 0.093 | 2.7 |
| Pt Ga IMP | 0.46 | 0.92 | 1.46 | 324 | 242 | 0.092 | 2.8 |
| Pt Mo BM | 0.47 | 0.94 | 1.48 | 348 | 261 | 0.100 | 2.6 |
| Pt Ga BM | 0.46 | 0.93 | 1.45 | 341 | 252 | 0.095 | 2.7 |
| Catalyst | Pt Mo IMP | Pt Mo BM | Pt Ga IMP | Pt Ga BM |
|---|---|---|---|---|
| n-Butane conversion (%) | 62 | 69 | 58 | 51 |
| Hydrocarbons distribution of reactor effluent (wt.%) | ||||
| CH4 | 3.3 | 1.5 | 4 | 1.5 |
| C2H6 + C3H8 + i-C4H10 | 8.8 | 17.2 | 17 | 15 |
| C2H4 + C3H6 + C4H8 | 3.1 | 2.4 | 3 | 5.5 |
| n-C4H10 | 38 | 31 | 42 | 49 |
| CO + CO2 + H2 + C2H6O | 2.3 | 1.4 | 2 | 2.4 |
| C5 + aliphatics | 3.5 | 1.5 | 3 | 1.9 |
| Aromatics | 41 | 45 | 29 | 24.7 |
| Aromatics selectivity (wt.%) | ||||
| Benzene | 2.5 | 2 | 5.3 | 3.5 |
| Toluene | 12.3 | 17.9 | 21.5 | 18.7 |
| Xylenes + ethylbenzene | 50.8 | 52.8 | 50.2 | 54.2 |
| Cn≥9 aromatics | 34.4 | 27.3 | 23 | 23.6 |
| Catalyst | Pt Mo IMP | Pt Mo BM | Pt Ga IMP | Pt Ga BM | Zn/CDM5 * |
|---|---|---|---|---|---|
| Reaction temperature (°C) | 475 | 475 | 450 | 450 | 480 |
| n-Butane conversion (%) | 65 | 86 | 74.7 | 76 | 62.7 |
| Hydrocarbons distribution of reactor effluent (wt.%) | |||||
| CH4 | 4.8 | 1.3 | 4.1 | 4.2 | 2.89 |
| C2H6 + C3H8 + i-C4H10 | 12.6 | 16.5 | 26.3 | 12.2 | 23.3 |
| C2H4 + C3H6 + C4H8 | 1.1 | 3.5 | 5 | 2 | 7.33 |
| n-C4H10 | 35 | 14 | 25.3 | 24 | 37.3 |
| CO + CO2 + H2 + C2H6O | 1.2 | 1.0 | 2 | 1.6 | 1.94 |
| C5 + aliphatics | 1.3 | 0.7 | 3.3 | 2 | 1.57 |
| Aromatics | 38 | 59 | 26 | 48 | 25.67 |
| Aromatics selectivity in the liquid product (wt.%) | |||||
| Benzene | 2.8 | 5.3 | 6.6 | 2.0 | 7.7 |
| Toluene | 21.2 | 15.7 | 26.4 | 14.0 | 37.6 |
| Xylenes + ethylbenzene | 52.7 | 60.0 | 49.0 | 47.0 | 38.0 |
| Cn≥9 aromatics | 23.3 | 19 | 18 | 37.0 | 16.7 |
| Coke (mg/g.cat.) | 101 | 80 | 120 | 100 | 160 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Zhu, L.; Zhang, J.; Huo, X.; Lai, W.; Lian, Y.; Fang, W. Co-Aromatization of n-Butane and Methanol over PtSnK-Mo/ZSM-5 Zeolite Catalysts: The Promotion Effect of Ball-Milling. Catalysts 2018, 8, 307. https://doi.org/10.3390/catal8080307
Yang K, Zhu L, Zhang J, Huo X, Lai W, Lian Y, Fang W. Co-Aromatization of n-Butane and Methanol over PtSnK-Mo/ZSM-5 Zeolite Catalysts: The Promotion Effect of Ball-Milling. Catalysts. 2018; 8(8):307. https://doi.org/10.3390/catal8080307
Chicago/Turabian StyleYang, Kang, Lingting Zhu, Jie Zhang, Xiuchun Huo, Weikun Lai, Yixin Lian, and Weiping Fang. 2018. "Co-Aromatization of n-Butane and Methanol over PtSnK-Mo/ZSM-5 Zeolite Catalysts: The Promotion Effect of Ball-Milling" Catalysts 8, no. 8: 307. https://doi.org/10.3390/catal8080307





