Controlled Synthesis of Heterostructured SnO2-CuO Composite Hollow Microspheres as Efficient Cu-Based Catalysts for the Rochow Reaction
Abstract
:1. Introduction
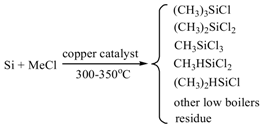
2. Results and Discussion
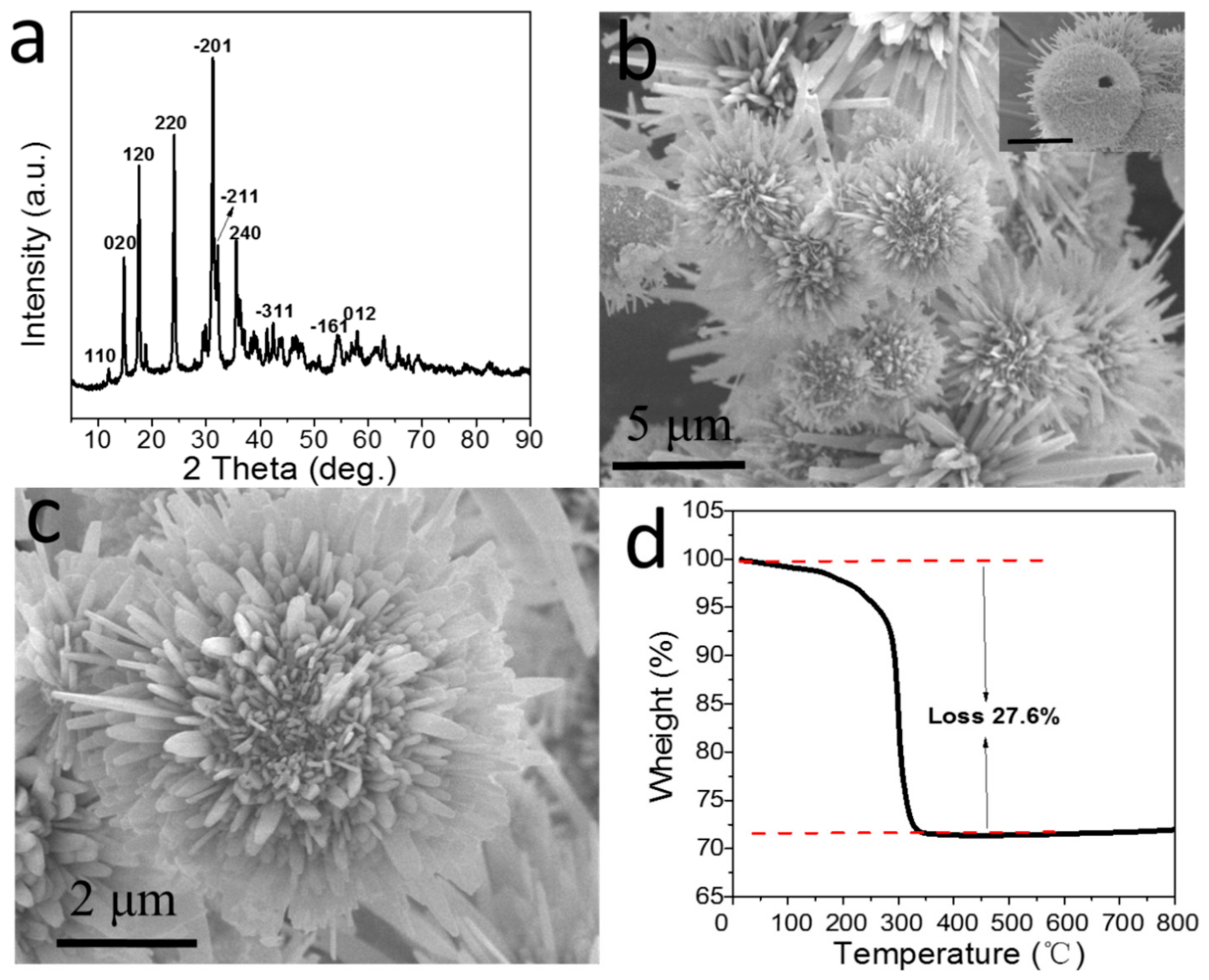
2.1. Characterization of H-SnO2-CuO Precursor Particle
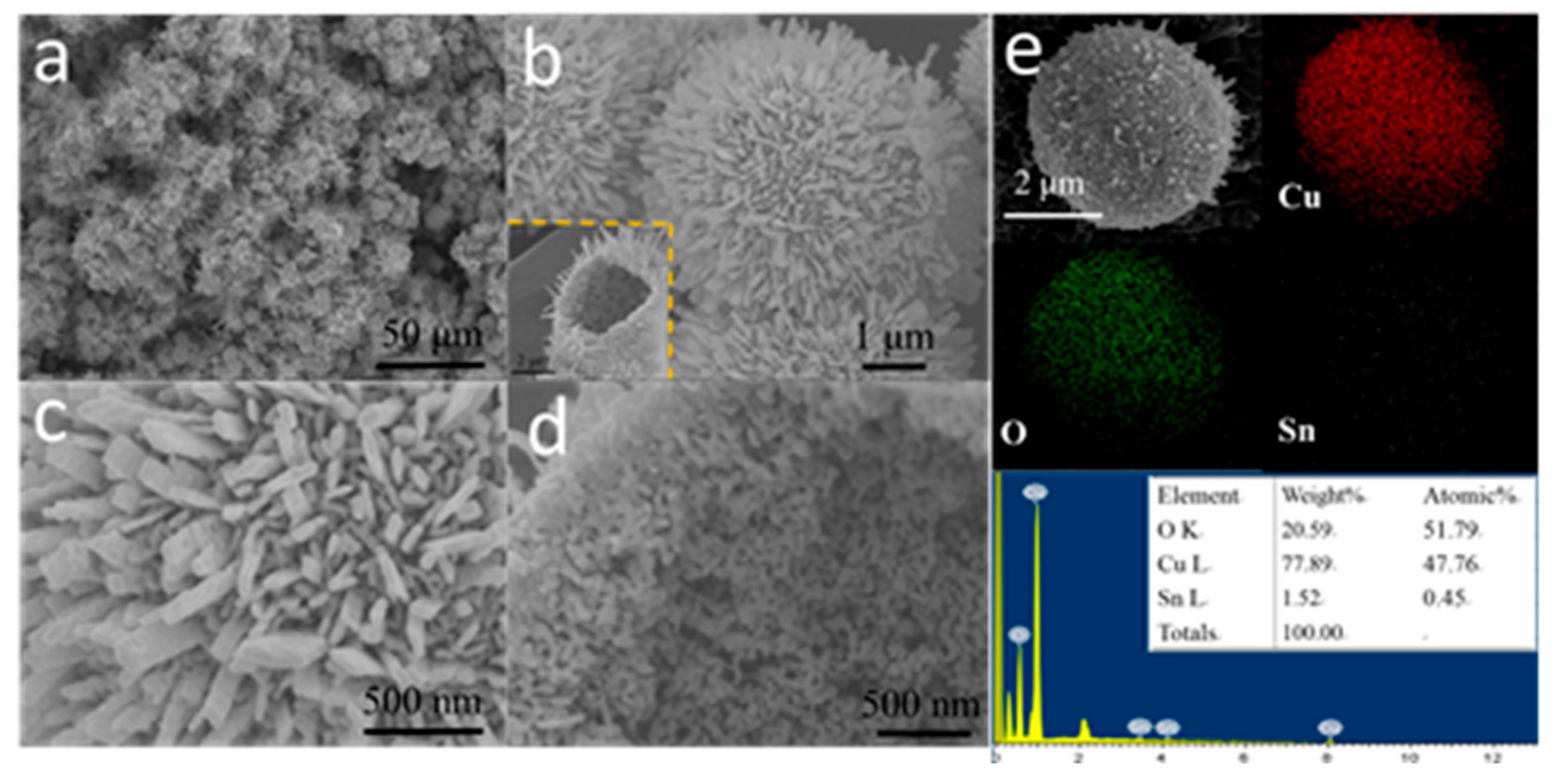
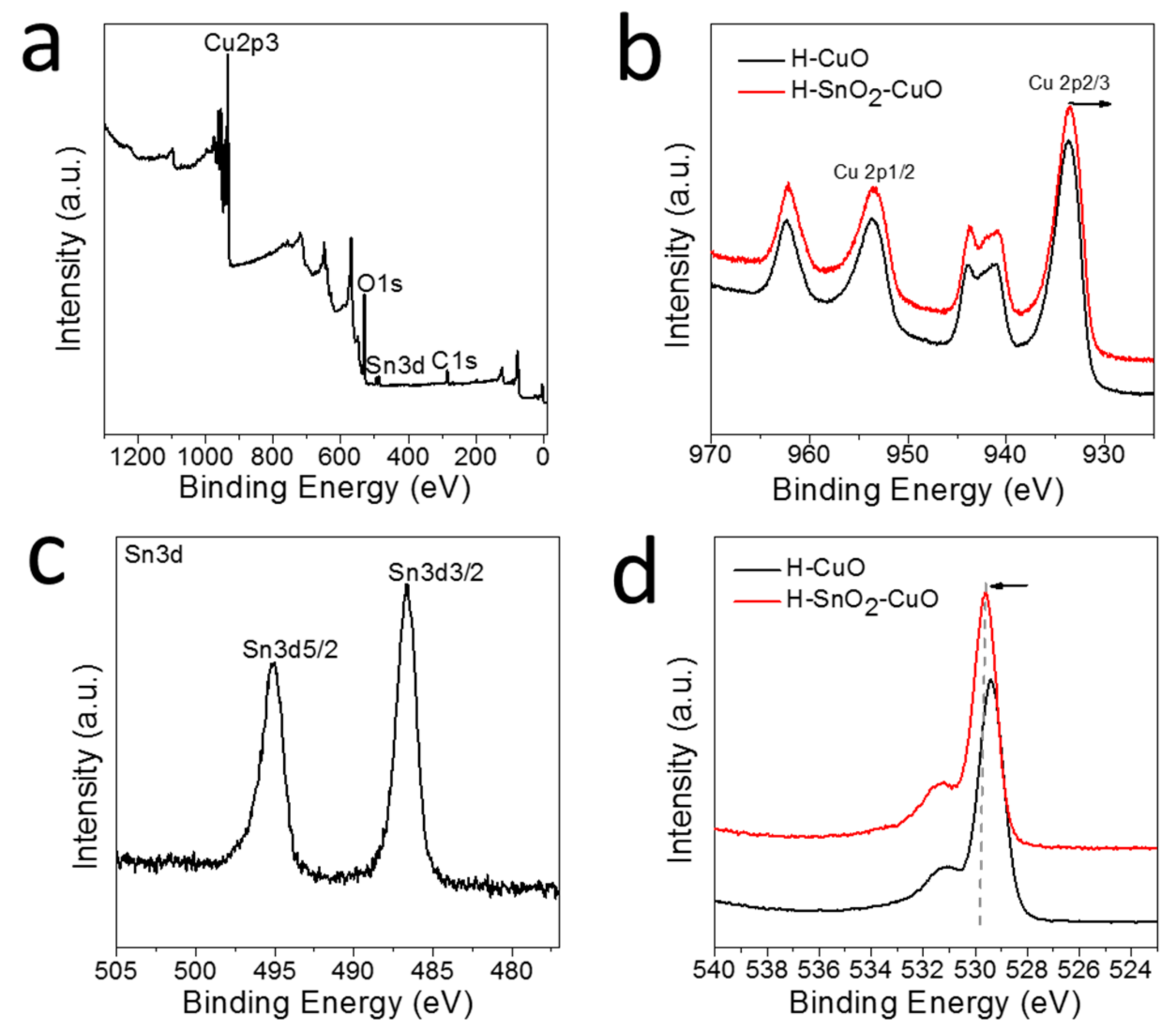
2.2. Characterization of H-SnO2-CuO
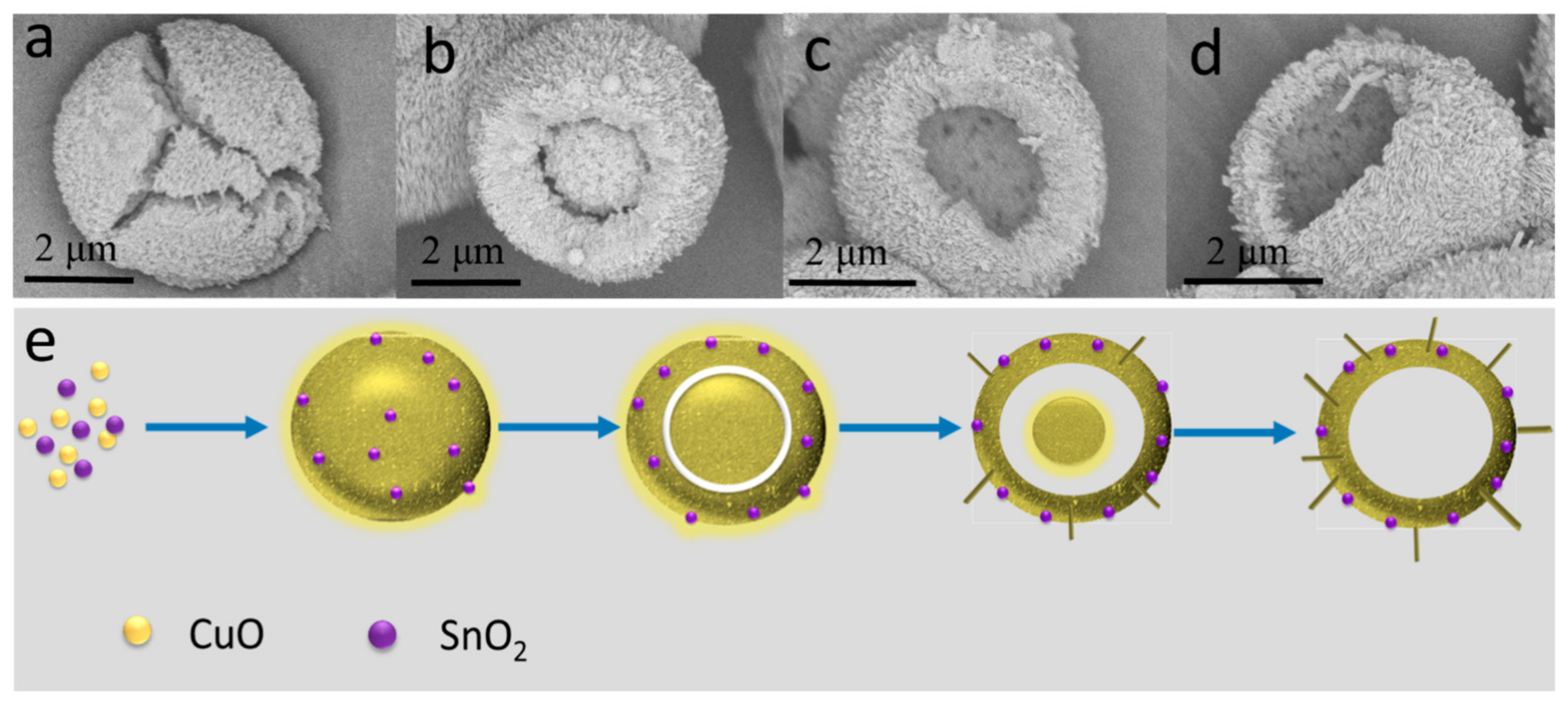
2.3. The Formation Mechanism of H-SnO2-CuO
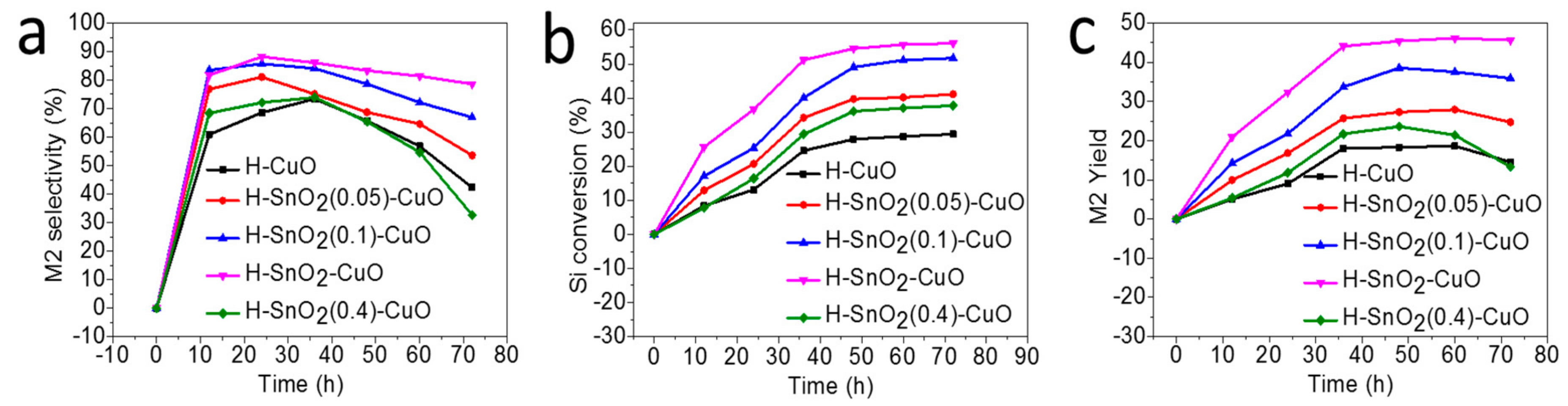
2.4. Catalytic Performance
3. Experimental
3.1. Material Synthesis
3.2. Material Characterization
3.3. Catalytic Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, H.; Jin, Z.L.; Hu, H.Y.; Lu, G.X.; Bi, Y.P. Fivefold enhanced photoelectrochemical properties of ZnO nanowire arrays modified with C3N4 quantum dots. Catalysts 2017, 7, 99. [Google Scholar] [CrossRef]
- Du, N.; Zhang, H.; Chen, B.D.; Wu, J.B.; Ma, X.Y.; Liu, Z.H.; Zhang, Y.Q.; Yang, D.R.; Huang, X.H.; Tu, J.P. Porous Co3O4 nanotubes derived from Co4(CO)12 clusters on carbon nanotube templates: A highly efficient material for Li-battery applications. Adv. Mater. 2007, 19, 4505–4509. [Google Scholar] [CrossRef]
- Daniela, N.; Ana, P.; Lidia, S.; Pedro, B.; Elvira, F.; Rodrigo, M.; Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Martins, R. Photocatalytic TiO2 nanorod spheres and arrays compatible with flexible applications. Catalysts 2017, 7, 60–77. [Google Scholar]
- Wu, Y.E.; Wang, D.S.; Li, Y.D. Understanding of the major reactions in solution synthesis of functional nanomaterials. Sci. China Mater. 2016, 59, 938–996. [Google Scholar]
- Dubau, L.; Nelayah, J.; Moldovan, S.; Ersen, O.; Bordet, P.; Drnec, J.; Asset, T.; Chattot, R.; Maillard, F. Defects do catalysis: CO monolayer oxidation and oxygen reduction reaction on hollow PtNi/C nanoparticles. ACS Catal. 2016, 6, 4673–4684. [Google Scholar] [CrossRef]
- Liu, H.Y.; Ma, H.; Joo, J.B.; Yin, Y.D. Contribution of multiple reflections to light utilization efficiency of submicron hollow TiO2 photocatalyst. Sci. China Mater. 2016, 59, 1017–1026. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Yu, F.; Zhang, Y.; Ye, B.C.; Li, Y.C. Facile synthesis of hollow MnFe2O4 nanoboxes based on galvanic replacement reaction for fast and sensitive VOCs sensor. Sens. Actuators B 2018, 258, 589–596. [Google Scholar] [CrossRef]
- He, Z.; Xia, D.; Huang, Y.; Tan, X.; He, C.; Hu, L.; He, H.; Zeng, J.; Xu, W.; Shu, D. 3D MnO2 hollow microspheres ozone-catalysis coupled with flat-plate membrane filtration for continuous removal of organic pollutants: Efficient heterogeneous catalytic system and membrane fouling control. J. Hazard. Mater. 2018, 344, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Wei, G.J.; Zhao, F.Z.; An, C.; Wang, H.; Li, J.Q.; An, C.H. Urchin-like FeOOH hollow microspheres decorated with MnO2 for enhanced supercapacitor performance. Sci. China Mater. 2018, 61, 48–56. [Google Scholar] [CrossRef]
- Wu, G.Q.; Liang, X.Y.; Zhang, L.J.; Tang, Z.Y.; Al-Mamun, M.; Zhao, H.J.; Su, X.T. Fabrication of highly stable metal oxide hollow nanospheres and their catalytic activity toward 4-nitrophenol reduction. ACS Appl. Mater. Interfaces 2017, 9, 18207–18214. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Z.; Zeng, G.; Chen, J.X.; Lu, C.Z.; Wen, Z.H. 3D graphene network encapsulating SnO2 hollow spheres as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 4535–4542. [Google Scholar] [CrossRef]
- Hassanpour, M.; Safardoust-Hojaghan, H.; Salavati-Niasari, M.; Yeganeh-Faal, A. Nano-sized CuO/ZnO hollow spheres: Synthesis, characterization and photocatalytic performance. J. Mater. Sci. Mater. Electron. 2017, 28, 14678–14684. [Google Scholar] [CrossRef]
- Tan, Q.Q.; Wang, P.F.; Liu, H.; Xu, Y.X.; Yang, J. Hollow MOx-RuO2 (M = Co, Cu, Fe, Ni, CuNi) nanostructures as highly efficient electrodes for supercapacitors. Sci. China Mater. 2016, 59, 323–336. [Google Scholar] [CrossRef]
- Hurd, D.T.; Rochow, E.G. On the mechanism of the reaction between methyl chloride and silicon-copper. J. Am. Chem. Soc. 1945, 67, 1057–1059. [Google Scholar] [CrossRef]
- Banholzer, W.F.; Lewis, N.; Ward, W. Active site formation in the direct process for methylchlorosilanes. J. Catal. 1986, 101, 405–415. [Google Scholar] [CrossRef]
- Luo, W.X.; Wang, G.R.; Wang, J.F.; Jin, Y. Modification of CuCl catalyst used in the direct synthesis reaction of methyl chlorosilane monomer. Chem. Eng. J. 2006, 34, 41–44. [Google Scholar]
- Gordon, A.D.; Hinch, B.J.; Strongin, D.R. Effects of individual promoters on the direct synthesis of methylchlorosilanes. J. Catal. 2009, 266, 291–298. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Che, H.W.; Wang, Y.L.; Gao, J.J.; She, X.L.; Sun, J.; Zhong, Z.Y.; Su, F.B. Flower-like CuO microspheres with enhanced catalytic performance for dimethyldichlorosilane synthesis. RSC Adv. 2012, 2, 2254–2256. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, Y.J.; Li, J.; Liu, H.Z.; Hu, X.; Zhong, Z.Y.; Su, F.B. Morphology–dependent catalytic properties of nano–cupric oxides in the Rochow reaction. Nano Res. 2018, 11, 804–819. [Google Scholar] [CrossRef]
- Ji, Y.J.; Jin, Z.Y.; Li, J.; Zhang, Y.; Liu, H.Z.; Shi, L.S.; Zhong, Z.Y.; Su, F.B. Rambutan-like hierarchically heterostructured CeO2-CuO hollow microspheres: Facile hydrothermal synthesis and applications. Nano Res. 2017, 10, 381–396. [Google Scholar] [CrossRef]
- Zou, S.Y.; Ji, Y.J.; Wang, G.N.; Zhu, Y.X.; Liu, H.Z.; Jia, L.H.; Guo, X.F.; Zhong, Z.Y.; Su, F.B. Heterojunctions generated in SnO2-CuO nanocatalysts for improved catalytic property in the Rochow reaction. RSC Adv. 2015, 5, 63355–63362. [Google Scholar] [CrossRef]
- Zou, S.Y.; Ji, Y.J.; Li, J.; Zhang, Y.; Jin, Z.Y.; Jia, L.H.; Guo, X.F.; Zhong, Z.Y.; Su, F.B. Novel leaf-like Cu-O-Sn nanosheets as highly efficient catalysts for the Rochow Reaction. J. Catal. 2016, 337, 1–13. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, J.J.; Gu, F.N.; Lu, X.P.; Liu, Y.J.; Li, H.F.; Zhong, Z.Y.; Su, F.B. One-pot synthesis of ordered mesoporous Ni−V−Al catalyst for CO methanation. J. Catal. 2015, 326, 127–138. [Google Scholar] [CrossRef]
- Yue, W.R.; Zhang, R.D.; Liu, N.; Chen, B.H. Selective catalytic oxidation of ammonia to nitrogen over orderly mesoporous CuFe2O4 with high specific surface area. Sci. Bull. 2014, 59, 3980–3986. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Sengele, A.; Keller, V.; Keller, N. Single-step synthesis of SnS2 nanosheet-decorated TiO2 anatase nanofibers as efficient photocatalysts for the degradation of gas-phase diethylsulfide. ACS Appl. Mater. Interfaces 2015, 7, 19324–19334. [Google Scholar] [CrossRef] [PubMed]
- Sellick, D.R.; Aranda, A.; García, T.; López, J.M.; Solsona, B.; Mastral, A.M.; Morgan, D.J.; Carley, A.F.; Taylor, S.H. Influence of the preparation method on the activity of ceria zirconia mixed oxides for naphthalene total oxidation. Appl. Catal. B Environ. 2013, 132–133, 98–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, Y.J.; Li, J.; Liu, H.Z.; Zhong, Z.Y.; Su, F.B. Hierarchical zinc-copper oxides hollow microspheres as active Rochow reaction catalysts: The formation and effect of charge transferable interfaces. J. Catal. 2017, 348, 233–245. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Vlugter, J.C. Mechanism and kinetics of the metal-catalyzed synthesis of methylchlorosilanes: III. The catalytically active form of the copper catalyst. J. Catal. 1965, 4, 123–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, Y.J.; Li, J.; Liu, H.Z.; Zhong, Z.Y.; Su, F.B. Promoting effect of In2O3 on CuO for the Rochow reaction: The formation of P–N junctions at the hetero-interfaces. J. Catal. 2017, 348, 110–124. [Google Scholar] [CrossRef]
- Ward, W.J.; Ritzer, A.; Carroll, K.M.; Flock, J.W. Catalysis of the Rochow direct process. J. Catal. 1986, 100, 240–249. [Google Scholar] [CrossRef]
- Floquet, N.; Yilmaz, S.; Falconer, J.L. Interaction of copper catalysts and Si(100) for the direct synthesis of methylchlorosilanes. J. Catal. 1994, 148, 348–368. [Google Scholar] [CrossRef]
- Luo, W.X.; Wang, G.R.; Wang, J.F. Effect of CuCl particle size on the reduction reaction by silicon in preparation of contact mass used for methylchlorosilane synthesis. Ind. Eng. Chem. Res. 2006, 45, 129–133. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Geertsema, B.J.H.; Vlugter, J.C. Mechanism and kinetics of the metal-catalyzed synthesis of methylchlorosilanes: II. The kinetics of the copper-catalyzed reaction of methyl chloride and silicon. J. Catal. 1965, 4, 43–55. [Google Scholar] [CrossRef]



| Sample | dCuO a (nm) | SBET b (m2 g−1) | Pore Volume c (cm3 g−1) | Sn/Cu Weight Ratio (×10−2) | |
|---|---|---|---|---|---|
| Bulk d | Surface e | ||||
| S-CuO | 19.4 | 18.1 | 0.007 | - | - |
| H-CuO | 42.1 | 26.5 | 0.15 | - | - |
| -SnO2(0.05)-CuO | 34.5 | 25.9 | 0.13 | 0.049 | 0.05 |
| H-SnO2(0.1)-CuO | 30.8 | 26.8 | 0.13 | 0.098 | 0.18 |
| H-SnO2-CuO | 22.4 | 25.4 | 0.15 | 0.199 | 0.42 |
| H-SnO2(0.4)-CuO | 22.9 | 27.1 | 0.12 | 0.398 | 0.38 |
| Sample | Product Composition (wt %) | CSi (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1H | M2H | LBR | HBR | ||
| S-SnO2 | - | - | - | - | - | - | - | - |
| S-CuO | 14.1 | 61.2 | 3.7 | 9.1 | 1.0 | 1.9 | 7.4 | 7.9 |
| H-CuO | 11.7 | 68.6 | 1.8 | 2.5 | 4.8 | 2.7 | 7.9 | 13.1 |
| H-SnO2(0.05)-CuO | 9.4 | 81.1 | 1.8 | 1.6 | 0.4 | 2.0 | 3.7 | 20.7 |
| H-SnO2(0.1)-CuO | 6.9 | 85.7 | 1.6 | 1.2 | 0.7 | 1.5 | 2.4 | 25.4 |
| H-SnO2-CuO | 5.1 | 88.2 | 1.0 | 0.9 | 1.0 | 0.9 | 2.9 | 36.7 |
| H-SnO2(0.4)-CuO | 15.0 | 72.1 | 2.2 | 4.3 | 0.9 | 1.6 | 3.9 | 16.4 |
| SnO2/CuO b | 14.0 | 67.8 | 2.1 | 9.3 | 0.3 | 1.0 | 7.5 | 10.1 |
| C-CAT | 12.0 | 78.0 | 4.0 | 0.8 | 0.6 | 0.5 | 3.1 | 16.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Ji, Y.; Zou, X.; Li, J.; Zhang, Y.; Wang, X.; Zhong, Z.; Su, F. Controlled Synthesis of Heterostructured SnO2-CuO Composite Hollow Microspheres as Efficient Cu-Based Catalysts for the Rochow Reaction. Catalysts 2018, 8, 144. https://doi.org/10.3390/catal8040144
Liu H, Ji Y, Zou X, Li J, Zhang Y, Wang X, Zhong Z, Su F. Controlled Synthesis of Heterostructured SnO2-CuO Composite Hollow Microspheres as Efficient Cu-Based Catalysts for the Rochow Reaction. Catalysts. 2018; 8(4):144. https://doi.org/10.3390/catal8040144
Chicago/Turabian StyleLiu, Hezhi, Yongjun Ji, Xiujing Zou, Jing Li, Yu Zhang, Xueguang Wang, Ziyi Zhong, and Fabing Su. 2018. "Controlled Synthesis of Heterostructured SnO2-CuO Composite Hollow Microspheres as Efficient Cu-Based Catalysts for the Rochow Reaction" Catalysts 8, no. 4: 144. https://doi.org/10.3390/catal8040144
APA StyleLiu, H., Ji, Y., Zou, X., Li, J., Zhang, Y., Wang, X., Zhong, Z., & Su, F. (2018). Controlled Synthesis of Heterostructured SnO2-CuO Composite Hollow Microspheres as Efficient Cu-Based Catalysts for the Rochow Reaction. Catalysts, 8(4), 144. https://doi.org/10.3390/catal8040144





