Dioxygen Activation by Laccases: Green Chemistry for Fine Chemical Synthesis
Abstract
:1. Introduction
2. Laccases for Pharmaceutical Green Chemistry
2.1. Laccase-Catalyzed Pharmaceuticals
2.1.1. Laccase-Synthesized Molecules with Anticancer Activity
2.1.2. Laccase-Synthesized Molecules with Antioxidant Activity
2.1.3. Laccase-Synthesized Molecules with Antibiotic Activity
2.1.4. Laccase-Synthesized Molecules with Antidiabetic Activity
3. Limitations for Laccase-Mediated Synthesis of Fine Chemicals at Large-Scale
3.1. Fermentation Yields of Native and Recombinant Laccase-Producers
3.2. Laccase Immobilization
4. Conclusions and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on solvent use in the pharmaceutical industry. Org. Process. Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, H.; Ang, E.L.; Zhao, H. Biocatalysis for the synthesis of pharmaceuticals and pharmaceutical intermediates. Bioorg. Med. Chem. 2018, 26, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Meyer, H.P. Biocatalysis as a Strategic Green Technology for the Chemical Industry. ChemCatChem 2014, 6, 918–920. [Google Scholar] [CrossRef]
- Sheldon, R.A. Engineering a more sustainable world through catalysis and green chemistry. J. R. Soc. Interface 2016, 13, 20160087. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- Wohlgemuth, R. Green Production of Fine Chemicals by Isolated Enzymes. In Biocatalysis for Green Chemistry and Chemical Process Development; Kazlauskas, R., Tao, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 1, pp. 277–298. ISBN 9780470437780. [Google Scholar]
- Choi, J.M.; Han, S.S.; Kim, H.S. Industrial applications of enzyme biocatalysis: Current status and future aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Schulze, B.; Wubbolts, M.G. Biocatalysis for industrial production of fine chemicals. Curr. Opin. Biotechnol. 1999, 10, 609–615. [Google Scholar] [CrossRef]
- Rose, A.S.; Bradley, A.R.; Valasatava, Y.; Duarte, J.M.; Prlić, A.; Rose, P.W. Web-based molecular graphics for large complexes. In Proceedings of the 21st International Conference on Web3D Technology, Anaheim, CA, USA, 22–24 July 2016; pp. 185–186. [Google Scholar]
- Rose, A.S.; Hildebrand, P.W. NGL Viewer: A web application for molecular visualization. Nucleic Acids Res. 2015, 43, W576–W579. [Google Scholar] [CrossRef] [PubMed]
- Matera, I.; Gullotto, A.; Tilli, S.; Ferraroni, M.; Scozzafava, A.; Briganti, F. Crystal structure of the blue multicopper oxidase from the white-rot fungus Trametes trogii complexed with p-toluate. Inorg. Chim. Acta 2008, 361, 4129–4137. [Google Scholar] [CrossRef]
- Solomon, E.I.; Baldwin, M.J.; Lowery, M.D. Electronic structures of active sites in copper proteins: Contributions to reactivity. Chem. Rev. 1992, 92, 521–542. [Google Scholar] [CrossRef]
- Augustine, A.J.; Kjaergaard, C.; Qayyum, M.; Ziegler, L.; Kosman, D.J.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. Systematic Perturbation of the Trinuclear Copper Cluster in the Multicopper Oxidases: The Role of Active Site Asymmetry in Its Reduction of O2 to H2O. J. Am. Chem. Soc. 2010, 132, 6057–6067. [Google Scholar] [CrossRef] [PubMed]
- Alessandra, P.; Cinzia, P.; Paola, G.; Vincenza, F.; Sannia, G. Heterologous laccase production and its role in industrial applications. Bioeng. Bugs 2010, 1, 252–262. [Google Scholar]
- Kudanga, T.; Nemadziva, B.; Le Roes-Hill, M. Laccase catalysis for the synthesis of bioactive compounds. Appl. Microbiol. Biotechnol. 2017, 101, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Ba, S.; Vinoth Kumar, V. Recent developments in the use of tyrosinase and laccase in environmental applications. Crit. Rev. Biotechnol. 2017, 37, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, B.; Rajesh, B.; Janardhan, A.; Kumar, A.P.; Narasimha, G. Fungal laccases and their applications in bioremediation. Enzym. Res. 2014, 2014, 163242. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Couto, S.; Toca Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Shumakovich, G.P.; Gorbacheva, M.A.; Shleev, S.V.; Yaropolov, A.I. “Blue” laccases. Biochemistry 2007, 72, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Brijwani, K.; Rigdon, A.; Vadlani, P.V. Fungal laccases: Production, function, and applications in food processing. Enzym. Res. 2010, 2010, 149748. [Google Scholar] [CrossRef] [PubMed]
- Kurniawati, S.; Nicell, J.A. A comprehensive kinetic model of laccase-catalyzed oxidation of aqueous phenol. Biotechnol. Prog. 2009, 25, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry Theory and Practice, 1st ed.; Oxford University Press: Oxford, UK, 2000; p. 152. ISBN 978-0198506980. [Google Scholar]
- Perveen, S.; Al-Taweel, A.M. Green Chemistry and Synthesis of Anticancer Molecule. In Green Chemistry; El-Din, H., Saleh, M., Eds.; IntechOpen: London, UK, 2018; Volume 1, pp. 51–72. ISBN 978-953-51-3848-8. [Google Scholar]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Wellington, K.W.; Kolesnikova, N.I. A laccase-catalysed one-pot synthesis of aminonaphthoquinones and their anticancer activity. Bioorg. Med. Chem. 2012, 20, 4472–4481. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.W.; Bokako, R.; Raseroka, N.; Steenkamp, P. A one-pot synthesis of 1,4-naphthoquinone-2,3-bis-sulfides catalysed by a commercial laccase. Green Chem. 2012, 14, 2567–2576. [Google Scholar] [CrossRef]
- Wellington, K.W.; Qwebani-Ogunleye, T.; Kolesnikova, N.I.; Brady, D.; de Koning, C.B. One-pot laccase-catalysed synthesis of 5,6-dihydroxylated benzo[b]furans and catechol derivatives, and their anticancer activity. Arch. Pharm. 2013, 346, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Qwebani-Ogunleye, T.; Kolesnikova, N.I.; Steenkamp, P.; de Koning, C.B.; Brady, D.; Wellington, K.W. A one-pot laccase-catalysed synthesis of coumestan derivatives and their anticancer activity. Bioorg. Med. Chem. 2017, 25, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, H.T.; Conrad, J.; Harms, K.; Nohr, D.; Beifuss, U. Laccase-catalyzed green synthesis and cytotoxic activity of novel pyrimidobenzothiazoles and catechol thioethers. RSC Adv. 2017, 7, 17427–17441. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Singhal, R.S. Laccase–gum Arabic conjugate for preparation of water-soluble oligomer of catechin with enhanced antioxidant activity. Food Chem. 2014, 150, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chebil, L.; Rhouma, G.; Chekir-Ghedira, L.; Ghoul, M. Enzymatic Polymerization of Rutin and Esculin and Evaluation of the Antioxidant Capacity of Polyrutin and Polyesculin. In Biotechnology; Ekinci, D., Ed.; IntechOpen Lomited: London, UK, 2015; Volume 1, ISBN 978-953-51-2040-7. [Google Scholar]
- Ezgi Ünlü, A.; Prasad, B.; Anavekar, K.; Bubenheim, P.; Liese, A. Investigation of a green process for the polymerization of catechin. Prep. Biochem. Biotechnol. 2017, 47, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, H.T.; Conrad, J.; Beifuss, U. Laccase-catalyzed synthesis of catechol thioethers by reaction of catechols with thiols using air as an oxidant. Green Chem. 2014, 16, 90–95. [Google Scholar] [CrossRef]
- Mikolasch, A.; Manda, K.; Schlüter, R.; Lalk, M.; Witt, S.; Seefeldt, S.; Hammer, E.; Schauer, F.; Jülich, W.D.; Lindequist, U. Comparative analyses of laccase-catalyzed amination reactions for production of novel β-lactam antibiotics. Biotechnol. Appl. Biochem. 2012, 59, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, H.T.; Conrad, J.; Beifuss, U. Laccase-Catalyzed Domino Reaction between Catechols and 6-Substituted 1,2,3,4-Tetrahydro-4-oxo-2-thioxo-5-pyrimidinecarbonitriles for the Synthesis of Pyrimidobenzothiazole Derivatives. J. Org. Chem. 2013, 78, 7986–8003. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-Y.; Oh, S.; Kim, E.; Imm, J.-Y. α-Glucosidase Inhibiton and Antiglycation Activity of Laccase-Catalyzed Catechin Polymers. J. Agric. Food Chem. 2013, 61, 4577–4584. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, M.; Jain, A.; Sharma, A.; Kuhad, R.C. Laccase-catalysed reaction between Meldrum’s acid and catechols/hydroquinones—An investigation. C. R. Chim. 2013, 16, 728–735. [Google Scholar] [CrossRef]
- Cannatelli, M.D.; Ragauskas, A.J. Laccase-catalyzed α-arylation of benzoylacetonitrile with substituted hydroquinones. Chem. Eng. Res. Des. 2015, 97, 128–134. [Google Scholar] [CrossRef]
- Cannatelli, M.D.; Ragauskas, A.J. Ecofriendly syntheses of phenothiazones and related structures facilitated by laccase—A comparative study. Tetrahedron Lett. 2016, 57, 3749–3753. [Google Scholar] [CrossRef]
- Hahn, V.; Mikolasch, A.; Kuhlisch, C.; Schauer, F. Laccase-mediated multi-step homo- and heteromolecular reactions of ortho-dihydroxylated aromatic compounds and mono- or diaminated substances resulting in CC, CO and CN bonds. J. Mol. Catal. B Enzym. 2015, 122, 56–63. [Google Scholar] [CrossRef]
- Habibi, D.; Rahimi, A.; Rostami, A.; Moradi, S. Green and mild laccase-catalyzed aerobic oxidative coupling of benzenediol derivatives with various sodium benzenesulfinates. Tetrahedron Lett. 2017, 58, 289–293. [Google Scholar] [CrossRef]
- Kidwai, M.; Jain, A.; Sharma, A.; Kuhad, R.C. First time reported enzymatic synthesis of new series of quinoxalines—A green approach. J. Mol. Catal. B Enzym. 2012, 74, 236–240. [Google Scholar] [CrossRef]
- Antibiotic Resistance. Available online: http://www.who.int/mediacentre/factsheets/antibiotic-resistance/en/ (accessed on 30 April 2018).
- Hajdok, S.; Leutbecher, H.; Greiner, G.; Conrad, J.; Beifuss, U. Laccase initiated oxidative domino reactions for the efficient synthesis of 3,4-dihydro-7,8-dihydroxy-2H-dibenzofuran-1-ones. Tetrahedron Lett. 2007, 48, 5073–5076. [Google Scholar] [CrossRef]
- Leutbecher, H.; Conrad, J.; Klaiber, I.; Beifuss, U. O-Heterocycles via Laccase-Catalyzed Domino Reactions with O2 as the Oxidant. Synlett 2005, 20, 3126–3130. [Google Scholar] [CrossRef]
- Bhalerao, U.T.; Muralikrishna, C.; Pandey, G. An Efficient Synthesis of Coumestans-A Probable Biogenetic Approach. Synth. Commun. 1989, 19, 1303–1307. [Google Scholar] [CrossRef]
- Nematollahi, D.; Habibi, D.; Rahmati, M.; Rafiee, M. A Facile Electrochemical Method for Synthesis of New Benzofuran Derivatives. J. Org. Chem. 2004, 69, 2637–2640. [Google Scholar] [CrossRef] [PubMed]
- Tabaković, I.; Grujić, Z.; Bejtović, Z. Electrochemical synthesis of heterocyclic compounds. XII. Anodic oxidation of catechol in the presence of nucleophiles. J. Heterocycl. Chem. 1983, 20, 635–638. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Enzymatic Synthesis and Antioxidant Properties of Poly(rutin). Biomacromolecules 2003, 4, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Desentis-Mendoza, R.M.; Hernández-Sánchez, H.; Moreno, A.; Rojas del c, E.; Chel-Guerrero, L.; Tamariz, J.; Jaramillo-Flores, M.E. Enzymatic Polymerization of Phenolic Compounds Using Laccase and Tyrosinase from Ustilago maydis. Biomacromolecules 2006, 7, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Draenert, R.; Seybold, U.; Grützner, E.; Bogner, J.R. Novel antibiotics: Are we still in the pre–post-antibiotic era? Infection 2015, 43, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I. A Brief History of the Antibiotic Era: Lessons Learned and Challenges for the Future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Agematu, H.; Tshuchida, T.; Kominato, K.; Shibamoto, N.; Yoshioka, T.; Nishida, H.; Okamoto, R. Enzymatic dimerization of penicillin X. J. Antibiot. 1993, 46, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Antošová, Z.; Sychrová, H. Yeast Hosts for the Production of Recombinant Laccases: A Review. Mol. Biotechnol. 2016, 58, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Ba, S.; Arsenault, A.; Hassani, T.; Jones, J.P.; Cabana, H. Laccase immobilization and insolubilization: From fundamentals to applications for the elimination of emerging contaminants in wastewater treatment. Crit. Rev. Biotechnol. 2013, 33, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Martínez-Morales, F.; Trejo-Hernández, M.R. Upgrading Laccase Production and Biochemical Properties: Strategies and Challenges. Biotechnol. Prog. 2017, 33, 1015–1034. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Shrivastava, R.; Agrawal, P.K. Bioprospecting and biotechnological applications of fungal laccase. 3 Biotech. 2016, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, W.; Bun Ng, T.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, expression regulation, and applications in pharmaceutical biodegradation. Front. Microbiol. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed]
- Tinoco-Valencia, R.; Gómez-Cruz, C.; Galindo, E.; Serrano-Carreón, L. Toward an understanding of the effects of agitation and aeration on growth and laccases production by Pleurotus ostreatus. J. Biotechnol. 2014, 177, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Revankar, M.S.; Lele, S.S. Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process. Biochem. 2006, 41, 581–588. [Google Scholar] [CrossRef]
- Wang, S.S.; Ning, Y.J.; Wang, S.N.; Zhang, J.; Zhang, G.Q.; Chen, Q.J. Purification, characterization, and cloning of an extracellular laccase with potent dye decolorizing ability from white rot fungus Cerrena unicolor GSM-01. Int. J. Biol. Macromol. 2017, 95, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.; Sharma, P.; George, N.; Chauhan, P.S.; Puri, N.; Gupta, N. An extracellular thermo-alkali-stable laccase from Bacillus tequilensis SN4, with a potential to biobleach softwood pulp. 3 Biotech. 2015, 5, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Ausec, L.; Berini, F.; Casciello, C.; Cretoiu, M.S.; van Elsas, J.D.; Marinelli, F.; Mandic-Mulec, I. The first acidobacterial laccase-like multicopper oxidase revealed by metagenomics shows high salt and thermo-tolerance. Appl. Microbiol. Biotechnol. 2017, 101, 6261–6276. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.O.; Durão, P.; Brissos, V.; Lindley, P.F. Laccases of prokaryotic origin: Enzymes at the interface of protein science and protein technology. Cell. Mol. Life Sci. 2015, 72, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, L.; Li, Q.; Liu, Y.; Yi, L.; Ma, L.; Zhai, C. High-level expression of a bacterial laccase, CueO from Escherichia coli K12 in Pichia pastoris GS115 and its application on the decolorization of synthetic dyes. Enzym. Microb. Technol. 2017, 103, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Tonin, F.; Melis, R.; Cordes, A.; Sanchez-Amat, A.; Pollegioni, L.; Rosini, E. Comparison of different microbial laccases as tools for industrial uses. New Biotechnol. 2016, 33, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, C.; Giacobelli, V.G.; Lettera, V.; Olivieri, G.; Cicatiello, P.; Sannia, G.; Piscitelli, A. A step forward in laccase exploitation: Recombinant production and evaluation of techno-economic feasibility of the process. J. Biotechnol. 2017, 259, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Mate, D.M.; Alcalde, M. Laccase engineering: From rational design to directed evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Ihssen, J.; Reiss, R.; Luchsinger, R.; Thöny-Meyer, L.; Richter, M. Biochemical properties and yields of diverse bacterial laccase-like multicopper oxidases expressed in Escherichia coli. Sci. Rep. 2015, 5, 10465. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, M.; Yamagishi, K.; Wang, J.; Tanaka, K.; Miyoshi, T.; Kamei, I.; Kondo, R.; Mori, T.; Kawagishi, H.; Hirai, H. Molecular breeding of lignin-degrading brown-rot fungus Gloeophyllum trabeum by homologous expression of laccase gene. AMB Express 2015, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.H.; Cho, M.K.; Kim, M.; Jung, S.M.; Seo, J.H. Enhanced lignin biodegradation by a laccase-overexpressed white-rot fungus polyporus brumalis in the pretreatment of wood chips. Appl. Biochem. Biotechnol. 2013, 171, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Li, G.; Wang, Y.; Nie, F.; Cheng, X.; Abdullah, M.; Lin, Y.; Cai, Y. Systematic Analysis of the Pleurotus ostreatus Laccase Gene (PoLac) Family and Functional Characterization of PoLac2 Involved in the Degradation of Cotton-Straw Lignin. Molecules 2018, 23, 880. [Google Scholar] [CrossRef] [PubMed]
- Songulashvili, G.; Jimenéz-Tobón, G.A.; Jaspers, C.; Penninckx, M.J. Immobilized laccase of Cerrena unicolor for elimination of endocrine disruptor micropollutants. Fungal Biol. 2012, 116, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Songulashvili, G.; Spindler, D.; Jimenéz-Tobón, G.A.; Jaspers, C.; Kerns, G.; Penninckx, M.J. Production of a high level of laccase by submerged fermentation at 120-L scale of Cerrena unicolor C-139 grown on wheat bran. C. R. Biol. 2015, 338, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, G.; Ng, T.B.; Lin, J.; Ye, X. Laccase production and differential transcription of laccase genes in Cerrena sp. in response to metal ions, aromatic compounds, and nutrients. Front. Microbiol. 2016, 6, 1558. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Wu, P.H.; Su, Y.C.; Wen, T.N.; Wei, Y.S.; Wang, N.C.; Hsu, C.A.; Wang, A.H.J.; Shyur, L.F. Biochemical characterization of a novel laccase from the basidiomycete fungus Cerrena sp. WR1. Protein Eng. Des. Sel. 2012, 25, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Songulashvili, G.; Flahaut, S.; Demarez, M.; Tricot, C.; Bauvois, C.; Debaste, F.; Penninckx, M.J. High yield production in seven days of Coriolopsis gallica 1184 laccase at 50 L scale; enzyme purification and molecular characterization. Fungal Biol. 2016, 120, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Galhaup, C.; Wagner, H.; Hinterstoisser, B.; Haltrich, D. Increased production of laccase by the wood-degrading basidiomycete Trametes pubescens. Enzym. Microb. Technol. 2002, 30, 529–536. [Google Scholar] [CrossRef]
- Afreen, S.; Anwer, R.; Singh, R.K.; Fatma, T. Extracellular laccase production and its optimization from Arthrospira maxima catalyzed decolorization of synthetic dyes. Saudi J. Biol. Sci. 2016. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, A.; Sondhi, S.; Sharma, P.; Gupta, N. An alkaline bacterial laccase for polymerization of natural precursors for hair dye synthesis. 3 Biotech. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mekmouche, Y.; Zhou, S.; Cusano, A.M.; Record, E.; Lomascolo, A.; Robert, V.; Simaan, A.J.; Rousselot-Pailley, P.; Ullah, S.; Chaspoul, F.; et al. Gram-scale production of a basidiomycetous laccase in Aspergillus niger. J. Biosci. Bioeng. 2014, 117, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Nishibori, N.; Masaki, K.; Tsuchioka, H.; Fujii, T.; Iefuji, H. Comparison of laccase production levels in pichia pastoris and Cryptococcus sp. S-2. J. Biosci. Bioeng. 2013, 115, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ng, T.B.; Lin, J.; Ye, X. A novel laccase from basidiomycete Cerrena sp.: Cloning, heterologous expression, and characterization. Int. J. Biol. Macromol. 2015, 77, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Macellaro, G.; Baratto, M.C.; Piscitelli, A.; Pezzella, C.; Fabrizi De Biani, F.; Palmese, A.; Piumi, F.; Record, E.; Basosi, R.; Sannia, G. Effective mutations in a high redox potential laccase from Pleurotus ostreatus. Appl. Microbiol. Biotechnol. 2014, 98, 4949–4961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Hong, Y.Z.; Xiao, Y.Z.; Cui, T.J.; Wang, X.T.; Pu, C.L. High Output of a Trametes Laccase in Pichia pastoris and Characterization of Recombinant Enzymes. Chin. J. Biotechnol. 2007, 23, 1055–1059. [Google Scholar] [CrossRef]
- Li, Q.; Ge, L.; Cai, J.; Pei, J.; Xie, J.; Zhao, L. Comparison of two laccases from Trametes versicolor for application in the decolorization of dyes. J. Microbiol. Biotechnol. 2014, 24, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Samak, N.A.; Hu, J.; Wang, K.; Guo, C.; Liu, C. Development of a Novel Micro-Aerobic Cultivation Strategy for High Potential CotA Laccase Production. Waste Biomass Valorization 2018, 9, 369–377. [Google Scholar] [CrossRef]
- Ihssen, J.; Schubert, M.; Thöny-Meyer, L.; Richter, M. Laccase catalyzed synthesis of iodinated phenolic compounds with antifungal activity. PLoS ONE. 2014, 9, e89924. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Spahn, C.; Minteer, S.D. Enzyme Immobilization in Biotechnology. Recent Pat. Eng. 2008, 2, 195–200. [Google Scholar] [CrossRef]
- Aguila, S.; Vazquez-Duhalt, R.; Covarrubias, C.; Pecchi, G.; Alderete, J.B. Enhancing oxidation activity and stability of iso-1-cytochrome c and chloroperoxidase by immobilization in nanostructured supports. J. Mol. Catal. B Enzym. 2011, 70, 81–87. [Google Scholar] [CrossRef]
- Campos-Terán, J.; Iñarritu, I.; Aburto, J.; Torres, E. Enhanced Functionality of Peroxidases by Its Immobilization at the Solid–Liquid Interface of Mesoporous Materials and Nanoparticles. In Proteins in Solution and at Interfaces; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 335–351. ISBN 9781118523063. [Google Scholar]
- Kumar, V.V.; Cabana, H. Towards high potential magnetic biocatalysts for on-demand elimination of pharmaceuticals. Bioresour. Technol. 2016, 200, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, S.; Rostami, A.; Salimi, A.; Pourshiani, O. Graphene oxide/CuFe2O4 nanocomposite as a novel scaffold for the immobilization of laccase and its application as a recyclable nanobiocatalyst for the green synthesis of arylsulfonyl benzenediols. Biochem. Eng. J. 2018, 133, 1–11. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü. Chitosan based metal-chelated copolymer nanoparticles: Laccase immobilization and phenol degradation studies. Int. Biodeterior. Biodegrad. 2017, 125, 235–242. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Knystautas, E.J.; Verma, M.; Surampalli, R.Y. Degradation of chlortetracycline using immobilized laccase on Polyacrylonitrile-biochar composite nanofibrous membrane. Sci. Total Environ. 2017, 605–606, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Hommes, G.; Gasser, C.A.; Howald, C.B.C.; Goers, R.; Schlosser, D.; Shahgaldian, P.; Corvini, P.F.X. Production of a robust nanobiocatalyst for municipal wastewater treatment. Bioresour. Technol. 2012, 115, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Hai, F.I.; Dosseto, A.; Richardson, C.; Price, W.E.; Nghiem, L.D. Continuous adsorption and biotransformation of micropollutants by granular activated carbon-bound laccase in a packed-bed enzyme reactor. Bioresour. Technol. 2016, 210, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Stemple, B.; Kumar, M.; Wei, N. Cell Surface Display Fungal Laccase as a Renewable Biocatalyst for Degradation of Persistent Micropollutants Bisphenol A and Sulfamethoxazole. Environ. Sci. Technol. 2016, 50, 8799–8808. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Otari, S.V.; Li, J.; Kim, D.R.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J. Hazard. Mater. 2018, 347, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-F.; Meng, G.; Cui, B.-K.; Si, J.; Dai, Y.-C. Chitosan crosslinked with genipin as supporting matrix for biodegradation of synthetic dyes: Laccase immobilization and characterization. Chem. Eng. Res. Des. 2018, 132, 664–676. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Knystautas, E.J.; Verma, M.; Surampalli, R.Y. Covalent Immobilization of Laccase onto Nanofibrous Membrane for Degradation of Pharmaceutical Residues in Water. ACS Sustain. Chem. Eng. 2017, 5, 10430–10438. [Google Scholar] [CrossRef]
- Arca-Ramos, A.; Kumar, V.V.; Eibes, G.; Moreira, M.T.; Cabana, H. Recyclable cross-linked laccase aggregates coupled to magnetic silica microbeads for elimination of pharmaceuticals from municipal wastewater. Environ. Sci. Pollut. Res. 2016, 23, 8929–8939. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, K.; Faramarzi, M.A.; Mahvi, A.H.; Gholami, M.; Esrafili, A.; Forootanfar, H.; Farzadkia, M. Elimination and detoxification of sulfathiazole and sulfamethoxazole assisted by laccase immobilized on porous silica beads. Int. Biodeterior. Biodegrad. 2015, 97, 107–114. [Google Scholar] [CrossRef]
- De Cazes, M.; Belleville, M.P.; Petit, E.; Llorca, M.; Rodríguez-Mozaz, S.; de Gunzburg, J.; Barceló, D.; Sanchez-Marcano, J. Design and optimization of an enzymatic membrane reactor for tetracycline degradation. Catal. Today 2014, 236, 146–152. [Google Scholar] [CrossRef]
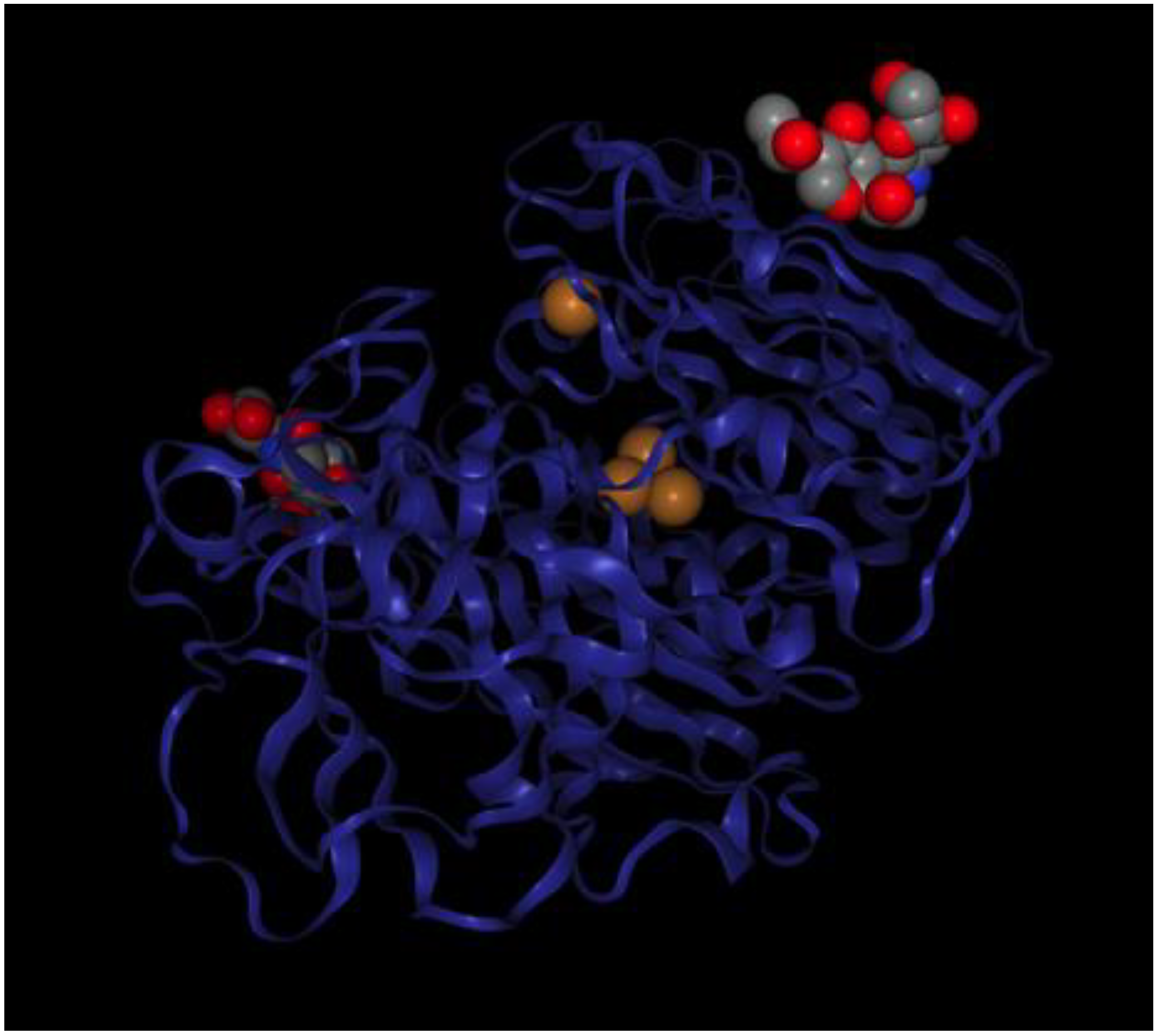
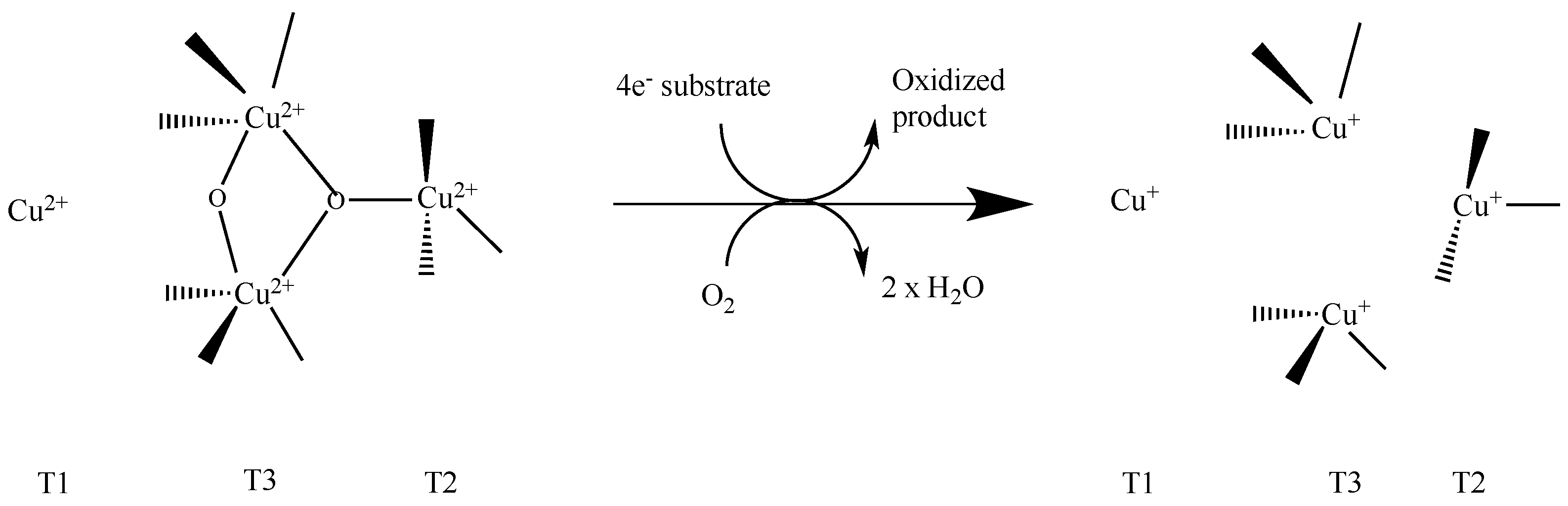
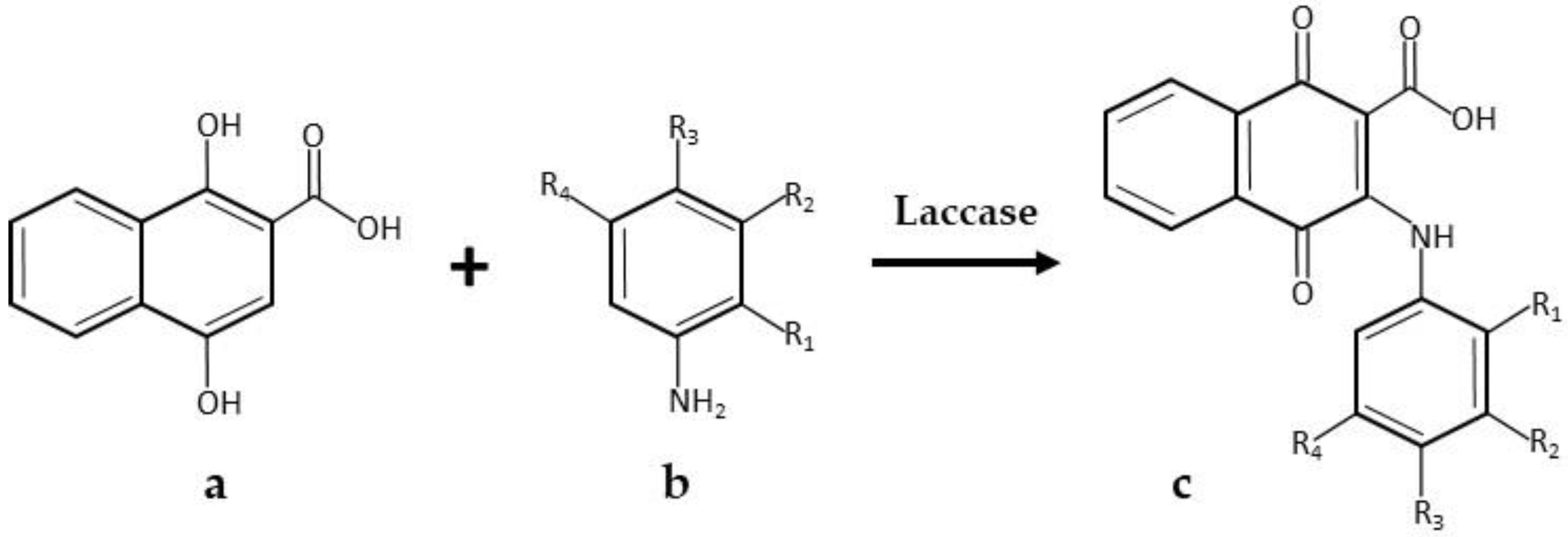
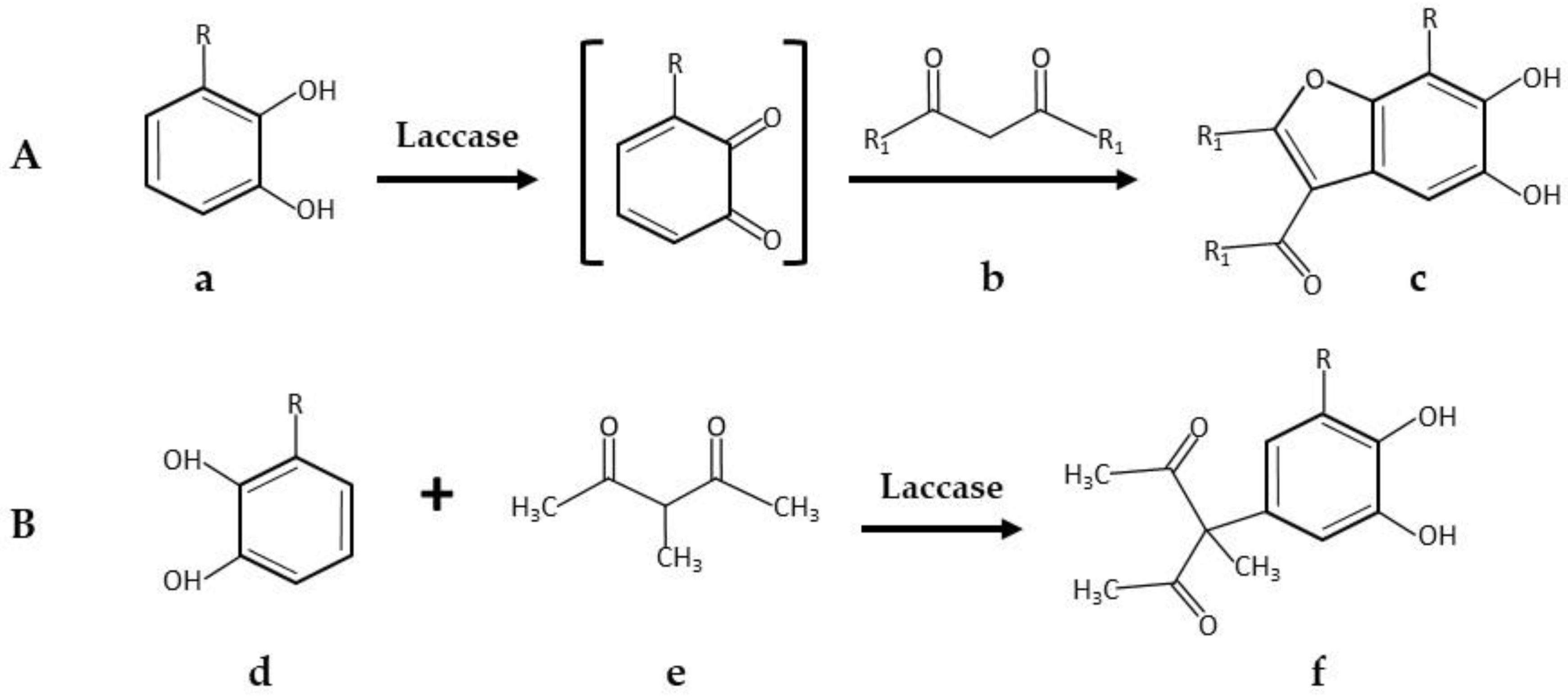
| Product | Laccase Source | Reaction Conditions and Results | Reference |
|---|---|---|---|
| Anticancer compounds | |||
| * Amino-naphtoquinones | Commercial Novozyme 51003, laccase from Myceliophthora thermophile, Novozymes | 1.0 M succinate-lactate buffer pH 4.5; 35 °C; 2220 U laccase; 1.2 mmol amine and 0.6 mmol 1,4-dihydroxy-2-naphthoic acid; 1 mL DMF as a co-solvent. 48 h, 77% yield | [30] |
| 0.01 M sodium phosphate buffer pH 6.0; 40 °C; stirring; 2775 U laccase; 1.2 mmol amine and 0.6 mmol 1,4-dihydroxy-2-naphthoic acid; DMF. 48 h, 85% yield | [30] | ||
| 1,4-Naphthoquinone-2,3-bis-sulfides | Commercial Novozyme 51003, laccase from Myceliophthora thermophile, Novozymes | 1 M succinate-lactate buffer pH 4.5; 35 °C; air by stirring; 3885 U laccase; 0.6 mmol 1,4-naphthohydroquinone and 1.8 mmol thiol. 48 h, 69% yield | [31] |
| 0.1 M potassium phosphate buffer pH 7.15; air by stirring; 6660 U laccase; 0.6 mmol 1,4 naphthohydroquinone and 1.8 mmol thiol. 48 h, 56% yield | [31] | ||
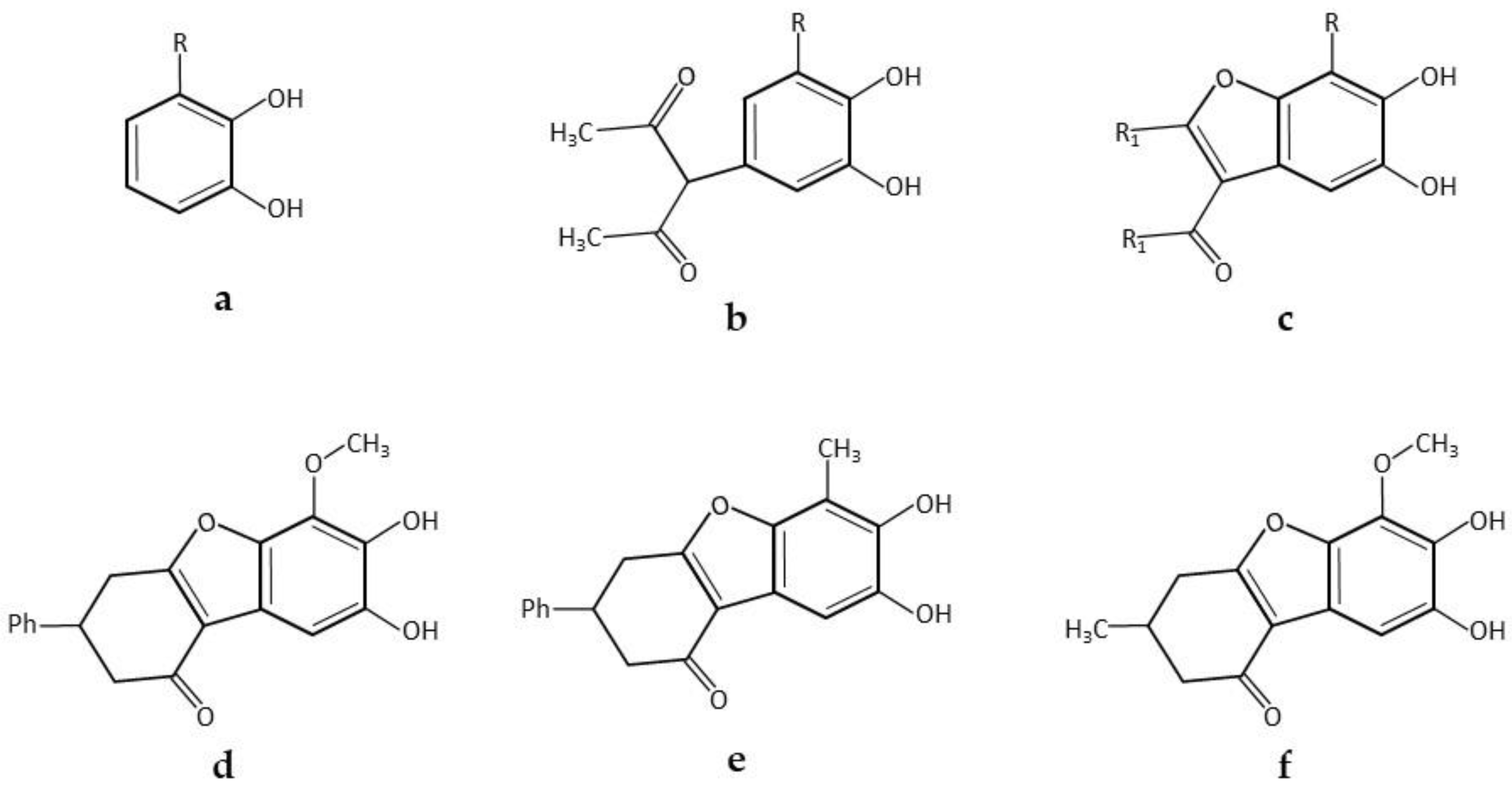
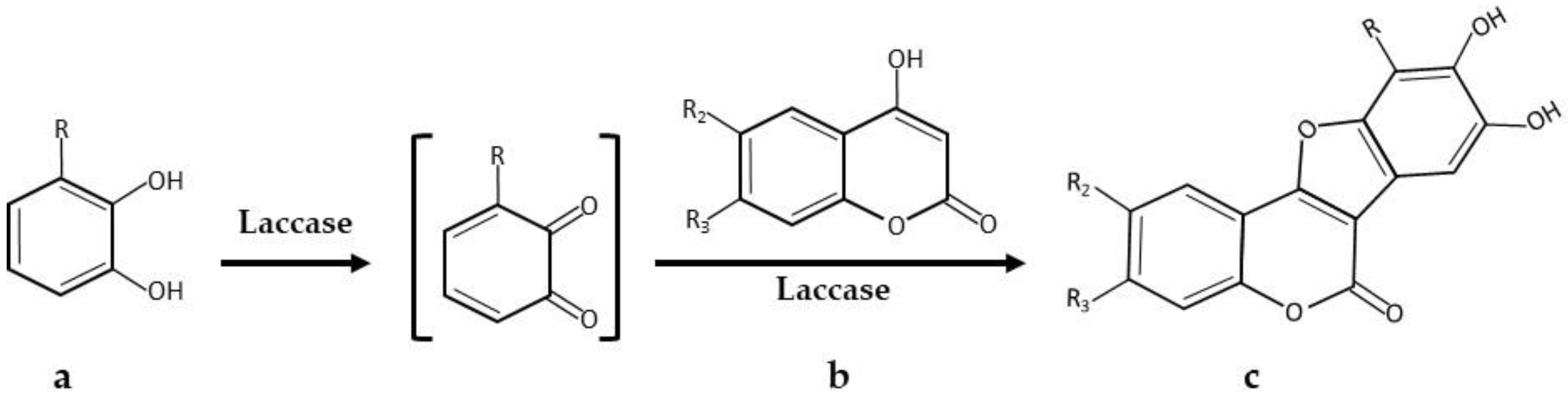
| * 5,6-Dihydroxylated benzo[b]furans and catechol derivatives | Commercial Suberase, laccase from Myceliophthora thermophila, Novozymes | 0.1 M phosphate buffer pH7.15; R.T.; air; 10,757.8 PCU/mL laccase; 2.0 mmol catechol and 2.0 mmol 1,3-dicarbonyl. 24 h, 98% yield | [32] |
| * Coumestans derivatives | Commercial Suberase, laccase from Myceliophthora thermophila, Novozymes | 0.1 M Phosphate buffer pH 7.15; R.T.; air; 10,757.8 PCU/mL laccase; 2.0 mM catechols and 2.0 mM coumarins. 24 h, 86% yield | [33] |
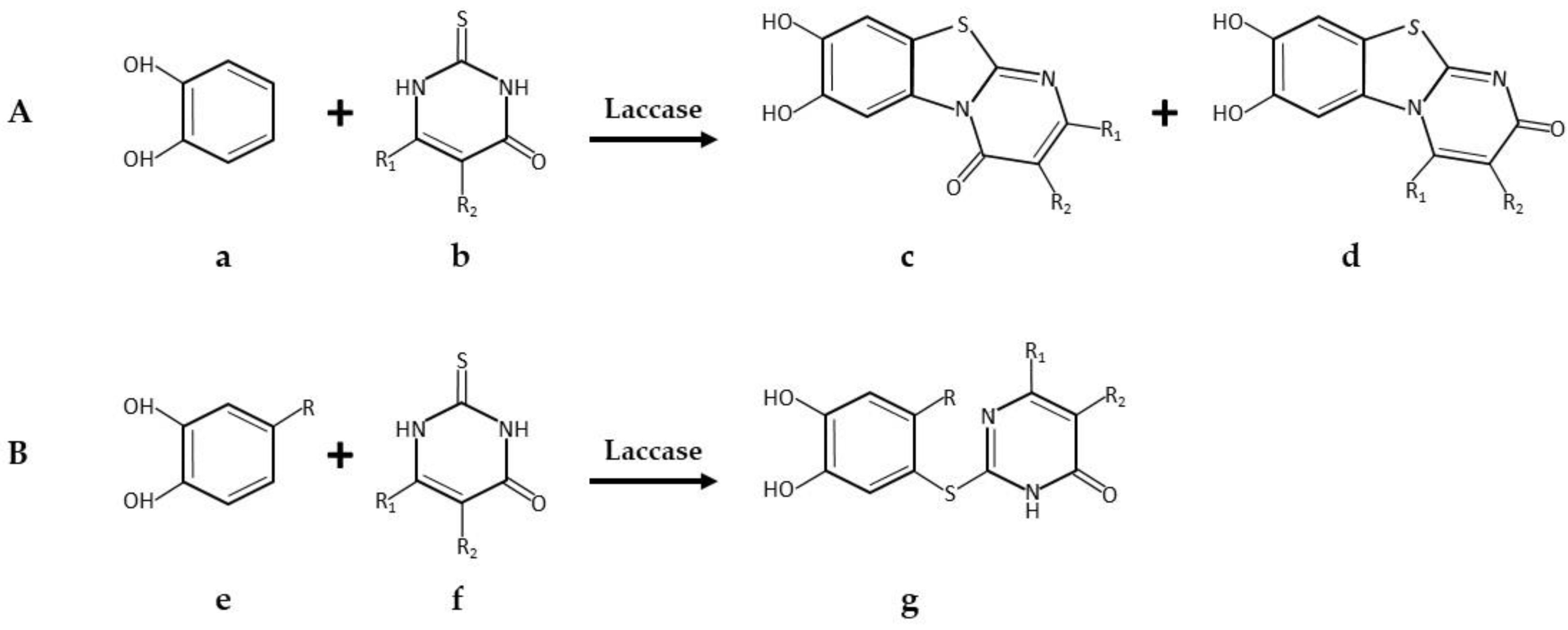
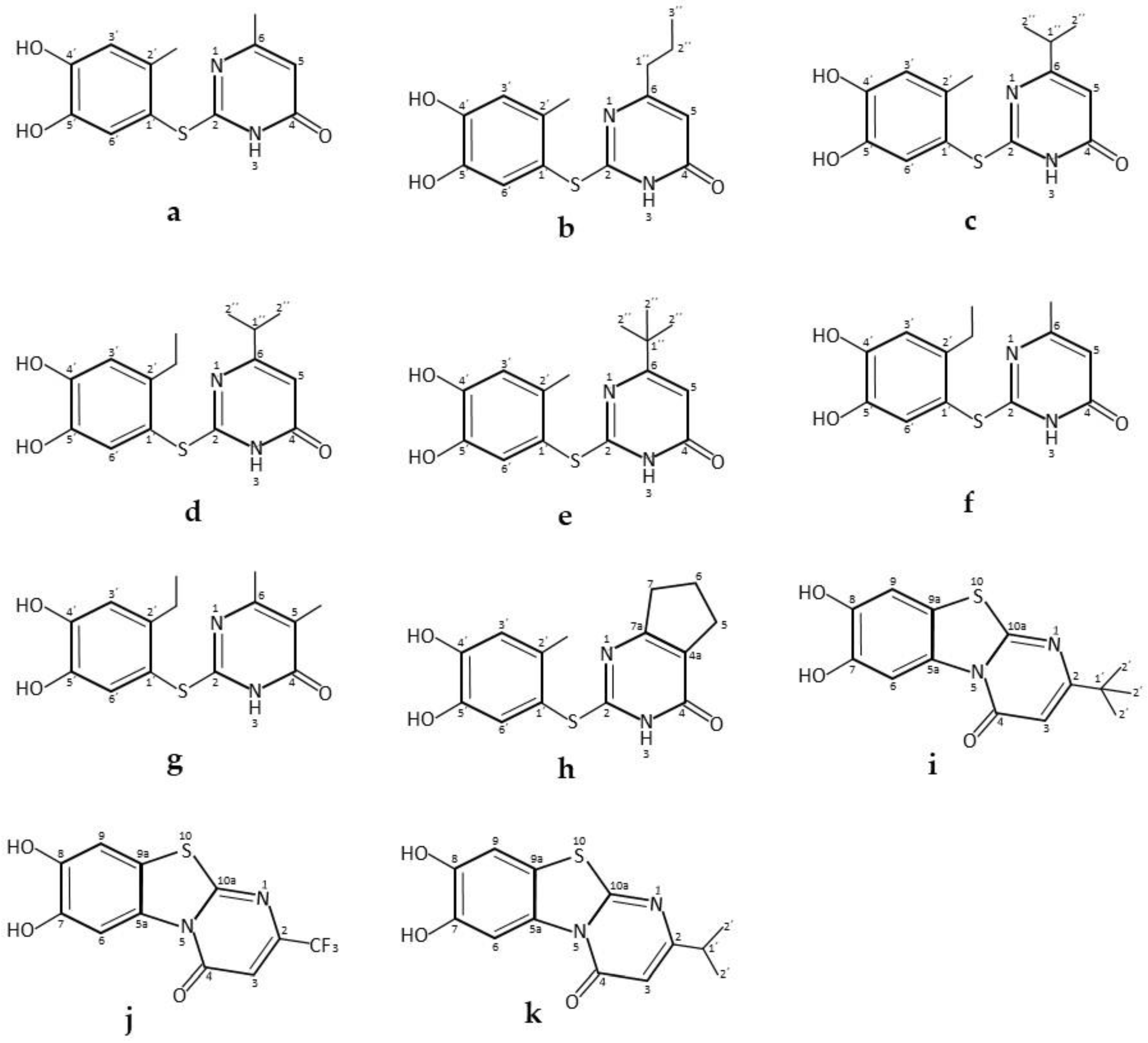
| * Pyrimido-benzothiazoles and catechol thioeters | Commercial laccase from Agaricus bisporus, ASA Spezialenzyme | 0.2 M phosphate buffer pH 6.0/10% ethanol; R.T.; air; 12 U laccase; 0.58 mmol catechol and 0.5 mmol 2,3-dihydro-2-thioxopyrimidin-4(1H)-ones. 14 h, 97% yield | [34] |
| 0.2 M phosphate buffer pH 6.0/10% ethanol; R.T.; air; 12 U laccase; 0.29 mmol catechol and 0.25 mmol 2,3-dihydro-2-thioxopyrimidin-4(1H)-ones. 14 h, 95% yield | [34] | ||
| Antioxidant compounds | |||
| * Catechin polymers | Commercial laccase from Trametes versicolor, Sigma Aldrich, conjugated with gum Arabic | 20 mM sodium citrate buffer pH 5.0/methanol in a ratio of 1.0:0.6; R.T.; 2300 U/mg gum Arabic conjugated laccase; 5 mmol catechin. 0.5 h | [35] |
| * Polyrutin | Commercial laccase from Trametes versicolor. | Methanol/water (30:70 v/v); 20 °C; air by stirring; 3 U/mL laccase; 50 g/L rutin. 24 h | [36] |
| * Polyesculin | Commercial laccase from Trametes versicolor | Methanol/water (30:70 v/v); 20 °C; air by stirring; 3 U/mL laccase; 50 g/L esculin. 72 h | [36] |
| * Catechin polymers | Commercial laccase from Trametes versicolor (2020), ASA Chemicals | 0.1 M acetate buffer pH 5.0 with 5% (v/v) of the natural deep eutectic solvents Betaine-Mannose (mole ratio 5:2), 30 °C, bubbling air; 125 U laccase, 17.23 mmol catechin (5 mg/mL). 1 h, 83% yield | [37] |
| Catechol thioeters | Non-commercial laccase from Agaricus bisporus | 0.2 M Phosphate buffer pH 6.0/Methanol 10%; R.T.; air; 150 U laccase; 1.25 mmol catechol and 1 mmol 2-mercaptobenzoxazole (thioeter). 16 h, 95% yield | [38] |
| Antimicrobial compounds | |||
| * ß-lactam antibiotics | Commercial laccase from Myceliophthora thermophila, Novozymes and commercial laccase from Trametes sp., ASA Spezialenzyme | Citrate-phosphate buffer (18 mM citrate, 165 mM phosphate) pH 7.0; R.T.; air by agitation; 10 mM β-lactams and 3 mM 2,5-dihydroxyphenylacetic acid. 4 h, 60% yield | [39] |
| Pyrimido-benzothiazole derivatives | Commercial laccase from Agaricus bisporus, ASA Spezialenzyme GmbH, Wolfenbüttel | 0.2 M phosphate buffer pH 6.0/methanol (85:15, v/v); 22 °C, air; 60 U laccase; 0.63 mmol catechol and 0.5 mmol. 15 h 1,2,3,4-tetrahydro-4-oxo-6-phenyl-2-thioxo-5-pyrimidinecarbonitrile. 15 h, 95% yield | [40] |
| Antiglycation compounds | |||
| * Catechin polymers | Commercial Denilite IIS, laccase from Aspergillus oryzae, Novozymes | 0.1 sodium acetate buffer pH 5.0/methanol 5%; R.T.; stirring 600 U laccase; 1 g (+)-Catechin hydrate. 24 h, >60% yield | [41] |
| New pharmacologically active substances | |||
| 1,3,9-Trioxa-fluoren-4-one derivatives | Non-commercial laccase from Pycnoporus cinnabarinus | 1 M phosphate/citrate buffer pH 7.0; 5 °C the first 2 h before increasing to R.T.; air by stirring; 400 U laccase; 1 mmol Meldrum’s acid and 2 mmol catechol. 12 h, 55% yield | [42] |
| Synthetic intermediates | |||
| Benzylic nitriles | Commercial laccase from Trametes villosa, Novo Nordisk | 0.1 M sodium phosphate buffer pH 7.0; 60 °C, bubbled O2; 10 U laccase; 1 mmol benzoylacetronitrile and 0.25 mmol methoxyhydroquinone. 24 h, 37% yield | [43] |
| 0.1 M sodium phosphate buffer pH 7.0; 60 °C, bubbled O2; 100 U laccase; 1 mmol benzoylacetronitrile and 0.25 mmol substituted hydroquinone. 24 h, 89% yield | [43] | ||
| Phenothiazones | Commercial laccase Not specified origin | 0.1 M sodium acetate buffer pH 5.0/methanol 10–15%; R.T.; 50 U laccase added after 2 h; 0.5 mmol 2-aminothiophenol and 0.625 mmol 1,4-quinone. 6 h, 61% yield | [44] |
| Ortho-dihydroxylated aromatic, mono-aminated and di-aminated compounds | Non-commercial laccase from Pycnoporus cinnabarinus. | 20 mM sodium acetate buffer pH 5.0; R.T.; air by agitation; 0.5 U laccase; 1 mM or 2 mM dihydroxylated compounds and 1 mM or 2 mM amines., 44% yield | [45] |
| Sulphonyl benzenediols | Commercial laccase from Tramates versicolor. | 0.1 M phosphate buffer pH 5.0; R.T.; O2; 40 U laccase; 1 mmol sodium sulphinate and 1 mmol benzenediols. 18 h, >99% yield | [46] |
| Quinoxalines | Non-commercial laccase from Ganoderma sp. rckk-02 | 1 M phosphate/citrate buffer pH 7.0; 25 °C; 200 U laccase; N,N′-dimethylethylenediamine and 1,2-dihydroxy benzene. 10 h, 61% yield | [47] |
| Microorganism | Activity Yield (U/mL) | Laccase Productivity (U/mL/d) | Fermentation Type | Inducer | Reference |
|---|---|---|---|---|---|
| Native fungal strains | |||||
| Cerrena unicolor strain C-139 | 250 and 450 | 35.7 and 32.1 | SmF, shake flask, 150 rpm, 30 °C | 1 mM Cu2+, wheat bran | [81] |
| Cerrena unicolor strain C-139 | 416.4 | 34.7 | SmF, 120-L STR (130–420 rpm), 30 °C | 1 mM Cu2+, wheat bran | [82] |
| Cerrena sp. strain HYB07 | 280 | 56 | SmF, shake flask, 200 rpm, 30 °C | 0.25 mM Cu2+ | [83] |
| Cerrena unicolor strain GSM- 01 | 2800 * | 350 | SmF, shake flask, 170 rpm, 28 °C | 1.0 mM Cu2+ | [69] |
| Cerrena sp. WR1 | 202 | 15.5 | SmF, 5-L STR (200 rpm), 25 °C | 2 mM 2,5-xylidine | [84] |
| Coriolopsis gallica 1184 | 200.9 | 28.7 | SmF, 50-L STR, (200–300 rpm), 30 °C | 0.2 mM Vanillin | [85] |
| Trametes pubescens | 333 and 743 | 19.7 and 25.7 | SmF, 20-L STR (100 rpm), 25 °C | 2 mM Cu2+ | [86] |
| white rot fungus WR-1 | 692 | 115 | SmF, shake flask, 150 rpm, 28 °C | 0.8 mM 2,5-xylidine, 1 mM Cu2+ | [68] |
| Native bacterial strains | |||||
| Arthrospira maxima | 56.9 | 14.2 | SmF, shake flask, swirled twice a day for mixing, 30 °C | 0.1 mM guaiacol, 2 mM Cu2+ | [87] |
| Bacillus subtilis DS | 820 a | 205 | SmF, shake flask, NA rpm, 37 °C | 0.15 mM vanillic acid, 0.15 mM MgSO4, 5 × 10−5 mM Cu2+ | [88] |
| Bacillus tequilensis SN4 | 1101 b | 275 | SmF, shake flask, 150 rpm, 30 °C | 0.65 mM MnSO4, 0.35 mM FeSO4, 3.5% Ethanol | [70] |
| Recombinant laccase source (Host) | |||||
| Trametes sp. C30 (Aspergillus niger) | 42 a | 6 | SmF, 0.5-L STR, (NA rpm), 28 °C | NA | [89] |
| Gaeumannomyces graminis (Cryptococcus sp. S-2) | 380 | 47.5 | SmF, 2-L STR, (NA rpm), 25 °C | 1.2 mM Cu2+ | [90] |
| Cerrena sp. HYB07 (Pichia pastoris) | 6.3 | 0.7 | SmF, shake flask, 200 rpm, 30 °C | 1 mM Cu2+ | [91] |
| Pleurotus ostreatus (Aspergillus niger) | 60 | 12 | SmF, shake flask, 150 rpm, 28 °C | 0.1 mM Cu2+, 0.2 mM ABTS | [92] |
| Pleurotus ostreatus (Pichia pastoris) | 60 | 15 | SmF, Fed-batch fermentation, NA rpm, 28 °C | glycerol | [75] |
| Trametes sp. 420 (Pichia pastoris) | 239 | 31.8 | SmF, Fed-batch fermentation, NA rpm, 28 °C | NA | [93] |
| Trametes versicolor (Pichia pastoris GS115) | 34.2 | 2.3 | SmF, shake flask, 180 rpm, 28 °C | 0.5 mM Cu2+ 0.6% (v/v) methanol | [94] |
| Escherichia coli K12 (Pichia pastoris GS115) | 41 | 8 | SmF, 5-L STR, NA rpm, 28 °C | 0.5 mM Cu2+ 0.6% (v/v) methanol | [73] |
| Bacillus subtilis 168 (Escherichia coli BL21) | 13.8 | 9.7 | SmF, shake flask, 200 rpm, 37 °C | 1 mM IPTG, micro-aerobic cultivation | [95] |
| Bacillus pumilus CotA (Escherichia coli) | 4.2 | 4.5 | SmF, shake flask, 160 rpm, 30 °C | 1 mM IPTG, 0.25 mM CuCl2, oxygen limitation | [96] |
| Immobilization System | Result | Reference |
|---|---|---|
| Graphene oxide/CuFe2O4/laccase nanocomposite | Recovered and reused up to 10 times with little loss of activity | [102] |
| Cross-linked protein-metal hybrid nanoflower | It showed high oxidation potential (265% that of the free enzyme), and retained the residual enzyme efficiency of up to 84.6% under repeated batch conditions of 10 cycles | [108] |
| Chitosan crosslinked with genipin | Improved pH, thermal, and storage stabilities of when compared with the free counterpart. The chitosan laccase system exhibited a residual activity of >55% after 11 cycles | [109] |
| polyacrylonitrile-biochar composite nanofibrous membrane | High conversion for after 8 h of reaction | [110] |
| Cu (II)-chelated chitosan-graft-poly (glycidyl methacrylate) nanoparticles | After eight cycles of continuous use, the immobilized enzyme retained above 50% | [103] |
| Polyacrylonitrile-biochar composite nanofibrous membrane | Retained more than 50% of its initial activity after 7 cycles of ABTS oxidation which indicated improved enzyme reusability | [104] |
| Fungal laccase immobilized on the surface of yeast cells | Retained 74% of initial activity after eight repeated batch reactions | [107] |
| Granular activated carbon (GAC) and the resulting GAC-bound laccase | Immobilized GAC outperformed regular granular activated carbon during continuous operation of packed-bed columns over two months | [106] |
| Magnetic mesoporous silica microbeads | Exhibited good operational stability, maintaining up to 70% of its initial activity after 10 successive batch reactions | [111] |
| Cross-linked enzyme aggregates (MAC-CLEAs) on magnetic nanoparticles and chitosan | The catalytic activity was maintained even after a hundred and fifty batch reactions | [101] |
| Porous silica beads | After ten cycles of removal experiments up to 82.6% of the initial activity | [112] |
| Ceramic membrane reactor | Able to reach a constant degradation rate of 0.34 mg of tetracycline per hour during 10 days | [113] |
| Fumed silica. | Enzyme activation by immobilization (164% activity). 80% residual activity after 7 days. | [105] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Guido, C.; Baez, A.; Torres, E. Dioxygen Activation by Laccases: Green Chemistry for Fine Chemical Synthesis. Catalysts 2018, 8, 223. https://doi.org/10.3390/catal8060223
Romero-Guido C, Baez A, Torres E. Dioxygen Activation by Laccases: Green Chemistry for Fine Chemical Synthesis. Catalysts. 2018; 8(6):223. https://doi.org/10.3390/catal8060223
Chicago/Turabian StyleRomero-Guido, Cynthia, Antonino Baez, and Eduardo Torres. 2018. "Dioxygen Activation by Laccases: Green Chemistry for Fine Chemical Synthesis" Catalysts 8, no. 6: 223. https://doi.org/10.3390/catal8060223
APA StyleRomero-Guido, C., Baez, A., & Torres, E. (2018). Dioxygen Activation by Laccases: Green Chemistry for Fine Chemical Synthesis. Catalysts, 8(6), 223. https://doi.org/10.3390/catal8060223





