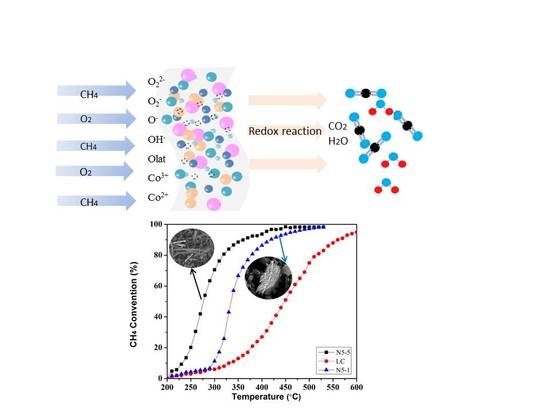
Active Component Migration and Catalytic Properties of Nitrogen Modified Composite Catalytic Materials
Abstract
1. Introduction
2. Results and Discussion
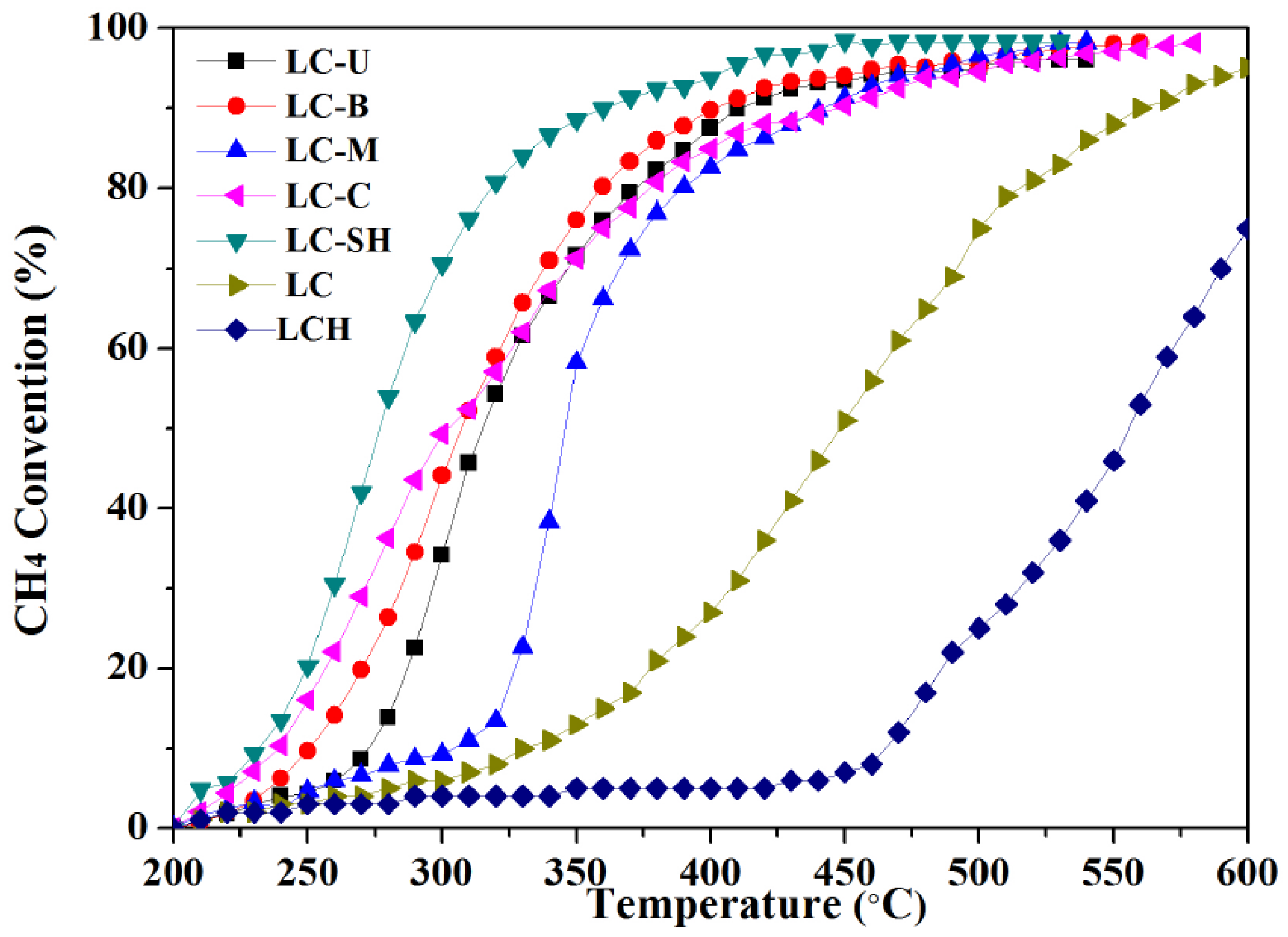
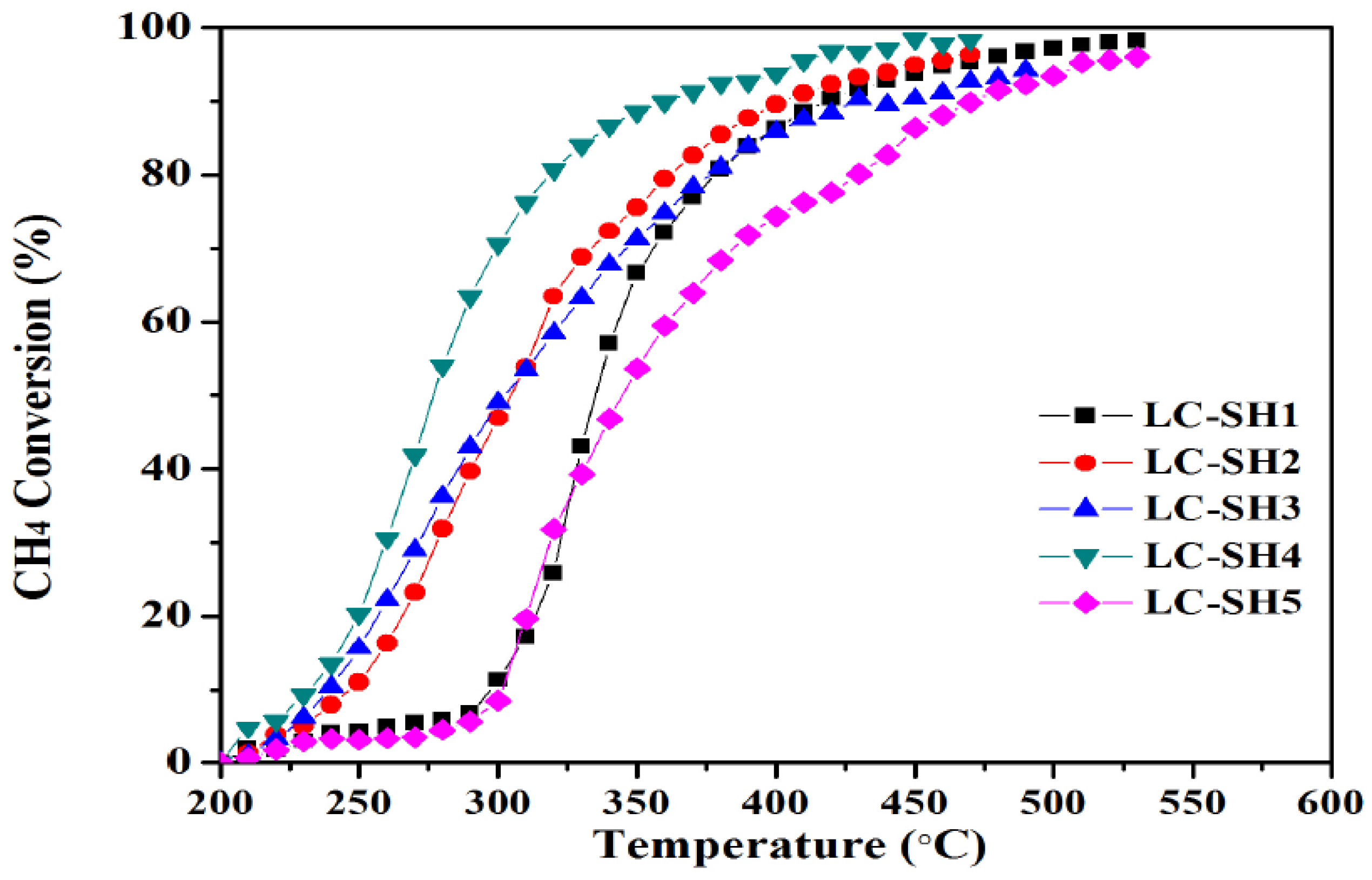
2.1. Catalytic Performance
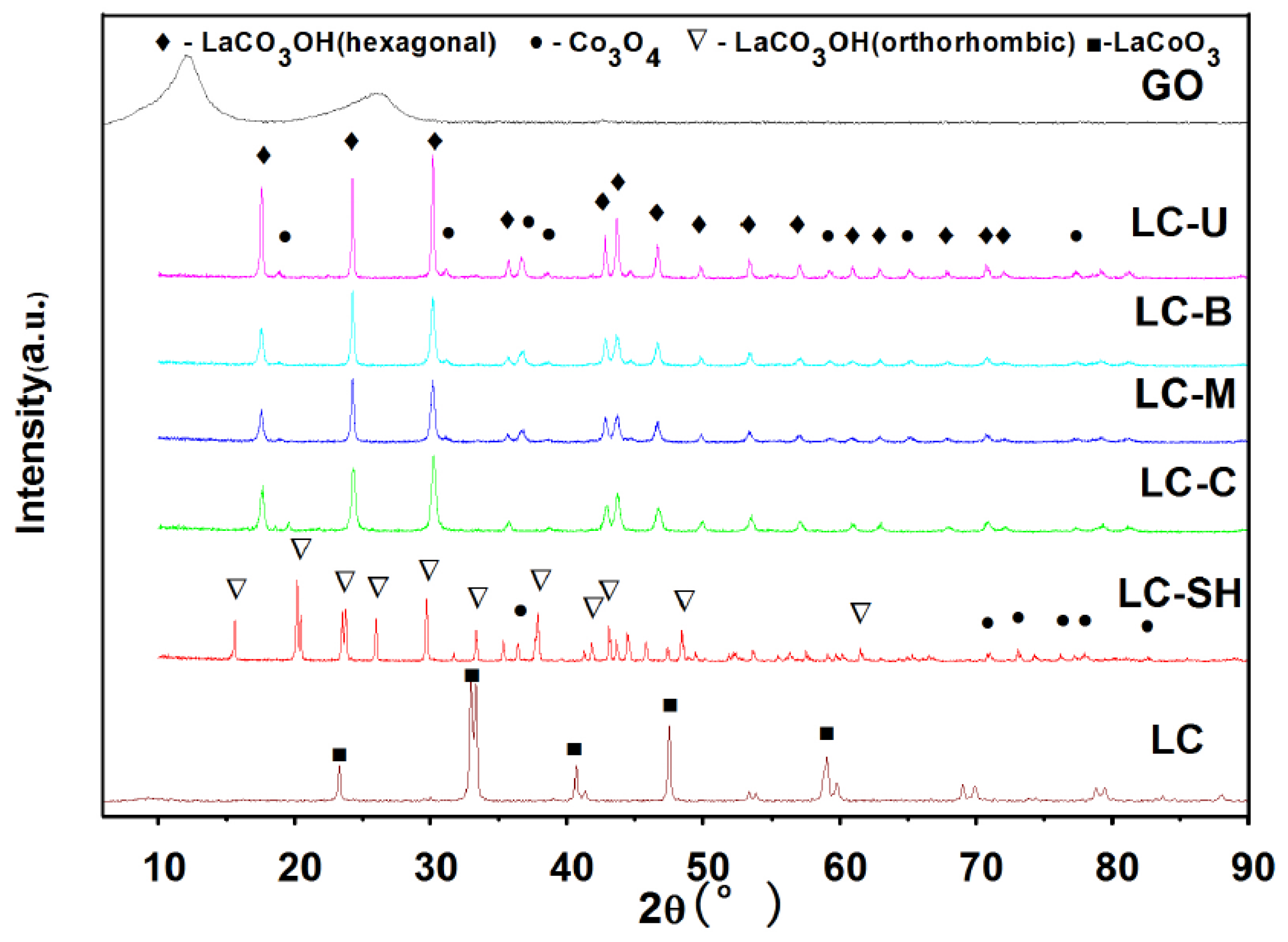
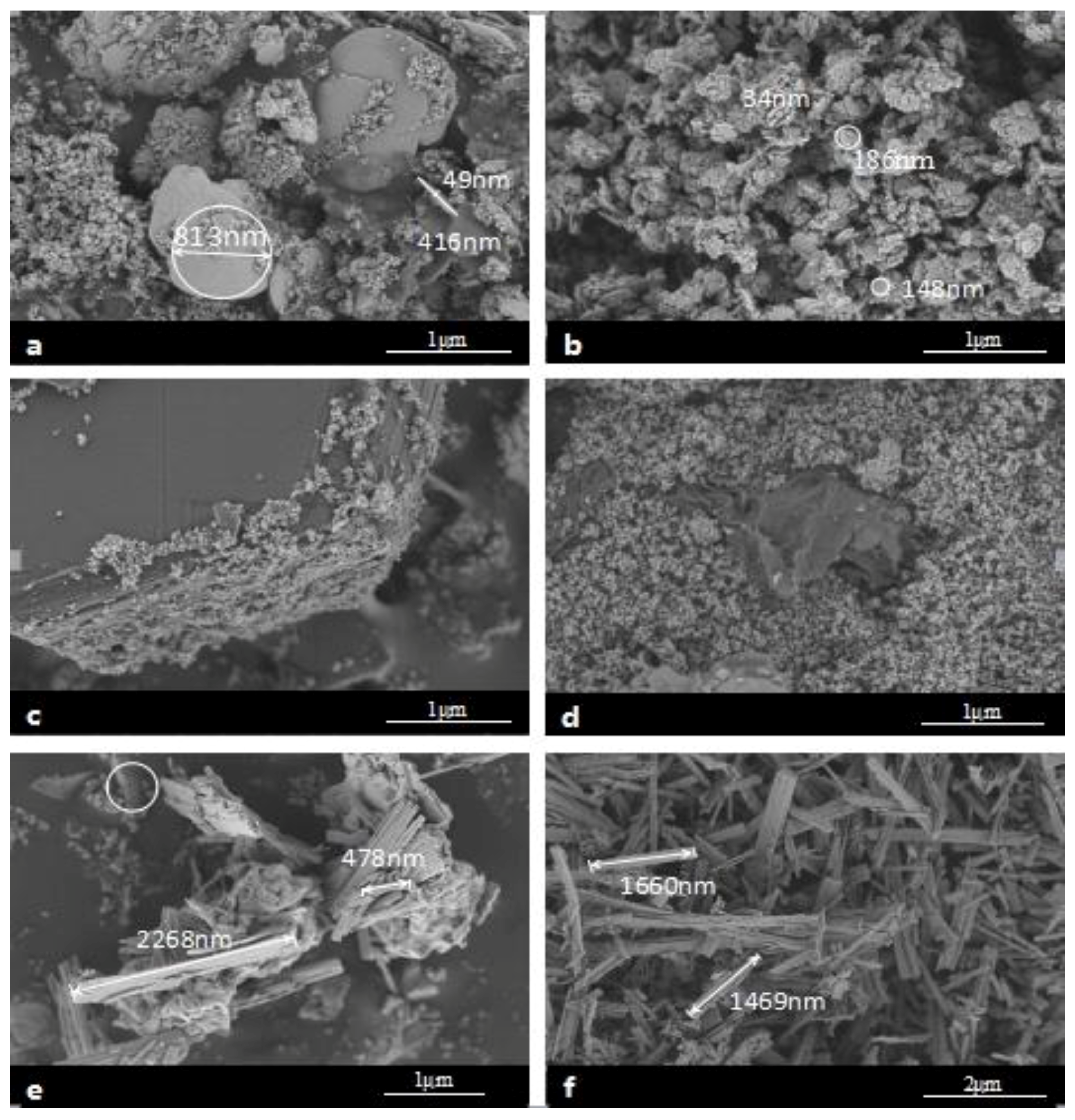
2.2. Physical Characterization
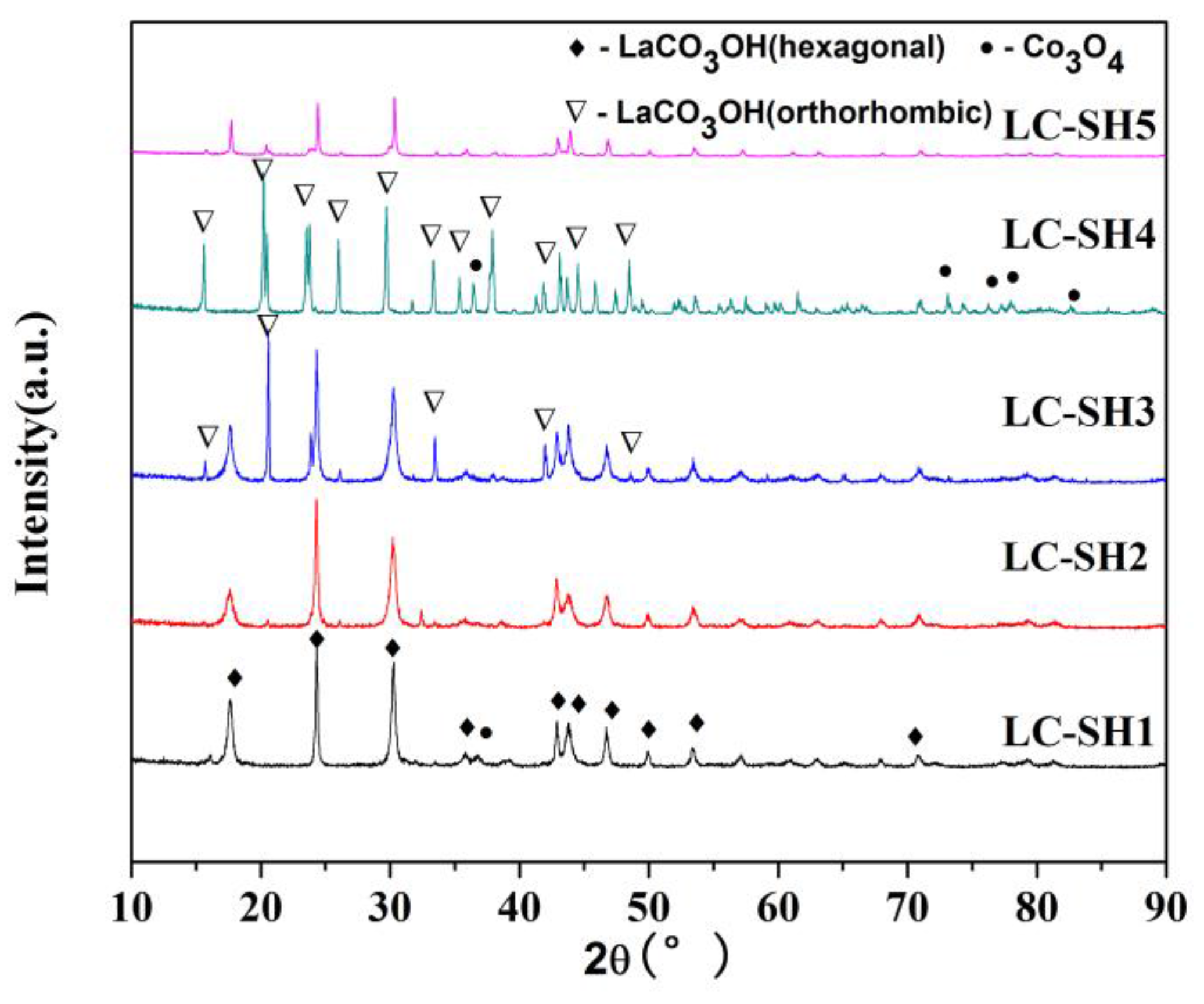
2.2.1. Modified Catalysts of Different Nitrogen Sources
2.2.2. Modified Catalysts of Different Added Amount Nitrogen Sources
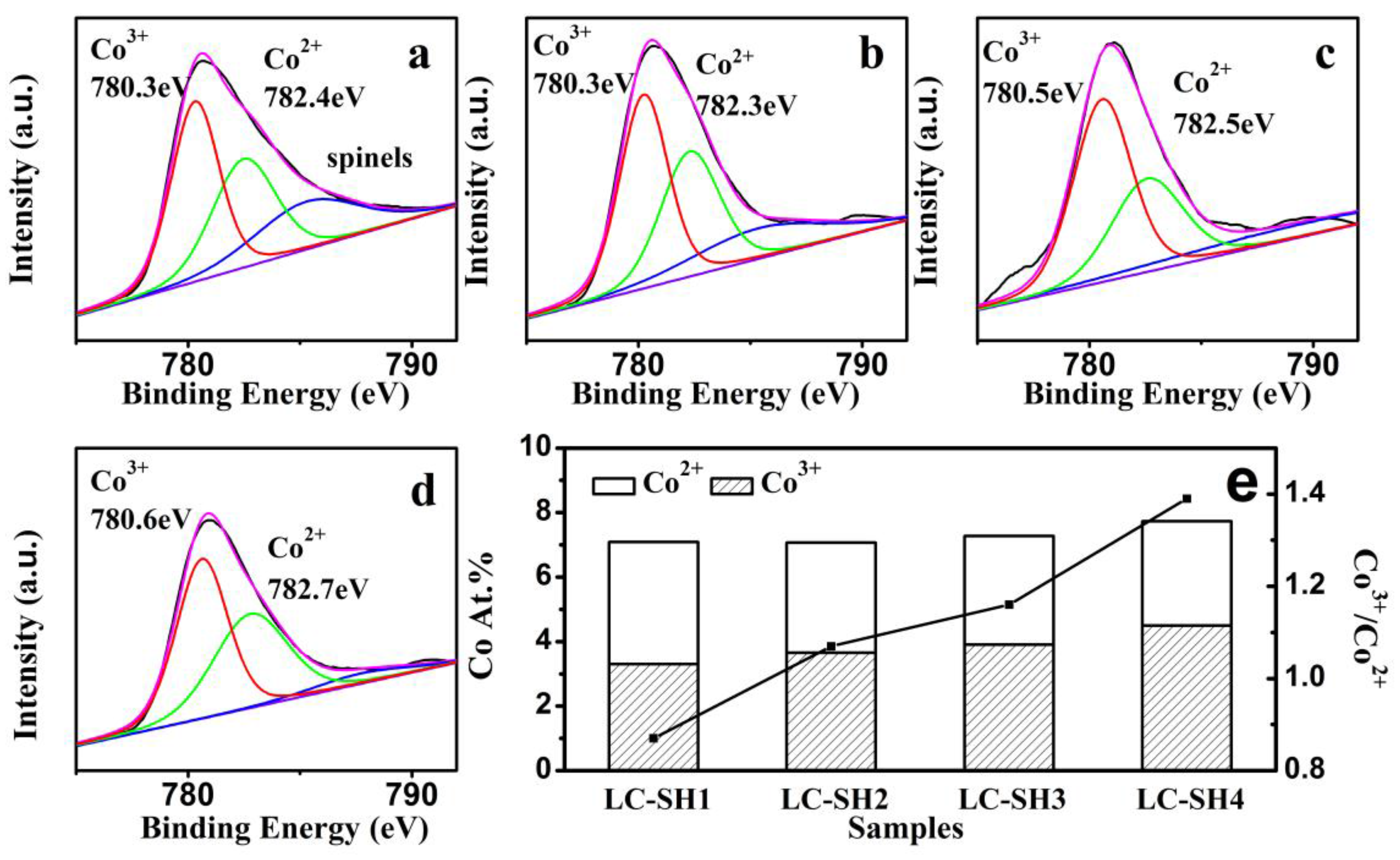
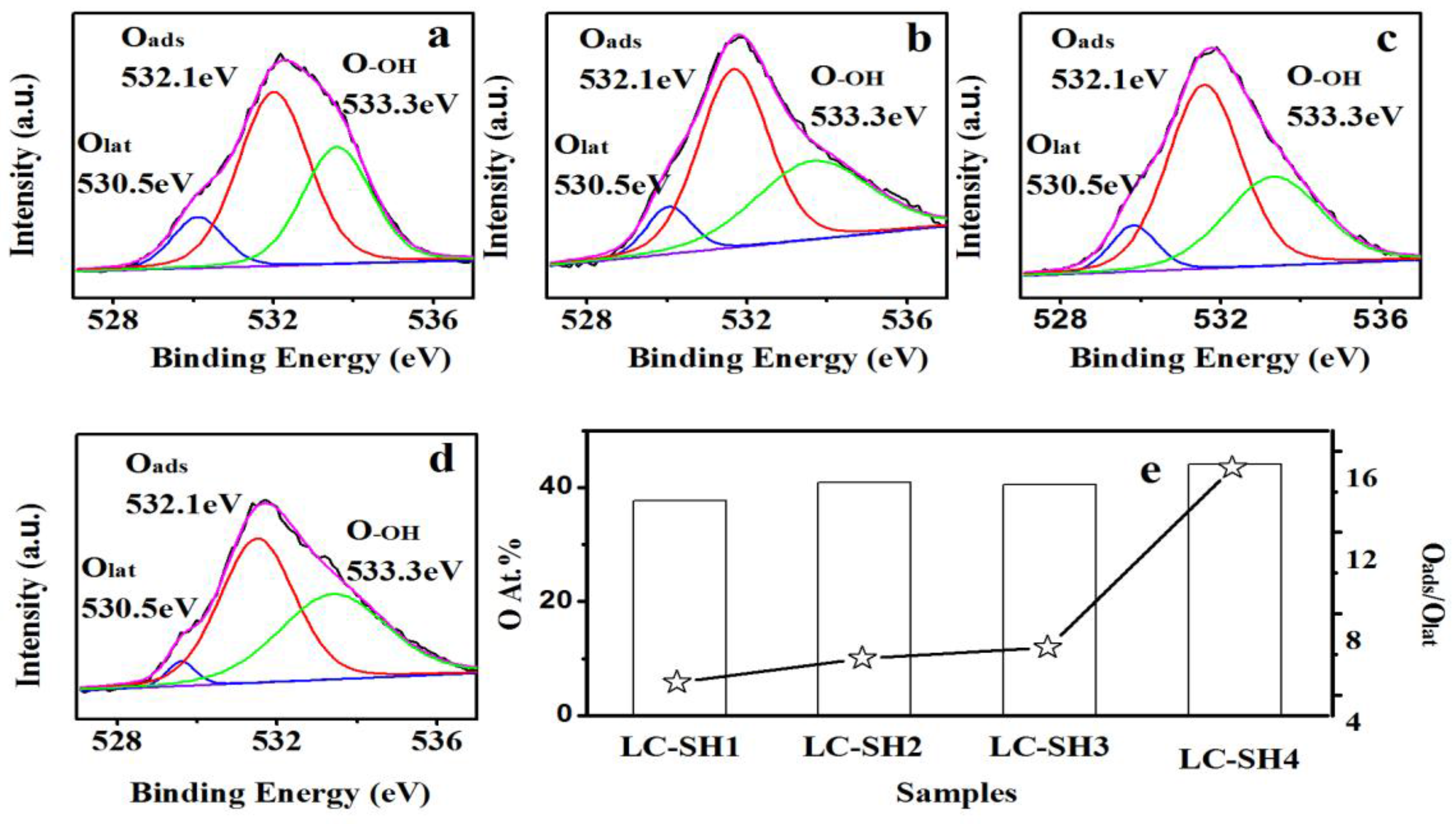
2.3. The Surface Composition of Composite Catalytic Materials
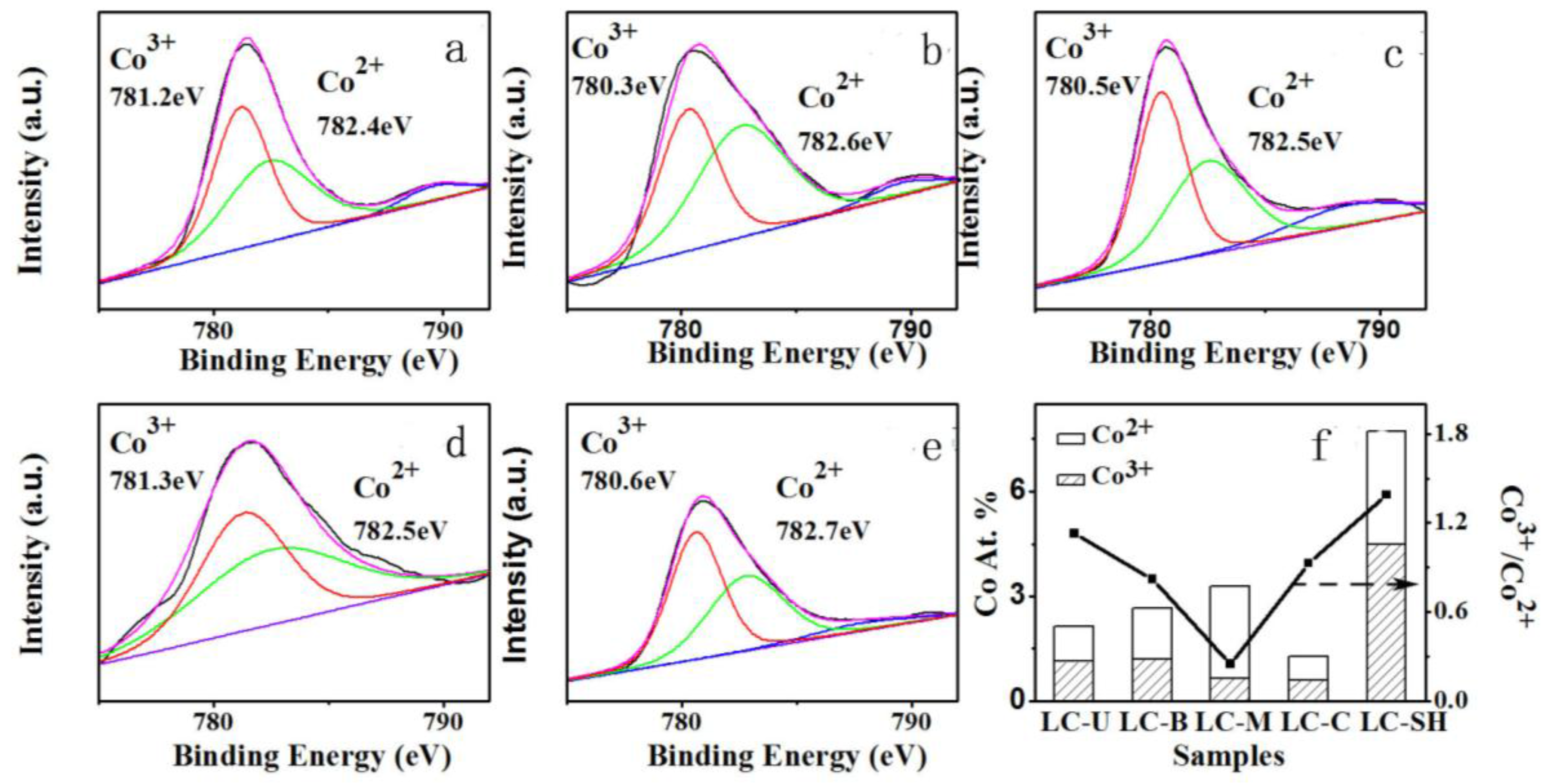
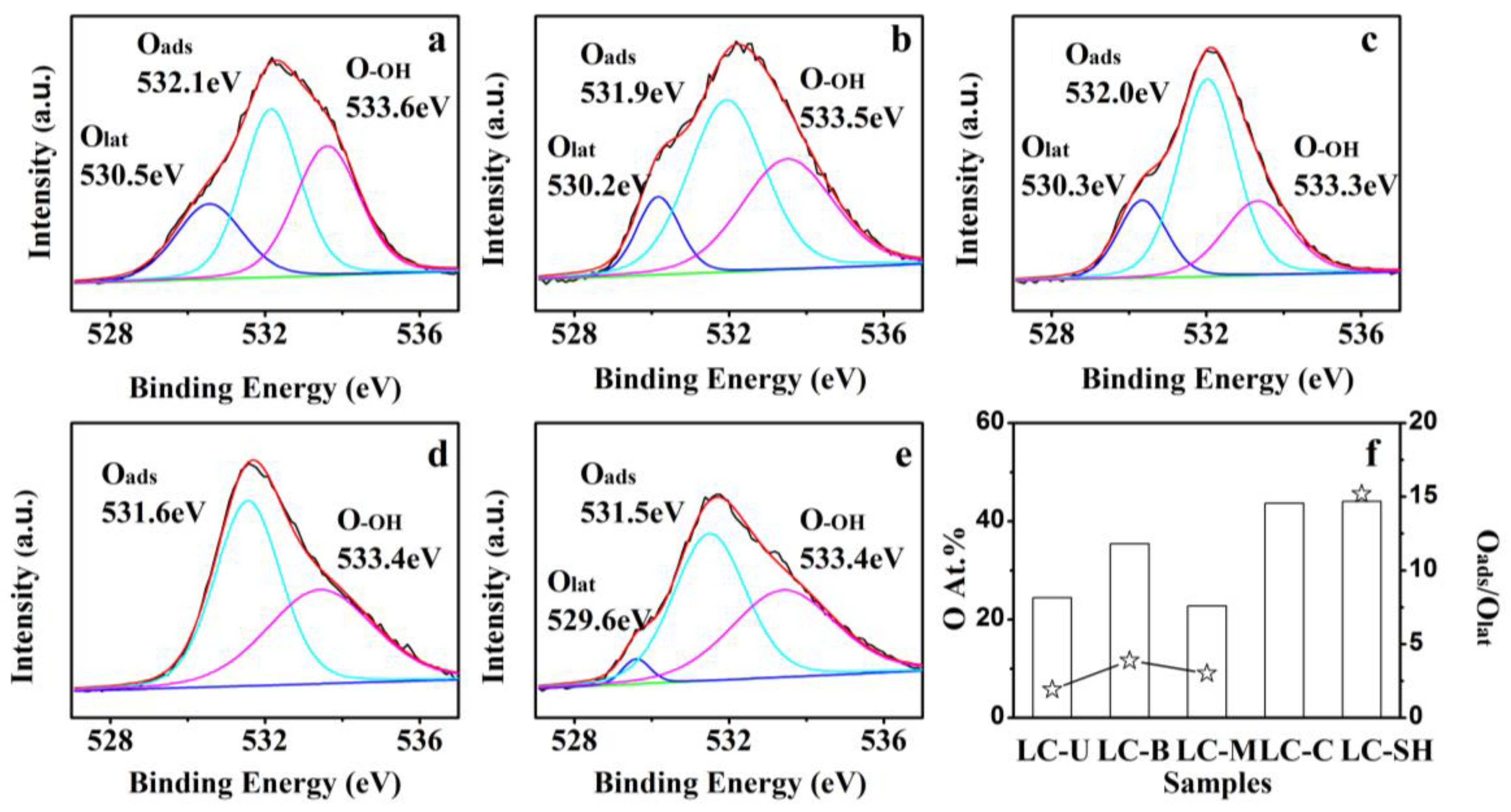
2.3.1. Modified Catalysts of Different Nitrogen Sources
2.3.2. Modified Catalyst of Different Added Amount Nitrogen Source
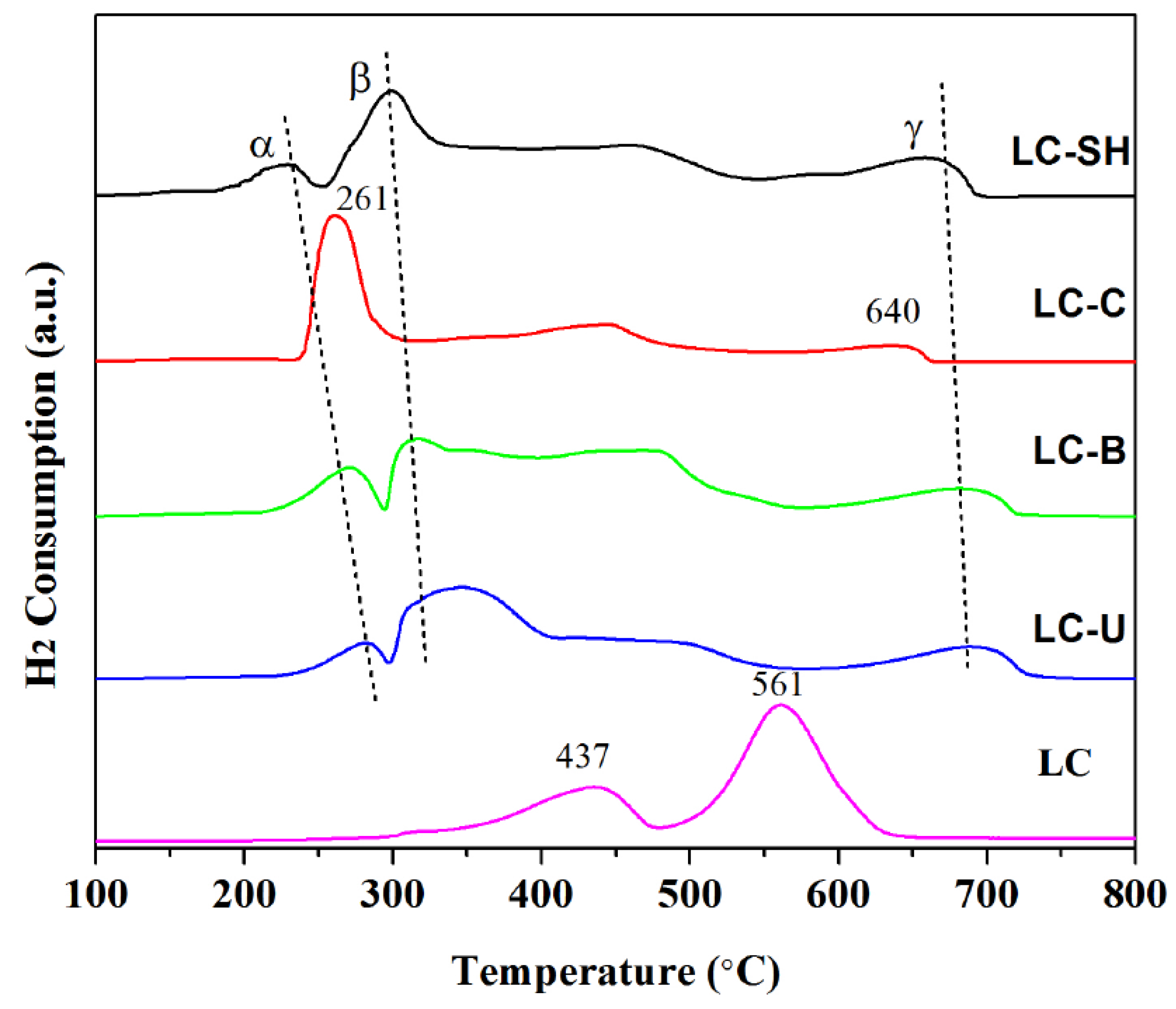
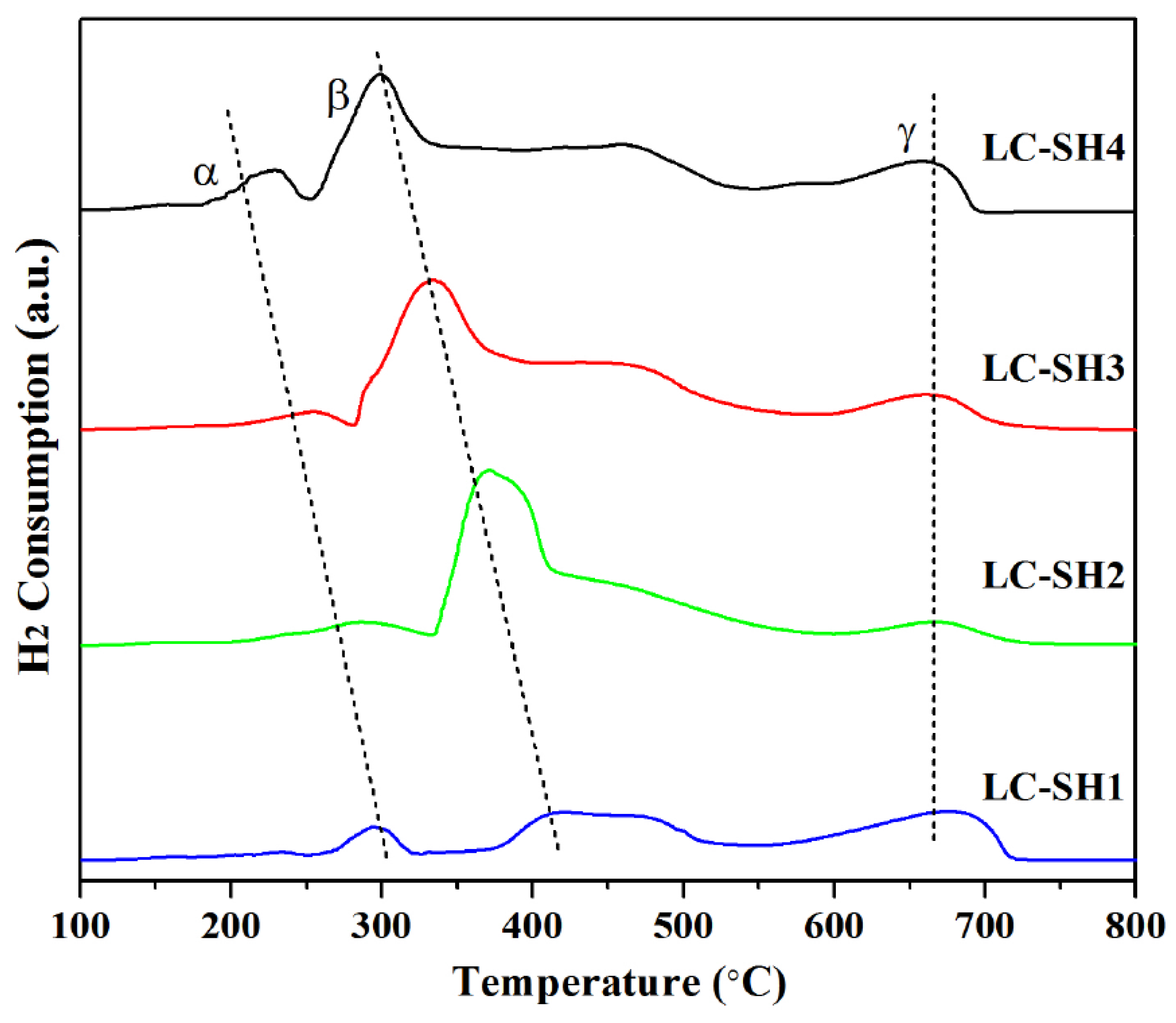
2.4. Reducibility
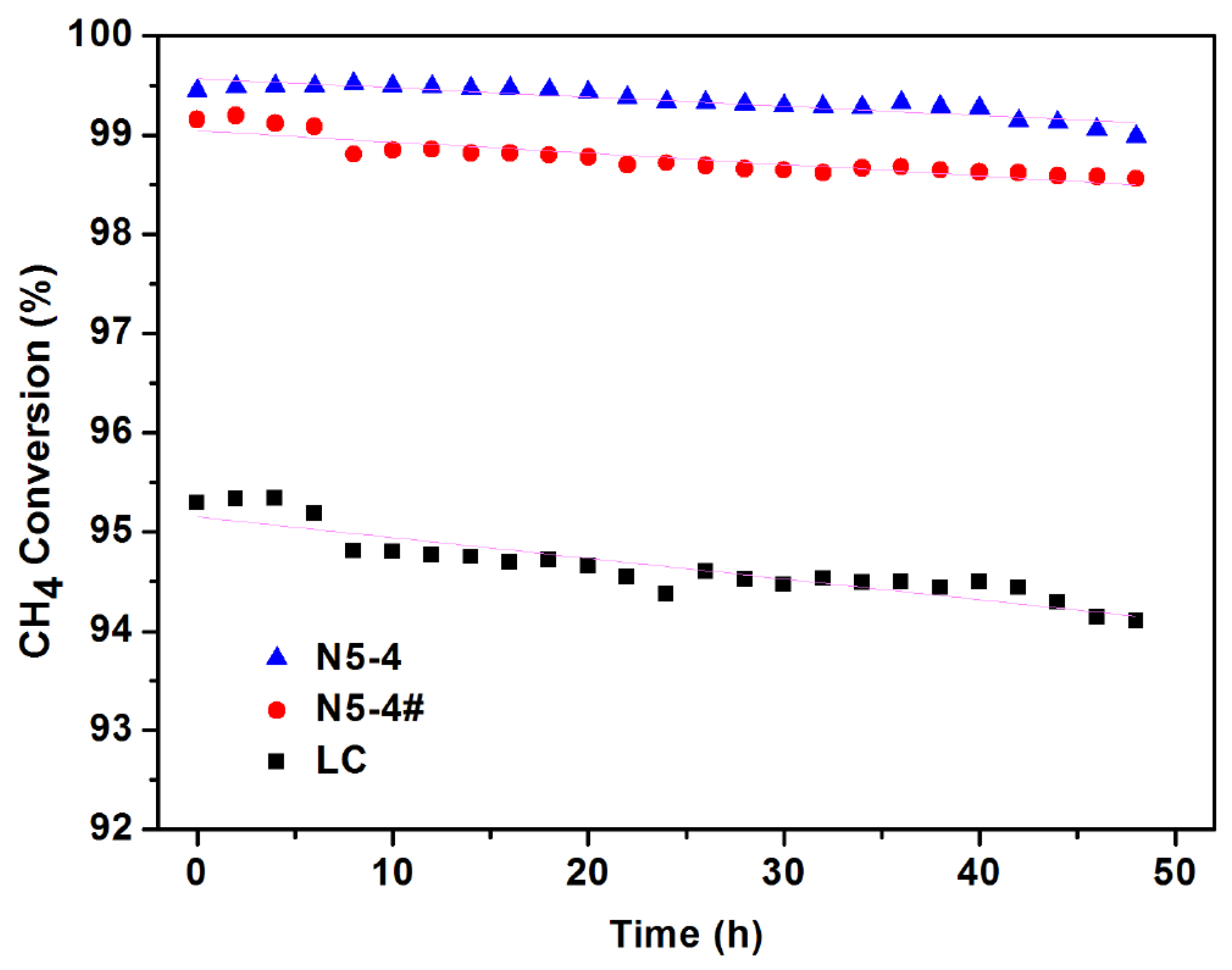
2.5. Catalyst Stability Test
2.6. Mechanism Analysis
2.6.1. Preparation Mechanism
2.6.2. Reaction Mechanism
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Evaluation
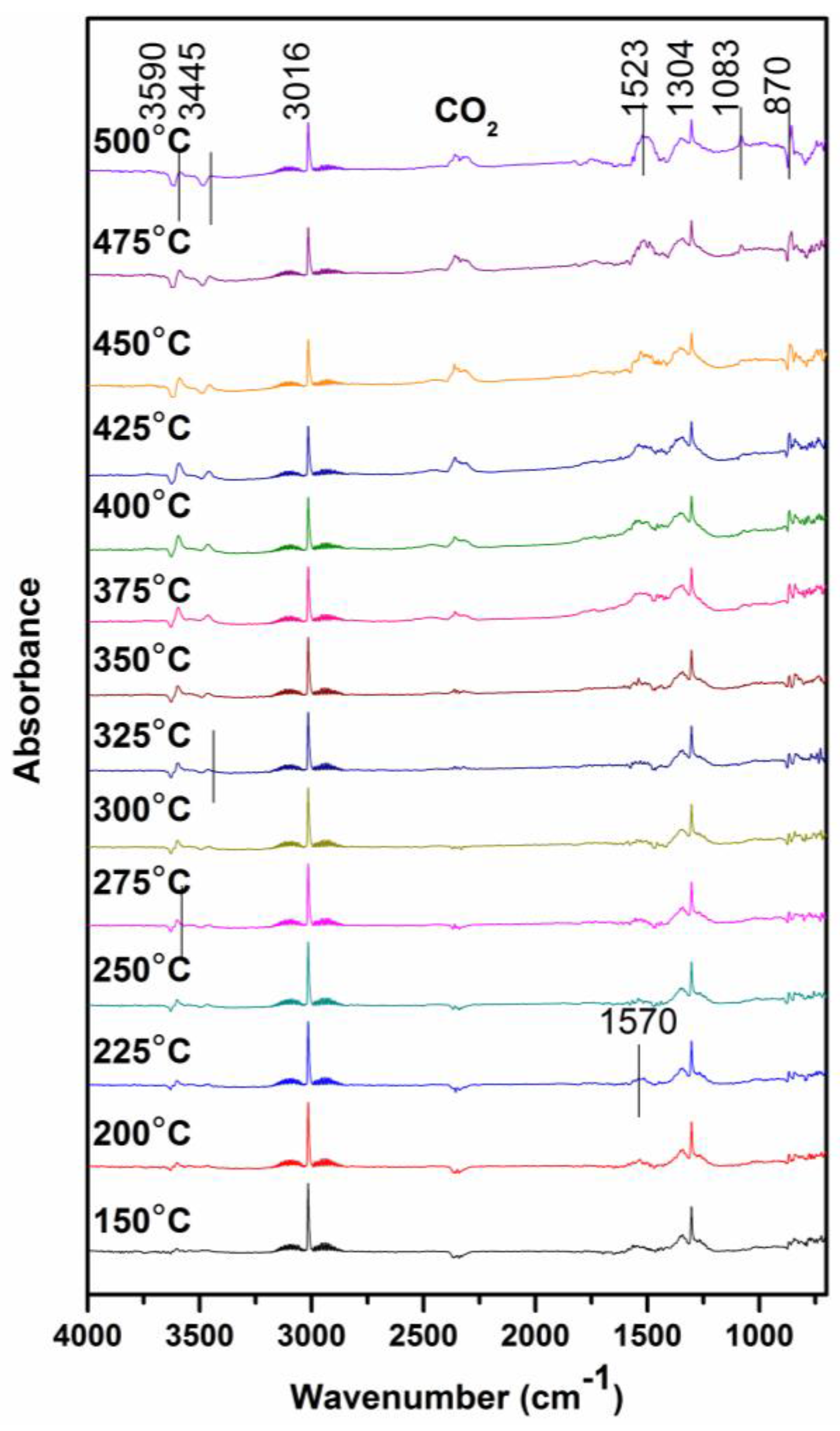
3.4. In Situ FITR Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, J.H.; Arandiyan, H.; Gao, X.; Li, J.H. Recent advances in catalysts for methane combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Y.X.; Deng, J.G.; Xie, S.H.; Zhao, X.T.; Zhang, Y.; Zhang, K.F.; Arandiyan, H.; Guo, G.S.; Dai, H.X. Enhanced catalytic performance for methane combustion of 3DOM CoFe2O4 by co-loading MnOx and Pd–Pt alloy nanoparticles. Appl. Surf. Sci. 2017, 403, 590–600. [Google Scholar] [CrossRef]
- Zuo, X.L.; Rui, Z.H.; Ji, B. Core-shell NiO@PdO nanoparticles supported on alumina as an advanced catalyst for methane oxidation. ACS Catal. 2017, 403, 590–600. [Google Scholar]
- Xu, P.; Wu, Z.X.; Deng, J.G.; Liu, Y.X.; Xie, S.H.; Guo, G.S.; Dai, H.X. Catalytic performance enhancement by alloying Pd with Pt on ordered mesoporous manganese oxide for methane combustion. Chin. J. Catal. 2017, 38, 92–105. [Google Scholar] [CrossRef]
- Tacchino, S.; Vella, L.D.; Specchia, S. Catalytic combustion of CH4 and H2 into micro-monoliths. Catal. Today 2010, 157, 440–445. [Google Scholar] [CrossRef]
- Gholami, R.; Alyani, M.; Smith, K.J. Deactivation of Pd catalysts by water during low temperature methane oxidation relevant to natural gas vehicle converters. Catalysts 2015, 5, 561–594. [Google Scholar] [CrossRef]
- Benedetto, A.D.; Landi, G.; Sarli, V.D. Methane catalytic combustion under pressure. Catal. Today 2012, 197, 206–213. [Google Scholar] [CrossRef]
- Barbato, P.S.; Benedetto, A.D.; Sarli, V.D. High-pressure methane combustion over a perovskite catalyst. Ind. Eng. Chem. Res. 2012, 51, 7547–7558. [Google Scholar] [CrossRef]
- Arandiyan, H.; Scott, J.; Yuan, W. Meso-molding three-dimensional macroporous perovskites: A new approach to generate high-performance nanohybrid catalysts. ACS Appl. Mater. Interfaces 2016, 8, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; He, F.; Huang, Z.; Zheng, A.Q.; Li, H.B.; Zhao, Z.L. La1−xSrxFeO3 perovskites as oxygen carriers for the partial oxidation of methane to syngas. Chin. J. Catal. 2014, 35, 1196–1205. [Google Scholar] [CrossRef]
- Xue, G.; Zhao, Y.; Liang, J.S.; Yuan, Y.K.; Wang, S.F.; Chen, D. Properties of La0.9Sr0.1MnO3 and tourmaline compound catalytic materials for methane combustion. J. Rare Earths 2014, 32, 837–841. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Chu, X.F.; Feng, W.C.; Zhou, C.P.; Wu, X.F.; Yuan, L.; Feng, S.H. Catalytic behavior of electrospinning synthesized La0.75Sr0.25MnO3, nanofibers in the oxidation of CO and CH4. Chem. Eng. J. 2014, 244, 27–32. [Google Scholar] [CrossRef]
- Ritzmann, A.; Pavone, M.; Munozgarcia, A.; Keith, J.; Carter, E. Ab initio DFT+U analysis of oxygen transport in LaCoO3: The effect of Co3+ magnetic states. J. Mater. Chem. A 2014, 2, 8060–8074. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sazonova, N.N.; Bobin, A.S.; Bobin, V.S.; Muzykantov, V.S.; Gubanova, E.L.; Alikina, G.M.; Lukashevich, A.I.; Rogov, V.A.; Ermakova, E.N.; et al. Partial oxidation of methane on Pt-supported lanthanide doped ceria–zirconia oxides: Effect of the surface/lattice oxygen mobility on catalytic performance. Catal. Today 2011, 169, 125–137. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Deng, J. Dual-templating synthesis of three-dimensionally ordered macroporous La0.6Sr0.4MnO3-supported Ag nanoparticles: Controllable alignments and super performance for the catalytic combustion of methane. Chem. Commun. 2013, 49, 10748–10750. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.B.; Yin, L.C.; Xie, Y.P.; Liu, G.; Ma, X.L.; Cheng, H.M.; Yu, Z.; Yin, L.C.; Xie, Y. Crystallinity-dependent substitutional nitrogen doping in ZnO and its improved visible light photocatalytic activity. J. Colloid Interface Sci. 2013, 400, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.L.; Zhang, X.Y.; Bai, X.; Lv, L.L.; Ye, Z.F.; Huang, X. Nitrogen-doped perovskite-type La2Ti2O7 decorated on graphene composites exhibiting efficient photocatalytic activity toward bisphenol A in water. J. Colloid Interface Sci. 2015, 450, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, T.; Shi, M. Polyelectrolyte-assisted one-step hydrothermal synthesis of Ag-reduced graphene oxide composite and its antibacterial properties. Mater. Sci. Eng. C 2012, 32, 2042–2047. [Google Scholar] [CrossRef]
- Gao, W.; Majumder, M.; Alemany, L.B. Engineered graphite oxide materials for application in water purification. ACS Appl. Mater. Interfaces 2011, 3, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xue, G.; Liang, J. Promotional effect of graphite oxide on surface properties and catalytic performance of La1−xSrxMnO3, (x = 0,0.1) for methane combustion. Catal. Commun. 2014, 45, 39–43. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, C.; Wang, X. Oxidation of SO2 to SO3 catalyzed by graphene oxide foams. J. Mater. Chem. 2011, 21, 13934–13941. [Google Scholar] [CrossRef]
- Zahed, B.; Hosseini-Monfared, H. A comparative study of silver-graphene oxide nanocomposites as a recyclable catalyst for the aerobic oxidation of benzyl alcohol: Support effect. Appl. Surf. Sci. 2015, 328, 536–547. [Google Scholar] [CrossRef]
- Nie, R.F.; Shi, J.J.; Du, W.C.; Ning, W.S.; Hou, Z.Y.; Xiao, F.S. A sandwich N-doped graphene/Co3O4 hybrid: An efficient catalyst for selective oxidation of olefins and alcohols. J. Mater. Chem. A 2013, 1, 9037–9045. [Google Scholar] [CrossRef]
- Djinović, P.; Črnivec, I.G.O.; Erjavec, B.; Albin, P. Influence of active metal loading and oxygen mobility on coke-free dry reforming of Ni-Co bimetallic catalysts. Appl. Catal. B Environ. 2012, 125, 259–270. [Google Scholar] [CrossRef]
- Ji, K.M.; Dai, H.X.; Deng, J.G.; Li, X.W.; W, Y.; Gao, B.Z.; Bai, G.M.; Au, C.T. A comparative study of bulk and 3DOM-structured Co3O4, Eu0.6Sr0.4FeO3, and Co3O4/Eu0.6Sr0.4FeO3: Preparation, characterization, and catalytic activities for toluene combustion. Appl. Catal. A Gen. 2012, 447, 41–48. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Kaliaguine, S. Oxygen mobility in LaCoO3 perovskites. Catal. Today 2006, 112, 99–102. [Google Scholar] [CrossRef]
- Huang, L.; Bassir, M.; Kaliaguine, S. Reducibility of Co3+ in perovskite-type LaCoO3 and promotion of copper on the reduction of Co3+ in perovskite-type oxides. Appl. Surf. Sci. 2005, 243, 360–375. [Google Scholar] [CrossRef]
- Wu, Z.X.; Deng, J.G.; Liu, Y.X.; Xie, S.H.; Jiang, Y.; Zhao, X.T.; Yang, J.; Arandiyan, H.; Guo, G.S.; Dai, H.X. Three-dimensionally ordered mesoporous Co3O4-supported Au–Pd alloy nanoparticles: High-performance catalysts for methane combustion. J. Catal. 2015, 332, 13–24. [Google Scholar] [CrossRef]
- Lu, J.; Song, J.J.; Niu, H.; Pan, L.; Zhang, X.W.; Wang, L.; Zou, J.J. Periodic density functional theory study of ethylene hydrogenation over Co3O4 (111) surface: The critical role of oxygen vacancies. Appl. Surf. Sci. 2016, 371, 61–66. [Google Scholar] [CrossRef]
- Li, H.F.; Lu, G.Z.; Qiao, D.S.; Wang, Y.Q.; Guo, Y.; Guo, Y.L. Catalytic methane combustion over Co3O4/CeO2 composite oxides prepared by modified citrate sol-gel method. Catal. Lett. 2011, 141, 452–458. [Google Scholar] [CrossRef]
- Petkovic, L.M.; Utgikar, V.; Rashkeev, S.N. Structural stability and catalytic activity of lanthanum-based perovskites. J. Phys. Chem. C 2011, 115, 8709–8715. [Google Scholar] [CrossRef]
- Pan, B.; Xie, Q.H.; Wang, H.M.; Wang, X.X. Synthesis and photocatalytic hydrogen production of a novel photocatalyst LaCO3OH. J. Mater. Chem. A 2013, 1, 6629–6634. [Google Scholar] [CrossRef]
- Han, Z.H.; Guo, N.; Tang, K.B. Hydrothermal crystal growth and characterization of cerium hydroxycarbonates. J. Cryst. Growth 2000, 219, 315–318. [Google Scholar] [CrossRef]
- Han, Z.; Yang, Q.; Lu, G.Q. Crystalline lanthanum hydroxycarbonates with controlled phases and varied morphologies prepared on non-crystalline substrates. J. Solid State Chem. 2004, 177, 3709–3714. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Sadovskaya, E.M.; Pinaeva, L.G.; Isupova, L.A. Influence of oxygen mobility on catalytic activity of La-Sr-Mn-O composites in the reaction of high temperature LC-BO decomposition. J. Catal. 2009, 267, 5–13. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]

| Samples | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| LC | 10.11 | 0.017 | 8.86 |
| LC-U | 12.00 | 0.030 | 9.91 |
| LC-B | 14.87 | 0.036 | 9.27 |
| LC-M | 10.37 | 0.042 | 9.82 |
| LC-C | 10.37 | 0.046 | 9.86 |
| LC-SH | 26.45 | 0.129 | 9.89 |
| Samples | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| LC-SH-1 | 12.62 | 0.089 | 8.94 |
| LC-SH-2 | 15.78 | 0.099 | 7.73 |
| LC-SH-3 | 20.37 | 0.104 | 8.90 |
| LC-SH-4 | 26.45 | 0.129 | 9.89 |
| LC-SH-5 | 10.13 | 0.074 | 7.14 |
| Samples | La (%) | Co (%) | O (%) | C (%) |
|---|---|---|---|---|
| LC-U | 1.97 | 2.75 | 31.51 | 63.77 |
| LC-B | 3.28 | 2.67 | 34.06 | 59.99 |
| LC-M | 1.75 | 4.86 | 33.58 | 58.99 |
| LC-C | 6.88 | 1.27 | 45.71 | 46.14 |
| LC-SH4 | 3.55 | 5.73 | 35.41 | 55.31 |
| Samples | La (%) | Co (%) | O (%) | C (%) |
|---|---|---|---|---|
| LC-SH1 | 6.80 | 7.09 | 40.91 | 45.20 |
| LC-SH2 | 5.66 | 7.07 | 40.57 | 46.71 |
| LC-SH3 | 5.85 | 1.87 | 37.68 | 54.60 |
| LC-SH4 | 3.55 | 5.73 | 35.41 | 55.31 |
| Samples | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| LC-fresh | 10.11 | 0.017 | 8.86 |
| LC-used | 4.46 | 0.003 | 9.87 |
| LC-SH4-fresh | 26.45 | 0.129 | 9.89 |
| LC-SH4-used | 24.67 | 0.091 | 10.09 |
| LC-SH4#-fresh | 21.00 | 0.081 | 11.77 |
| LC-SH4#-used | 19.38 | 0.033 | 12.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Gui, P.; Zheng, L.; Li, J.; Xue, G.; Liang, J. Active Component Migration and Catalytic Properties of Nitrogen Modified Composite Catalytic Materials. Catalysts 2018, 8, 125. https://doi.org/10.3390/catal8040125
Li M, Gui P, Zheng L, Li J, Xue G, Liang J. Active Component Migration and Catalytic Properties of Nitrogen Modified Composite Catalytic Materials. Catalysts. 2018; 8(4):125. https://doi.org/10.3390/catal8040125
Chicago/Turabian StyleLi, Miaomiao, Peng Gui, Luning Zheng, Jiaang Li, Gang Xue, and Jinsheng Liang. 2018. "Active Component Migration and Catalytic Properties of Nitrogen Modified Composite Catalytic Materials" Catalysts 8, no. 4: 125. https://doi.org/10.3390/catal8040125
APA StyleLi, M., Gui, P., Zheng, L., Li, J., Xue, G., & Liang, J. (2018). Active Component Migration and Catalytic Properties of Nitrogen Modified Composite Catalytic Materials. Catalysts, 8(4), 125. https://doi.org/10.3390/catal8040125