Dehydrogenation Catalysts for Synthesis of O-Phenylphenol via Cu/Ni/Mg/Al Hydrotalcite-Like Compounds as Precursors
Abstract
:1. Introduction
2. Results and Discussions
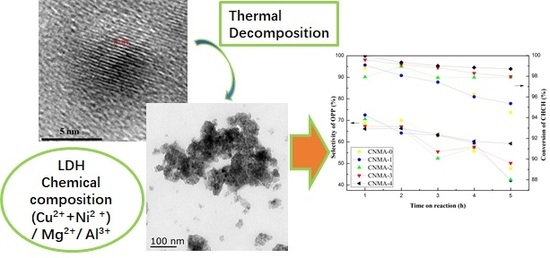
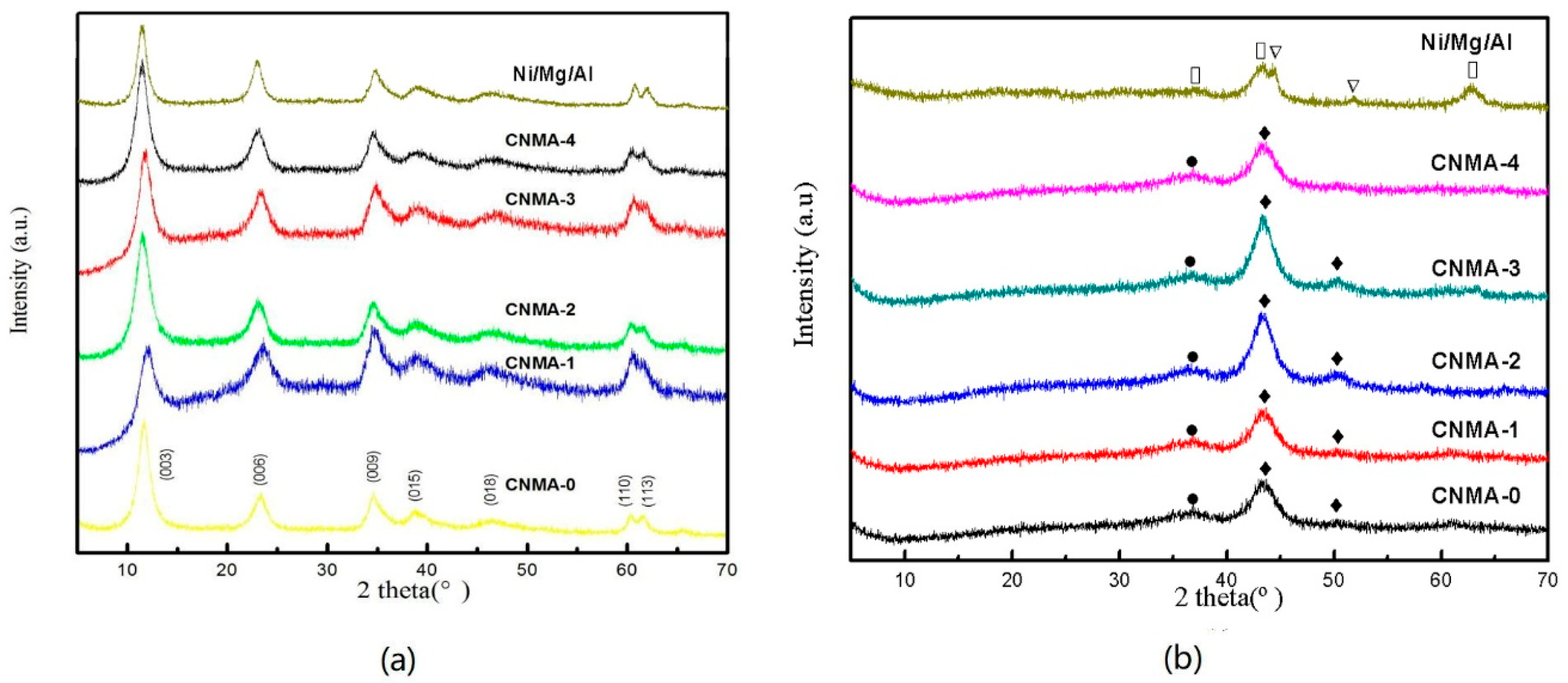
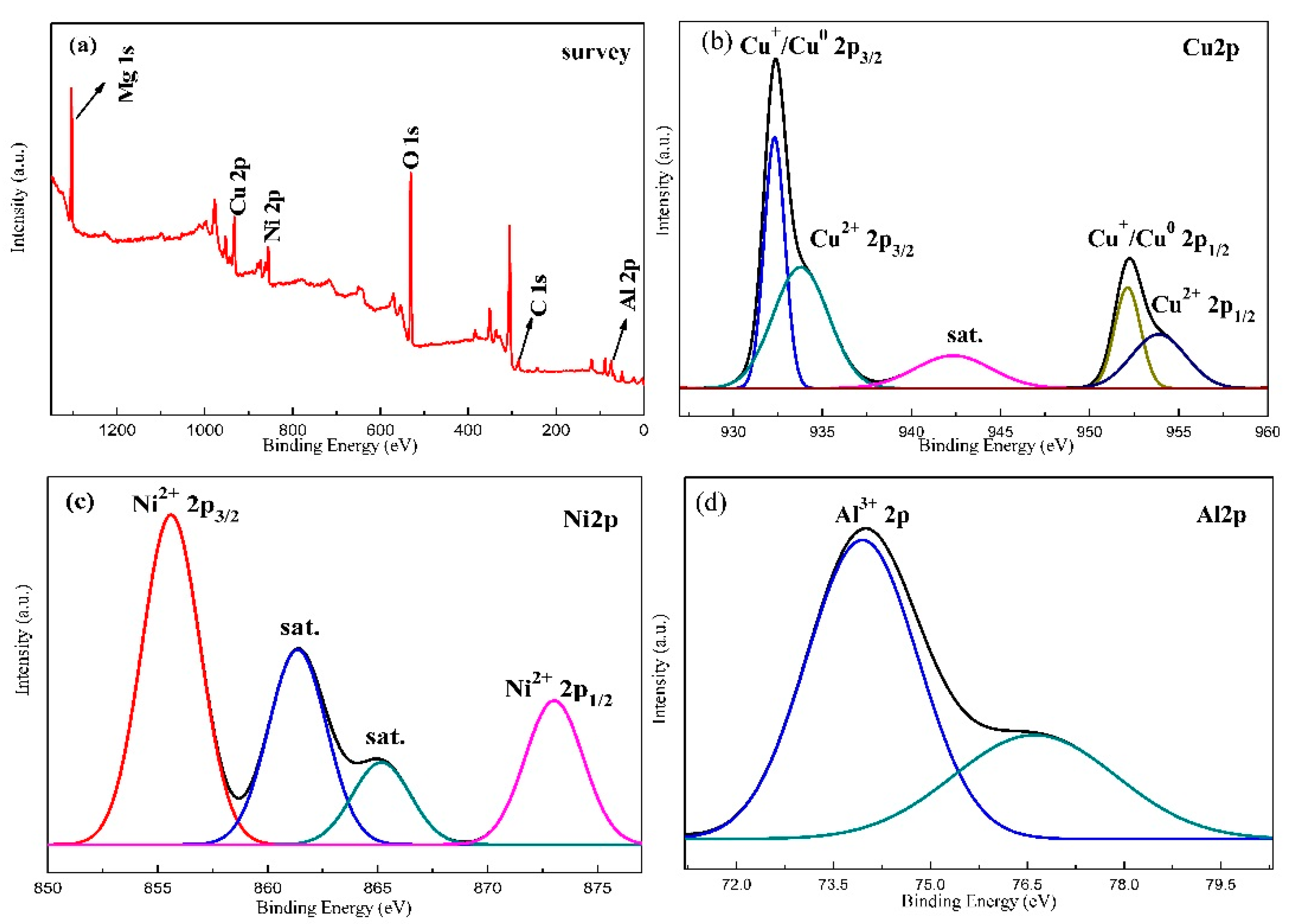
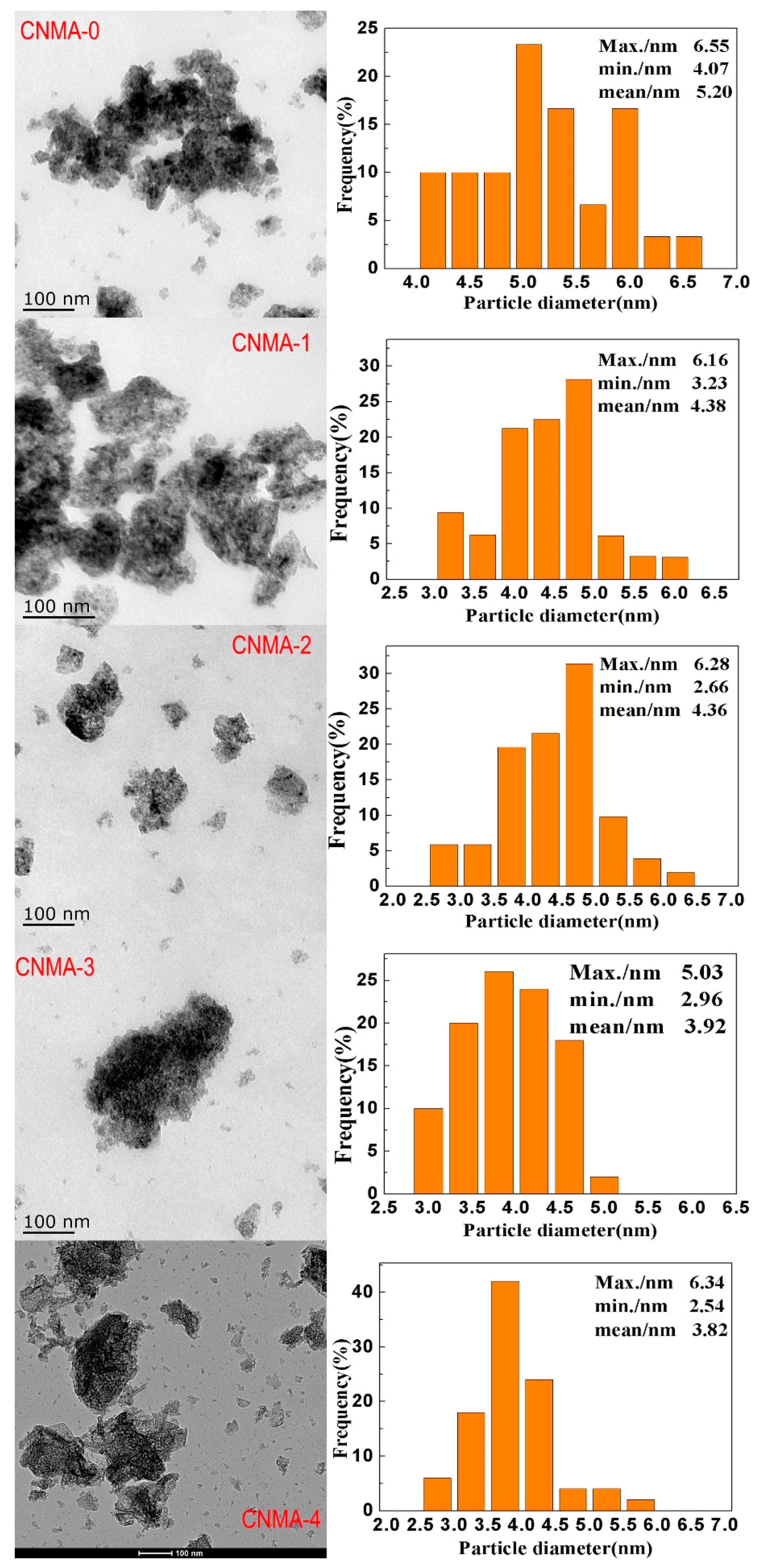
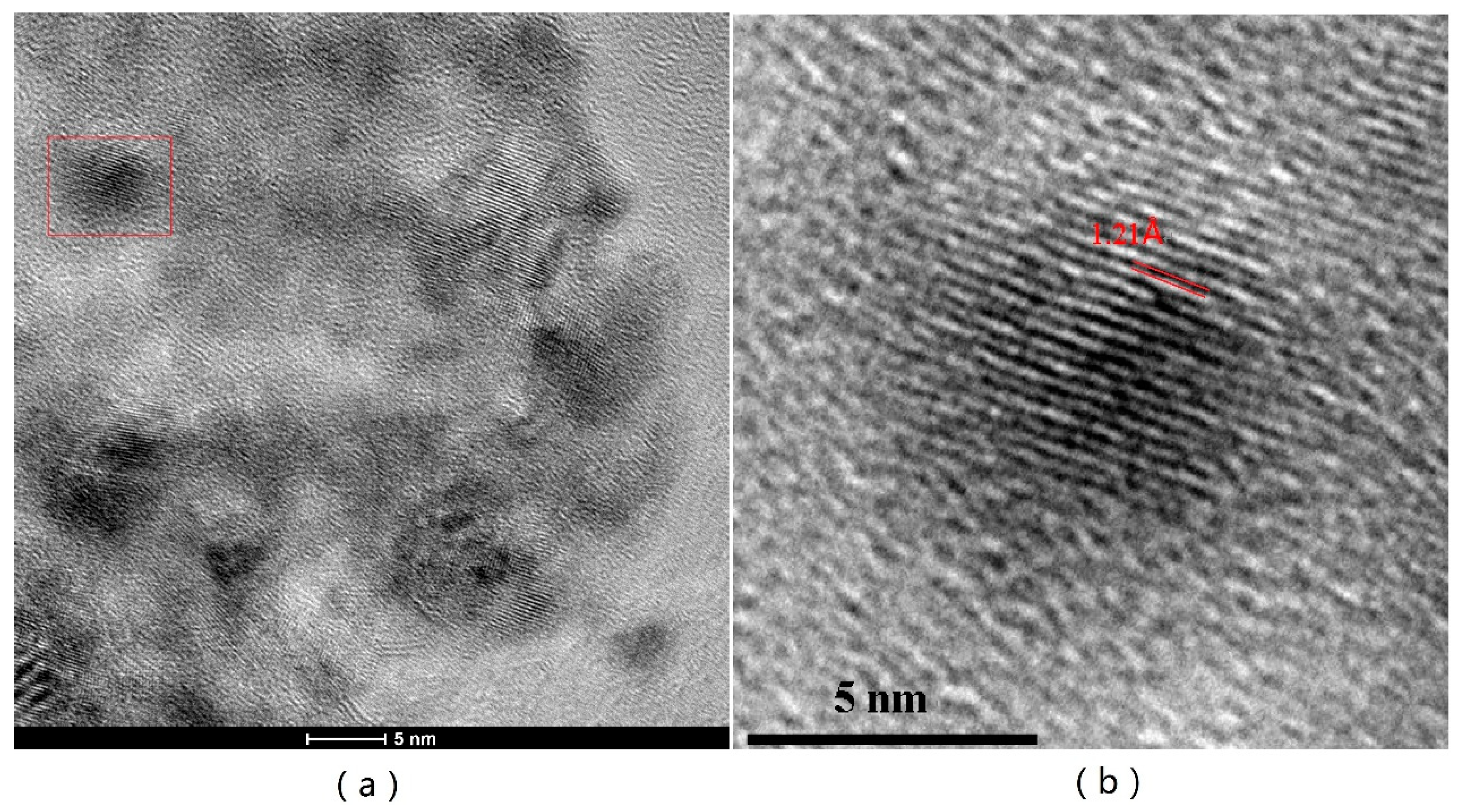
2.1. Characterization of Structural Properties
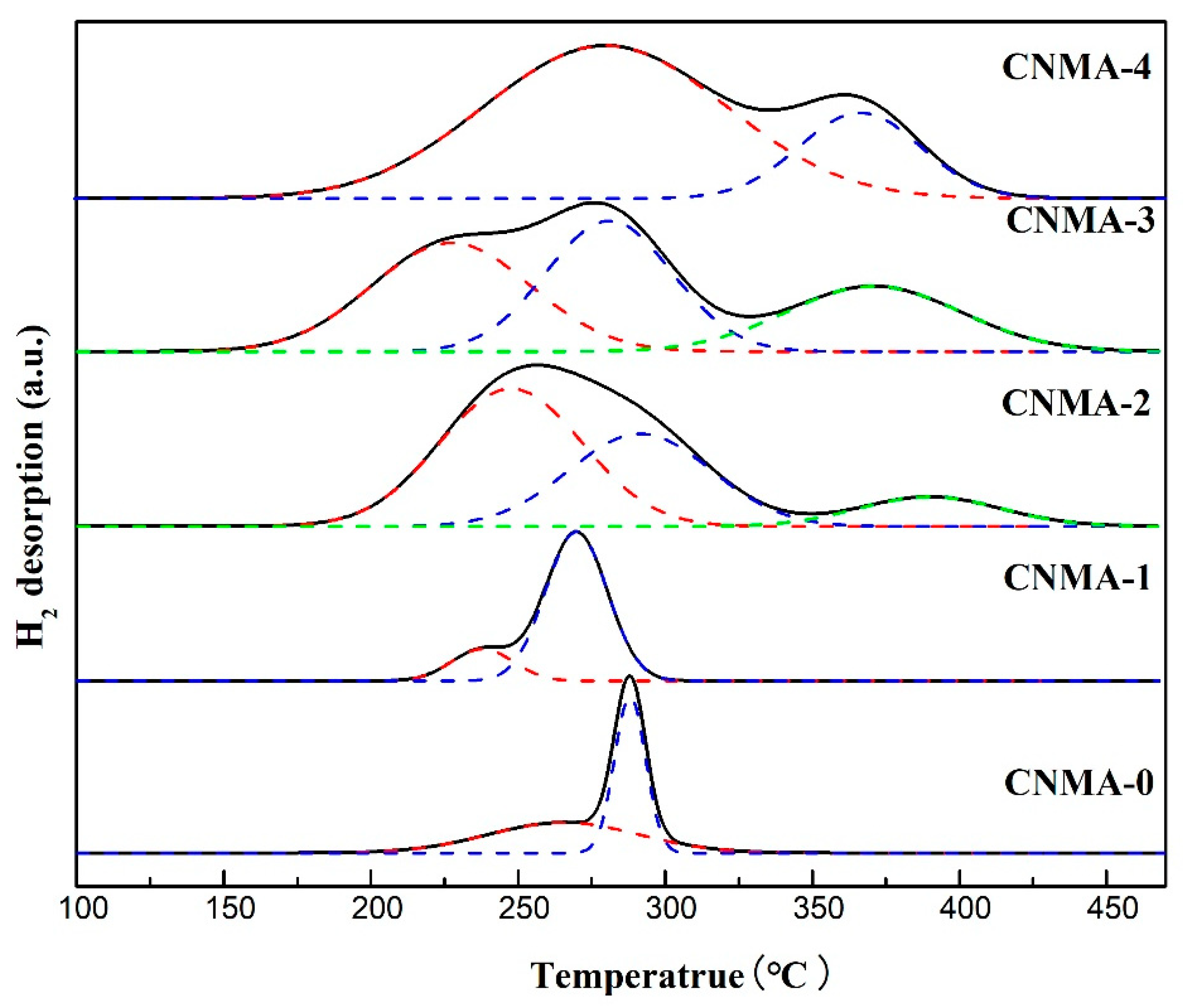
2.2. H2-TPR
2.3. CO2-TPD
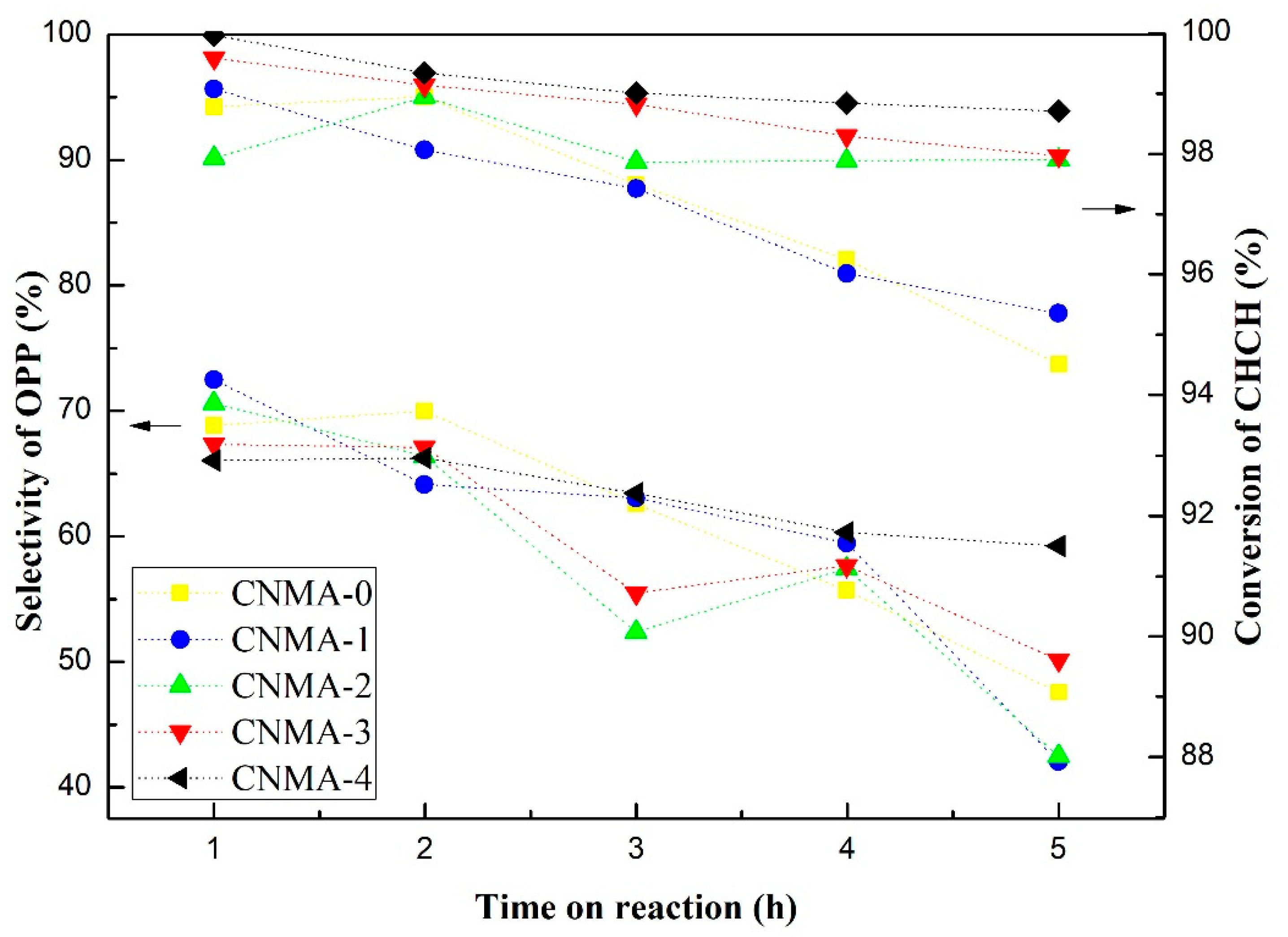
2.4. Catalytic Performance
3. Experimental
3.1. Preparation of Samples
3.2. Sample Characterization
3.3. Catalytic Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hu, R.; Xiao, X.; Tu, S.; Zuo, X.; Nan, J. Synthesis of flower-like heterostructured β-Bi2O3/Bi2O2CO3 microspheres using Bi2O2CO3 self-sacrifice precursor and its visible-light-induced photocatalytic degradation of o-phenylphenol. Appl. Catal. B Environ. 2015, 163, 510–519. [Google Scholar] [CrossRef]
- Bomhard, E.M.; Brendler-Schwaab, S.Y.; Freyberger, A.; Herbold, B.A.; Leser, K.H.; Richter, M. O-phenylphenol and its sodium and potassium salts: A toxicological assessment. Crit. Rev. Toxicol. 2002, 32, 551–626. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, T.; Xu, Y.; Ye, T.; Wang, R.; Yang, Z.; Jia, Z.; Ju, S. Cu/mg/al hydrotalcite-like hydroxide catalysts for o-phenylphenol synthesis. Appl. Clay. Sci. 2016, 126, 207–214. [Google Scholar] [CrossRef]
- Goto, H.; Shibamoto, N.; Tanaka, S. Process for Preparing O-Phenylphenol. U.S. Patents 4088702, 29 November 1977. [Google Scholar]
- Imafuku, K.; Oda, J.; Itoh, K.; Matsumura, H. Dehydrogenation of 2-(1-cyclohexenyl) cyclohexanone with palladium catalyst. Bull. Chem. Soc. Jpn. 1974, 47, 1201–1202. [Google Scholar] [CrossRef]
- Matsumura, H.; Imafuku, K.; Takano, I.; Matsuura, S. Rearrangements in the palladium-catalyzed dehydrogenation of cyclohexylphenols to phenylphenols. Bull. Chem. Soc. Jpn. 1971, 44, 567. [Google Scholar] [CrossRef]
- Jin, D.; Zhu, B.; Hou, Z.; Fei, J.; Lou, H.; Zheng, X. Dimethyl ether synthesis via methanol and syngas over rare earth metals modified zeolite y and dual Cu-Mn-Zn catalysts. Fuel 2007, 86, 2707–2713. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Zhang, Y.-S.; Li, C.; Yu, C.; Tran, T.; Guo, F.; Yang, Y.; Yu, J.; Xu, G. Synthesis, characterization and activity evaluation of Cu-based catalysts derived from layered double hydroxides (ldhs) for denox reaction. Chem. Eng. J. 2017, 330, 1082–1090. [Google Scholar] [CrossRef]
- Patankar, S.C.; Yadav, G.D. Cascade engineered synthesis of 2-ethyl-1-hexanol from n-butanal and 2-methyl-1-pentanol from n-propanal using combustion synthesized Cu/Mg/Al mixed metal oxide trifunctional catalyst. Catal. Today 2017, 291, 223–233. [Google Scholar] [CrossRef]
- Jiang, Z.; Kong, L.; Chu, Z.; France, L.J.; Xiao, T.; Edwards, P.P. Catalytic combustion of propane over mixed oxides derived from CuxMg3-xAl hydrotalcites. Fuel 2012, 96, 257–263. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Beltramini, J.N.; Lin, C.-X.; Xu, Z.-P.; Lu, G.Q.; Tanksale, A. Selective oxidation of biorenewable glycerol with molecular oxygen over Cu-containing layered double hydroxide-based catalysts. Catal. Sci. Technol. 2011, 1, 111–122. [Google Scholar] [CrossRef]
- Shi, G.; Jin, K.; Su, L.; Shen, J. Synthesis of Ni/SiO2 catalysts and their performances in glycerol hydrogenolysis to 1,2-pranpanediol. J. Mol. Catal. 2015, 29, 403–413. [Google Scholar]
- Shi, G.; Su, L.; Jin, K. New bulk nickel phosphide catalysts for glycerol hydrogenolysis to 1,2-propanediol. Catal. Commun. 2015, 59, 180–183. [Google Scholar] [CrossRef]
- Alejandre, A.; Medina, F.; Rodriguez, X.; Salagre, P.; Cesteros, Y.; Sueiras, J.E. Cu/Ni/Al layered double hydroxides as precursors of catalysts for the wet air oxidation of phenol aqueous solutions. Appl. Catal. B Environ. 2001, 30, 195–207. [Google Scholar] [CrossRef]
- Marino, F.; Baronetti, G.; Jobbagy, M.; Laborde, M. Cu-ni-k/gamma-al2o3 supported catalysts for ethanol steam reforming formation of hydrotalcite-type compounds as a result of metal-support interaction. Appl. Catal. A Gen. 2003, 238, 41–54. [Google Scholar] [CrossRef]
- Mott, D.; Galkowski, J.; Wang, L.; Luo, J.; Zhong, C.-J. Synthesis of size-controlled and shaped copper nanoparticles. Langmuir 2007, 23, 5740–5745. [Google Scholar] [CrossRef] [PubMed]
- Reichle, W.T.; Kang, S.Y.; Everhardt, D.S. The nature of the thermal decomposition of a catalytically active anionic clay mineral. J. Catal. 1986, 101, 352–359. [Google Scholar] [CrossRef]
- Kannan, S.; Dubey, A.; Knozinger, H. Synthesis and characterization of cumgal ternary hydrotalcites as catalysts for the hydroxylation of phenol. J. Catal. 2005, 231, 381–392. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, C.; Ye, X.; Wu, Y. Catalysis of hydrotalcite-like compounds in liquid phase oxidation: (i) phenol hydroxylation. Appl. Catal. A Gen. 1998, 168, 365–372. [Google Scholar] [CrossRef]
- Dubey, A.; Kannan, S.; Velu, S.; Suzuki, K. Catalytic hydroxylation of phenol over CuM(II)M(III) ternary hydrotalcites, where M(II) = Ni or Co and M(III) = Al, Cr or Fe. Appl. Catal. A Gen. 2003, 238, 319–326. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Wang, P.; Kosaka, T.; Takaki, K. Autothermal reforming of CH4 over supported Ni catalysts prepared from Mg-Al hydrotalcite-like anionic clay. J. Catal. 2004, 221, 43–54. [Google Scholar] [CrossRef]
- De Bokx, P.; Wassenberg, W.; Geus, J. Interaction of nickel ions with a γ-Al2O3 support during deposition from aqueous solution. J. Catal. 1987, 104, 86–98. [Google Scholar] [CrossRef]
- Ni, Z.; Chen, A.; Fang, C.; Wang, L.; Yu, W. Enhanced nox adsorption using calcined Zn/Mg/Ni/Al hydrotalcite-like compounds. J. Phys. Chem. Solids 2009, 70, 632–638. [Google Scholar] [CrossRef]
- Costantino, U.; Curini, M.; Montanari, F.; Nocchetti, M.; Rosati, O. Hydrotalcite-like compounds as catalysts in liquid phase organic synthesis: I. Knoevenagel condensation promoted by [Ni0.73Al0.27(OH)2](CO3)0.135. J. Mol. Catal. A Chem. 2003, 195, 245–252. [Google Scholar] [CrossRef]
- Jitianu, M.; Bãlãsoiu, M.; Marchidan, R.; Zaharescu, M.; Crisan, D.; Craiu, M. Thermal behaviour of hydrotalcite-like compounds: Study of the resulting oxidic forms. Int. J. Inorg. Mater. 2000, 2, 287–300. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V. Infrared spectroscopic identification of species arising from reactive adsorption of carbon oxides on metal oxide surfaces. Mater. Chem. 1982, 7, 89–126. [Google Scholar] [CrossRef]
- Anillo, A.A.; Villa-Garcı́a, M.A.A.; Llavona, R.; Suárez, M.; Rodrı́guez, J. Textural properties of α-titanium(iv) phenylphosphonate: Influence of preparation conditions. Mater. Res. Bull. 1999, 34, 627–640. [Google Scholar] [CrossRef]
- Kubiak, G.D.; Sitz, G.O.; Zare, R.N. Recombinative desorption dynamics: Molecular hydrogen from Cu(110) and Cu(111). J. Chem. Phys. 1985, 83, 2538–2551. [Google Scholar] [CrossRef]
- Guo, X.; Mao, D.; Lu, G.; Wang, S.; Wu, G. Glycine–nitrate combustion synthesis of CuO–ZnO–ZrO2 catalysts for methanol synthesis from CO2 hydrogenation. J. Catal. 2010, 271, 178–185. [Google Scholar] [CrossRef]
- Li, D.; Koike, M.; Chen, J.; Nakagawa, Y.; Tomishige, K. Preparation of Ni–Cu/Mg/Al catalysts from hydrotalcite-like compounds for hydrogen production by steam reforming of biomass tar. Int. J. Hydrog. Energy 2014, 39, 10959–10970. [Google Scholar] [CrossRef]
- Rynkowski, J.M.; Paryjczak, T.; Lenik, M. On the nature of oxidic nickel phases in NiO/γ-Al2O3 catalysts. Appl. Catal. A Gen. 1993, 106, 73–82. [Google Scholar] [CrossRef]
- Flores, J.H.; Peixoto, D.P.B.; Appel, L.G.; de Avillez, R.R.; Silva, M.I.P.D. The influence of different methanol synthesis catalysts on direct synthesis of dme from syngas. Catal. Today 2011, 172, 218–225. [Google Scholar] [CrossRef]
- Shi, G.; Fang, D.; Shen, J. Hydroisomerization of model FCC naphtha over sulfided Co(Ni)–Mo(W)/MCM-41 catalysts. Mesoporous. Mater. 2009, 120, 339–345. [Google Scholar] [CrossRef]
- Dębek, R.; Radlik, M.; Motak, M.; Galvez, M.E.; Turek, W.; Da Costa, P.; Grzybek, T. Ni-containing Ce-promoted hydrotalcite derived materials as catalysts for methane reforming with carbon dioxide at low temperature—On the effect of basicity. Catal. Today 2015, 257, 59–65. [Google Scholar] [CrossRef]
- Marcu, I.-C.; Tichit, D.; Fajula, F.; Tanchoux, N. Catalytic valorization of bioethanol over Cu-Mg-Al mixed oxide catalysts. Catal. Today 2009, 147, 231–238. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Balasamy, R.J.; Tope, B.B.; Khurshid, A.; Al-Ali, A.A.S.; Atanda, L.A.; Sagata, K.; Asamoto, M.; Yahiro, H.; Nomura, K.; Sano, T.; et al. Ethylbenzene dehydrogenation over FeOx/(Mg,Zn)(Al)O catalysts derived from hydrotalcites: Role of MgO as basic sites. Appl. Catal. A Gen. 2011, 398, 113–122. [Google Scholar] [CrossRef]
- Wang, L.-C.; Liu, Q.; Chen, M.; Liu, Y.-M.; Cao, Y.; Fan, K.-N. Structural evolution and catalytic properties of nanostructured cu/zro2 catalysts prepared by oxalate gel-coprecipitation technique. J. Phys. Chem. C 2007, 111, 16549–16557. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, T.; Xu, Y.; Hu, P.; Ye, T.; Jia, Z.; Ju, S. Cu/Mg/Al/Zr non-noble metal catalysts for o-phenylphenol synthesis. RSC Adv. 2016, 6, 6737–6746. [Google Scholar] [CrossRef]
- Jiang, Z.; Hao, Z.; Yu, J.; Hou, H.; Hu, C.; Su, J. Catalytic combustion of methane on novel catalysts derived from Cu-Mg/Al-hydrotalcites. Catal. Lett. 2005, 99, 157–163. [Google Scholar] [CrossRef]
- Auer, S.M.; Gredig, S.V.; Köppel, R.A.; Baiker, A. Synthesis of methylamines from CO2, H2 and NH3 over Cu-Mg-Al mixed oxides. J. Mol. Catal. A Chem. 1999, 141, 193–203. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, T.; Li, K.; Cao, Y.; Zeng, Y. Dehydrogenation Catalysts for Synthesis of O-Phenylphenol via Cu/Ni/Mg/Al Hydrotalcite-Like Compounds as Precursors. Catalysts 2018, 8, 186. https://doi.org/10.3390/catal8050186
Wang J, Zhang T, Li K, Cao Y, Zeng Y. Dehydrogenation Catalysts for Synthesis of O-Phenylphenol via Cu/Ni/Mg/Al Hydrotalcite-Like Compounds as Precursors. Catalysts. 2018; 8(5):186. https://doi.org/10.3390/catal8050186
Chicago/Turabian StyleWang, Jilong, Tianchi Zhang, Kai Li, Yanan Cao, and Yongping Zeng. 2018. "Dehydrogenation Catalysts for Synthesis of O-Phenylphenol via Cu/Ni/Mg/Al Hydrotalcite-Like Compounds as Precursors" Catalysts 8, no. 5: 186. https://doi.org/10.3390/catal8050186
APA StyleWang, J., Zhang, T., Li, K., Cao, Y., & Zeng, Y. (2018). Dehydrogenation Catalysts for Synthesis of O-Phenylphenol via Cu/Ni/Mg/Al Hydrotalcite-Like Compounds as Precursors. Catalysts, 8(5), 186. https://doi.org/10.3390/catal8050186