Three-Dimensional Heteroatom-Doped Nanocarbon for Metal-Free Oxygen Reduction Electrocatalysis: A Review
Abstract
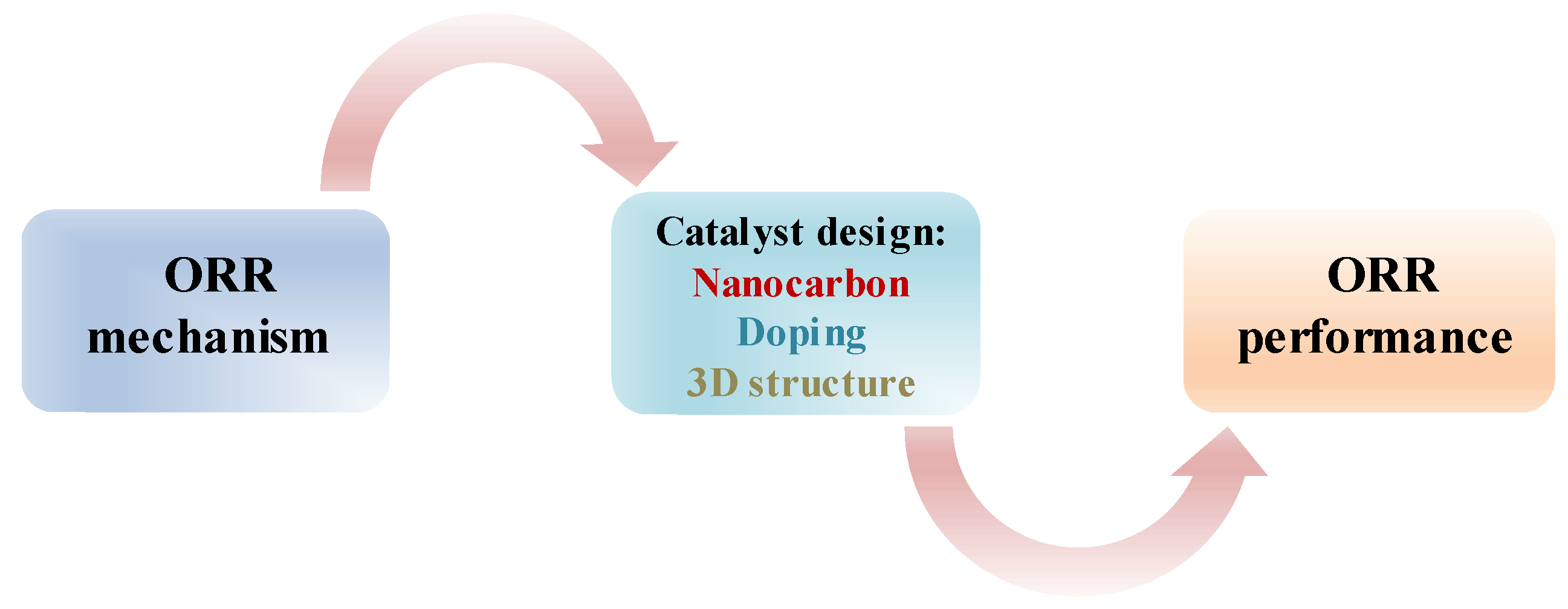
:1. Introduction
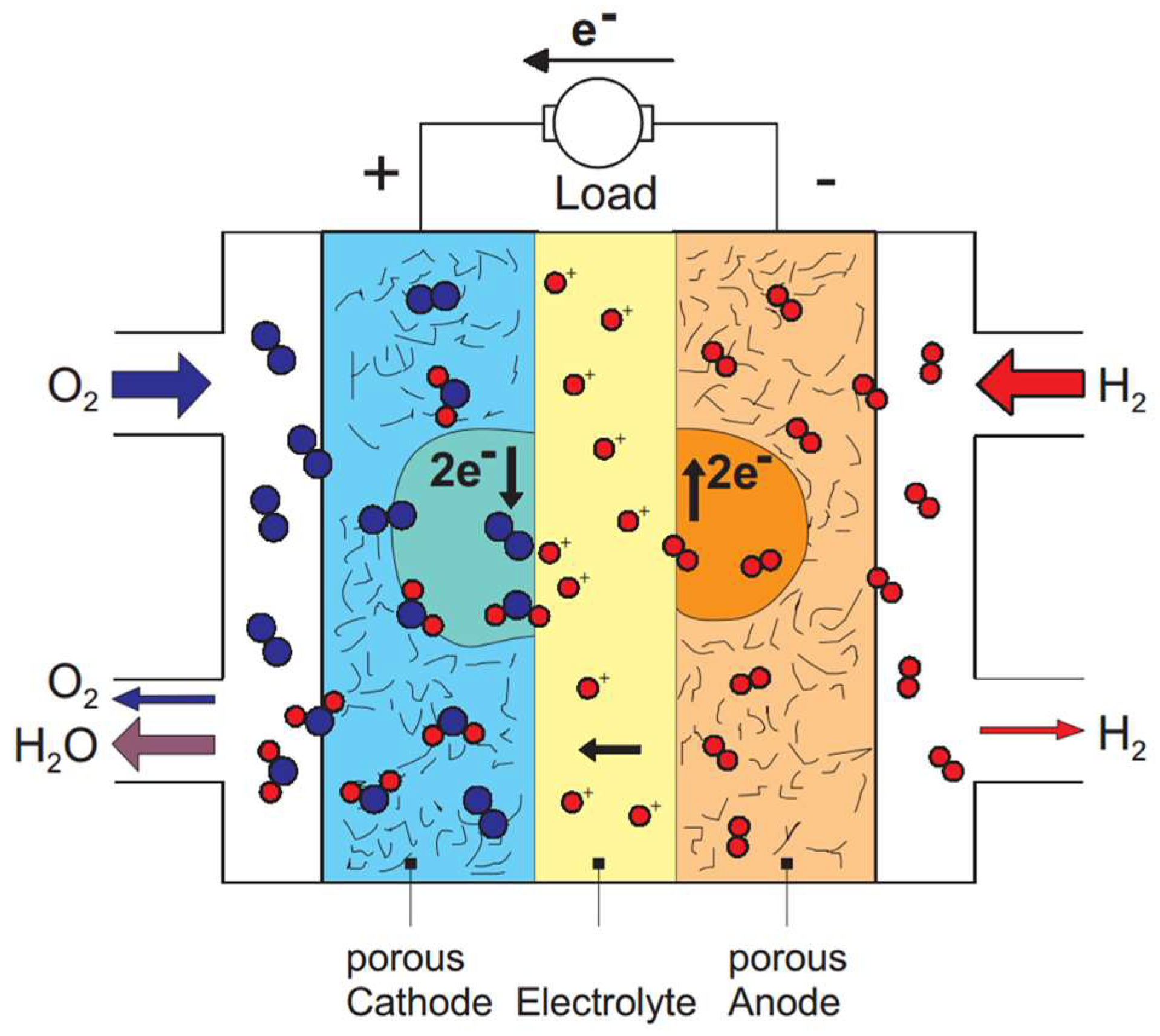
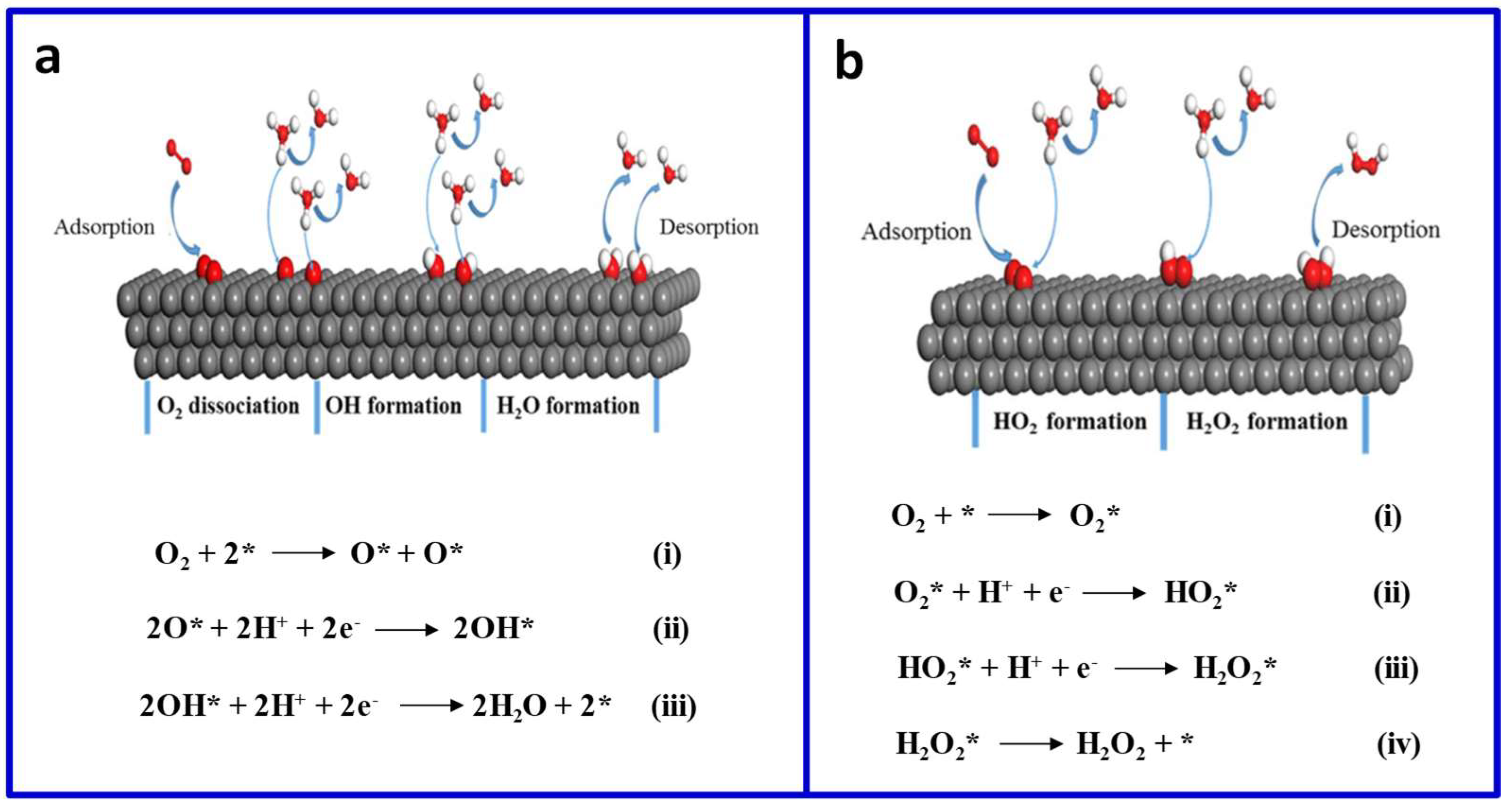
2. The Mechanisms for ORR
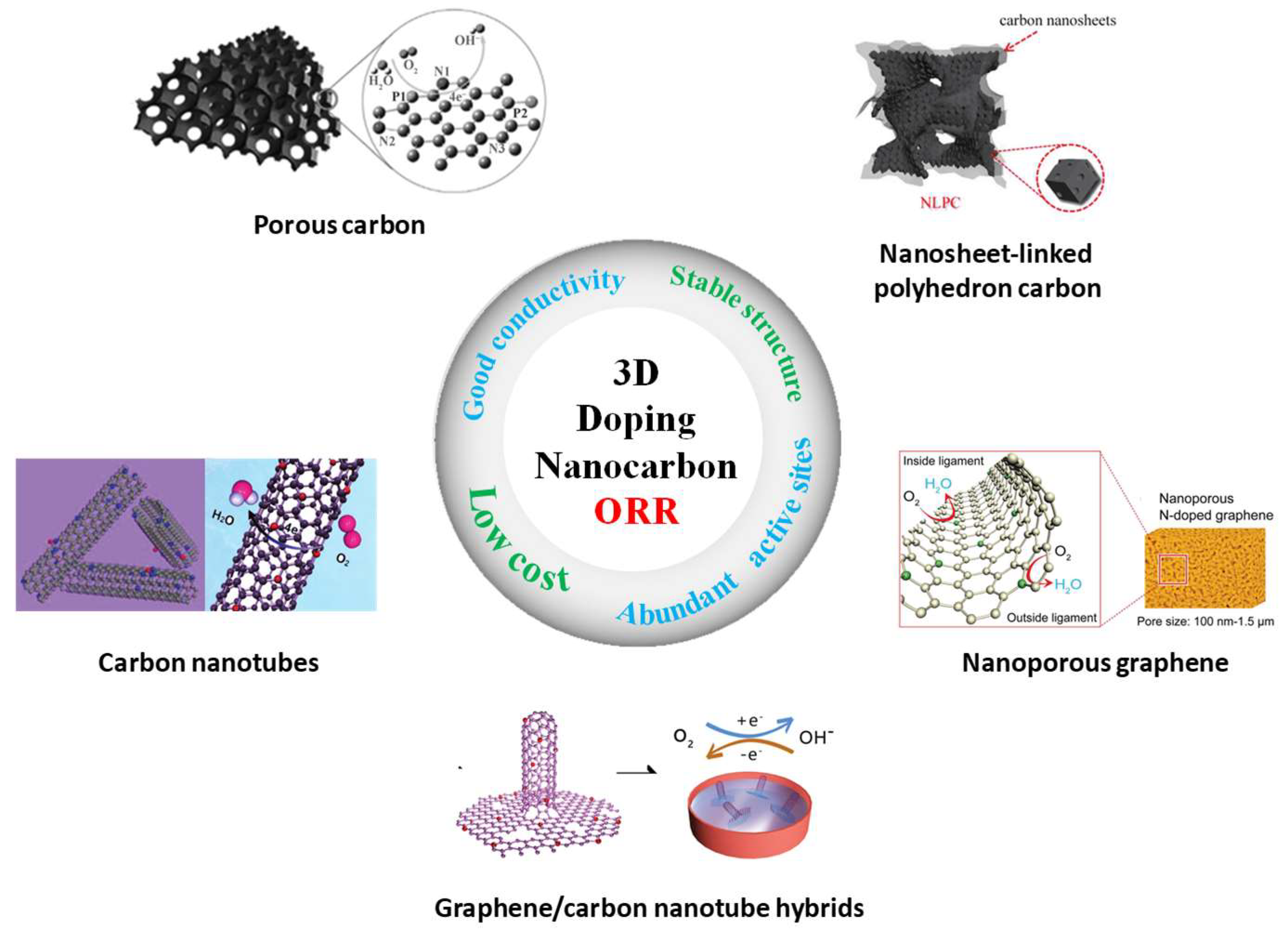
3. 3D Heteroatom-Doped Nanocarbon Electrocatalysts for ORR
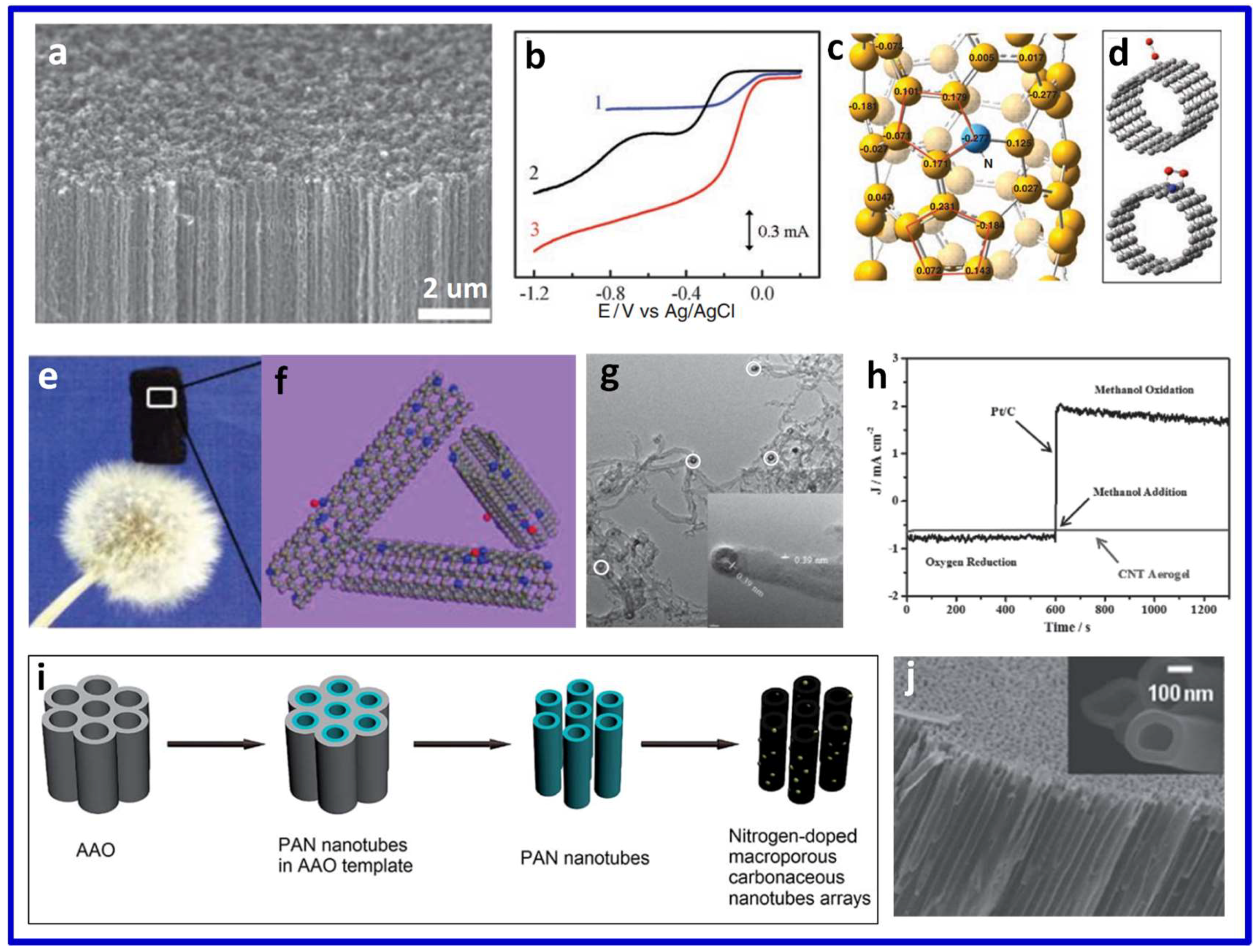
3.1. Heteroatom-Doped 3D CNTs for ORR
3.1.1. Single Heteroatom-Doped 3D CNTs
3.1.2. Multiple Heteroatom-Co-Doped 3D CNTs
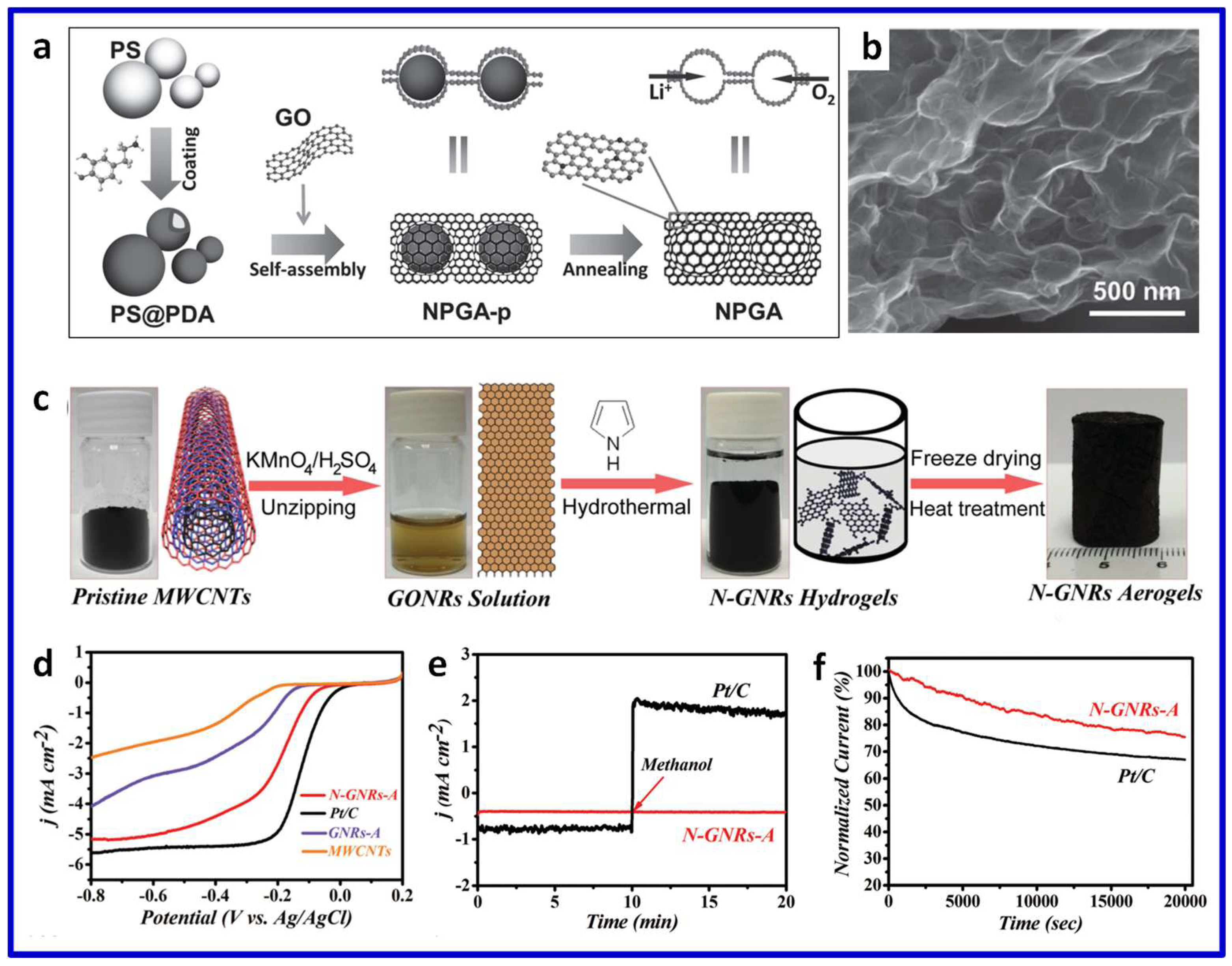
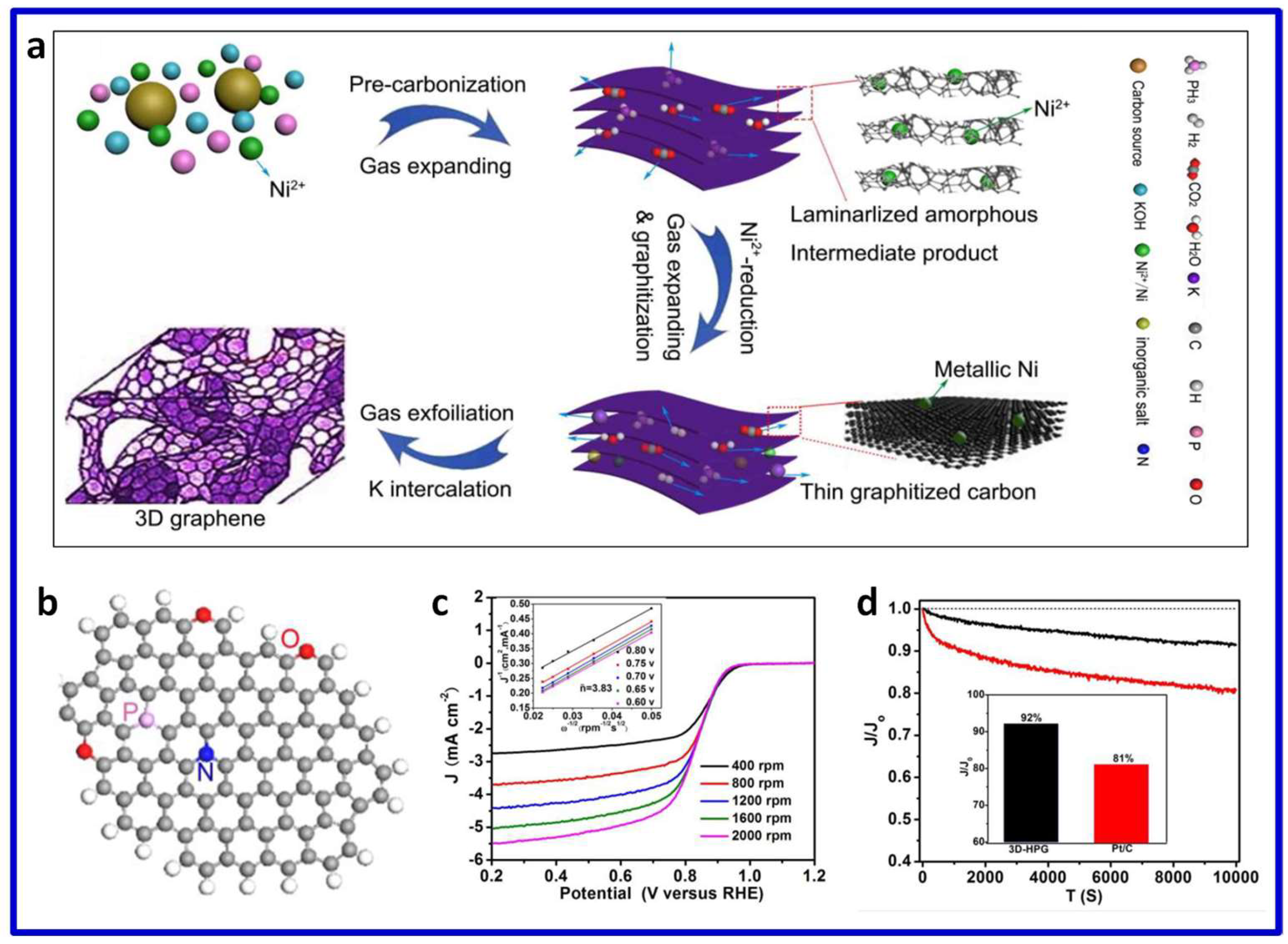
3.2. Heteroatom-Doped 3D Graphene for ORR
3.2.1. Single Heteroatom-Doped 3D Graphene
3.2.2. Multiple Heteroatom-Co-Doped 3D Graphene
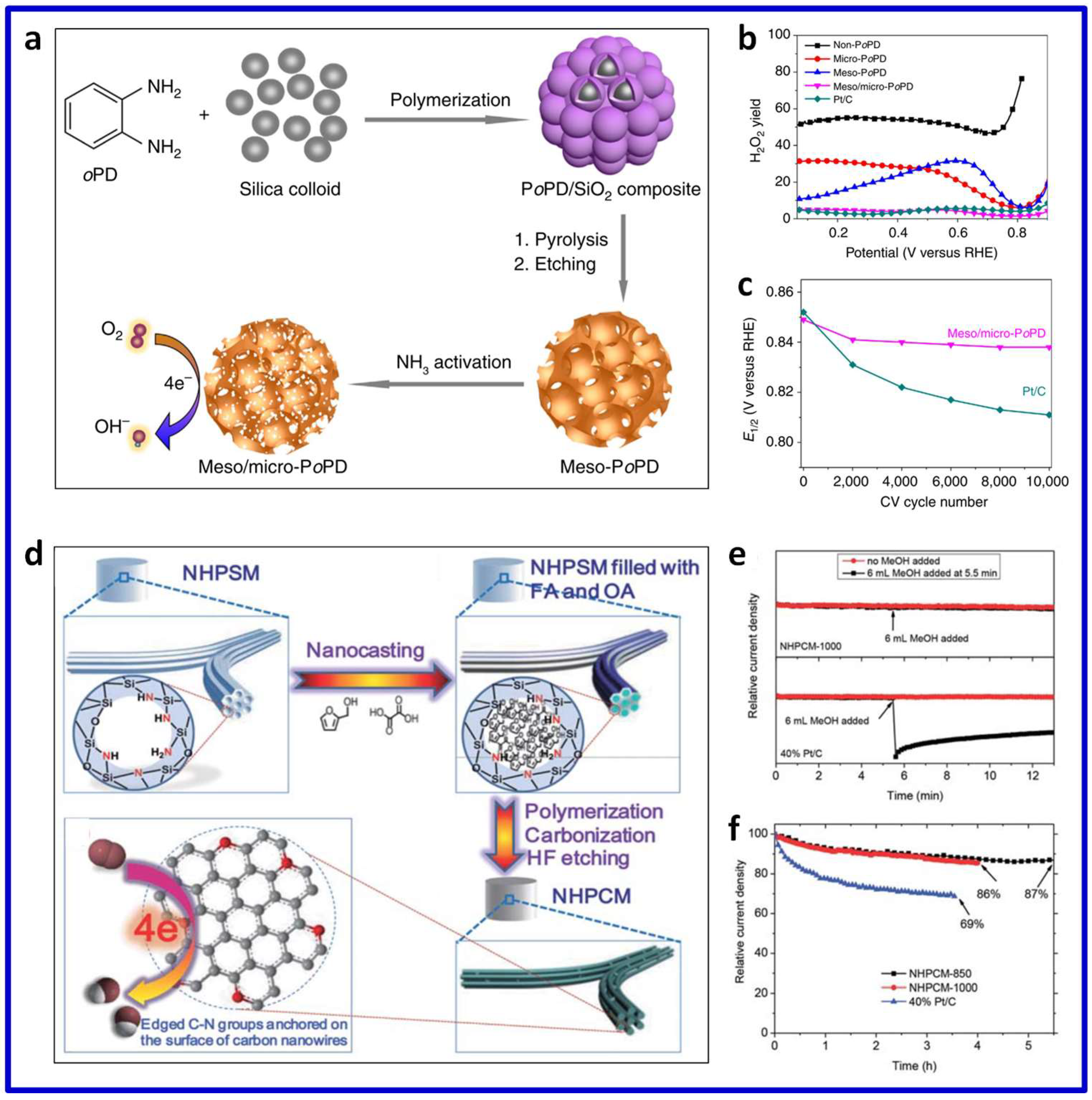
3.3. Heteroatom-Doped 3D Porous Carbon for ORR
3.3.1. Single Heteroatom-Doped 3D Porous Carbon
3.3.2. Multiple Heteroatom-Co-Doped 3D Porous Carbon
3.4. Nanocarbon Hybrid Materials for ORR
3.5. Other Kinds of Nanocarbon Materials for ORR
4. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Tanaka, S.; Ito, S.; Tetreault, N.; Manabe, K.; Nishino, H.; Nazeeruddin, M.K.; Gratzel, M. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 2014, 5, 3834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent Advances in Layered Ti3C2Tx MXene for Electrochemical Energy Storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-Performance Anode Materials for Rechargeable Lithium-Ion Batteries. Electrochem. Energy Rev. 2018, 1, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.L.; Dou, S.X. Next-Generation Batteries. Adv. Mater. 2017, 29, 1705871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodenough, J.B. Electrochemical energy storage in a sustainable modern society. Energy Environ. Sci. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal-air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Hou, P.X.; Liu, C. Heteroatom-Doped Carbon Nanotube and Graphene-Based Electrocatalysts for Oxygen Reduction Reaction. Small 2017, 13, 1702002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myles, T.; Bonville, L.; Maric, R. Catalyst, Membrane, Free Electrolyte Challenges, and Pathways to Resolutions in High Temperature Polymer Electrolyte Membrane Fuel Cells. Catalysts 2017, 7, 16. [Google Scholar] [CrossRef]
- Suthirakun, S.; Ammal, S.C.; Munoz-Garcia, A.B.; Xiao, G.; Chen, F.; zur Loye, H.C.; Carter, E.A.; Heyden, A. Theoretical investigation of H2 oxidation on the Sr2Fe1.5Mo0.5O6 (001) perovskite surface under anodic solid oxide fuel cell conditions. J. Am. Chem. Soc. 2014, 136, 8374–8386. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhu, C.; Song, J.; Du, D.; Lin, Y. Metal-Organic Framework-Derived Non-Precious Metal Nanocatalysts for Oxygen Reduction Reaction. Adv. Energy Mater. 2017, 7, 1700363. [Google Scholar] [CrossRef]
- Marcel, R. Perovskite Electrocatalysts for the Oxygen Reduction Reaction in Alkaline Media. Catalysts 2017, 7, 154. [Google Scholar] [CrossRef]
- Li, Q.; Cao, R.; Cho, J.; Wu, G. Nanocarbon Electrocatalysts for Oxygen Reduction in Alkaline Media for Advanced Energy Conversion and Storage. Adv. Energy Mater. 2014, 4, 1301415. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Norskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Banis, M.N.; Liu, J.; Riese, A.; Li, X.; Li, R.; Ye, S.; Knights, S.; Sun, X. Extremely stable platinum nanoparticles encapsulated in a zirconia nanocage by area-selective atomic layer deposition for the oxygen reduction reaction. Adv. Mater. 2015, 27, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, D.; Zhu, H.; Zhang, S.; Markovic, N.M.; Stamenkovic, V.R.; Sun, S. FePt and CoPt nanowires as efficient catalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 2013, 125, 3449–3552. [Google Scholar]
- Tan, Y.; Xu, C.; Chen, G.; Zheng, N.; Xie, Q. A graphene–platinum nanoparticles–ionic liquid composite catalyst for methanol-tolerant oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 6923–6927. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, X.; Evans, D.G.; Yang, W. Well-dispersed Co3O4/Co2MnO4 nanocomposites as a synergistic bifunctional catalyst for oxygen reduction and oxygen evolution reactions. Nanoscale 2013, 5, 5312–5315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Lai, Y.J.; Song, L.; Zhou, Z.Y.; Liu, J.G.; Wang, Q.; Yang, X.D.; Chen, C.; Shi, W.; Zheng, Y.P.; et al. S-Doping of an Fe/N/C ORR Catalyst for Polymer Electrolyte Membrane Fuel Cells with High Power Density. Angew. Chem. Int. Ed. Engl. 2015, 54, 9907–9910. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.L.; Guo, S. Towards high-efficiency nanoelectrocatalysts for oxygen reduction through engineering advanced carbon nanomaterials. Chem. Soc. Rev. 2016, 45, 1273–1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Fang, B.; Zhang, D.; Li, A.; Wilkinson, D.P.; Ignaszak, A.; Zhang, L.; Zhang, J. A Review of Carbon-Composited Materials as Air-Electrode Bifunctional Electrocatalysts for Metal–Air Batteries. Electrochem. Energy Rev. 2018, 1, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Dai, L.; Li, L.S. Nitrogen-doped colloidal graphene quantum dots and their size-dependent electrocatalytic activity for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 18932–18935. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M.; Wong, C.-P. Facile Synthesis of Nitrogen-Doped Graphene via Pyrolysis of Graphene Oxide and Urea, and its Electrocatalytic Activity toward the Oxygen-Reduction Reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Dai, L. Carbon-based catalysts for metal-free electrocatalysis. Curr. Opin. Electrochem. 2017, 4, 18–25. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Jin, Y.; Qiao, S.Z. Nanostructured metal-free electrochemical catalysts for highly efficient oxygen reduction. Small 2012, 8, 3550–3566. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Guo, C.; Zheng, Y.; Qiao, S.Z. Surface and Interface Engineering of Noble-Metal-Free Electrocatalysts for Efficient Energy Conversion Processes. Acc. Chem. Res. 2017, 50, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, L. Heteroatom-Doped Graphitic Carbon Catalysts for Efficient Electrocatalysis of Oxygen Reduction Reaction. ACS Catal. 2015, 5, 7244–7253. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q. Nanocarbon for Oxygen Reduction Electrocatalysis: Dopants, Edges, and Defects. Adv. Mater. 2017, 29, 1604103. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Y.; Jiang, C.; Lu, L. Structural effects of a carbon matrix in non-precious metal O2-reduction electrocatalysts. Chem. Soc. Rev. 2016, 45, 2396–2409. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Deng, D.; Bao, X. Nanocarbons and their hybrids as catalysts for non-aqueous lithium–oxygen batteries. J. Energy Chem. 2016, 25, 957–966. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Han, T.H.; Cho, M.H. Metal-Free Carbon-Based Materials: Promising Electrocatalysts for Oxygen Reduction Reaction in Microbial Fuel Cells. Int. J. Mol. Sci. 2016, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Zamani, P.; Yu, A.; Chen, Z. The application of graphene and its composites in oxygen reduction electrocatalysis: A perspective and review of recent progress. Energy Environ. Sci. 2016, 9, 357–390. [Google Scholar] [CrossRef]
- Du, R.; Zhang, N.; Zhu, J.; Wang, Y.; Xu, C.; Hu, Y.; Mao, N.; Xu, H.; Duan, W.; Zhuang, L.; et al. Nitrogen-Doped Carbon Nanotube Aerogels for High-Performance ORR Catalysts. Small 2015, 11, 3903–3908. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhou, X.; Xu, N.; Bai, Z.; Qiao, J.; Zhang, J. Template-free synthesis of three-dimensional nanoporous N-doped graphene for high performance fuel cell oxygen reduction reaction in alkaline media. Appl. Energy 2016, 175, 405–413. [Google Scholar] [CrossRef]
- Jiang, H.; Zhu, Y.; Feng, Q.; Su, Y.; Yang, X.; Li, C. Nitrogen and phosphorus dual-doped hierarchical porous carbon foams as efficient metal-free electrocatalysts for oxygen reduction reactions. Chem. Eur. J. 2014, 20, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nie, H.; Chen, X.A.; Chen, X.; Huang, S. Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction. J. Power Sources 2013, 236, 238–249. [Google Scholar] [CrossRef]
- Liu, J.; Song, P.; Ning, Z.; Xu, W. Recent Advances in Heteroatom-Doped Metal-Free Electrocatalysts for Highly Efficient Oxygen Reduction Reaction. Electrocatalysis 2015, 6, 132–147. [Google Scholar] [CrossRef]
- Ma, R.; Ma, Y.; Dong, Y.; Lee, J.-M. Recent Advances in Heteroatom-Doped Graphene Materials as Efficient Electrocatalysts towards the Oxygen Reduction Reaction. Nano Adv. 2016, 1, 50–61. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Z. Mechanisms of Oxygen Reduction Reaction on Nitrogen-Doped Graphene for Fuel Cells. J. Phys. Chem. C 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel Cells–Fundamentals and Applications. Fuel Cells 2001, 1, 5–39. [Google Scholar] [CrossRef]
- Liew, K.B.; Daud, W.R.W.; Ghasemi, M.; Leong, J.X.; Lim, S.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrog. Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Gu, W.; Hu, L.; Li, J.; Wang, E. Recent Advancements in Transition Metal-Nitrogen-Carbon Catalysts for Oxygen Reduction Reaction. Electroanalysis 2018. [Google Scholar] [CrossRef]
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef]
- Shui, J.; Wang, M.; Du, F.; Dai, L. N-doped carbon nanomaterials are durable catalysts for oxygen reduction reaction in acidic fuel cells. Sci. Adv. 2015, 1, 1400129. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Li, X.; Shan, H.; Yan, B.; Dong, L.; Cao, Y.; Li, D. Controllable oxygenic functional groups of metal-free cathodes for high performance lithium ion batteries. J. Mater. Chem. A 2015, 3, 11376–11386. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Shan, H.; Yan, B.; Li, D.; Langford, C.; Sun, X. Scalable synthesis of functionalized graphene as cathodes in Li-ion electrochemical energy storage devices. Appl. Energy 2016, 175, 512–521. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Zhang, H.-T.; Sun, X.-Z.; Ma, Y.-W. Three dimensional graphene networks for supercapacitor electrode materials. New Carbon Mater. 2015, 30, 193–206. [Google Scholar] [CrossRef]
- Lin, Z.; Zeng, Z.; Gui, X.; Tang, Z.; Zou, M.; Cao, A. Carbon Nanotube Sponges, Aerogels, and Hierarchical Composites: Synthesis, Properties, and Energy Applications. Adv. Energy Mater. 2016, 6, 1600554. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Torad, N.L.; Kimura, T.; Yamauchi, Y. Tailored design of functional nanoporous carbon materials toward fuel cell applications. Nano Today 2014, 9, 305–323. [Google Scholar] [CrossRef]
- Daems, N.; Sheng, X.; Vankelecom, F.J.; Pescarmona, P.P. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4085–4110. [Google Scholar] [CrossRef]
- Ito, Y.; Qiu, H.J.; Fujita, T.; Tanabe, Y.; Tanigaki, K.; Chen, M. Bicontinuous nanoporous N-doped graphene for the oxygen reduction reaction. Adv. Mater. 2014, 26, 4145–4150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, L.; Xiao, Z.; Chen, R.; Jiang, Z.; Wang, S. 3D Carbon Electrocatalysts In Situ Constructed by Defect-Rich Nanosheets and Polyhedrons from NaCl-Sealed Zeolitic Imidazolate Frameworks. Adv. Funct. Mater. 2018, 28, 1705356. [Google Scholar] [CrossRef]
- Tian, G.L.; Zhao, M.Q.; Yu, D.; Kong, X.Y.; Huang, J.Q.; Zhang, Q.; Wei, F. Nitrogen-doped graphene/carbon nanotube hybrids: In situ formation on bifunctional catalysts and their superior electrocatalytic activity for oxygen evolution/reduction reaction. Small 2014, 10, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Xiao, X.; Peng, X.; Jin, H.; Li, T.; Zhang, C.; Gao, B.; Hu, B.; Huo, K.; Zhou, J. Freestanding mesoporous VN/CNT hybrid electrodes for flexible all-solid-state supercapacitors. Adv. Mater. 2013, 25, 5091–5097. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 2011, 50, 7132–7135. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xue, Y.; Dai, L. Vertically Aligned Carbon Nanotube Arrays Co-doped with Phosphorus and Nitrogen as Efficient Metal-Free Electrocatalysts for Oxygen Reduction. J. Phys. Chem. Lett. 2012, 3, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, Q.; Dai, L. Highly efficient metal-free growth of nitrogen-doped single-walled carbon nanotubes on plasma-etched substrates for oxygen reduction. J. Am. Chem. Soc. 2010, 132, 15127–15129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jia, R.; Zheng, J.; Zhao, J.; Li, L.; Song, J.; Zhu, Z. Nitrogen-promoted self-assembly of N-doped carbon nanotubes and their intrinsic catalysis for oxygen reduction in fuel cells. ACS Nano 2011, 5, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Liu, H.; Ren, G.; Li, Y.; Lu, X.; Zhu, Y. Metal-free porous nitrogen-doped carbon nanotubes for enhanced oxygen reduction and evolution reactions. Sci. Bull. 2016, 61, 889–896. [Google Scholar] [CrossRef]
- She, X.; Yang, D.; Jing, D.; Yuan, F.; Yang, W.; Guo, L.; Che, Y. Nitrogen-doped one-dimensional (1D) macroporous carbonaceous nanotube arrays and their application in electrocatalytic oxygen reduction reactions. Nanoscale 2014, 6, 11057–11061. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Du, F.; Liu, Y.; Perez, A., Jr.; Supp, M.; Ramakrishnan, T.S.; Dai, L.; Jiang, L. 3-D carbon nanotube structures used as high performance catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2010, 132, 15839–15841. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Benipal, N.; Chadderdon, D.J.; Huo, J.; Jiang, Y.; Qiu, Y.; Han, X.; Hu, Y.H.; Shanks, B.H.; Li, W. Carbon nanotubes as catalysts for direct carbohydrazide fuel cells. Carbon 2015, 89, 142–147. [Google Scholar] [CrossRef]
- Guo, M.-Q.; Huang, J.-Q.; Kong, X.-Y.; Peng, H.-J.; Shui, H.; Qian, F.-Y.; Zhu, L.; Zhu, W.-C.; Zhang, Q. Hydrothermal synthesis of porous phosphorus-doped carbon nanotubes and their use in the oxygen reduction reaction and lithium-sulfur batteries. New Carbon Mater. 2016, 31, 352–362. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, F.; Wang, H.; Yu, H.; Tan, J.; Zhu, L. Novel phosphorus-doped multiwalled nanotubes with high electrocatalytic activity for O2 reduction in alkaline medium. Catal. Commun. 2011, 16, 35–38. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, S.P.; Wang, R.; Shi, K.; Shen, P.K. One-pot synthesis of a nitrogen and phosphorus-dual-doped carbon nanotube array as a highly effective electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 15448–15453. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. 2D materials. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yin, Z.; Zhang, H. Three-dimensional graphene materials: Preparation, structures and application in supercapacitors. Energy Environ. Sci. 2014, 7, 1850–1865. [Google Scholar] [CrossRef]
- Hu, C.; Liu, D.; Xiao, Y.; Dai, L. Functionalization of graphene materials by heteroatom-doping for energy conversion and storage. Prog. Nat. Sci. Mater. 2018. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Shan, H.; Fan, L.; Wu, C.; Li, D.; Lu, S. Superior Cathode Performance of Nitrogen-Doped Graphene Frameworks for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 10643–10651. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Li, X.; Cui, Y.; Xiong, D.; Yan, B.; Li, D.; Lushington, A.; Sun, X. Sulfur/Nitrogen Dual-doped Porous Graphene Aerogels Enhancing Anode Performance of Lithium Ion Batteries. Electrochim. Acta 2016, 205, 188–197. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2011, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.; Sanetuntikul, J.; Shanmugam, S. Boron and phosphorous-doped graphene as a metal-free electrocatalyst for the oxygen reduction reaction in alkaline medium. RSC Adv. 2015, 5, 53637–53643. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Zhuang, X.; Li, S.; Wu, D.; Zhang, F.; Feng, X. Low-temperature synthesis of nitrogen/sulfur co-doped three-dimensional graphene frameworks as efficient metal-free electrocatalyst for oxygen reduction reaction. Carbon 2013, 62, 296–301. [Google Scholar] [CrossRef]
- Xue, Y.; Yu, D.; Dai, L.; Wang, R.; Li, D.; Roy, A.; Lu, F.; Chen, H.; Liu, Y.; Qu, J. Three-dimensional B,N-doped graphene foam as a metal-free catalyst for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2013, 15, 12220–12226. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Du, R.; Zhu, J.; Mao, Y.; Xue, C.; Zhang, N.; Hou, Y.; Zhang, J.; Yi, T. Three-dimensional nitrogen-doped graphene nanoribbons aerogel as a highly efficient catalyst for the oxygen reduction reaction. Small 2015, 11, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Dou, Z.; Yang, Y.; Xue, J.; Zhu, Z.; Cui, L. Fabrication of functionalized 3D graphene with controllable micro/meso-pores as a superior electrocatalyst for enhanced oxygen reduction in both acidic and alkaline solutions. RSC Adv. 2016, 6, 79459–79469. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.B.; Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Xiong, D.; Li, X.; Sun, Y.; Yan, B.; Li, D.; Lawes, S.; Cui, Y.; Sun, X. Tailored lithium storage performance of graphene aerogel anodes with controlled surface defects for lithium-ion batteries. Appl. Surf. Sci. 2016, 364, 651–659. [Google Scholar] [CrossRef]
- Kabir, S.; Artyushkova, K.; Serov, A.; Atanassov, P. Role of Nitrogen Moieties in N-Doped 3D-Graphene Nanosheets for Oxygen Electroreduction in Acidic and Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 11623–11632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yu, C.; Liu, S.; Yang, J.; Fan, X.; Huang, H.; Qiu, J. 3D Porous N-Doped Graphene Frameworks Made of Interconnected Nanocages for Ultrahigh-Rate and Long-Life Li–O2 Batteries. Adv. Funct. Mater. 2015, 25, 6913–6920. [Google Scholar] [CrossRef]
- Shi, J.-L.; Tang, C.; Huang, J.-Q.; Zhu, W.; Zhang, Q. Effective exposure of nitrogen heteroatoms in 3D porous graphene framework for oxygen reduction reaction and lithium–sulfur batteries. J. Energy Chem. 2018, 27, 167–175. [Google Scholar] [CrossRef]
- Lu, X.; Li, Z.; Yin, X.; Wang, S.; Liu, Y.; Wang, Y. Controllable synthesis of three-dimensional nitrogen-doped graphene as a high performance electrocatalyst for oxygen reduction reaction. Int. J. Hydrog. Energy 2017, 42, 17504–17513. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Xu, X.; Yang, Z.; Liu, Z.; Zhang, L.; Xu, X.; Chen, Y.; Huang, S. Sulfur-doped porous reduced graphene oxide hollow nanosphere frameworks as metal-free electrocatalysts for oxygen reduction reaction and as supercapacitor electrode materials. Nanoscale 2014, 6, 13740–13747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chu, M.; Yang, L.; Deng, W.; Tan, Y.; Ma, M.; Xie, Q. Synthesis and oxygen reduction properties of three-dimensional sulfur-doped graphene networks. Chem. Commun. 2014, 50, 6382–6385. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiu, Y.; Hu, P.A. Three Dimensional P-doped Graphene Synthesized by Eco-Friendly Chemical Vapor Deposition for Oxygen Reduction Reactions. J. Nanosci. Nanotechnol. 2016, 16, 6216–6222. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed. Engl. 2012, 51, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Su, Y.; Liu, D.; He, X. Three-dimensional N,B-doped graphene aerogel as a synergistically enhanced metal-free catalyst for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2015, 17, 25440–25448. [Google Scholar] [CrossRef] [PubMed]
- Amiinu, I.S.; Zhang, J.; Kou, Z.; Liu, X.; Asare, O.K.; Zhou, H.; Cheng, K.; Zhang, H.; Mai, L.; Pan, M.; et al. Self-Organized 3D Porous Graphene Dual-Doped with Biomass-Sponsored Nitrogen and Sulfur for Oxygen Reduction and Evolution. ACS Appl. Mater. Interfaces 2016, 8, 29408–29418. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.M.; Fajardo, S.; Arévalo, M.D.C.; García, G.; Pastor, E. S- and N-Doped Graphene Nanomaterials for the Oxygen Reduction Reaction. Catalysts 2017, 7, 278. [Google Scholar] [CrossRef]
- Chabu, J.M.; Wang, L.; Tang, F.-Y.; Zeng, K.; Sheng, J.; Walle, M.D.; Deng, L.; Liu, Y.-N. Synthesis of Three-Dimensional Nitrogen and Sulfur Dual-Doped Graphene Aerogels as an Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. ChemElectroChem 2017, 4, 1885–1890. [Google Scholar] [CrossRef]
- Wu, M.; Dou, Z.; Chang, J.; Cui, L. Nitrogen and sulfur co-doped graphene aerogels as an efficient metal-free catalyst for oxygen reduction reaction in an alkaline solution. RSC Adv. 2016, 6, 22781–22790. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Huang, J.; Zhou, Y.; Xu, K.; Zhao, N.; Cheng, X. Soft template-assisted method for synthesis of nitrogen and sulfur co-doped three-dimensional reduced graphene oxide as an efficient metal free catalyst for oxygen reduction reaction. Carbon 2017, 122, 237–246. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, S.; Xia, M.; Rehman, S.; Mu, S.; Kou, Z.; Zhang, Z.; Chen, Z.; Gao, F.; Hou, Y. N-P-O co-doped high performance 3D graphene prepared through red phosphorous-assisted “cutting-thin” technique: A universal synthesis and multifunctional applications. Nano Energy 2016, 28, 346–355. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Feng, X.; Mullen, K. Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction. Angew. Chem. Int. Ed. Engl. 2010, 49, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Nie, H.; Yang, Z.; Zhang, J.; Jin, Z.; Lu, Y.; Xiao, Z.; Huang, S. Sulfur-nitrogen co-doped three-dimensional carbon foams with hierarchical pore structures as efficient metal-free electrocatalysts for oxygen reduction reactions. Nanoscale 2013, 5, 3283–3288. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Zhuang, X.; Bruller, S.; Feng, X.; Mullen, K. Hierarchically porous carbons with optimized nitrogen doping as highly active electrocatalysts for oxygen reduction. Nat. Commun. 2014, 5, 4973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Ma, R.; Zhou, Z.; Liu, G.; Zhou, Y.; Liu, Q.; Kaskel, S.; Wang, J. An In Situ Source-Template-Interface Reaction Route to 3D Nitrogen-Doped Hierarchical Porous Carbon as Oxygen Reduction Electrocatalyst. Adv. Mater. Interfaces 2015, 2, 1500199. [Google Scholar] [CrossRef]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bi, J.; Zhao, Y.; Yang, L.; Zhang, C.; Ma, Y.; Wu, Q.; Wang, X.; Hu, Z. Nitrogen-doped carbon nanocages as efficient metal-free electrocatalysts for oxygen reduction reaction. Adv. Mater. 2012, 24, 5593–5597. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, Y.; Fan, H.; Wei, X.; Hu, C.; Luo, H.; Qu, L. Large scale production of biomass-derived N-doped porous carbon spheres for oxygen reduction and supercapacitors. J. Mater. Chem. A 2014, 2, 3317–3324. [Google Scholar] [CrossRef]
- Huang, H.; Wei, X.; Gao, S. Nitrogen-Doped Porous Carbon Derived from Malachium Aquaticum Biomass as a Highly Efficient Electrocatalyst for Oxygen Reduction Reaction. Electrochim. Acta 2016, 220, 427–435. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Liu, S.; Zhang, X.; Wu, T.; Ge, X.; Zang, Y.; Zhao, H.; Wang, G. Shrimp-shell derived carbon nanodots as carbon and nitrogen sources to fabricate three-dimensional N-doped porous carbon electrocatalysts for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2016, 18, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, C.; Zhao, Y.; Amiinu, I.S.; Zhou, H.; Liu, X.; Tang, Y.; Mu, S. Three dimensional few-layer porous carbon nanosheets towards oxygen reduction. Appl. Catal. B-Environ. 2017, 211, 148–156. [Google Scholar] [CrossRef]
- Zhong, H.X.; Wang, J.; Zhang, Y.W.; Xu, W.L.; Xing, W.; Xu, D.; Zhang, Y.F.; Zhang, X.B. ZIF-8 derived graphene-based nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts. Angew. Chem. Int. Ed. Engl. 2014, 53, 14235–14239. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shang, L.; Bian, T.; Shi, R.; Waterhouse, G.I.; Zhao, Y.; Zhou, C.; Wu, L.Z.; Tung, C.H.; Zhang, T. Nitrogen-Doped Porous Carbon Nanosheets Templated from g-C3N4 as Metal-Free Electrocatalysts for Efficient Oxygen Reduction Reaction. Adv. Mater. 2016, 28, 5080–5086. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, Z.; Li, Y.; Yi, L.; Hu, H. Self-assembly of N doped 3D porous carbon frameworks from carbon quantum dots and its application for oxygen reduction reaction. J. Mater. Sci.-Mater. Electron. 2017, 28, 12660–12669. [Google Scholar] [CrossRef]
- Li, X.; Xue, X.; Fu, Y. Carbon Quantum Dots Derived N-Doped Porous Carbon Frameworks with High Electrocatalytic for Oxygen Reduction Reaction. Nano 2017, 12, 1750093. [Google Scholar] [CrossRef]
- Luo, E.; Xiao, M.; Ge, J.; Liu, C.; Xing, W. Selectively doping pyridinic and pyrrolic nitrogen into a 3D porous carbon matrix through template-induced edge engineering: Enhanced catalytic activity towards the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 21709–21714. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, K.; Song, S.; Tsiakaras, P. 3D interconnected hierarchically porous N-doped carbon with NH3 activation for efficient oxygen reduction reaction. Appl. Catal. B-Environ. 2017, 210, 57–66. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Q.; Jiang, D.; Huang, Y. Facile synthesis of B, N co-doped three-dimensional porous graphitic carbon toward oxygen reduction reaction and oxygen evolution reaction. Catal. Commun. 2017, 100, 89–92. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.; Hao, J.; Liu, Y.; Li, W.; Li, J. N and P co-functionalized three-dimensional porous carbon networks as efficient metal-free electrocatalysts for oxygen reduction reaction. Carbon 2017, 122, 64–73. [Google Scholar] [CrossRef]
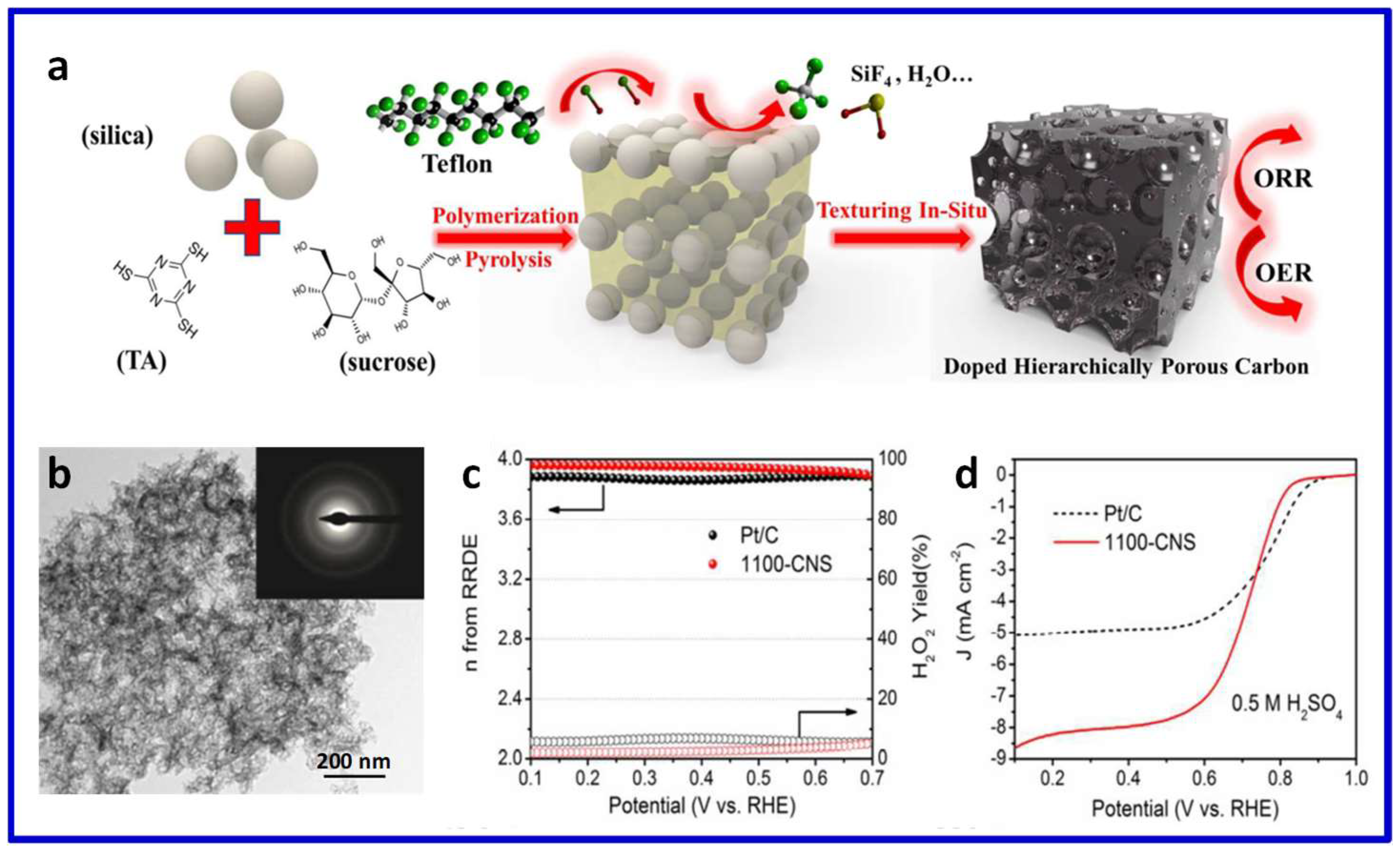
- Pei, Z.; Li, H.; Huang, Y.; Xue, Q.; Huang, Y.; Zhu, M.; Wang, Z.; Zhi, C. Texturing in situ: N,S-enriched hierarchically porous carbon as a highly active reversible oxygen electrocatalyst. Energy Environ. Sci. 2017, 10, 742–749. [Google Scholar] [CrossRef]
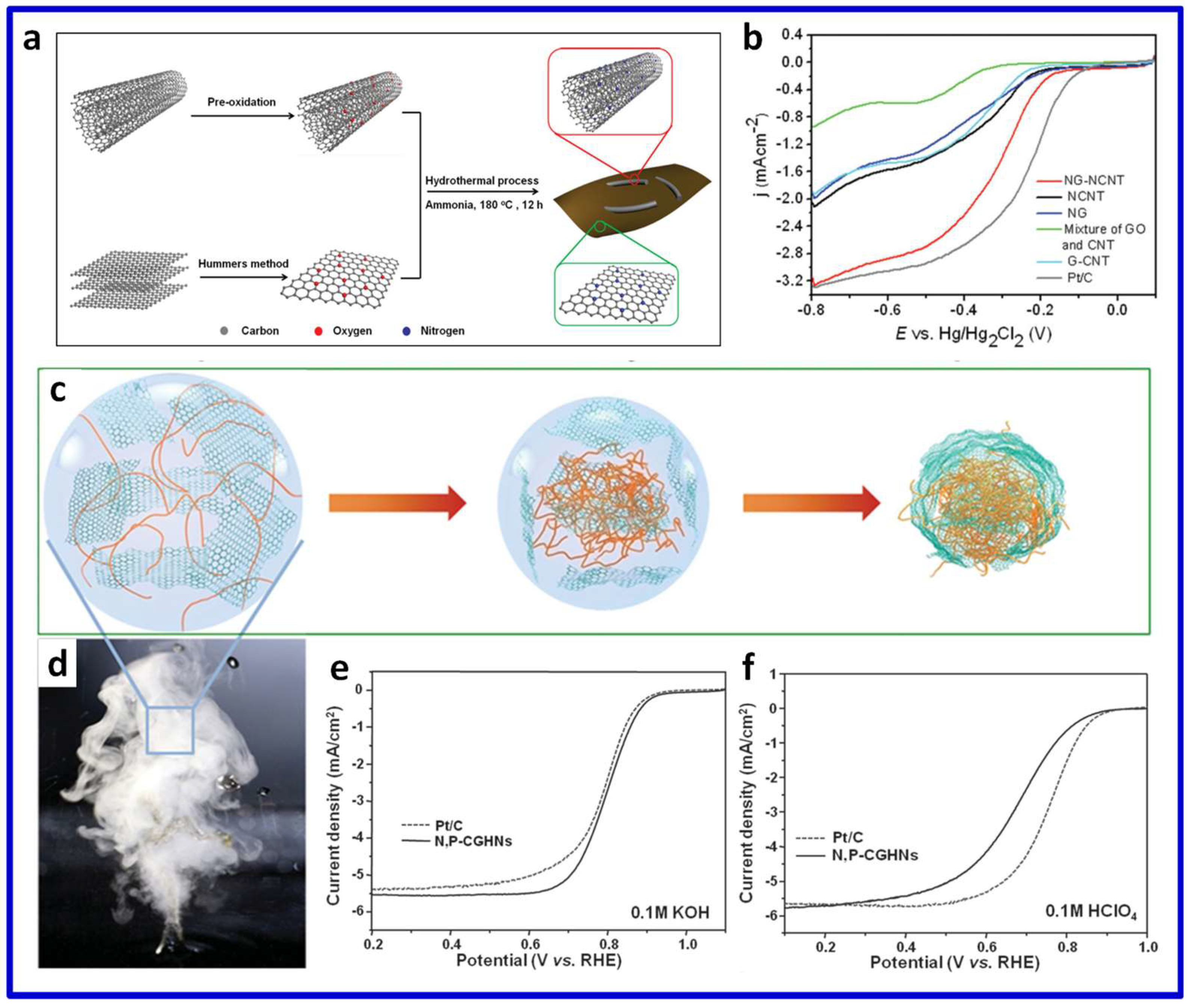
- Chen, P.; Xiao, T.Y.; Qian, Y.H.; Li, S.S.; Yu, S.H. A nitrogen-doped graphene/carbon nanotube nanocomposite with synergistically enhanced electrochemical activity. Adv. Mater. 2013, 25, 3192–3196. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Jo, K.; Lee, T.; Yun, T.; Cho, J.; Kim, B.-S. Facile synthesis of hybrid graphene and carbon nanotubes as a metal-free electrocatalyst with active dual interfaces for efficient oxygen reduction reaction. J. Mater. Chem. A 2013, 1, 9603–9607. [Google Scholar] [CrossRef] [Green Version]
- Higgins, D.C.; Hoque, M.A.; Hassan, F.; Choi, J.-Y.; Kim, B.; Chen, Z. Oxygen Reduction on Graphene–Carbon Nanotube Composites Doped Sequentially with Nitrogen and Sulfur. ACS Catal. 2014, 4, 2734–2740. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, Z.; Chen, J.Y.; Wang, X. Nitrogen-Doped Carbon Nanotubes and Graphene Nanohybrid for Oxygen Reduction Reaction in Acidic, Alkaline and Neutral Solutions. J. Nano Res. 2015, 30, 50–58. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Quan, X.; Chen, S.; Zhao, H.; Yu, H. Nitrogen and sulfur co-doped graphene/carbon nanotube as metal-free electrocatalyst for oxygen evolution reaction: The enhanced performance by sulfur doping. Electrochim. Acta 2016, 204, 169–175. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, L.; Huang, W.; Zhang, L.; Zhao, J.; Fan, Q.; Huang, W. Three-Dimensional Nitrogen-Doped Carbon Nanotubes/Graphene Structure Used as a Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. J. Phys. Chem. C 2011, 115, 24592–24597. [Google Scholar] [CrossRef]
- Yang, J.; Sun, H.; Liang, H.; Ji, H.; Song, L.; Gao, C.; Xu, H. A Highly Efficient Metal-Free Oxygen Reduction Electrocatalyst Assembled from Carbon Nanotubes and Graphene. Adv. Mater. 2016, 28, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, Y.; Wang, Z.; Lei, Y.; Wang, B.; Wu, N.; Han, C.; Xie, S.; Gou, Y. Three-dimensional (3D) interconnected networks fabricated via in-situ growth of N-doped graphene/carbon nanotubes on Co-containing carbon nanofibers for enhanced oxygen reduction. Nano Res. 2015, 9, 317–328. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Wang, H.; Xie, L.; Liang, Y.; Wei, F.; Idrobo, J.C.; Pennycook, S.J.; Dai, H. An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes. Nat. Nanotechnol. 2012, 7, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qu, L.; Shi, G.; Liu, J.; Chen, J.; Dai, L. N,P-Codoped Carbon Networks as Efficient Metal-free Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Angew. Chem. Int. Ed. Engl. 2016, 55, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Ji, S.; Lv, W.; Wang, R. Harvesting a 3D N-Doped Carbon Network from Waste Bean Dregs by Ionothermal Carbonization as an Electrocatalyst for an Oxygen Reduction Reaction. Materials 2017, 10, 1366. [Google Scholar] [CrossRef] [PubMed]
- Mulyadi, A.; Zhang, Z.; Dutzer, M.; Liu, W.; Deng, Y. Facile approach for synthesis of doped carbon electrocatalyst from cellulose nanofibrils toward high-performance metal-free oxygen reduction and hydrogen evolution. Nano Energy 2017, 32, 336–346. [Google Scholar] [CrossRef]
- Li, L.; Manthiram, A. O- and N-Doped Carbon Nanowebs as Metal-Free Catalysts for Hybrid Li-Air Batteries. Adv. Energy Mater. 2014, 4, 1301795. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, D.; Liu, S.; Chen, S.; Hanif, M.; Hou, H. Free-standing nitrogen-doped carbon nanotubes at electrospun carbon nanofibers composite as an efficient electrocatalyst for oxygen reduction. Electrochim. Acta 2014, 138, 318–324. [Google Scholar] [CrossRef]
- Meng, F.; Li, L.; Wu, Z.; Zhong, H.; Li, J.; Yan, J. Facile preparation of N-doped carbon nanofiber aerogels from bacterial cellulose as an efficient oxygen reduction reaction electrocatalyst. Chin. J. Catal. 2014, 35, 877–883. [Google Scholar] [CrossRef]
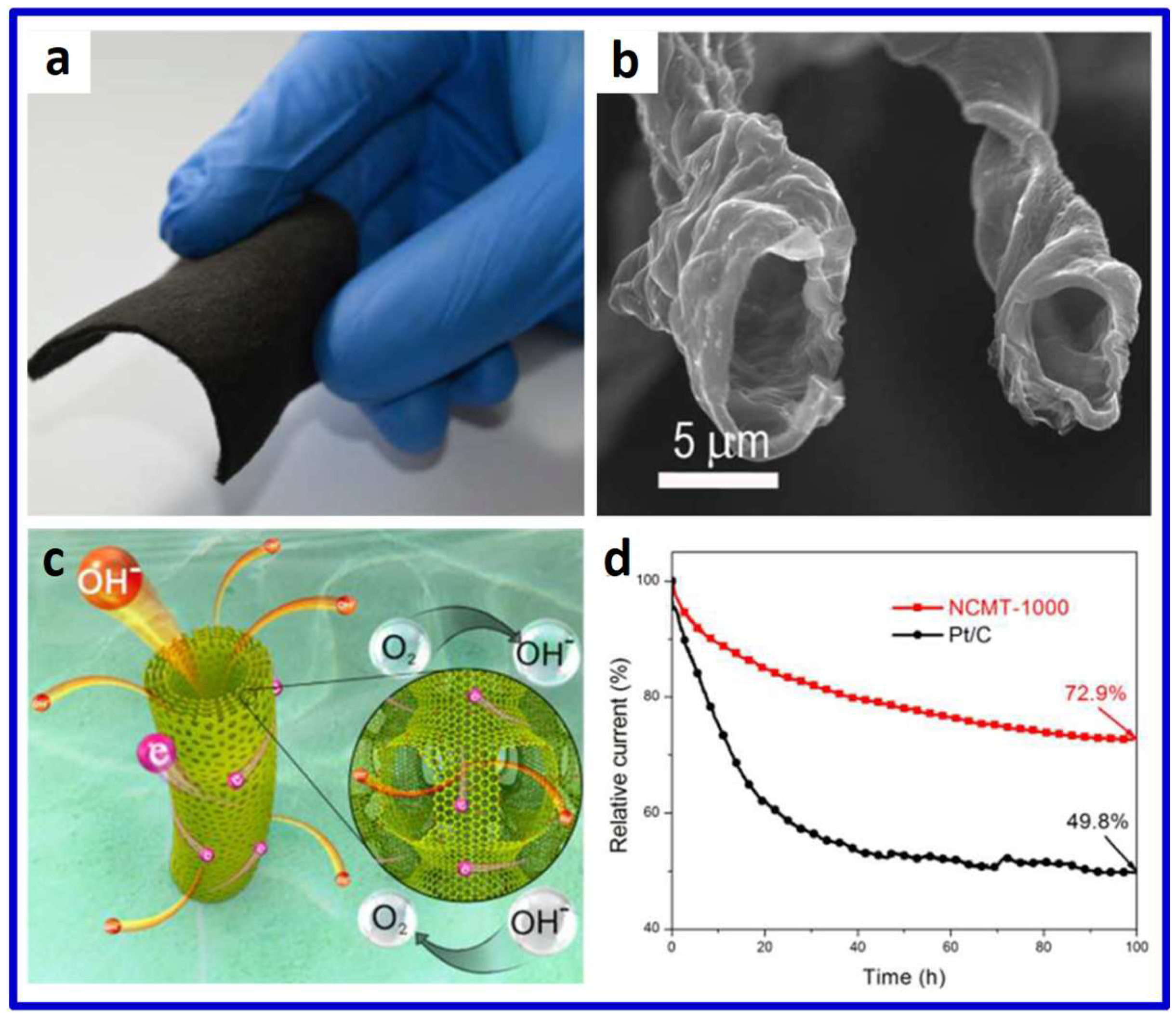
- Li, J.-C.; Hou, P.-X.; Zhao, S.-Y.; Liu, C.; Tang, D.-M.; Cheng, M.; Zhang, F.; Cheng, H.-M. A 3D bi-functional porous N-doped carbon microtube sponge electrocatalyst for oxygen reduction and oxygen evolution reactions. Energy Environ. Sci. 2016, 9, 3079–3084. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, R.; Wang, J.; Zhu, J.; Xiao, W.; Xuan, C.; Lei, W.; Wang, D. Nitrogen and sulfur co-doping of 3D hollow-structured carbon spheres as an efficient and stable metal free catalyst for the oxygen reduction reaction. Nanoscale 2016, 8, 19086–19092. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Song, B.; Wei, X.; Liu, Y.; Tan, Q.; Chong, S.; Chen, Y.; Yang, X.-D.; Yang, W.-H.; Liu, Y. Mesoporous 3D nitrogen-doped yolk-shelled carbon spheres for direct methanol fuel cells with polymer fiber membranes. Carbon 2018, 129, 613–620. [Google Scholar] [CrossRef]
- Guo, D.; Wei, H.; Chen, X.; Liu, M.; Ding, F.; Yang, Z.; Yang, Y.; Wang, S.; Yang, K.; Huang, S. 3D hierarchical nitrogen-doped carbon nanoflower derived from chitosan for efficient electrocatalytic oxygen reduction and high performance lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 18193–18206. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, D.; Li, X.; Fan, L.; Bai, Z. Three-Dimensional Heteroatom-Doped Nanocarbon for Metal-Free Oxygen Reduction Electrocatalysis: A Review. Catalysts 2018, 8, 301. https://doi.org/10.3390/catal8080301
Xiong D, Li X, Fan L, Bai Z. Three-Dimensional Heteroatom-Doped Nanocarbon for Metal-Free Oxygen Reduction Electrocatalysis: A Review. Catalysts. 2018; 8(8):301. https://doi.org/10.3390/catal8080301
Chicago/Turabian StyleXiong, Dongbin, Xifei Li, Linlin Fan, and Zhimin Bai. 2018. "Three-Dimensional Heteroatom-Doped Nanocarbon for Metal-Free Oxygen Reduction Electrocatalysis: A Review" Catalysts 8, no. 8: 301. https://doi.org/10.3390/catal8080301
APA StyleXiong, D., Li, X., Fan, L., & Bai, Z. (2018). Three-Dimensional Heteroatom-Doped Nanocarbon for Metal-Free Oxygen Reduction Electrocatalysis: A Review. Catalysts, 8(8), 301. https://doi.org/10.3390/catal8080301



