Active and Stable Methane Oxidation Nano-Catalyst with Highly-Ionized Palladium Species Prepared by Solution Combustion Synthesis
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
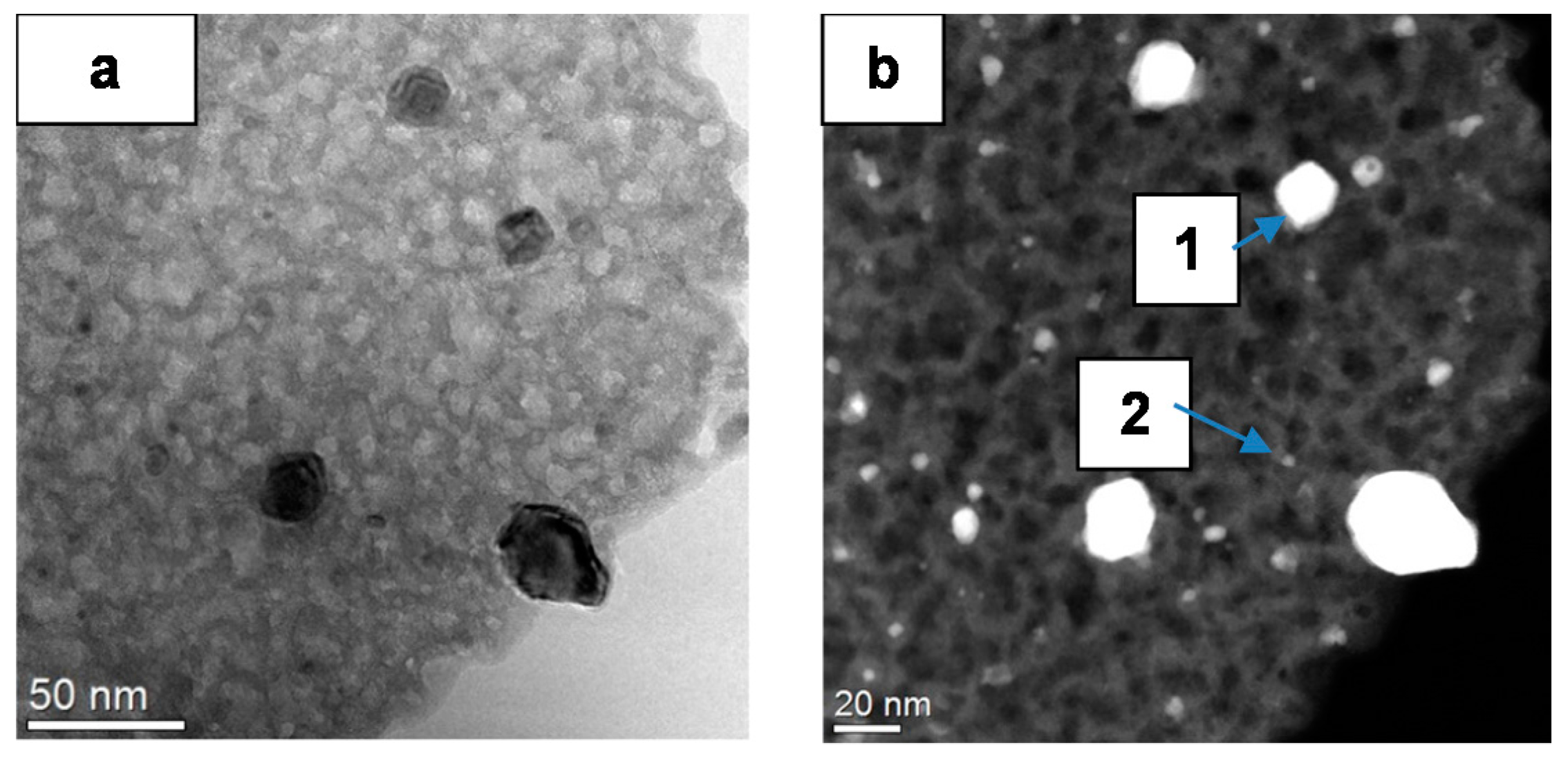
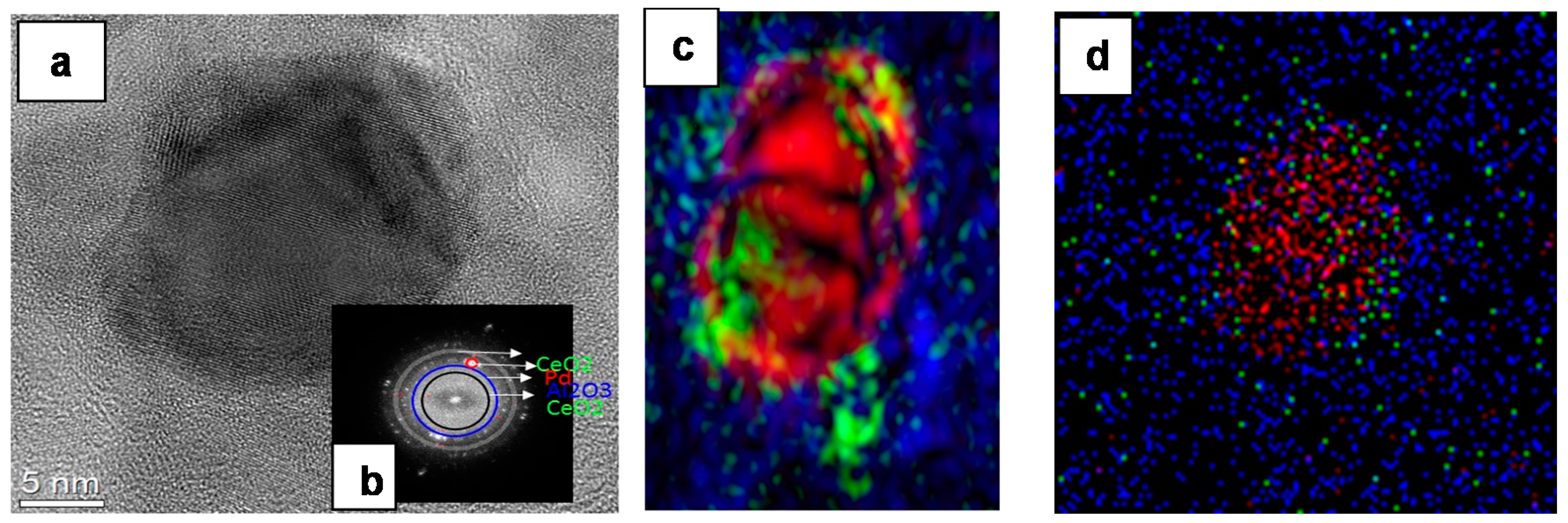
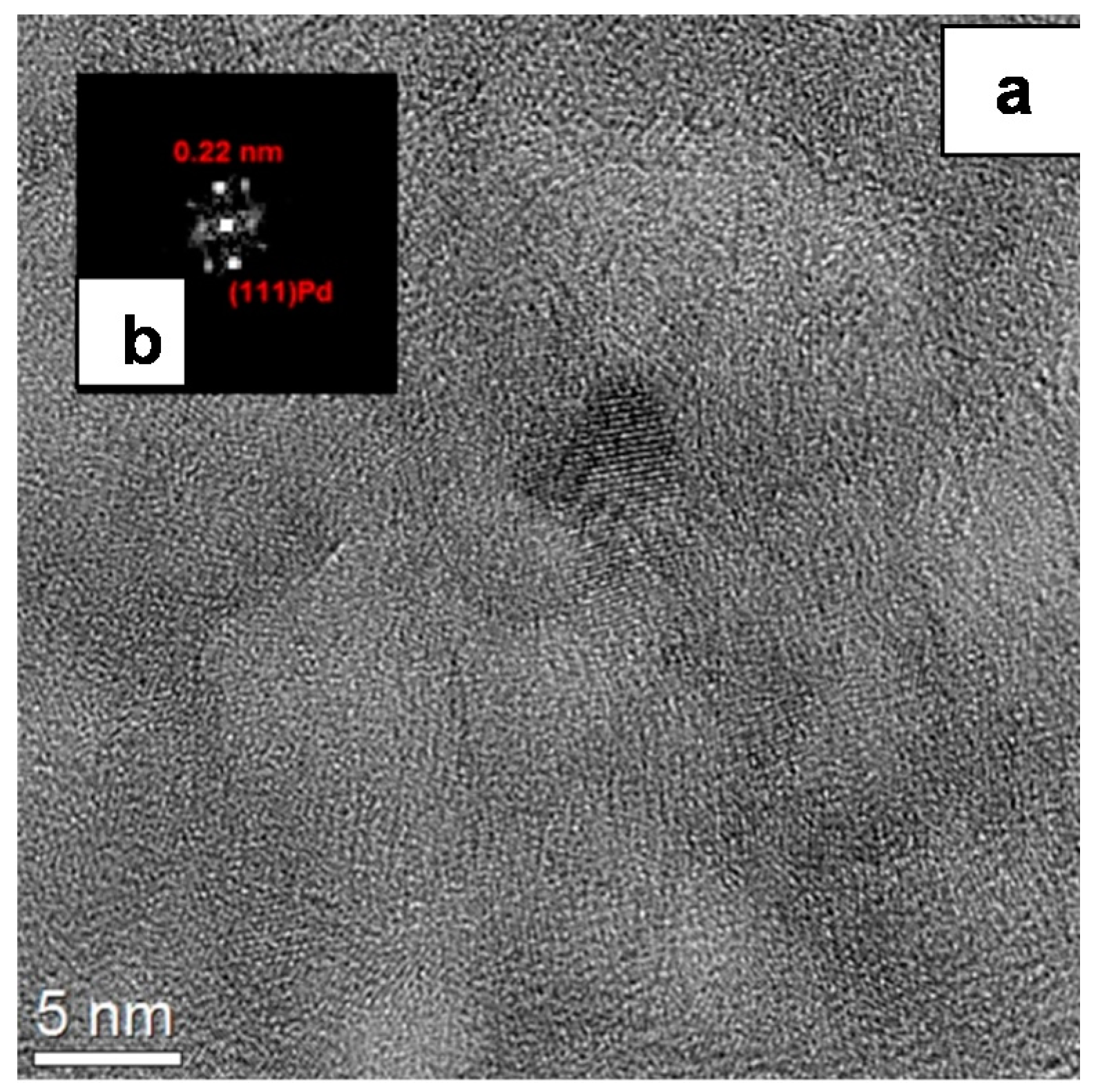
2.1.1. Catalyst Surface Morphology
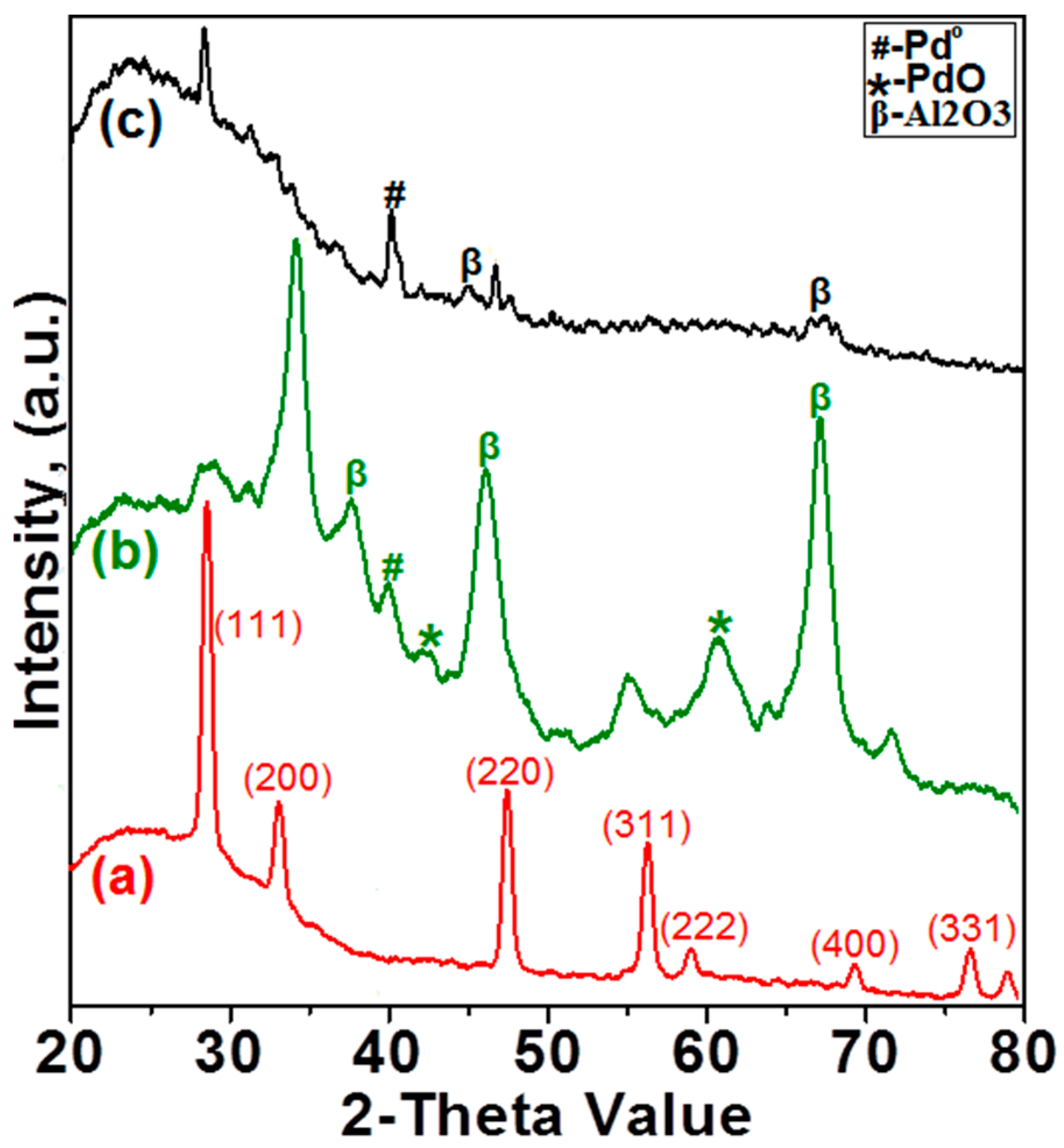
2.1.2. X-ray Diffraction Analysis
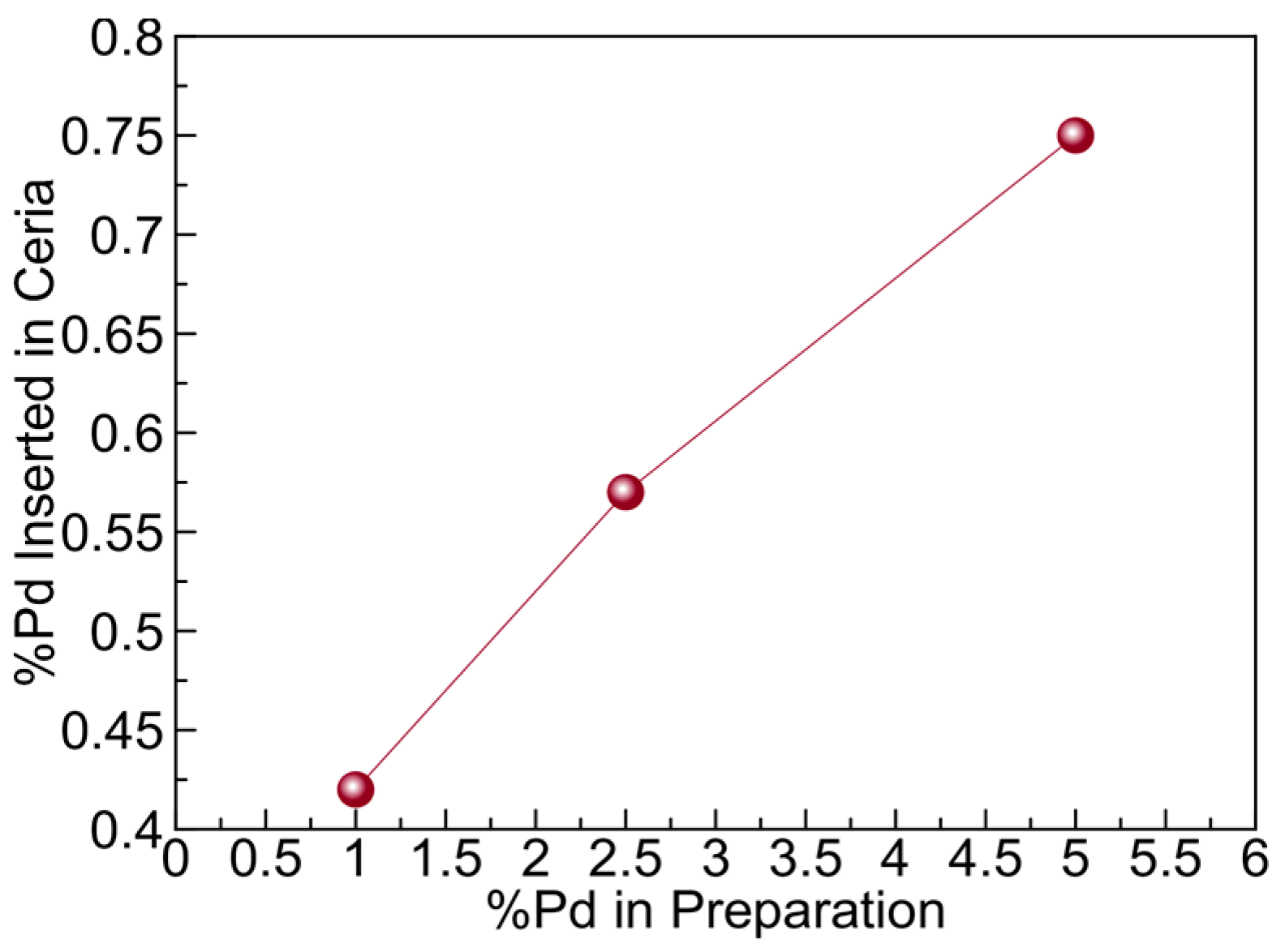
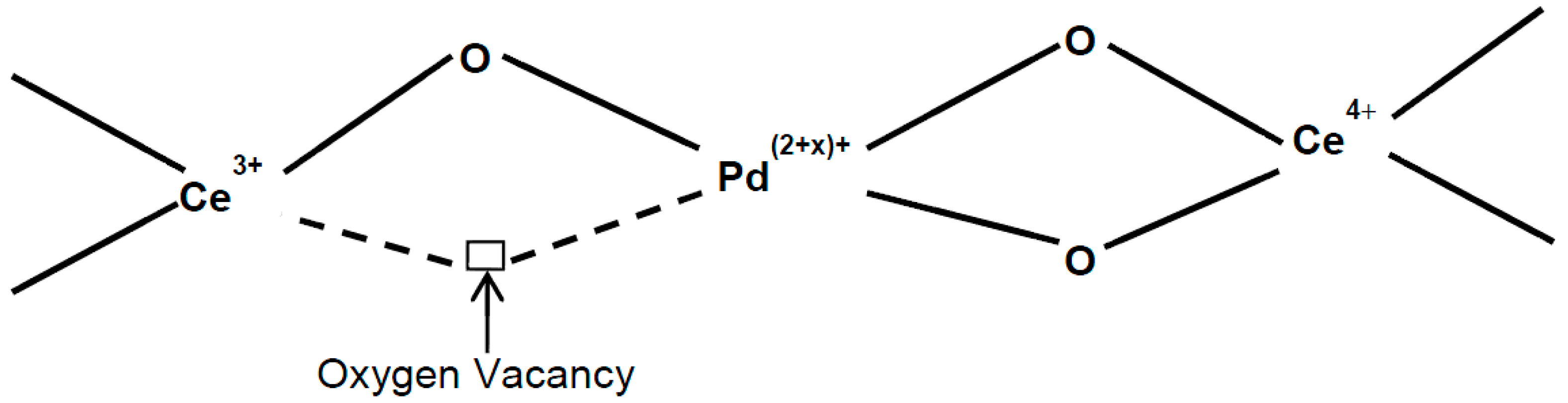
2.1.3. X-ray Photoelectron Spectroscopy (XPS) Studies
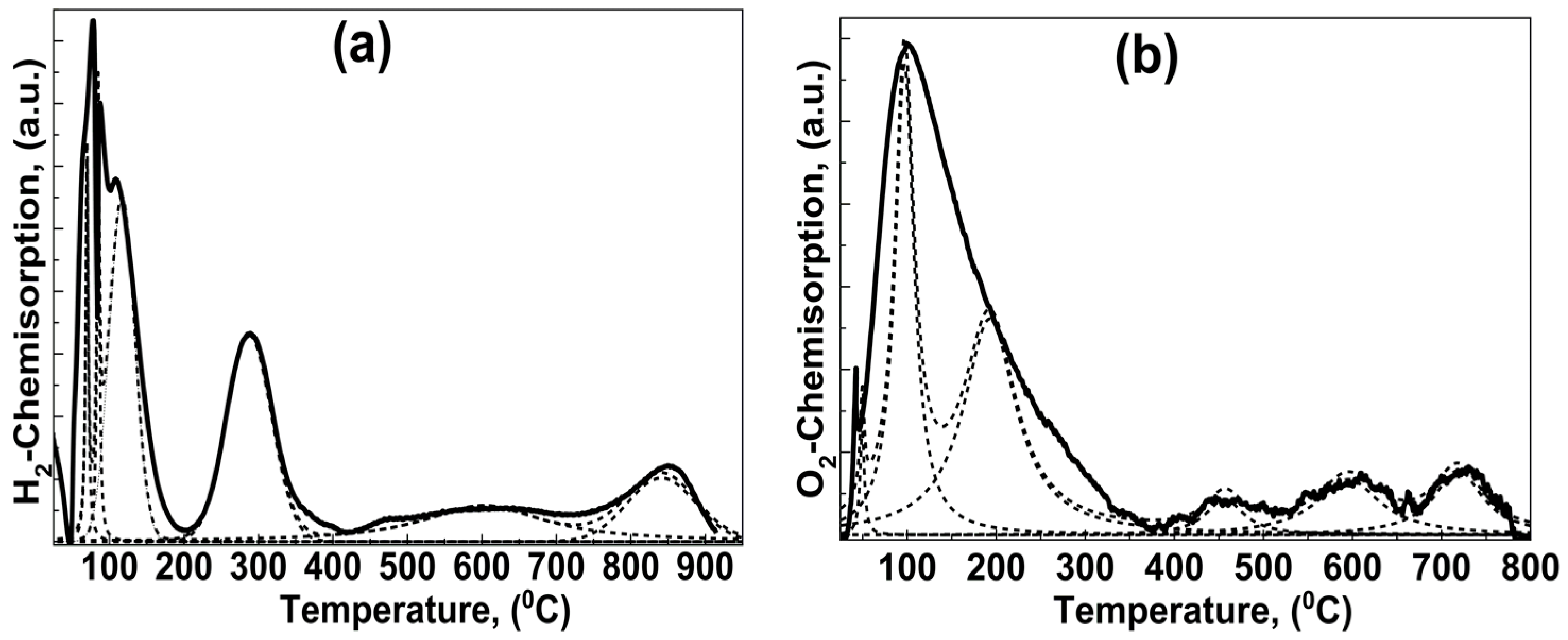
2.1.4. Sequential Temperature Programmed Reduction (TPR) and Temperature Programmed Oxidation (TPO)
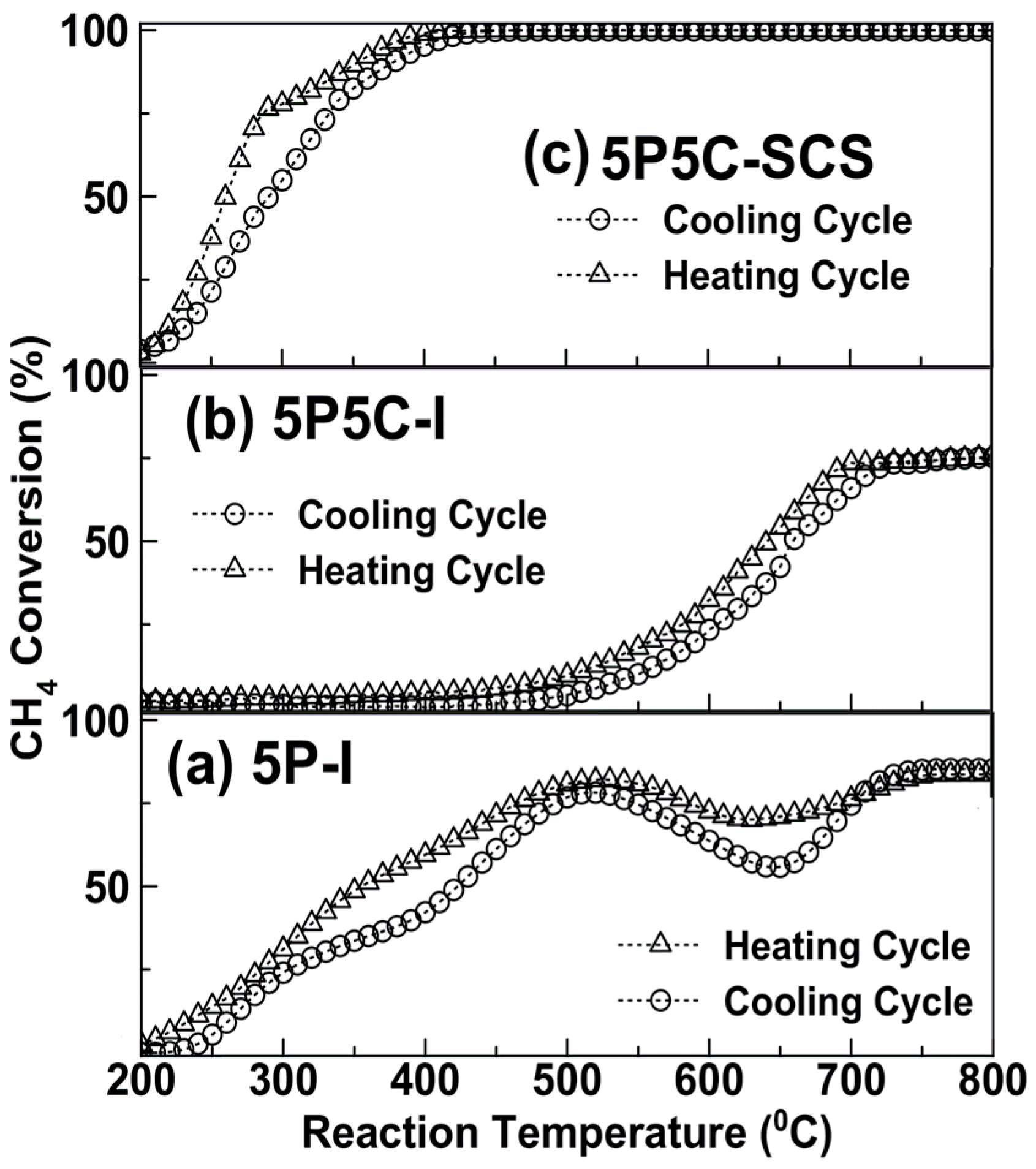
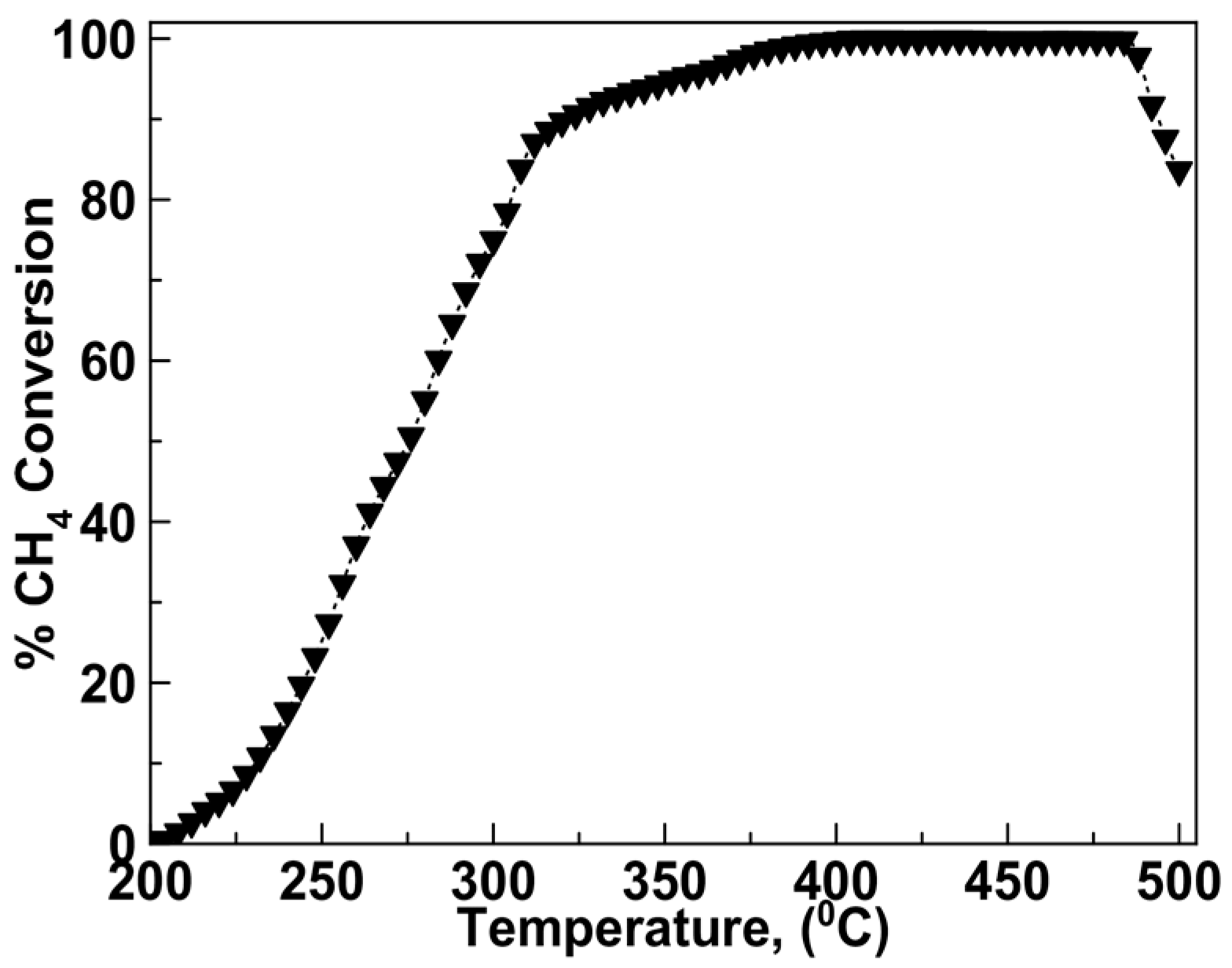
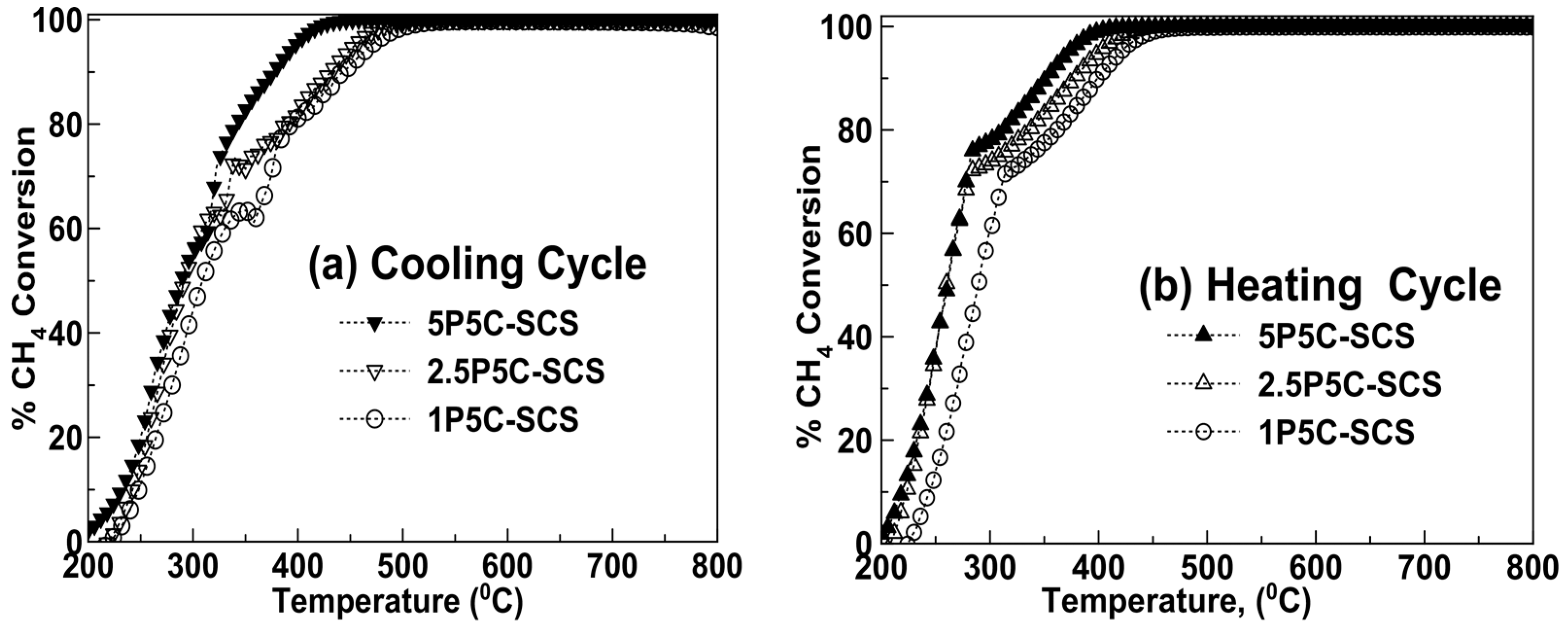
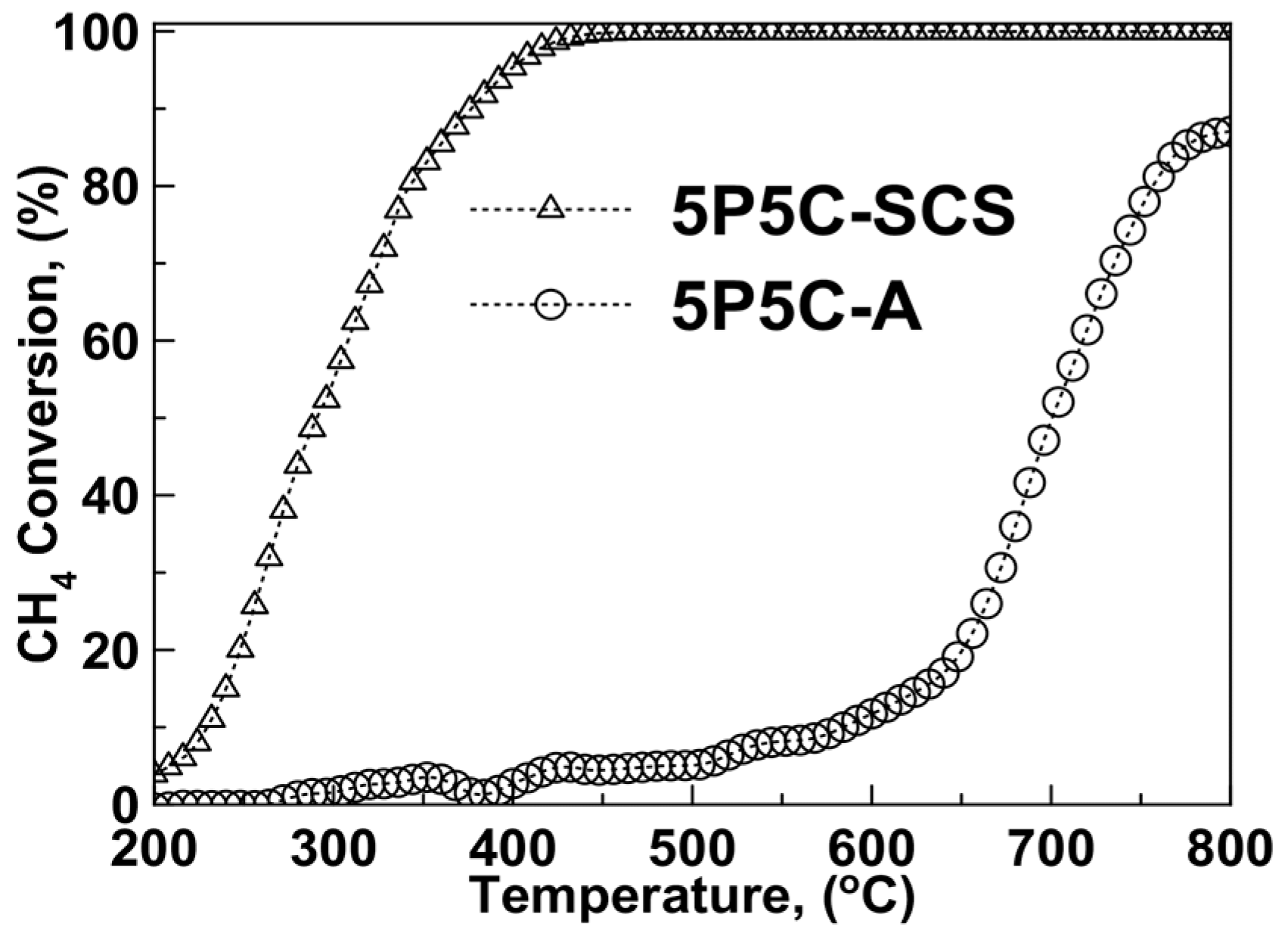
2.2. Catalytic Activity
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Catalytic Activity Measurements
3.4. Effect of Reductive Pretreatment on Catalytic Activity
3.5. Effects of the Type of Alumina Support on Catalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rivero-Mendoza, D.E.; Stanley, J.N.; Scott, J.; Aguey-Zinsou, K.-F. An alumina-supported Ni-La-based catalyst for producing synthetic natural gas. Catalysts 2016, 6, 170. [Google Scholar] [CrossRef]
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Samimi, F.; Rahimpour, M.R.; Shariati, A. Development of an Efficient Methanol Production Process for Direct CO2 Hydrogenation over a Cu/ZnO/Al2O3 Catalyst. Catalysts 2017, 7, 332. [Google Scholar] [CrossRef]
- Persson, K.; Jansson, K.; Järås, S.G. Characterisation and microstructure of Pd and bimetallic Pd–Pt catalysts during methane oxidation. J. Catal. 2007, 245, 401–414. [Google Scholar] [CrossRef]
- Ciuparu, D.; Lyubovsky, M.R.; Altman, E.; Pfefferle, L.D.; Datye, A. Catalytic combustion of methane over palladium-based catalysts. Catal. Rev. 2002, 44, 593–649. [Google Scholar] [CrossRef]
- Gélin, P.; Urfels, L.; Primet, M.; Tena, E. Complete oxidation of methane at low temperature over Pt and Pd catalysts for the abatement of lean-burn natural gas fuelled vehicles emissions: Influence of water and sulphur containing compounds. Catal. Today 2003, 83, 45–57. [Google Scholar] [CrossRef]
- Gholami, R.; Alyani, M.; Smith, K.J. Deactivation of Pd catalysts by water during low temperature methane oxidation relevant to natural gas vehicle converters. Catalysts 2015, 5, 561–594. [Google Scholar] [CrossRef]
- Lapisardi, G.; Urfels, L.; Gélin, P.; Primet, M.; Kaddouri, A.; Garbowski, E.; Toppi, S.; Tena, E. Superior catalytic behaviour of Pt-doped Pd catalysts in the complete oxidation of methane at low temperature. Catal. Today 2006, 117, 564–568. [Google Scholar] [CrossRef]
- Persson, K.; Ersson, A.; Jansson, K.; Iverlund, N.; Järås, S. Influence of co-metals on bimetallic palladium catalysts for methane combustion. J. Catal. 2005, 231, 139–150. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Pfefferle, L.D. Combustion of Methane over Palladium-Based Catalysts: Support Interactions. J. Phys. Chem. C 2012, 116, 8571–8578. [Google Scholar] [CrossRef]
- Chin, Y.-H.; Buda, C.; Neurock, M.; Iglesia, E. Consequences of Metal–Oxide Interconversion for C–H Bond Activation during CH4 Reactions on Pd Catalysts. J. Am. Chem. Soc. 2013, 135, 15425–15442. [Google Scholar] [CrossRef] [PubMed]
- Venezia, A.M.; Di Carlo, G.; Pantaleo, G.; Liotta, L.F.; Melaet, G.; Kruse, N. Oxidation of CH4 over Pd supported on TiO2-doped SiO2: Effect of Ti(IV) loading and influence of SO2. Appl. Catal. B 2009, 88, 430–437. [Google Scholar] [CrossRef]
- Meng, L.; Lin, J.-J.; Pu, Z.-Y.; Luo, L.-F.; Jia, A.-P.; Huang, W.-X.; Luo, M.-F.; Lu, J.-Q. Identification of active sites for CO and CH4 oxidation over PdO/Ce1−xPdxO2−δ catalysts. Appl. Catal. B 2012, 119–120, 117–122. [Google Scholar] [CrossRef]
- Zhu, G.; Han, J.; Zemlyanov, D.Y.; Ribeiro, F.H. Temperature Dependence of the Kinetics for the Complete Oxidation of Methane on Palladium and Palladium Oxide. J. Phys. Chem. B 2005, 109, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Domingos, D.; Rodrigues, L.M.T.S.; Frety, R.; Brandao, S.T. Combustion of Methane Using Palladium Catalysts Supported in Alumina or Zirconia. Combust. Sci. Technol. 2014, 186, 518–528. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Vegten, N.V.; Baiker, A. Insight into the structure of supported palladium catalysts during the total oxidation of methane. Chem. Commun. 2007, 4635–4637. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.G.; Gazquez, J.L. Oxygen vacancy model in strong metal-support interaction. J. Catal. 1987, 104, 120–135. [Google Scholar] [CrossRef]
- Van Vegten, N.; Maciejewski, M.; Krumeich, F.; Baiker, A. Structural properties, redox behaviour and methane combustion activity of differently supported flame-made Pd catalysts. Appl. Catal. B 2009, 93, 38–49. [Google Scholar] [CrossRef]
- Matam, S.K.; Aguirre, M.; Weidenkaff, A.; Ferri, D. Revisiting the problem of active sites for methane combustion on Pd/Al2O3 by operando XANES in a lab-scale fixed-bed reactor. J. Phys. Chem. C 2010, 114, 9439–9443. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Hobson, M.C.; Kennelly, T.; Waterman, E.M. Catalytic chemistry of supported palladium for combustion of methane. Appl. Catal. A 1992, 81, 227–237. [Google Scholar] [CrossRef]
- Xiao, L.-H.; Sun, K.-P.; Xu, X.-L.; Li, X.-N. Low-temperature catalytic combustion of methane over Pd/CeO2 prepared by deposition–precipitation method. Catal. Commun. 2005, 6, 796–801. [Google Scholar] [CrossRef]
- Colussi, S.; Trovarelli, A.; Groppi, G.; Llorca, J. The effect of CeO2 on the dynamics of Pd–PdO transformation over Pd/Al2O3 combustion catalysts. Catal. Commun. 2007, 8, 1263–1266. [Google Scholar] [CrossRef]
- Colussi, S.; Gayen, A.; Farnesi Camellone, M.; Boaro, M.; Llorca, J.; Fabris, S.; Trovarelli, A. Nanofaceted Pd–O Sites in Pd–Ce Surface Superstructures: Enhanced Activity in Catalytic Combustion of Methane. Angew. Chem. Int. Ed. 2009, 48, 8481–8484. [Google Scholar] [CrossRef] [PubMed]
- Mayernick, A.D.; Janik, M.J. Methane oxidation on Pd–Ceria: A DFT study of the mechanism over PdxCe1−xO2, Pd, and PdO. J. Catal. 2011, 278, 16–25. [Google Scholar] [CrossRef]
- Cargnello, M.; Jaén, J.J.D.; Garrido, J.C.H.; Bakhmutsky, K.; Montini, T.; Gámez, J.J.C.; Gorte, R.J.; Fornasiero, P. Exceptional Activity for Methane Combustion over Modular Pd@CeO2 Subunits on Functionalized Al2O3. Science 2012, 337, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Bakhmutsky, K.; Wieder, N.L.; Cargnello, M.; Galloway, B.; Fornasiero, P.; Gorte, R.J. A versatile route to core-shell catalysts: Synthesis of dispersible M@oxide (M = Pd, Pt; Oxide = TiO2, ZrO2) nanostructures by self-assembly. ChemSusChem 2012, 5, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Monai, M.; Montini, T.; Melchionna, M.; Duchoň, T.; Kúš, P.; Chen, C.; Tsud, N.; Nasi, L.; Prince, K.C.; Veltruská, K.; et al. The effect of sulfur dioxide on the activity of hierarchical Pd-based catalysts in methane combustion. Appl. Catal. B 2017, 202, 72–83. [Google Scholar] [CrossRef]
- Chen, C.; Yeh, Y.-H.; Cargnello, M.; Murray, C.B.; Fornasiero, P.; Gorte, R.J. Methane Oxidation on Pd@ZrO2/Si–Al2O3 Is Enhanced by Surface Reduction of ZrO2. ACS Catal. 2014, 4, 3902–3909. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Ciuparu, D.; Pfefferle, L.D. Combustion of Methane over Palladium-Based Catalysts: Catalytic Deactivation and Role of the Support. J. Phys. Chem. C 2012, 116, 8587–8593. [Google Scholar] [CrossRef]
- Onn, T.M.; Arroyo-Ramirez, L.; Monai, M.; Oh, T.-S.; Talati, M.; Fornasiero, P.; Gorte, R.J.; Khader, M.M. Modification of Pd/CeO2 catalyst by Atomic Layer Deposition of ZrO2. Appl. Catal. B 2016, 197, 280–285. [Google Scholar] [CrossRef]
- Aruna, S.T.; Mukasyan, A.S. Combustion synthesis and nanomaterials. Curr. Opin. Solid State Mater. Sci. 2008, 12, 44–50. [Google Scholar] [CrossRef]
- González-Cortés, S.L.; Imbert, F.E. Fundamentals, properties and applications of solid catalysts prepared by solution combustion synthesis (SCS). Appl. Catal. A 2013, 452, 117–131. [Google Scholar] [CrossRef]
- Suresh, K.; Patil, K.C.; Rao, K.J. Perspectives in Solid State Chemistry; Narosa Publishing House: New Delhi, India, 1995. [Google Scholar]
- Monai, M.; Montini, T.; Chen, C.; Fonda, E.; Gorte, R.J.; Fornasiero, P. Methane Catalytic Combustion over Hierarchical Pd@CeO2/Si-Al2O3: Effect of the Presence of Water. ChemCatChem 2015, 7, 2038–2046. [Google Scholar] [CrossRef]
- Specchia, S.; Finocchio, E.; Busca, G.; Palmisano, P.; Specchia, V. Surface chemistry and reactivity of ceria–zirconia-supported palladium oxide catalysts for natural gas combustion. J. Catal. 2009, 263, 134–145. [Google Scholar] [CrossRef]
- Priolkar, K.R.; Bera, P.; Sarode, P.R.; Hegde, M.S.; Emura, S.; Kumashiro, R.; Lalla, N.P. Formation of Ce1−xPdxO2−δ Solid Solution in Combustion-Synthesized Pd/CeO2 Catalyst: XRD, XPS, and EXAFS Investigation. Chem. Mater. 2002, 14, 2120–2128. [Google Scholar] [CrossRef]
- Bera, P.; Patil, K.C.; Jayaram, V.; Subbanna, G.N.; Hegde, M.S. Ionic Dispersion of Pt and Pd on CeO2 by Combustion Method: Effect of Metal–Ceria Interaction on Catalytic Activities for NO Reduction and CO and Hydrocarbon Oxidation. J. Catal. 2000, 196, 293–301. [Google Scholar] [CrossRef]
- Colussi, S.; Gayen, A.; Boaro, M.; Llorca, J.; Trovarelli, A. Influence of Different Palladium Precursors on the Properties of Solution-Combustion-Synthesized Palladium/Ceria Catalysts for Methane Combustion. ChemCatChem 2015, 7, 2222–2229. [Google Scholar] [CrossRef]
- Gil, S.; Garcia-Vargas, M.J.; Liotta, F.L.; Pantaleo, G.; Ousmane, M.; Retailleau, L.; Giroir-Fendler, A. Catalytic Oxidation of Propene over Pd Catalysts Supported on CeO2, TiO2, Al2O3 and M/Al2O3 Oxides (M = Ce, Ti, Fe, Mn). Catalysts 2015, 5, 671–689. [Google Scholar] [CrossRef]
- Hong, J.W.; Lee, Y.W.; Kim, M.; Kang, S.W.; Han, S.W. One-pot synthesis and electrocatalytic activity of octapodal Au–Pd nanoparticles. Chem. Commun. 2011, 47, 2553–2555. [Google Scholar] [CrossRef] [PubMed]
- Wu, C. Solvothermal synthesis of N-doped CeO2 microspheres with visible light-driven photocatalytic activity. Mater. Lett. 2015, 139, 382–384. [Google Scholar] [CrossRef]
- Chiu, P.-C.; Ku, Y.; Wu, Y.-L.; Wu, H.-C.; Kuo, Y.-L.; Tseng, Y.-H. Characterization and evaluation of prepared Fe2O3/Al2O3 oxygen carriers for chemical looping process. Aerosol Air Qual. Res. 2014, 14, 981–990. [Google Scholar] [CrossRef]
- Seo, C.; Yi, E.; Nahata, M.; Laine, R.M.; Schwank, J.W. Facile, one-pot synthesis of Pd@CeO2 core@ shell nanoparticles in aqueous environment by controlled hydrolysis of metalloorganic cerium precursor. Mater. Lett. 2017, 206, 105–108. [Google Scholar] [CrossRef]
- Mistri, R.; Rahaman, M.; Llorca, J.; Priolkar, K.R.; Colussi, S.; Ray, B.C.; Gayen, A. Liquid phase selective oxidation of benzene over nanostructured CuxCe1−xO2−δ (0.03 ≤ x ≤ 0.15). J. Mol. Catal. A Chem. 2014, 390, 187–197. [Google Scholar] [CrossRef]
- Th, P.; Zimmermann, R.; Steiner, P.; Hüfner, S. The electronic structure of PdO found by photoemission (UPS and XPS) and inverse photoemission (BIS). J. Phys. Condens. Matter. 1997, 9, 3987–3999. [Google Scholar] [CrossRef]
- Brun, M.; Berthet, A.; Bertolini, J.C. XPS, AES and Auger parameter of Pd and PdO. J. Electron. Spectrosc. Relat. Phenom. 1999, 104, 55–60. [Google Scholar] [CrossRef]
- Shinde, V.M.; Madras, G. Kinetic studies of ionic substituted copper catalysts for catalytic hydrogen combustion. Catal. Today 2012, 198, 270–279. [Google Scholar] [CrossRef]
- Huang, H.; Ye, X.; Huang, H.; Zhang, L.; Leung, D.Y.C. Mechanistic study on formaldehyde removal over Pd/TiO2 catalysts: Oxygen transfer and role of water vapor. Chem. Eng. J. 2013, 230, 73–79. [Google Scholar] [CrossRef]
- Ihm, S.-K.; Jun, Y.-D.; Kim, D.-C.; Jeong, K.-E. Low-temperature deactivation and oxidation state of Pd/γ-Al2O3 catalysts for total oxidation of n-hexane. Catal. Today 2004, 93–95, 149–154. [Google Scholar] [CrossRef]
- Aznárez, A.; Korili, S.A.; Gil, A. The promoting effect of cerium on the characteristics and catalytic performance of palladium supported on alumina pillared clays for the combustion of propene. Appl. Catal. A 2014, 474, 95–99. [Google Scholar] [CrossRef]
- Bi, Y.; Lu, G. Catalytic CO oxidation over palladium supported NaZSM-5 catalysts. Appl. Catal. B 2003, 41, 279–286. [Google Scholar] [CrossRef]
- Venezia, A.M.; Di Carlo, G.; Liotta, L.F.; Pantaleo, G.; Kantcheva, M. Effect of Ti(IV) loading on CH4 oxidation activity and SO2 tolerance of Pd catalysts supported on silica SBA-15 and HMS. Appl. Catal. B 2011, 106, 529–539. [Google Scholar] [CrossRef]
- Vita, A.; Cristiano, G.; Italiano, C.; Pino, L.; Specchia, S. Syngas production by methane oxy-steam reforming on Me/CeO2 (Me = Rh, Pt, Ni) catalyst lined on cordierite monoliths. Appl. Catal. B 2015, 162, 551–563. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Morgan, B.J.; Watson, G.W. The origin of the enhanced oxygen storage capacity of Ce1−x(Pd/Pt)xO2. Phys. Chem. Chem. Phys. 2011, 13, 4279–4284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Wang, S.; Liu, W.; Liu, X.; Guo, J.; Yang, Y. Mesoporous CeO2 nanoparticles assembled by hollow nanostructures: Formation mechanism and enhanced catalytic properties. CrystEngComm 2014, 16, 8777–8785. [Google Scholar] [CrossRef]
- Harrison, B.; Diwell, A.F.; Hallett, C. Promoting Platinum Metals by Ceria. Platin. Met. Rev. 1988, 32, 73–83. [Google Scholar]
- Huang, M.; Fabris, S. Role of surface peroxo and superoxo species in the low-temperature oxygen buffering of ceria: Density functional theory calculations. Phys. Rev. B 2007, 75, 081404. [Google Scholar] [CrossRef]
- Fouladvand, S.; Schernich, S.; Libuda, J.; Grönbeck, H.; Pingel, T.; Olsson, E.; Skoglundh, M.; Carlsson, P.-A. Methane oxidation over Pd supported on ceria–alumina under rich/lean cycling conditions. Top. Catal. 2013, 56, 410–415. [Google Scholar] [CrossRef]
- Su, Y.-Q.; Filot, I.A.; Liu, J.-X.; Hensen, E.J. Stable Pd-doped Ceria Structures for CH4 Activation and CO Oxidation. ACS Catal. 2017. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-Q.; Liu, J.-X.; Filot, I.A.; Hensen, E.J. Theoretical study of ripening mechanisms of Pd clusters on Ceria. Chem. Mater. 2017, 29, 9456–9462. [Google Scholar] [CrossRef] [PubMed]
- Primavera, A.; Trovarelli, A.; de Leitenburg, C.; Dolcetti, G.; Llorca, J. Reactivity and characterization of Pd-containing ceria-zirconia catalysts for methane combustion. Stud. Surf. Sci. Catal. 1998, 87–92. [Google Scholar] [CrossRef]
- De Rogatis, L.; Cargnello, M.; Gombac, V.; Lorenzut, B.; Montini, T.; Fornasiero, P. Embedded phases: A way to active and stable catalysts. ChemSusChem 2010, 3, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.M.; Yu, K.M.K.; Fu, Q.J.; Thompsett, D.; Petch, M.I.; Tsang, S.C. Engineering Pt in ceria for a maximum metal—Support interaction in catalysis. J. Am. Chem. Soc. 2005, 127, 18010–18011. [Google Scholar] [CrossRef] [PubMed]
- Tedsree, K.; Li, T.; Jones, S.; Chan, C.W.A.; Yu, K.M.K.; Bagot, P.A.; Marquis, E.A.; Smith, G.D.; Tsang, S.C.E. Hydrogen production from formic acid decomposition at room temperature using a Ag-Pd core-shell nanocatalyst. Nat. Nanotechnol. 2011, 6, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Colussi, S.; Gayen, A.; Llorca, J.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Catalytic performance of solution combustion synthesized alumina-and ceria-supported Pt and Pd nanoparticles for the combustion of propane and dimethyl ether (DME). Ind. Eng. Chem. Res. 2012, 51, 7510–7517. [Google Scholar] [CrossRef]
- Burch, R.; Urbano, F.J. Investigation of the active state of supported palladium catalysts in the combustion of methane. Appl. Catal. A 1995, 124, 121–138. [Google Scholar] [CrossRef]
- Kumar, A.; Ashok, A.; Bhosale, R.R.; Saleh, M.A.H.; Almomani, F.A.; Al-Marri, M.; Khader, M.M.; Tarlochan, F. In situ DRIFTS Studies on Cu, Ni and CuNi catalysts for Ethanol Decomposition Reaction. Cataly. Lett. 2016, 146, 778–787. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal., B 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Kumar, A.; Mukasyan, A.S.; Wolf, E.E. Combustion synthesis of Ni, Fe and Cu multi-component catalysts for hydrogen production from ethanol reforming. Appl. Catal. A 2011, 401, 20–28. [Google Scholar] [CrossRef]
- Ali, S.; Al-Marri, M.J.; Abdelmoneim, A.G.; Kumar, A.; Khader, M.M. Catalytic evaluation of nickel nanoparticles in methane steam reforming. Int. J. Hydrogen Energy 2016, 41, 22876–22885. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Saleh, M.A.H.; Ghosh, U.K.; Al-Marri, M.; Almomani, F.A.; Khader, M.M.; Tarlochan, F. Cobalt oxide nanopowder synthesis using cellulose assisted combustion technique. Ceram. Int. 2016, 42, 12771–12777. [Google Scholar] [CrossRef]
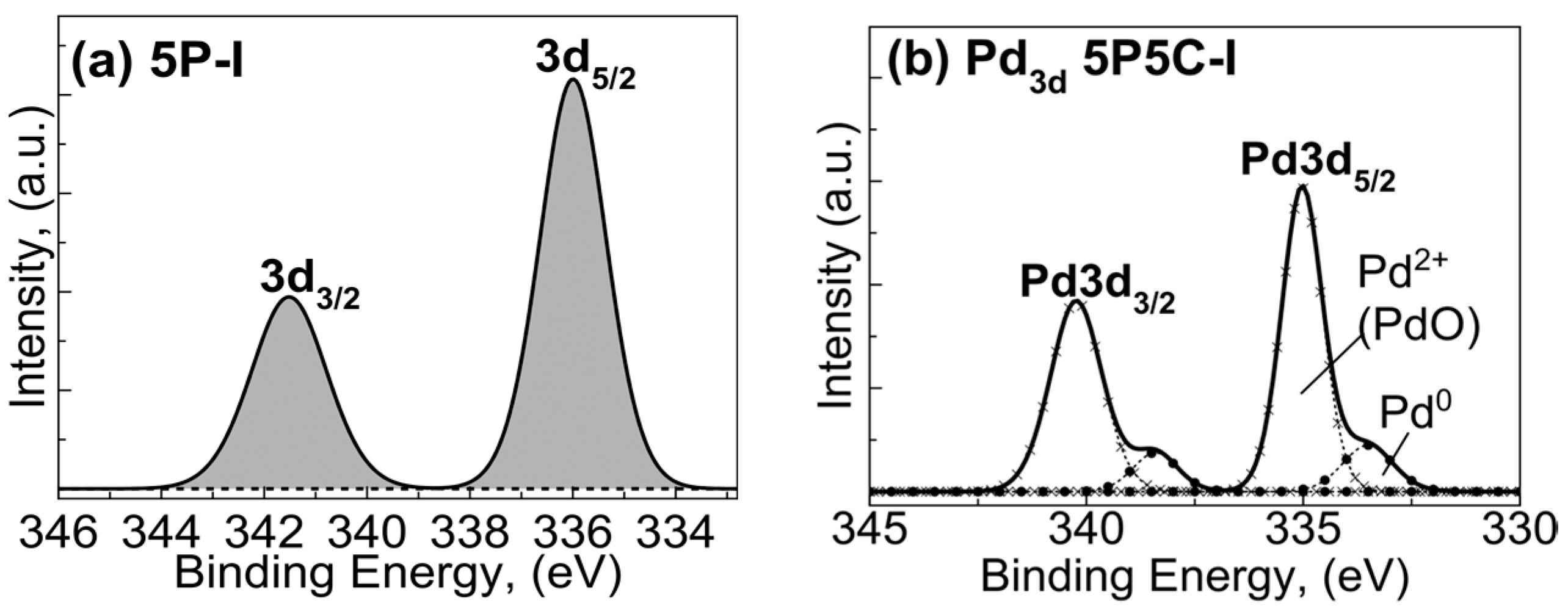
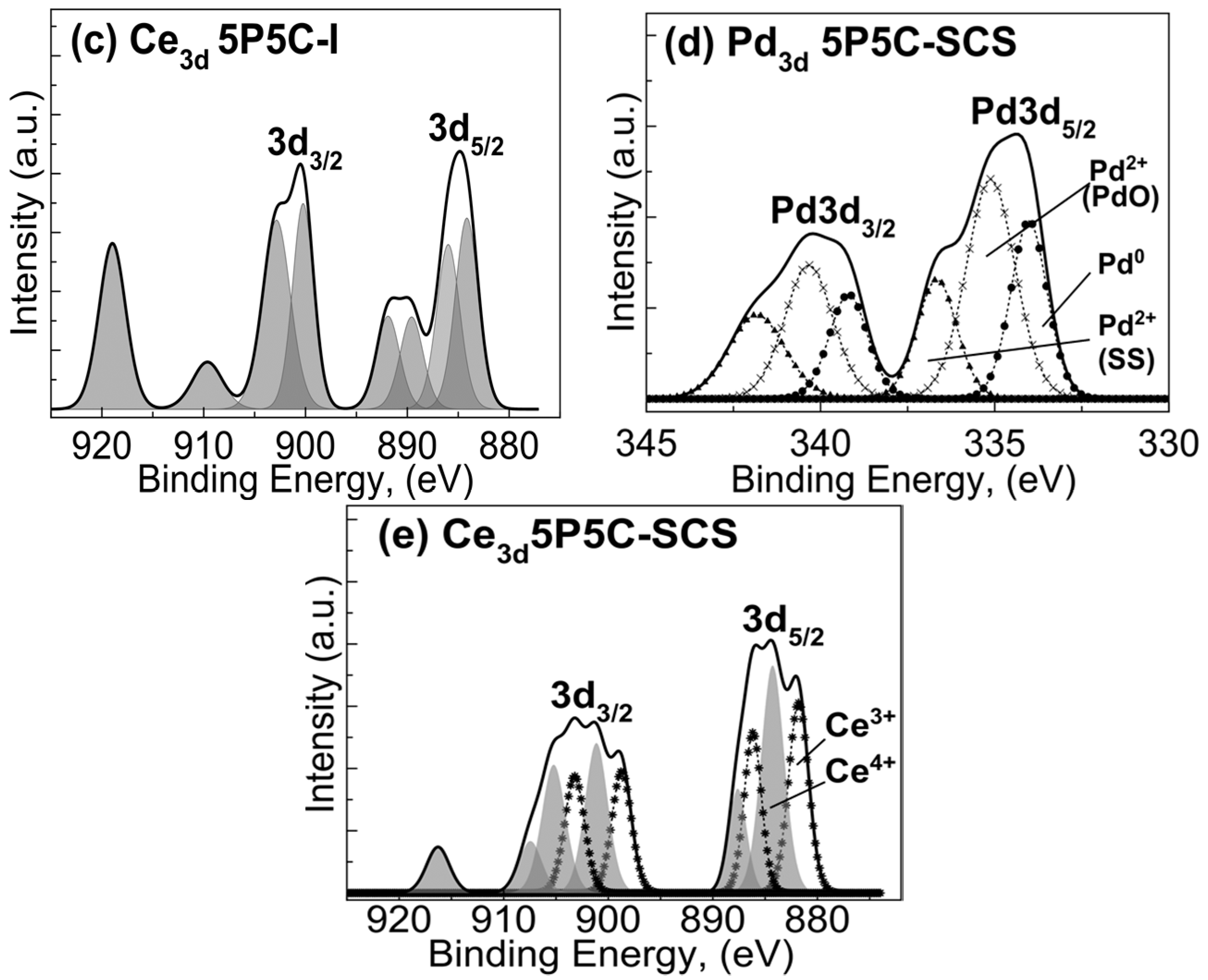
| 1P5C-SCS | Ce(3d5/2) | Pd(3d5/2) | Al(2p3/2) | O1s | |||
| 9.84 ± 0.64 | 1.29 ± 0.27 | 44.75 ± 0.4 | 43.81 ± 0.5 | ||||
| Ce(III) | Ce(IV) | Pd0 | Pd2+(PdO) | Pd(2+x)+ b | |||
| 43.44 ± 2.8 | 56.56 ± 2.8 | 29.17 ± 3.2 | 36.84 ± 2.6 | 32.98 ± 5.4 | |||
| 2.5P5C-SCS | Ce(3d5/2) | Pd(3d5/2) | 43.236 ± 0.4 | 41.65 ± 0.4 | |||
| 12.82 ± 0.82 | 2.28 ± 0.14 | ||||||
| Ce(III) | Ce(IV) | Pd0 | Pd2+(PdO) | Pd(2+x)+ b | |||
| 49.92 ± 4.6 | 50.07 ± 4.6 | 30.36 ± 2.7 | 46.16 ± 4.7 | 24.29 ± 1.7 | |||
| 5P5C-SCS | Ce(3d5/2) | Pd(3d5/2) | 45.10 ± 0.3 | 42.92 ± 0.5 | |||
| 8.82 ± 0.26 | 2.95 ± 0.25 | ||||||
| Ce(III) | Ce(IV) | Pd0 | Pd2+(PdO) | Pd(2+x)+ b | |||
| 41.17 ± 1.8 | 58.42 ± 1.8 | 32.63 ± 5.5 | 41.95 ± 3.2 | 25.71 ± 2.3 | |||
| 5P5C-I | Ce(3d5/2) | Pd(3d5/2) | 47.23 ± 0.4 | 44.62 ± 0.4 | |||
| 3.63 ± 1.3 | 4.52 ± 0.23 | ||||||
| Ce(III) | Ce(IV) | Pd0 | Pd2+ | Pd(2+x)+ b | |||
| 0 | 100 | 13.2 | 86.8 | 0 | |||
| Sample | Pd (wt%) a | S.A. (m2 g−1) b | Rate of Methane Decomposition c (µmol (g Pd)−1 s−1) |
|---|---|---|---|
| 5P-I | 4.84 | - | 57.06 |
| 5P5C-I | 4.52 | 54.28 | 60.82 |
| 5P5C-SCS | 2.95 | 100.56 | 1077.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khader, M.M.; Al-Marri, M.J.; Ali, S.; Abdelmoneim, A.G. Active and Stable Methane Oxidation Nano-Catalyst with Highly-Ionized Palladium Species Prepared by Solution Combustion Synthesis. Catalysts 2018, 8, 66. https://doi.org/10.3390/catal8020066
Khader MM, Al-Marri MJ, Ali S, Abdelmoneim AG. Active and Stable Methane Oxidation Nano-Catalyst with Highly-Ionized Palladium Species Prepared by Solution Combustion Synthesis. Catalysts. 2018; 8(2):66. https://doi.org/10.3390/catal8020066
Chicago/Turabian StyleKhader, Mahmoud M., Mohammed J. Al-Marri, Sardar Ali, and Ahmed G. Abdelmoneim. 2018. "Active and Stable Methane Oxidation Nano-Catalyst with Highly-Ionized Palladium Species Prepared by Solution Combustion Synthesis" Catalysts 8, no. 2: 66. https://doi.org/10.3390/catal8020066
APA StyleKhader, M. M., Al-Marri, M. J., Ali, S., & Abdelmoneim, A. G. (2018). Active and Stable Methane Oxidation Nano-Catalyst with Highly-Ionized Palladium Species Prepared by Solution Combustion Synthesis. Catalysts, 8(2), 66. https://doi.org/10.3390/catal8020066




