Stereoselective Enzymatic Reduction of 1,4-Diaryl-1,4-Diones to the Corresponding Diols Employing Alcohol Dehydrogenases
Abstract
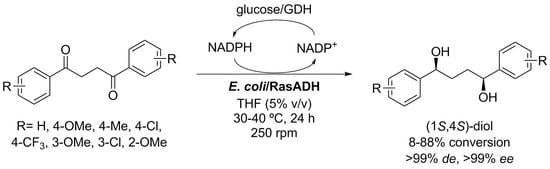
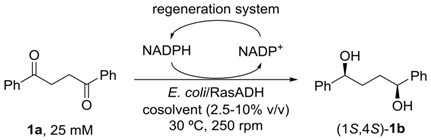
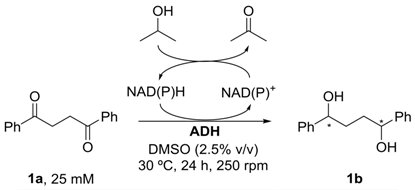
1. Introduction
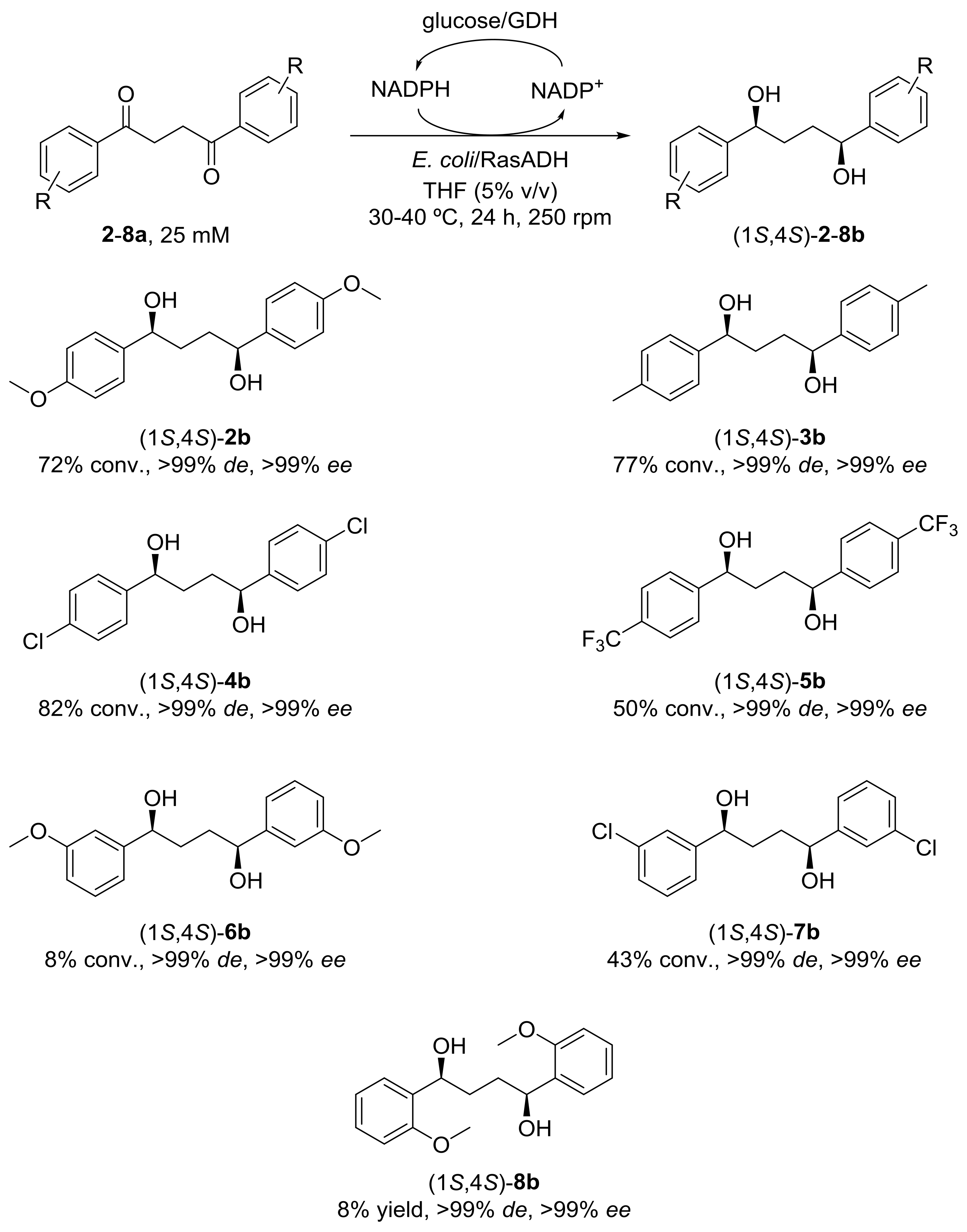
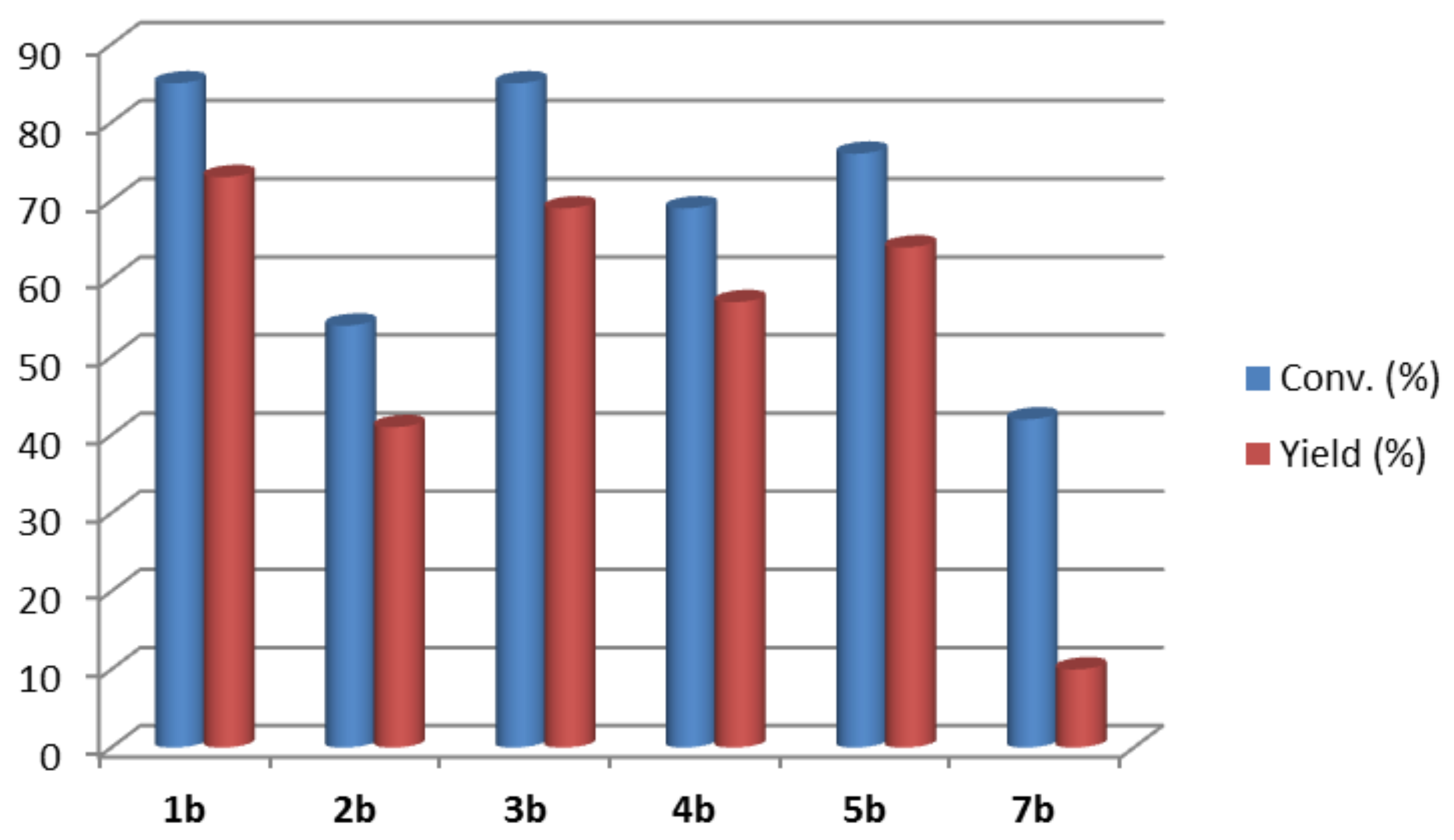
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Materials and Methods
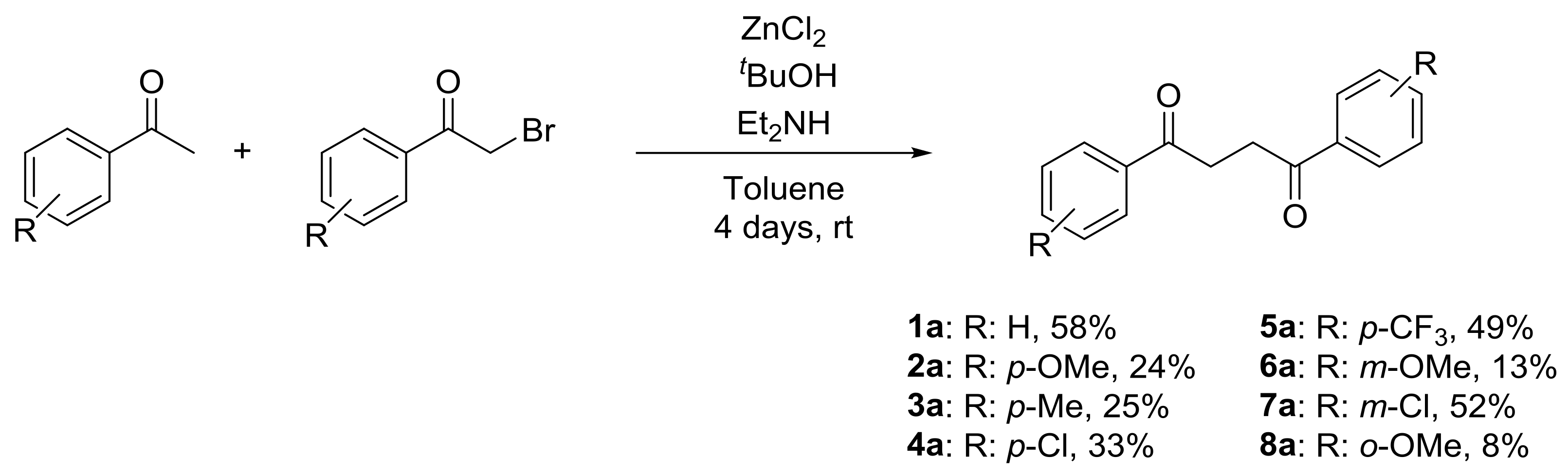
4.2. General Procedure for the Synthesis of 1,4-Diaryl-1,4-Diketones 1–8a
4.3. General Procedure for the Synthesis of Racemic 1,4-Diaryl-1,4-Diols 1–8b
4.4. General Procedure for the Synthesis of Racemic 4-Hydroxy-1,4-Bis(2-methoxyphenyl)butan-1-one 8c
4.5. General Procedure for the Enzymatic Conversion of 1,4-Diaryl-1,4-Diols 1–8b Using Overexpressed E. coli/RasADH
4.6. General Procedure for the Bioreduction of 1,4-Diphenylbutane-1,4-Dione 1a Using Commercial Alcohol Dehydrogenases
4.7. Preparative Bioreductions of 1,4-Diarylbutane-1,4-Diones 1–5a, 7a, and 8a Using Overexpressed E. coli/RasADH
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robinson, A.; Aggarwal, V. Asymmetric total synthesis of solandelactone E: Stereocontrolled synthesis of the 2-ene-1,4-diol core through a lithiation–borylation–allylation sequence. Angew. Chem. 2010, 122, 6823–6825. [Google Scholar] [CrossRef]
- Seebach, D.; Beck, A.K.; Heckel, A. TADDOLs, Their Derivatives, and TADDOL Analogues: Versatile Chiral Auxiliaries. Angew. Chem. Int. Ed. 2001, 40, 92–138. [Google Scholar] [CrossRef]
- Denmark, S.E.; Chang, W.-T.T.; Houk, K.N.; Liu, P. Development of chiral bis-hydrazone ligands for the enantioselective cross-coupling reactions of aryldimethylsilanolates. J. Org. Chem. 2015, 80, 313–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sweet, J.A.; Lam, K.-C.; Rheingold, A.; McGrath, D.V. Chiral amine–imine ligands based on trans-2,5-disubstituted pyrrolidines and their application in the palladium-catalyzed allylic alkylation. Tetrahedron Asymmetry 2009, 20, 1672–1682. [Google Scholar] [CrossRef]
- Melchiorre, P.; Jorgensen, K.A. Direct enantioselective Michael addition of aldehydes to vinyl ketones catalyzed by chiral amines. J. Org. Chem. 2003, 68, 4151–4157. [Google Scholar] [CrossRef] [PubMed]
- Foubelo, F.; Nájera, C.; Yus, M. Catalytic asymmetric transfer hydrogenation of ketones: Recent advances. Tetrahedron Asymmetry 2015, 26, 769–790. [Google Scholar] [CrossRef]
- Hynes, J.T.; Klinman, J.P.; Limbach, H.-H.; Schowen, R.L. (Eds.) Hydrogen Transfer Reactions; Wiley-VCH: Weinheim, Germany, 2007; Volumes 1–4. [Google Scholar]
- Zheng, L.-S.; Llopis, Q.; Echeverria, P.-G.; Férand, C.; Guillamot, G.; Phansavath, P.; Ratovelomanana-Vidal, V. Asymmetric transfer hydrogenation of (hetero)arylketones with tethered Rh(III)−N-(p-tolylsulfonyl)-1,2-diphenylethylene-1,2-diamine complexes: Scope and limitations. J. Org. Chem. 2017, 82, 5607–5615. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Helal, C.J. Reduction of carbonyl compounds with chiral oxazaborolidine catalyst: A new paradigm for enantioselective catalysis and a powerful new synthetic method. Angew. Chem. Int. Ed. 1998, 37, 1986–2012. [Google Scholar] [CrossRef]
- Mathre, D.J.; Thomson, A.S.; Douglas, A.W.; Hoogsteen, K.; Carroll, J.D.; Corley, E.G.; Grabowski, E.J.J. A practical process for the preparation of tetrahydro-1-methyl-3,3-diphenyl-1H,3H-pyrrolo[1,2-c][1,3,2]oxazaborole-borane. A highly enantioselective stoichiometric and catalytic reducing agent. J. Org. Chem. 1993, 58, 2880–2888. [Google Scholar] [CrossRef]
- Li, X.; Zhao, G.; Cao, W.-G. An efficient method for catalytic asymmetric reduction of diketones and application of synthesis to chiral 2,5-diphenylpirrolidine and 2,5-diphenylthiolane. Chin. J. Chem. 2006, 24, 1402–1405. [Google Scholar] [CrossRef]
- Aldous, D.J.; Dutton, W.M.; Steel, P.G. A simple enantioselective preparation of (2S,5S)-2,5-diphenylpyrrolidine and related diaryl amines. Tetrahedron Asymmetry 2000, 11, 2455–2462. [Google Scholar] [CrossRef]
- Hudlicky, T.; Reed, J.W. Applications of biotransformations and biocatalysis to complexity generation in organic synthesis. Chem. Soc. Rev. 2009, 38, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Clouthier, C.M.; Pelletier, J.M. Expanding the organic toolbox: A guide to integrating biocatalysis in synthesis. Chem. Soc. Rev. 2012, 41, 1585–1605. [Google Scholar] [CrossRef] [PubMed]
- Milner, S.E.; Maguire, A.R. Recent trends in whole cell and isolated enzymes in enantioselective synthesis. Arkivoc 2012, 2012, 321–382. [Google Scholar]
- Torrelo, G.; Hanefeld, U.; Hollmann, F. Biocatalysis. Catal. Lett. 2015, 145, 309–345. [Google Scholar] [CrossRef]
- Albarrán-Velo, J.; González-Martínez, D.; Gotor-Fernández, V. Stereoselective Biocatalysis. A mature technology for the asymmetric synthesis of pharmaceutical building blocks. Biocatal. Biotransform. 2018, 36, 102–130. [Google Scholar] [CrossRef]
- Mattson, A.; Öhrner, N.; Hult, K.; Norin, T. Resolution of diols with C2-symmetry by lipase catalysed transesterification. Tetrahedron Asymmetry 1993, 4, 925–930. [Google Scholar] [CrossRef]
- Nagai, H.; Morimoto, T.; Achiwa, K. Facile enzymatic synthesis of optically active 2,5-hexanediol derivatives and its application to the preparation of optically pure cyclic sulfate for chiral ligands. Synlett 1994, 1994, 289–290. [Google Scholar] [CrossRef]
- Caron, G.; Kazlauskas, R.J. Isolation of racemic 2,4-pentanediol and 2,5-hexanediol from commercial mixtures of racemic and meso isomers by way of cyclic sulfites. Tetrahedron Asymmetry 1994, 6, 657–664. [Google Scholar] [CrossRef]
- Persson, B.A.; Larsson, A.L.E.; Le Ray, M.; Bäckvall, J.-E. Ruthenium- and enzyme-catalyzed dynamic kinetic resolution of secondary alcohols. J. Am. Chem. Soc. 1999, 121, 1645–1650. [Google Scholar] [CrossRef]
- Persson, B.A.; Huerta, F.; Bäckvall, J.-E. Dynamic kinetic resolution of secondary diols via coupled ruthenium and enzyme catalysis. J. Org. Chem. 1999, 64, 5237–5240. [Google Scholar] [CrossRef]
- Edin, M.; Bäckvall, J.-E. On the mechanism of the unexpected facile formation of meso-diacetate products in enzymatic acetylation of alkanediols. J. Org. Chem. 2003, 68, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Martín-Matute, B.; Edin, M.; Bäckvall, J.-E. Highly efficient synthesis of enantiopure diacetylated C2-symmetric diols by ruthenium- and enzyme-catalyzed dynamic kinetic asymmetric transformation (DYKAT). Chem. Eur. J. 2006, 12, 6053–6061. [Google Scholar] [CrossRef] [PubMed]
- Borén, L.; Leijondahl, K.; Bäckvall, J.-E. Dynamic kinetic asymmetric transformation of 1,4-diols and the preparation of trans-2,5-disubstituted pyrrolidines. Tetrahedron Lett. 2009, 50, 3237–3240. [Google Scholar] [CrossRef]
- Martín-Matute, B.; Bäckvall, J.-E. Ruthenium- and enzyme-catalyzed dynamic kinetic asymmetric transformation of 1,4-diols: Synthesis of γ-hydroxy ketones. J. Org. Chem. 2004, 69, 9191–9195. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lihammar, R.; Bäckvall, J.-E. Investigation of the impact of water on the enantioselectivity displayed by CALB in the kinetic resolution of δ-functionalized alkan-2-ol derivatives. Chem. Eur. J. 2014, 20, 13517–13521. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, F.; Arends, I.W.C.E.; Holtmann, D. Enzymatic reductions for the chemist. Green Chem. 2011, 13, 2285–2313. [Google Scholar] [CrossRef]
- Magano, J.; Dunetz, J.R. Large-scale carbonyl reductions in the pharmaceutical industry. Org. Process Res. Dev. 2012, 16, 1156–1184. [Google Scholar] [CrossRef]
- Nealon, C.M.; Musa, M.M.; Patel, J.M.; Phillips, R.S. Controlling substrate specificity and stereospecificity of alcohol dehydrogenases. ACS Catal. 2015, 5, 2100–2114. [Google Scholar] [CrossRef]
- Kratzer, R.; Woodley, J.M.; Nidetzky, B. Rules for biocatalyst and reaction engineering to implement effective, NAD(P)H-dependent, whole cell bioreductions. Biotechnol. Adv. 2015, 33, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Kurina-Sanz, M.; Bisogno, F.R.; Lavandera, I.; Orden, A.A.; Gotor, V. Promiscuous substrate-binding explains the enzymatic stereo- and regiocontrolled synthesis of enantiopure hydroxy ketones and diols. Adv. Synth. Catal. 2009, 351, 1842–1848. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Wu, X. Dicarbonyl reduction by single enzyme for the preparation of chiral diols. Chem. Soc. Rev. 2012, 41, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, K.; Edegger, K.; Kroutil, W.; Liese, A. Overcoming the thermodynamic limitation in asymmetric hydrogen transfer reactions catalyzed by whole cells. Biotechnol. Bioeng. 2006, 95, 192–198. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo, G.; Lavandera, I.; Faber, K.; Kroutil, W. Enzymatic reduction of ketones in “micro-aqueous” media catalyzed by ADH-A from Rhodococcus ruber. Org. Lett. 2007, 9, 2163–2166. [Google Scholar] [CrossRef] [PubMed]
- Machielsen, R.; Leferink, N.G.H.; Hendriks, A.; Brouns, S.J.J.; Hennemann, H.-G.; Daussmann, T.; Oost, J. Laboratory evolution of Pyrococcus furiosus alcohol dehydrogenase to improve the production of (2S,5S)-hexanediol at moderate temperatures. Extremophiles 2008, 12, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Katzberg, M.; Bertau, M.; Hummel, W. Highly efficient and stereoselective biosynthesis of (2S,5S)-hexanediol with a dehydrogenase from Saccharomyces cerevisiae. Org. Biomol. Chem. 2010, 8, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Mizar, P.; Wirth, T. Flexible stereoselective functionalizations of ketones through umpolung with hypervalent iodine reagents. Angew. Chem. Int. Ed. 2014, 53, 5993–5997. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Henry, J.B.; Li, Y.; Mount, A.R.; Slawin, A.M.Z.; Woollins, J.D. Synthesis of novel 2,5-diarylselenophenes from selenation of 1,4-diarylbutane-1,4-diones or methanol/arylacetylenes. Org. Biomol. Chem. 2010, 8, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Nevar, N.M.; Kel’in, A.V.; Kulinkovich, O.G. One step preparation of 1,4-diketones from methyl ketones and α-bromomethyl ketones in the presence of ZnCl2 • t-BuOH·• Et2NR as a condensation agent. Synthesis 2000, 9, 1259–1262. [Google Scholar] [CrossRef]
- Lavandera, I.; Kern, A.; Ferreira-Silva, B.; Glieder, A.; de Wildeman, S.; Kroutil, W. Stereoselective bioreduction of bulky-bulky ketones by a novel ADH from Ralstonia sp. J. Org. Chem. 2008, 73, 6003–6005. [Google Scholar] [CrossRef] [PubMed]
- Leuchs, S.; Greiner, L. Alcohol dehydrogenase from Lactobacillus brevis: A versatile robust catalyst for enantioselective transformations. Chem. Biochem. Eng. Q. 2011, 25, 267–281. [Google Scholar]
- Lavandera, I.; Kern, A.; Resch, V.; Ferreira-Silva, B.; Glieder, A.; Fabian, W.M.F.; de Wildeman, S.; Kroutil, W. One-way biohydrogen transfer for oxidation of sec-alcohols. Org. Lett. 2008, 10, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Laivenieks, M.; Zeikus, J.G.; Phillips, R.S. Mutation of cysteine-295 to alanine in secondary alcohol dehydrogenase from Thermoanaerobacter ethanolicus affects the enantioselectivity and substrate specificity of ketone reductions. Bioorg. Med. Chem. 2001, 9, 1659–1666. [Google Scholar] [CrossRef]
- Stampfer, W.; Kosjek, B.; Moitzi, C.; Kroutil, W.; Faber, K. Biocatalytic asymmetric hydrogen transfer. Angew. Chem. Int. Ed. 2002, 41, 1014–1017. [Google Scholar] [CrossRef]
- Abu, R.; Woodley, J. Application of enzyme coupling reactions to shift thermodynamically limited biocatalytic reactions. ChemCatChem 2015, 7, 3094–3105. [Google Scholar] [CrossRef]
- Man, H.; Kędziora, K.; Kulig, J.; Frank, A.; Lavandera, I.; Gotor-Fernández, V.; Rother, D.; Hart, S.; Turkenburg, J.P.; Grogan, G. Structures of alcohol dehydrogenases from Ralstonia and Sphingobium spp. reveal the molecular basis for their recognition of ‘bulky–bulky’ ketones. Top. Catal. 2014, 57, 356–365. [Google Scholar] [CrossRef]
- García-Cerrada, S.; Redondo-Gallego, L.; Martínez-Olid, F.; Rincón, J.A.; García-Losada, P. Practical manufacture of 4-alkyl-4-aminocyclohexylalcohols using ketoreductases. Org. Process Res. Dev. 2017, 21, 779–784. [Google Scholar] [CrossRef]
- Ceylan, M.; Gürdere, M.B.; Budak, Y.; Kazaz, C.; Seçen, H. One-step preparation of symmetrical 1,4-diketones from α-halo ketones in the presence of Zn-I2 as a condensation agent. Synthesis 2004, 35, 1750–1754. [Google Scholar] [CrossRef]
- Fujita, K.; Shinokubo, H.; Oshima, K. Highly diastereoselective tandem reduction–allylation reactions of 1,4-diketones with zirconocene–olefin complexes. Angew. Chem. Int. Ed. 2003, 42, 2550–2552. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Feng, Z.-J.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J. Visible-light-induced C‒S bond activation: Facile access to 1,4-diketones from β-ketosulfones. Chem: Eur. J. 2014, 20, 3045–3049. [Google Scholar] [CrossRef] [PubMed]
- Domin, D.; Benito-Garagorri, D.; Mereiter, K.; Hametner, C.; Fröhlich, J.; Kirchner, K. Synthesis and characterization of new chiral palladium β-diimine complexes. J. Organomet. Chem. 2007, 692, 1048–1057. [Google Scholar] [CrossRef]
| Entry | Regeneration System | Cosolvent | % (v/v) | t (h) | Conversion (%) b | de b,c | ee (%) b,d |
|---|---|---|---|---|---|---|---|
| 1 | Glucose/GDH | DMSO | 2.5 | 24 | 56 | 99:1 | >99 |
| 2 | Glucose/GDH | DMSO | 2.5 | 48 | 82 | 99:1 | >99 |
| 3 | Glucose/GDH | - | - | 48 | 72 | 99:1 | >99 |
| 4 | IPA | - | - | 48 | 72 | 89:11 | >99 |
| 5 | Glucose/GDH | EtOH | 2.5 | 24 | 57 | 99:1 | >99 |
| 6 | Glucose/GDH | 1,4-dioxane | 2.5 | 24 | 72 | 99:1 | >99 |
| 7 | Glucose/GDH | 1,4-dioxane | 5 | 24 | 78 | >99 | >99 |
| 8 | Glucose/GDH | 1,4-dioxane | 10 | 24 | 79 | 99:1 | >99 |
| 9 | Glucose/GDH | 1,4-dioxane | 10 | 48 | 80 | 99:1 | >99 |
| 10 | Glucose/GDH | MTBE | 2.5 | 24 | 70 | >99 | >99 |
| 11 | Glucose/GDH | MTBE | 5 | 24 | 83 | >99 | >99 |
| 12 | Glucose/GDH | MTBE | 10 | 24 | 77 | >99 | >99 |
| 13 | Glucose/GDH | MTBE | 10 | 48 | 77 | >99 | >99 |
| 14 | Glucose/GDH | THF | 2.5 | 24 | 72 | 99:1 | >99 |
| 15 | Glucose/GDH | THF | 5 | 24 | 88 | >99 | >99 |
| 16 | Glucose/GDH | THF | 10 | 24 | 80 | 99:1 | >99 |
| 17 | Glucose/GDH | THF | 10 | 48 | 82 | 99:1 | >99 |
| Entry | ADH | Conversion (%) b | de b,c | ee (%) b,d |
|---|---|---|---|---|
| 1 | P1-B02 | 85 | >99 | >99 |
| 2 | P1-B10 | 79 | >99 | >99 |
| 3 | P1-B12 | 75 | 99:1 | >99 |
| 4 | P2-D03 | 89 | 87:13 | >99 e |
| 5 | P2-D11 | 69 | 98:2 | >99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourelle-Insua, Á.; De Gonzalo, G.; Lavandera, I.; Gotor-Fernández, V. Stereoselective Enzymatic Reduction of 1,4-Diaryl-1,4-Diones to the Corresponding Diols Employing Alcohol Dehydrogenases. Catalysts 2018, 8, 150. https://doi.org/10.3390/catal8040150
Mourelle-Insua Á, De Gonzalo G, Lavandera I, Gotor-Fernández V. Stereoselective Enzymatic Reduction of 1,4-Diaryl-1,4-Diones to the Corresponding Diols Employing Alcohol Dehydrogenases. Catalysts. 2018; 8(4):150. https://doi.org/10.3390/catal8040150
Chicago/Turabian StyleMourelle-Insua, Ángela, Gonzalo De Gonzalo, Iván Lavandera, and Vicente Gotor-Fernández. 2018. "Stereoselective Enzymatic Reduction of 1,4-Diaryl-1,4-Diones to the Corresponding Diols Employing Alcohol Dehydrogenases" Catalysts 8, no. 4: 150. https://doi.org/10.3390/catal8040150
APA StyleMourelle-Insua, Á., De Gonzalo, G., Lavandera, I., & Gotor-Fernández, V. (2018). Stereoselective Enzymatic Reduction of 1,4-Diaryl-1,4-Diones to the Corresponding Diols Employing Alcohol Dehydrogenases. Catalysts, 8(4), 150. https://doi.org/10.3390/catal8040150