Pyrene-Based Conjugated Polymer/Bi2MoO6 Z-Scheme Hybrids: Facile Construction and Sustainable Enhanced Photocatalytic Performance in Ciprofloxacin and Cr(VI) Removal under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
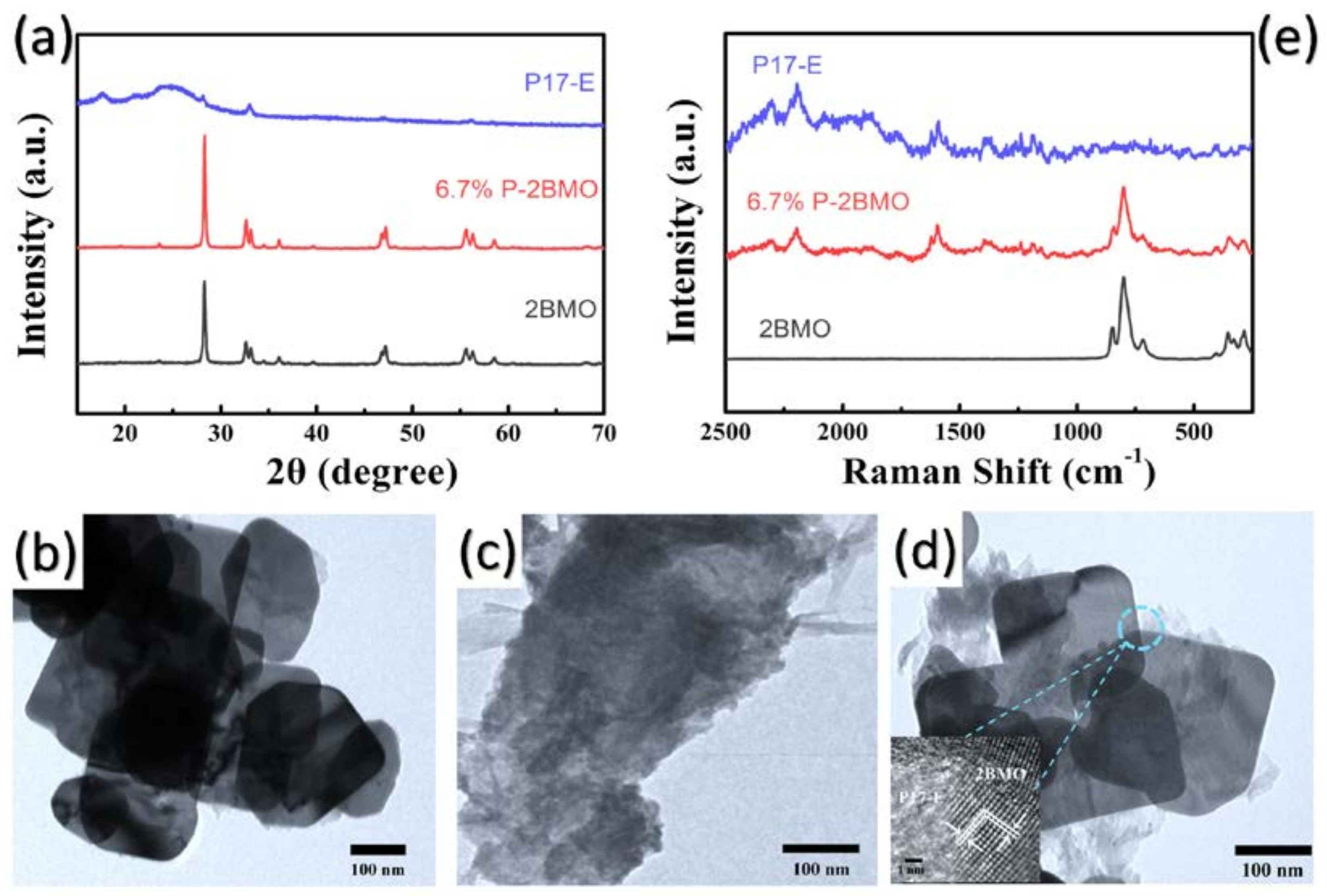
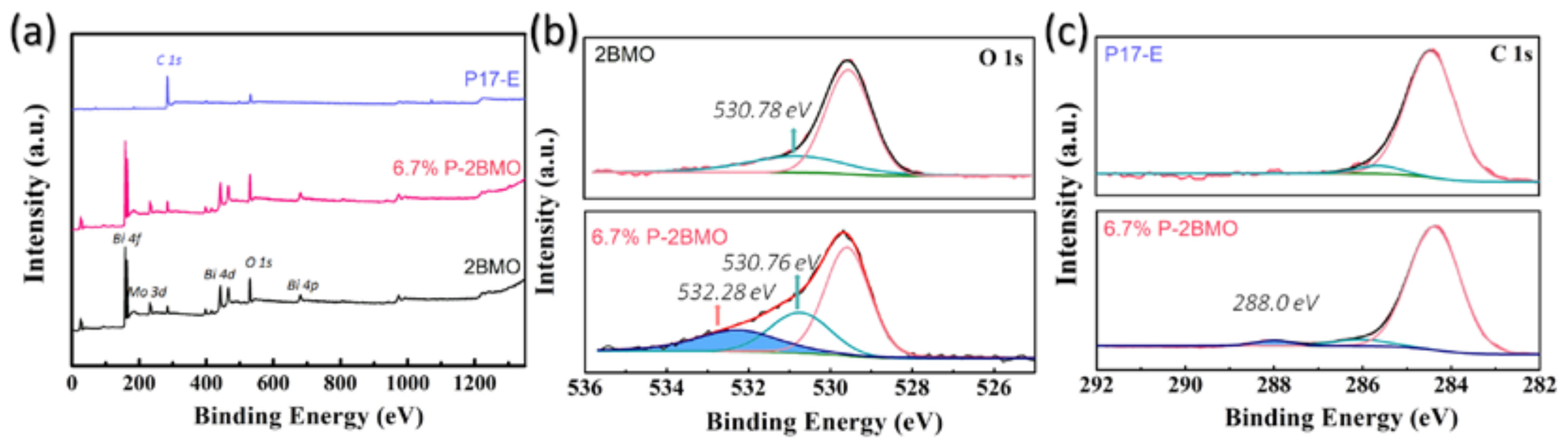
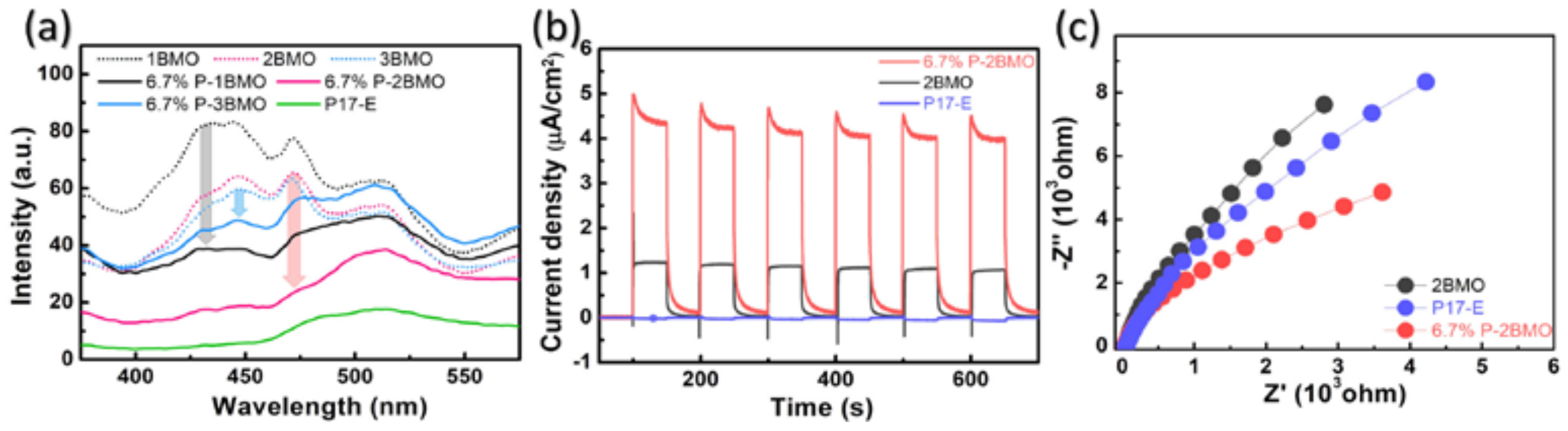
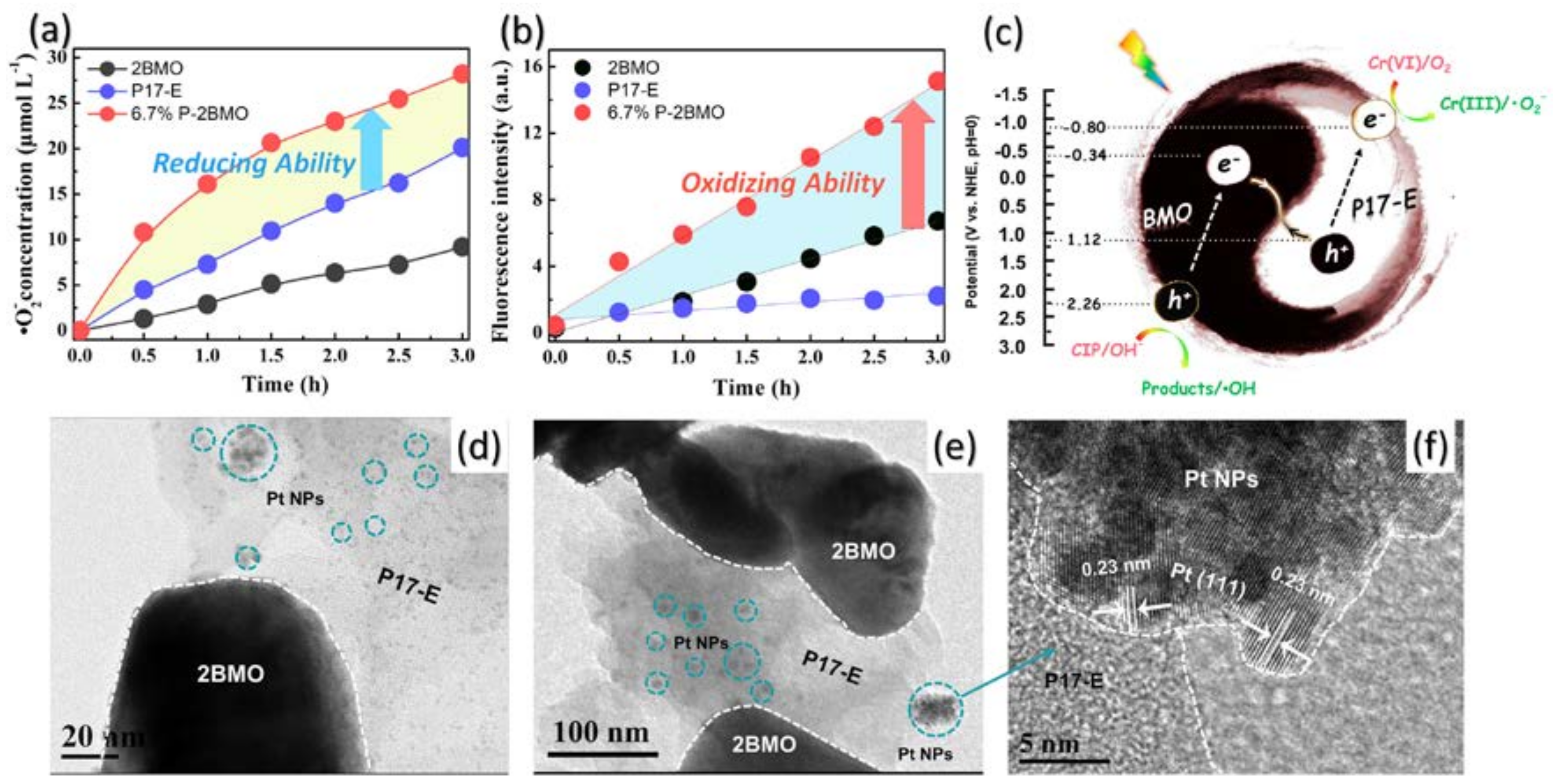
2.1. Characterization of the Samples
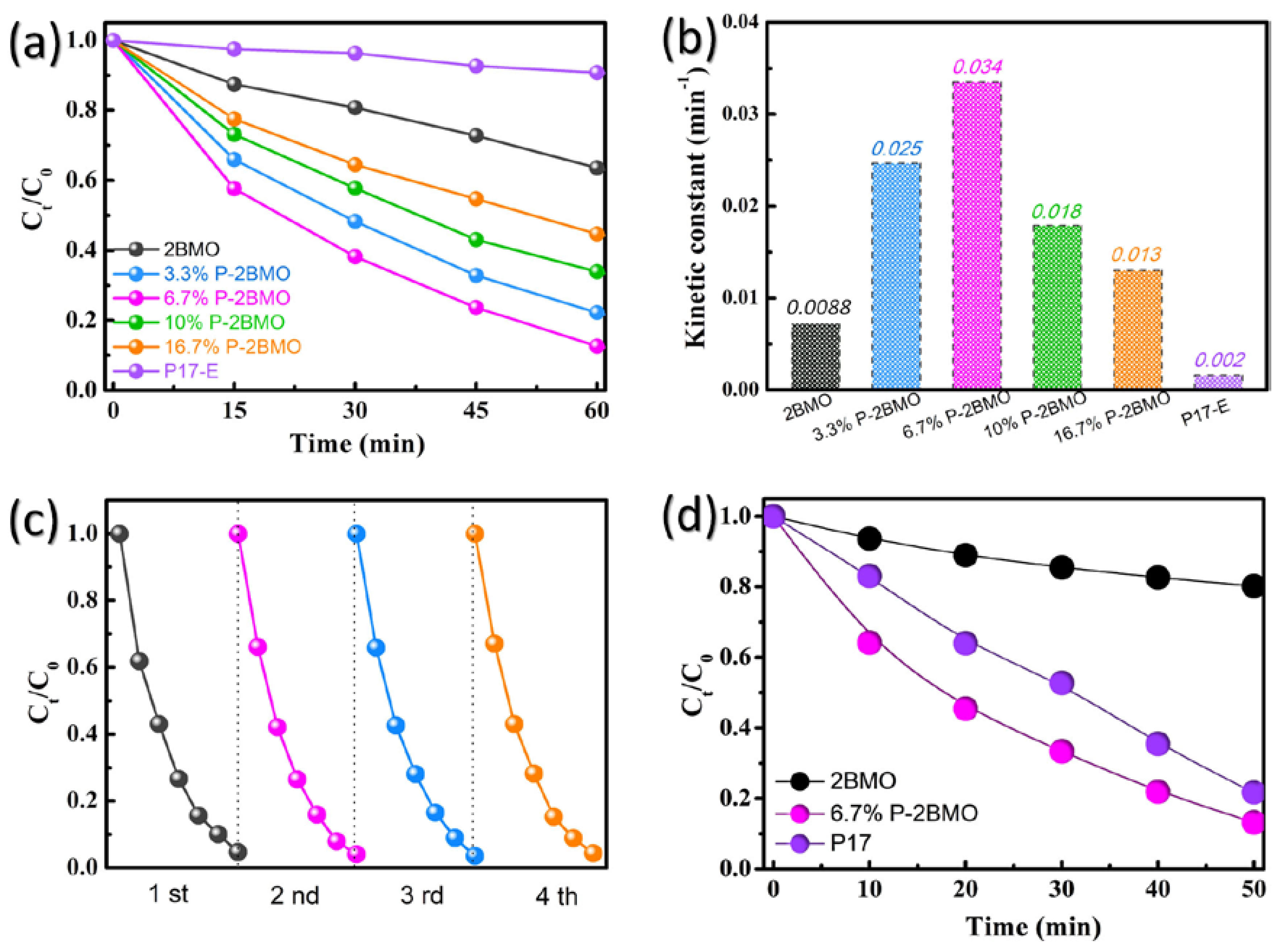
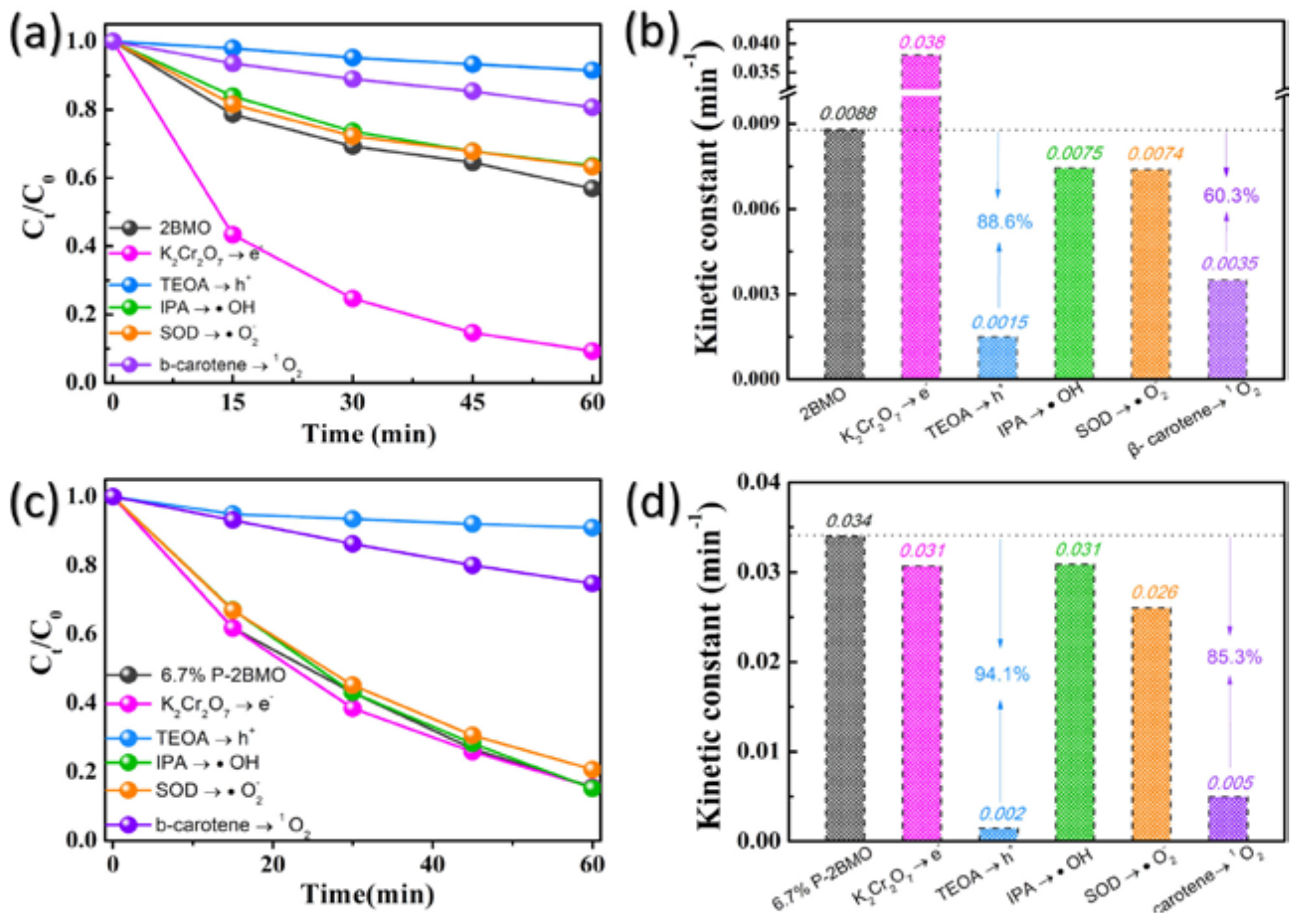
2.2. Photocatalytic Performance of P-2BMO
2.3. Strategy Evaluation
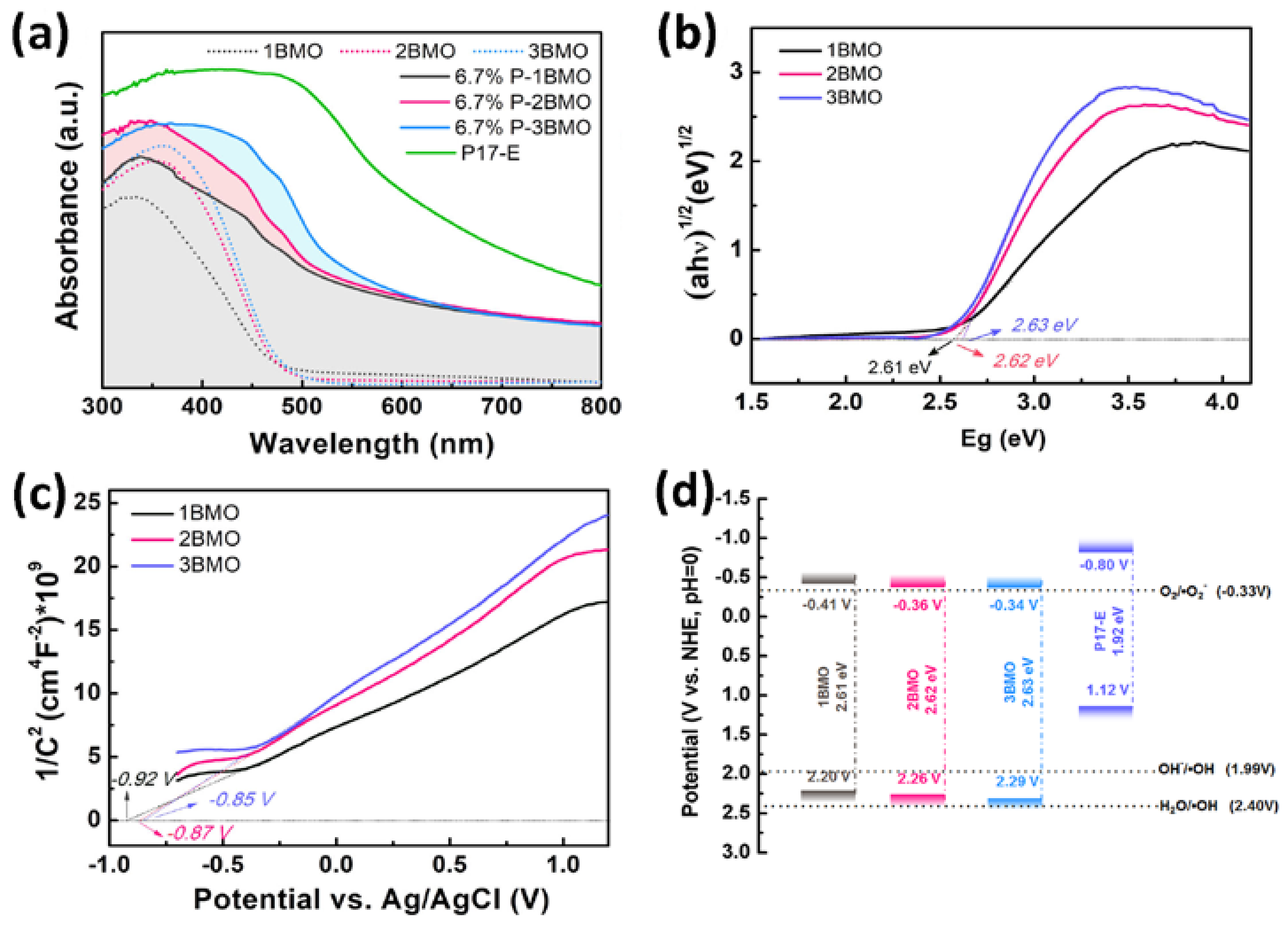
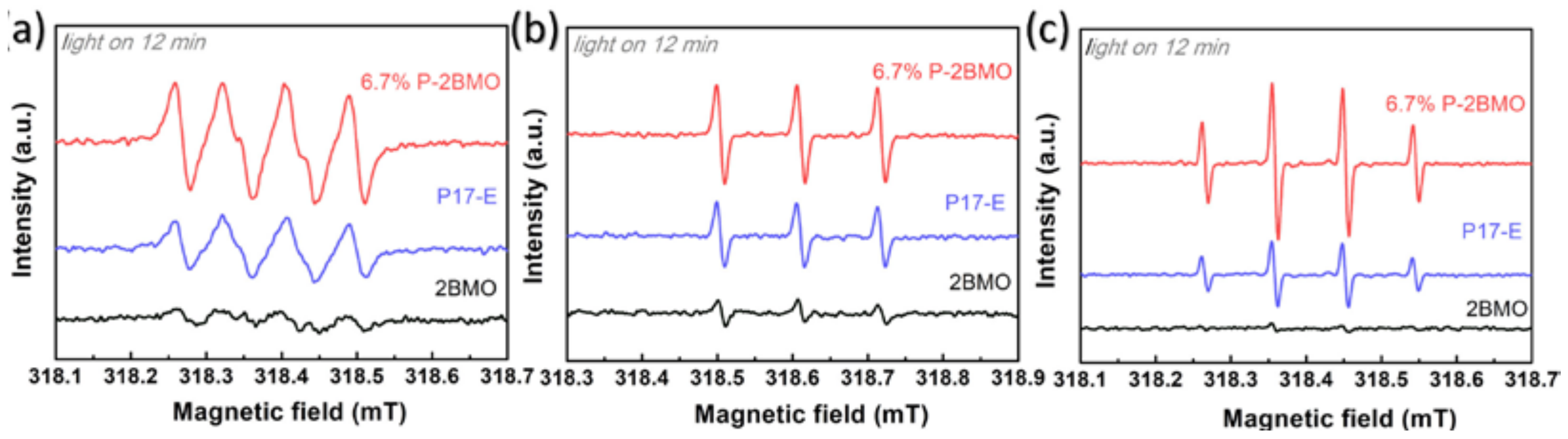
2.4. Insight of the Photocatalytic Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalysts
3.3. Characterizations
3.4. Measurement of Photocatalytic Activity
3.5. Detection of Reactive Species
3.6. Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Marin, M.L.; Santos-Juanes, L.; Arques, A.; Amat, A.M.; Miranda, M.A. Organic photocatalysts for the oxidation of pollutants and model compounds. Chem. Rev. 2012, 112, 1710–1750. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Alex, O.I.; Paul, F. Heterogeneous photocatalysis: recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Peng, T.; Song, S. Recent advances in dye-sensitized semiconductor systems for photocatalytic hydrogen production. J. Mater. Chem. A 2016, 4, 2365–2402. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.; Han, T.; Du, F.; Zhao, Y.; Li, X.; Sun, Y. Visualization of the formation and 3D porous structure of Ag doped MnO2 aerogel monoliths with high photocatalytic activity. ACS Sustain. Chem. Eng. 2016, 4, 6277–6287. [Google Scholar] [CrossRef]
- Low, J.; Yu, M.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Ai, Z.; Jia, F.; Zhang, L. Oxygen vacancy-mediated photocatalysis of BiOCl: reactivity, selectivity, and perspectives. Angew. Chem. Int. Ed. 2018, 57, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Chen, Z.; Li, X.; Sun, Y.; Gao, S.; Yan, W.; Wang, C.; Zhang, Q.; Lin, Y.; Luo, Y.; et al. Defect-mediated electron-hole separation in one-unit-cell ZnIn2S4 layers for boosted solar-driven CO2 reduction. J. Am. Chem. Soc. 2017, 139, 7586–7594. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, L.; Teng, B.; Fan, M. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ. Sci. Technol. 2015, 49, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, J.; Jaroniec, M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Shen, X.; Wu, J.; Ji, Z.; Wang, J.; Kong, L.; Liu, M.; Song, C. Fabrication of an all solid Z-scheme photocatalyst g-C3N4/GO/AgBr with enhanced visible light photocatalytic activity. Appl. Catal. A 2017, 539, 104–113. [Google Scholar] [CrossRef]
- Xia, P.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 2D/2D g-C3N4/MnO2 nanocomposite as a direct Z-scheme photocatalyst for enhanced photocatalytic activity. ACS Sustain. Chem. Eng. 2018, 6, 965–973. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Fang, Y.; Zhang, M.; Liu, K.; Dong, B. A nonmetal plasmonic Z-Scheme Photocatalyst with UV- to NIR-driven photocatalytic protons reduction. Adv. Mater. 2017, 29, 1606688. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Jiang, Y.; Li, C.; Fan, W.; Yan, X.; Yan, M.; Shi, W. In-situ synthesis of direct solid-state Z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl. Catal. B 2016, 180, 663–673. [Google Scholar] [CrossRef]
- Li, H.; Hu, T.; Zhang, R.; Liu, J.; Hou, W. Preparation of solid-state Z-scheme Bi2MoO6/MO (M = Cu, Co3/4, or Ni) heterojunctions with internal electric field-improved performance in photocatalysis. Appl. Catal. B 2016, 188, 313–323. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, F.; He, Q.; Huo, L.; Wu, Y.; Parker, T.C.; Ma, W.; Sun, Y.; Wang, C.; Zhu, D.; et al. High-performance electron acceptor with thienyl side chains for organic photovoltaics. J. Am. Chem. Soc. 2016, 138, 4955–4961. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, Z.; Yip, H.; Zhang, H.; Jiang, X.; Xue, Q.; Hu, Z.; Hu, Z.; Shen, Y.; Wang, M.; et al. Amino-functionalized conjugated polymer as an efficient electron transport layer for high-performance planar-heterojunction perovskite solar cells. Adv. Energy Mater. 2016, 6, 1501534. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Gong, X.; Heeger, A.J. Low bandgap semiconducting polymers for polymeric photovoltaics. Chem. Soc. Rev. 2016, 45, 4825–4846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kopetzki, D.; Seeberger, P.H.; Antonietti, M.; Vilela, F. Surface area control and photocatalytic activity of conjugated microporous poly(benzothiadiazole) networks. Angew. Chem. Int. Ed. 2013, 52, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; He, Z.; Wu, Y.; Cheng, X.; Wu, H.; Li, Y.; Cao, Y.; Wong, W. High-performance polymer solar cells based on a 2D-conjugated polymer with an alkylthio side-chain. Energy Environ. Sci. 2016, 9, 885–891. [Google Scholar] [CrossRef]
- Ghosh, S.; Kouamé, N.A.; Ramos, L.; Remita, S.; Dazzi, A.; Deniset-Besseau, A.; Beaunier, P.; Goubard, F.; Aubert, P.; Remita, H. Conducting polymer nanostructures for photocatalysis under visible light. Nat. Mater. 2015, 14, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ma, B.C.; Zhang, L.; Lin, S.; Ghasimi, S.; Landfester, K.; Zhang, K.A.; Wang, X. Molecular engineering of conjugated polybenzothiadiazoles for enhanced hydrogen production by photosynthesis. Angew. Chem. Int. Ed. 2016, 55, 9202–9206. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhang, X.; Wang, X.; Ding, X.; Huang, D.; Chen, H. Molecular structure design of conjugated microporous poly(dibenzo[b, d]thiophene 5,5-dioxide) for optimized photocatalytic NO removal. J. Catal. 2018, 357, 188–194. [Google Scholar] [CrossRef]
- Sprick, R.S.; Jiang, J.X.; Bonillo, B.; Ren, S.; Ratvijitvech, T.; Guiglion, P.; Zwijnenburg, M.A.; Adams, D.J.; Cooper, A.I. Tunable organic photocatalysts for visible-light-driven hydrogen evolution. J. Am. Chem. Soc. 2015, 137, 3265–3270. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.A.; Fang, Y.; Zhang, Y.; Wang, X. Photocatalytic oxygen evolution from functional triazine-based polymers with tunable band structures. Angew. Chem. Int. Ed. 2018, 57, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xiang, Y.; Qu, Y.; Ding, X.; Chen, H. Novel in situ fabrication of conjugated microporous poly(benzothiadiazole)-Bi2MoO6 Z-scheme heterojunction with enhanced visible light photocatalytic activity. J. Catal. 2017, 345, 319–328. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, J.; Hou, M.; Xiang, Y.; Chen, H. Robust visible/near-infrared light driven hydrogen generation over Z-scheme conjugated polymer/CdS hybrid. Appl. Catal. B 2018, 224, 871–876. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Xiao, J.; Wang, S.; Huang, D.; Ding, X.; Xiang, Y.; Chen, H. Synthesis of 1,4-diethynylbenzene-based conjugated polymer photocatalysts and their enhanced visible/near-infrared-light-driven hydrogen production activity. J. Catal. 2017, 350, 64–71. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhao, X.; Zhu, Y. Controllable synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocatalytic activities. Appl. Catal. B 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Wang, S.; Ding, X.; Zhang, X.; Pang, H.; Hai, X.; Zhan, G.; Zhou, W.; Song, H.; Zhang, L.; Chen, H.; et al. In situ carbon homogeneous doping on ultrathin bismuth molybdate: A dual-purpose strategy for efficient molecular oxygen activation. Adv. Funct. Mater. 2017, 27, 1703923. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Hou, W.; Du, N.; Zhang, R.; Tao, X. Synthesis and charactserization of g-C3N4/Bi2MoO6 heterojunctions with enhanced visible light photocatalytic activity. Appl. Catal. B 2014, 160, 89–97. [Google Scholar] [CrossRef]
- Tian, J.; Hao, P.; Wei, N.; Cui, H.; Liu, H. 3D Bi2MoO6 nanosheet/TiO2 nanobelt heterostructure: enhanced photocatalytic activities and photoelectochemistry performance. ACS. Catal. 2015, 5, 4530–4536. [Google Scholar] [CrossRef]
- Dai, W.; Yu, J.; Xu, H.; Hu, X.; Luo, X.; Yang, L.; Tu, X. Synthesis of hierarchical flower-like Bi2MoO6 microspheres as efficient photocatalyst for photoreduction of CO2 into solar fuels under visible light. CrystEngComm 2016, 18, 3472–3480. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Li, H.; Xu, H.; Li, H.; Chen, R. The synergistic role of carbon quantum dots for the improved photocatalytic performance of Bi2MoO6. Nanoscale 2015, 7, 11433–11443. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, W. Advanced chemical compositions and nanoarchitectures of bismuth based complex oxides for solar photocatalytic application. RSC Adv. 2014, 4, 47136–47152. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.; He, Y.; Guo, Y.; Zhang, T.; Zhang, Y. Mediator-free direct Z-scheme photocatalytic system: BiVO4/g-C3N4 organic-inorganic hybrid photocatalyst with highly efficient visible-light-induced photocatalytic activity. Dalton Trans. 2015, 44, 4297–4307. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Baruah, A.; Tonda, S.; Kumar, B.; Shanker, V.; Sreedhar, B. Cost-effective and eco-friendly synthesis of novel and stable N-doped ZnO/g-C3N4 core-shell nanoplates with excellent visible-light responsive photocatalysis. Nanoscale 2014, 6, 4830–4842. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, K.; Song, L.; Zhao, M.; Zhang, Z. Enhanced photocarrier separation in hierarchical graphitic-C3N4-supported CuInS2 for noble-metal-free Z-scheme photocatalytic water splitting. ACS Appl. Mater. Interfaces 2017, 9, 24577–24583. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Sun, C.; Wu, S.; Zhu, Y. Enhancement of photocatalytic performance via a P3HT-g-C3N4 heterojunction. J. Mater. Chem. A 2015, 3, 2741–2747. [Google Scholar] [CrossRef]
- Kongmark, C.; Martis, V.; Pirovano, C.; Löfberg, A.; Beek, W.; Sankar, G.; Rubbens, A.; Cristol, S.; Vannier, R.N.; Bordes-Richard, E. Synthesis of γ-Bi2MoO6 catalyst studied by combined high-resolution powder diffraction, XANES and Raman spectroscopy. Catal. Today 2010, 157, 257–262. [Google Scholar] [CrossRef]
- Ding, X.; Ho, W.; Shang, J.; Zhang, L. Self doping promoted photocatalytic removal of no under visible light with Bi2MoO6: Indispensable role of superoxide ions. Appl. Catal. B 2016, 182, 316–325. [Google Scholar] [CrossRef]
- Hull, R.V.; Li, L.; Xing, Y.; Chusuei, C.C. Pt nanoparticle binding on functionalized multiwalled carbon nanotubes. Chem. Mater. 2006, 18, 1780–1788. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Mallouk, T.E.; Pan, L.; Dickey, E.C. Individual single-walled nanotubes and hydrogels made by oxidative exfoliation of carbon nanotube Ropes. J. Am. Chem. Soc. 2003, 1252, 9761–9769. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Niu, C.; Zhang, L.; Liang, C.; Guo, H.; Zeng, G. Photocatalytic degradation of ciprofloxacin by a novel Z-scheme CeO2-Ag/AgBr photocatalyst: Influencing factors, possible degradation pathways, and mechanism insight. J. Catal. 2018, 358, 141–154. [Google Scholar] [CrossRef]
- Bai, Y.; Ye, L.; Chen, T.; Wang, L.; Shi, X.; Zhang, X.; Chen, D. Facet-dependent photocatalytic N2 fixation of bismuth-rich Bi5O7I nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 27661–27668. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, K.; Zhang, L. Enhanced photocatalytic removal of sodium pentachlorophenate with self-doped Bi2WO6 under visible light by generating more superoxide ions. Environ. Sci. Technol. 2014, 48, 5823–5831. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, L.; Ma, H.; Zhang, H.; Guo, L.H. Quantitative analysis of reactive oxygen species photogenerated on metal oxide nanoparticles and their bacteria toxicity: the role of superoxide radicals. Environ. Sci. Technol. 2017, 51, 10137–10145. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, S.; Yong, D.; Zhang, X.; Li, S.; Shao, W.; Sun, X.; Pan, B.; Xie, Y. Giant electron-hole interactions in confined layered structures for molecular oxygen activation. J. Am. Chem. Soc. 2017, 139, 4737–4742. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, Z.; Li, Y.; Ho, W.K.; Lee, S.C. Immobilization of polymeric g-C3N4 on structured ceramic foam for efficient visible light photocatalytic air purification with real indoor illumination. Environ. Sci. Technol. 2014, 48, 10345–10353. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Xiang, Y.; Wang, X.; Li, S.; Chen, H.; Ding, X. Pyrene-Based Conjugated Polymer/Bi2MoO6 Z-Scheme Hybrids: Facile Construction and Sustainable Enhanced Photocatalytic Performance in Ciprofloxacin and Cr(VI) Removal under Visible Light Irradiation. Catalysts 2018, 8, 185. https://doi.org/10.3390/catal8050185
Yang X, Xiang Y, Wang X, Li S, Chen H, Ding X. Pyrene-Based Conjugated Polymer/Bi2MoO6 Z-Scheme Hybrids: Facile Construction and Sustainable Enhanced Photocatalytic Performance in Ciprofloxacin and Cr(VI) Removal under Visible Light Irradiation. Catalysts. 2018; 8(5):185. https://doi.org/10.3390/catal8050185
Chicago/Turabian StyleYang, Xianglong, Yonggang Xiang, Xuepeng Wang, Shu Li, Hao Chen, and Xing Ding. 2018. "Pyrene-Based Conjugated Polymer/Bi2MoO6 Z-Scheme Hybrids: Facile Construction and Sustainable Enhanced Photocatalytic Performance in Ciprofloxacin and Cr(VI) Removal under Visible Light Irradiation" Catalysts 8, no. 5: 185. https://doi.org/10.3390/catal8050185
APA StyleYang, X., Xiang, Y., Wang, X., Li, S., Chen, H., & Ding, X. (2018). Pyrene-Based Conjugated Polymer/Bi2MoO6 Z-Scheme Hybrids: Facile Construction and Sustainable Enhanced Photocatalytic Performance in Ciprofloxacin and Cr(VI) Removal under Visible Light Irradiation. Catalysts, 8(5), 185. https://doi.org/10.3390/catal8050185




