Critical Review of Low-Temperature CO Oxidation and Hysteresis Phenomenon on Heterogeneous Catalysts
Abstract
1. Introduction
2. Origin of Hysteresis Phenomenon in Low-Temperature CO Oxidation
3. Effect of Catalysts and Reaction Parameters/Conditions on Hysteresis Behavior
4. Analysis of Reviewed Literature
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heck, R.M.; Farrauto, R.J. Automobile exhaust catalysts. Appl. Catal. A Gen. 2001, 221, 443–457. [Google Scholar] [CrossRef]
- Heck, R.M.; Farrauto, R.J.; Gulati, S.T. Catalytic Air Pollution Control; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Kurz, H. Physiology of Angiogenesis. J. Neurooncol. 2000, 50, 17–35. [Google Scholar] [CrossRef]
- Shelef, M.; McCabe, R.W. Twenty-five years after introduction of automotive catalysts: What next? Catal. Today 2000, 62, 35–50. [Google Scholar] [CrossRef]
- Carlsson, P.-A.; Skoglundh, M. Low-temperature oxidation of carbon monoxide and methane over alumina and ceria supported platinum catalysts. Appl. Catal. B Environ. 2011, 101, 669–675. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, P. A Review on CO Oxidation Over Copper Chromite Catalyst. Catal. Rev. 2012, 54, 224–279. [Google Scholar] [CrossRef]
- Manos, M.; James, A.D.; Amit, A.G.; Rahul, P.N.; Calvin, H.B.; Hu, Z.; Brian, C. Atomic-Scale Design of iron Fisher-Tropsch Catalysts: Acombined Computional Chemistry, Experimental, and Microkinetic Modeling Approach; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 2005. [Google Scholar]
- Wang, L.; Yang, L.; Zhang, Y.; Ding, W.; Chen, S.; Fang, W.; Yang, Y. Promoting effect of an aluminum emulsion on catalytic performance of Cu-based catalysts for methanol synthesis from syngas. Fuel Process. Technol. 2010, 91, 723–728. [Google Scholar] [CrossRef]
- Grabow, L.C.; Gokhale, A.A.; Evans, S.T.; Dumesic, J.A.; Mavrikakis, M. Mechanism of the Water Gas Shift Reaction on Pt: First Principles, Experiments, and Microkinetic Modeling. J. Phys. Chem. C 2008, 112, 4608–4617. [Google Scholar] [CrossRef]
- Armor, J.N. The multiple roles for catalysis in the production of H2. Appl. Catal. A Gen. 1999, 176, 159–176. [Google Scholar] [CrossRef]
- Kummer, J.T. Use of noble metals in automobile exhaust catalysts. J. Phys. Chem. 1986, 90, 4747–4752. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W. Recent advances in organocatalytic asymmetric Michael reactions. Catal. Sci. Technol. 2012, 2, 42–53. [Google Scholar] [CrossRef]
- Yadav, R.; Saoud, k.; Rasouli, F.; Hajaligol, M. Formation and Deposition of Sputtered Nanoscale Particles in Cigarette Manufacture. U.S. Patent 8,051,859, 8 August 2005. [Google Scholar]
- Health Hazard Evaluation Determination Report: HHE-77-50-419, The Glass Detail, Cincinnati, Ohio, 45202; U.S. Department of Health, Education, and Welfare, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health: Washington, DC, USA, 1977.
- Henein, N.A.; Tagomori, M.K. Cold-start hydrocarbon emissions in port-injected gasoline engines. Prog. Energy Combust. Sci. 1999, 25, 563–593. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Hickey, N. Automotive catalytic converters: Current status and some perspectives. Catal. Today 2003, 77, 419–449. [Google Scholar] [CrossRef]
- Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [CrossRef]
- Vannic, M.A. Handbook of Heterogeneous Catalysis. Herausgegeben von G. Ertl, H. Knözinger undJ. Weitkamp, WILEY-VCH, Weinheim, 1997. 2500 S., geb., 24900.00 DM-ISBN 3-527-29212-8. Angew. Chem. 1997, 109, 2808–2810. [Google Scholar] [CrossRef]
- Saoud, K.M. Supported gold nanocatalyst for low temperature CO oxidation and combustion of volatile organic compounds (VOC). In Proceedings of the Qatar Foundation Annual Research Forum Doha, Doha, Qatar, 13 December 2010. [Google Scholar]
- Valange, S.; Védrine, J. General and Prospective Views on Oxidation Reactions in Heterogeneous Catalysis. Catalysts 2018, 8, 483. [Google Scholar] [CrossRef]
- Comotti, M.; Weidenthaler, C.; Li, W.-C.; Schüth, F. Comparison of gold supported catalysts obtained by using different allotropic forms of titanium dioxide. Top. Catal. 2007, 44, 275–284. [Google Scholar] [CrossRef]
- Beltramini, J.N.; Trimm, D.L. Role of germanium in bimetallic reforming catalysts. React. Kinet. Catal. Lett. 1988, 37, 293–299. [Google Scholar] [CrossRef]
- Oh, S.; Hoflund, G. Low-temperature catalytic carbon monoxide oxidation over hydrous and anhydrous palladium oxide powders. J. Catal. 2007, 245, 35–44. [Google Scholar] [CrossRef]
- Saoud, K.M. Carbon Monoxide Oxidation on Nanoparticle Catalysts and Gas Phase Reactions of Small Molecules and Volatile Organics with Metal Cations. Ph.D, Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2005. [Google Scholar]
- Boccuzzi, F.; Chiorino, A.; Tsubota, S.; Haruta, M. The oxidation and scrambling of CO with oxygen at room temperature on Au/ZnO. Catal. Lett. 1994, 29, 225–234. [Google Scholar] [CrossRef]
- Escamilla-Perea, L.; Nava, R.; Pawelec, B.; Rosmaninho, M.G.; Peza-Ledesma, C.L.; Fierro, J.L.G. SBA-15-supported gold nanoparticles decorated by CeO2: Structural characteristics and CO oxidation activity. Appl. Catal. A Gen. 2010, 381, 42–53. [Google Scholar] [CrossRef]
- Keshipour, S.; Mirmasoudi, S.S. Cross-linked chitosan aerogel modified with Au: Synthesis, characterization and catalytic application. Carbohydr. Polym. 2018, 196, 494–500. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, W.; Ye, Y.; Huang, Y.; Xu, Y.; Yuan, Y.; Wu, F.; Li, J. Adsorption Synthesis of Iron Oxide-Supported Gold Catalyst under Self-Generated Alkaline Conditions for Efficient Elimination of Carbon Monoxide. Catalysts 2018, 8, 357. [Google Scholar] [CrossRef]
- Reveles, J.U.; Saoud, K.M.; El-Shall, M.S. Water inhibits CO oxidation on gold cations in the gas phase. Structures and binding energies of the sequential addition of CO, H2O, O2, and N2 onto Au+. Phys. Chem. Chem. Phys. 2016, 18, 28606–28616. [Google Scholar] [CrossRef] [PubMed]
- Haaland, D. Simultaneous measurement of CO oxidation rate and surface coverage on Pt/Al2O3 using infrared spectroscopy: Rate hysteresis and CO island formation*1. J. Catal. 1982, 76, 450–465. [Google Scholar] [CrossRef]
- Bond, G.C.; Thompson, D.T. Gold-catalysed oxidation of carbon monoxide. Gold Bull. 2000, 33, 41–50. [Google Scholar] [CrossRef]
- Epling, W.S.; Hoflund, G.B.; Weaver, J.F.; Tsubota, S.; Haruta, M. Surface Characterization Study of Au/α-Fe2O3 and Au/Co3O4 Low-Temperature CO Oxidation Catalysts. J. Phys. Chem. 1996, 100, 9929–9934. [Google Scholar] [CrossRef]
- Haruta, M. Novel catalysis of gold deposited on metal oxides. Catal. Surv. Jpn. 1997, 1, 61–73. [Google Scholar] [CrossRef]
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Montes, M.; Genet, M.; Hodnett, B.K.; Stone, W.E.; Delmon, B. X-Ray Photoelectron Spectroscopy of Sulfur Containing Ni/SiO2 Catalysts. Bull. Soc. Chim. Belg. 2010, 95, 1–12. [Google Scholar] [CrossRef]
- Yang, Y.; Saoud, K.M.; Abdelsayed, V.; Glaspell, G.; Deevi, S.; El-Shall, M.S. Vapor phase synthesis of supported Pd, Au, and unsupported bimetallic nanoparticle catalysts for CO oxidation. Catal. Commun. 2006, 7, 281–284. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Saoud, K.M.; El-Shall, M.S. Vapor phase synthesis and characterization of bimetallic alloy and supported nanoparticle catalysts. J. Nanopart. Res. 2006, 8, 519–531. [Google Scholar] [CrossRef]
- El-Shall, M.S.; Saoud, K.M. Vapor Phase Synthesis of Gold Nanoparticle Catalysts for Low Temperature CO Oxidation. In Proceedings of the ACS 56th Southeast Regional Meeting, Research Triangle Park, NC, USA, 10–13 November 2004. [Google Scholar]
- Glaspell, G.; Abdelsayed, V.; Saoud, K.M.; El-Shall, M.S. Vapor-phase synthesis of metallic and intermetallic nanoparticles and nanowires: Magnetic and catalytic properties. Pure Appl. Chem. 2006, 78, 1667–1689. [Google Scholar] [CrossRef]
- Liu, W.; Flytzanistephanopoulos, M. Total Oxidation of Carbon Monoxide and Methane over Transition Metal Fluorite Oxide Composite Catalysts. J. Catal. 1995, 153, 304–316. [Google Scholar] [CrossRef]
- Luo, M.-F.; Zhong, Y.-J.; Yuan, X.-X.; Zheng, X.-M. TPR and TPD studies of CuOCeO2 catalysts for low temperature CO oxidation. Appl. Catal. A Gen. 1997, 162, 121–131. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Soria, J.; Cataluña, R.; Conesa, J.C.; Corberán, V.C. Influence of Ceria Dispersion on the Catalytic Performance of Cu/(CeO2)/Al2O3 Catalysts for the CO Oxidation Reaction. In Catalysis and Automotive Pollution Control IV, Proceedings of the Fourth International Symposium (CAPoC4); Elsevier: Amsterdam, The Netherlands, 1998; pp. 591–600. [Google Scholar] [CrossRef]
- Mergler, Y.J.; Hoebink, J.; Nieuwenhuys, B.E. CO Oxidation over a Pt/CoOx/SiO2Catalyst: A Study Using Temporal Analysis of Products. J. Catal. 1997, 167, 305–313. [Google Scholar] [CrossRef]
- Biabani-Ravandi, A.; Rezaei, M.; Fattah, Z. Study of Fe-Co mixed metal oxide nanoparticles in the catalytic low-temperature CO oxidation. Process Saf. Environ. Prot. 2013, 91, 489–494. [Google Scholar] [CrossRef]
- Yu, H.; Zhong, S.; Zhu, B.; Huang, W.; Zhang, S. Synthesis and CO Oxidation Activity of 1D Mixed Binary Oxide CeO(2)-LaO (x) Supported Gold Catalysts. Nanoscale Res. Lett. 2017, 12, 579. [Google Scholar] [CrossRef]
- Bonzel, H.P.; Ku, R. Carbon Monoxide Oxidation on a Pt(110) Single Crystal Surface. J. Vac. Sci. Technol. 1972, 9, 663–667. [Google Scholar] [CrossRef]
- Cant, N. Steady-state oxidation of carbon monoxide over supported noble metals with particular reference to platinum. J. Catal. 1978, 54, 372–383. [Google Scholar] [CrossRef]
- Dabill, D. The oxidation of hydrogen and carbon monoxide mixtures over platinum. J. Catal. 1978, 53, 164–167. [Google Scholar] [CrossRef]
- Hegedus, L.L.; Oh, S.H.; Baron, K. Multiple steady states in an isothermal, integral reactor: The catalytic oxidation of carbon monoxide over platinum-alumina. AlChE J. 1977, 23, 632–642. [Google Scholar] [CrossRef]
- Hoebink, J.H.B.J.; Nievergeld, A.J.L.; Marin, G.B. CO oxidation in a fixed bed reactor with high frequency cycling of the feed. Chem. Eng. Sci. 1999, 54, 4459–4468. [Google Scholar] [CrossRef]
- Hori, G. Transient kinetics in CO oxidation on platinum. J. Catal. 1975, 38, 335–350. [Google Scholar] [CrossRef]
- Salomons, S.; Hayes, R.E.; Votsmeier, M.; Drochner, A.; Vogel, H.; Malmberg, S.; Gieshoff, J. On the use of mechanistic CO oxidation models with a platinum monolith catalyst. Appl. Catal. B Environ. 2007, 70, 305–313. [Google Scholar] [CrossRef]
- Voltz, S.E.; Morgan, C.R.; Liederman, D.; Jacob, S.M. Kinetic Study of Carbon Monoxide and Propylene Oxidation on Platinum Catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1973, 12, 294–301. [Google Scholar] [CrossRef]
- Salomons, S.; Votsmeier, M.; Hayes, R.E.; Drochner, A.; Vogel, H.; Gieshof, J. CO and H2 oxidation on a platinum monolith diesel oxidation catalyst. Catal. Today 2006, 117, 491–497. [Google Scholar] [CrossRef]
- Carlsson, P.; Osterlund, L.; Thormahlen, P.; Palmqvist, A.; Fridell, E.; Jansson, J.; Skoglundh, M. A transient in situ FTIR and XANES study of CO oxidation over Pt/AlO catalysts. J. Catal. 2004, 226, 422–434. [Google Scholar] [CrossRef]
- Chakrabarty, T.; Silveston, P.L.; Hudgins, R.R. Hysteresis phenomena in co oxidation over platinum-alumina catalyst. Cana. J. Chem. Eng. 1984, 62, 651–660. [Google Scholar] [CrossRef]
- Imbihl, R.; Ertl, G. Oscillatory Kinetics in Heterogeneous Catalysis. Chem. Rev. 1995, 95, 697–733. [Google Scholar] [CrossRef]
- Hysteresis. Available online: https://en.wikipedia.org/wiki/Hysteresis (accessed on 12 October 2018).
- Noori, H.R. Examples of Hysteresis Phenomena in Biology. In SpringerBriefs in Applied Sciences and Technology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 35–45. [Google Scholar] [CrossRef]
- Visintin, A. Differential Models of Hysteresis. In Applied Mathematical Sciences; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar] [CrossRef]
- Haul, R.S.J.; Gregg, K.S.W. Sing: Adsorption, Surface Area and Porosity. 2. Auflage, Academic Press, London 1982. 303 Seiten, Preis: $ 49.50. Berichte Bunsengesellschaft Physikalische Chemie 1982, 86, 957. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Lampert, J.K.; Hobson, M.C.; Waterman, E.M. Thermal decomposition and reformation of PdO catalysts; support effects. Appl. Catal. B Environ. 1995, 6, 263–270. [Google Scholar] [CrossRef]
- Subbotin, A.N.; Gudkov, B.S.; Yakerson, V.I. Temperature hysteresis phenomena in heterogeneous catalysis. Russ. Chem. Bull. 2000, 49, 1373–1379. [Google Scholar] [CrossRef]
- Kalinkin, A.V.; Boreskov, G.K.; Savchenko, V.I.; Dadayan, K.A. Study of CO oxidation on the (111) face of nickel during Ni-NiO phase transition. React. Kinet. Catal. Lett. 1980, 13, 111–114. [Google Scholar] [CrossRef]
- Orlik, S.N.; Koval, G.L.; Fesenko, A.V.; Korneichuk, G.P.; Yablonskii, G.S. Kinetics of CO oxidation on a palladium-containing catalyst. Theor. Exp. Chem. 1979, 15, 59–61. [Google Scholar] [CrossRef]
- Gudkov, B.S.; Subbotin, A.N.; Yakerson, V.I. On the phenomena of temperature hysteresis in hydrogenation reactions over heterogeneous catalysts. React. Kinet. Catal. Lett. 1999, 68, 125–132. [Google Scholar] [CrossRef]
- Hauptmann, W.; Votsmeier, M.; Gieshoff, J.; Drochner, A.; Vogel, H. Inverse hysteresis during the NO oxidation on Pt under lean conditions. Appl. Catal. B Environ. 2009, 93, 22–29. [Google Scholar] [CrossRef]
- Oh, S.; Baron, K.; Sloan, E.; Hegedus, L. Effects of catalyst particle size on multiple steady states. J. Catal. 1979, 59, 272–277. [Google Scholar] [CrossRef]
- Schmitz, R.A. Multiplicity, Stability, and Sensitivity of States in Chemically Reacting Systems—A Review. In Chemical Reaction Engineering Reviews; Aerican Chemical Sociaty: Washington, DC, USA, 1975; pp. 156–211. [Google Scholar] [CrossRef]
- Smith, T.G.; Zahradnik, J.; Carberry, J.J. Non-isothermal inter-intraphase effectiveness factors for negative order kinetics—CO oxidation over Pt. Chem. Eng. Sci. 1975, 30, 763–767. [Google Scholar] [CrossRef]
- Subbotin, A.N.; Gudkov, B.S.; Dykh, Z.L.; Yakerson, V.I. Temperature hysteresis in CO oxidation on catalysts of various nature. React. Kinet. Catal. Lett. 1999, 66, 97–104. [Google Scholar] [CrossRef]
- Usachev, N.Y.; Gorevaya, I.A.; Belanova, E.P.; Kazakov, A.V.; Kharlamov, V.V. Hysteresis phenomenon in CO and hydrogen oxidation on Cu-Ce-Zr-O systems. Mendeleev Commun. 2004, 14, 79–80. [Google Scholar] [CrossRef]
- Wei, J.; Becker, E.R. The Optimum Distribution of Catalytic Material on Support Layers in Automotive Catalysis. In Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1975; pp. 116–132. [Google Scholar] [CrossRef]
- Ye, S.; Yap, Y.H.; Kolaczkowski, S.T.; Robinson, K.; Lukyanov, D. Catalyst ‘light-off’ experiments on a diesel oxidation catalyst connected to a diesel engine—Methodology and techniques. Chem. Eng. Res. Des. 2012, 90, 834–845. [Google Scholar] [CrossRef]
- Engel, T.; Ertl, G. Elementary Steps in the Catalytic Oxidation of Carbon Monoxide on Platinum Metals. In Advance Catalysis; Academic Press: Cambridge, MA, USA, 1979; Volume 28, pp. 1–78. [Google Scholar] [CrossRef]
- Salanov, A.N.; Savchenko, V.I. TD and AES studies of the interaction of oxygen with Rh(100). React. Kinet. Catal. Lett. 1985, 29, 101–109. [Google Scholar] [CrossRef]
- Abedi, A.; Hayes, R.; Votsmeier, M.; Epling, W.S. Inverse Hysteresis Phenomena During CO and C3H6 Oxidation over a Pt/Al2O3 Catalyst. Catal. Lett. 2012, 142, 930–935. [Google Scholar] [CrossRef]
- Busca, G.; Cristiani, C.; Forzatti, P.; Groppi, G. Surface characterization of Ba-?-alumina. Catal. Lett. 1995, 31, 65–74. [Google Scholar] [CrossRef]
- Dalle Nogare, D.; Salemi, S.; Biasi, P.; Canu, P. Taking advantage of hysteresis in methane partial oxidation over Pt on honeycomb monolith. Chem. Eng. Sci. 2011, 66, 6341–6349. [Google Scholar] [CrossRef]
- McCarty, J.G. Kinetics of PdO combustion catalysis. Catal. Today 1995, 26, 283–293. [Google Scholar] [CrossRef]
- Amin, A.; Abedi, A.; Hayes, R.; Votsmeier, M.; Epling, W. Methane oxidation hysteresis over Pt/Al2O3. Appl. Catal. A Gen. 2014, 478, 91–97. [Google Scholar] [CrossRef]
- Dadi, R.K.; Luss, D.; Balakotaiah, V. Dynamic hysteresis in monolith reactors and hysteresis effects during co-oxidation of CO andC2H6. Chem. Eng. J. 2016, 297, 325–340. [Google Scholar] [CrossRef]
- Etheridge, J.E.; Watling, T.C. Is reactor light-off data sufficiently discriminating between kinetic parameters to be used for developing kinetic models of automotive exhaust aftertreatment catalysts? The effect of hysteresis induced by strong self inhibition. Chem. Eng. J. 2015, 264, 376–388. [Google Scholar] [CrossRef]
- Sun, M.; Croiset, E.B.; Hudgins, R.R.; Silveston, P.L.; Menzinger, M. Steady-State Multiplicity and Superadiabatic Extinction Waves in the Oxidation of CO/H2Mixtures over a Pt/Al2O3-Coated Monolith. Ind. Eng. Chem. Res. 2003, 42, 37–45. [Google Scholar] [CrossRef]
- Bourane, A.; Bianchi, D. Oxidation of CO on a Pt/Al2O3 Catalyst: From the Surface Elementary Steps to Lighting-Off Tests. J. Catal. 2002, 209, 126–134. [Google Scholar] [CrossRef]
- Russell, A.; Epling, W.S.; Hess, H.; Chen, H.-Y.; Henry, C.; Currier, N.; Yezerets, A. Spatially-Resolved Temperature and Gas Species Changes in a Lean-Burn Engine Emissions Control Catalyst. Ind. Eng. Chem. Res. 2010, 49, 10311–10322. [Google Scholar] [CrossRef]
- Chakrabarty, T.; Hudgins, R.R.; Silveston, P.L. Criterion for simultaneous interphase temperature and concentration gradients. J. Chem. Eng. Jpn. 1982, 15, 237–239. [Google Scholar] [CrossRef]
- Depcik, C.; Loya, S.; Srinivasan, A.; Wentworth, T.; Stagg-Williams, S. Adaptive Global Carbon Monoxide Kinetic Mechanism over Platinum/Alumina Catalysts. Catalysts 2013, 3, 517–542. [Google Scholar] [CrossRef]
- Poenitzsch, L.; Wilde, M.; Tetenyi, P.; Dobrovolszky, M.; Paal, Z. ChemInform Abstract: Sulfur Adsorption, Desorption, and Exchange on Platinum/Alumina, Rhenium/Alumina, and Platinum-Rhenium/Alumina Catalysts. ChemInform 2010, 23. [Google Scholar] [CrossRef]
- Pakharukov, I.Y.; Stakheev, A.Y.; Beck, I.E.; Zubavichus, Y.V.; Murzin, V.Y.; Parmon, V.N.; Bukhtiyarov, V.I. Concentration Hysteresis in the Oxidation of Methane over Pt/γ-Al2O3: X-ray Absorption Spectroscopy and Kinetic Study. ACS Catal. 2015, 5, 2795–2804. [Google Scholar] [CrossRef]
- Dubbe, H.; Eigenberger, G.; Nieken, U. Hysteresis Phenomena on Pt- and Pd-Diesel Oxidation Catalysts: Experimental Observations. Top. Catal. 2016, 59, 1054–1058. [Google Scholar] [CrossRef]
- Zhao, R.; Hao, Q.; Bin, F.; Kang, R.; Dou, B. Influence of Ce/Zr Ratio on the Synergistic Effect over CuCe1-xZrxOy/ZSM-5 Catalysts for the Self-Sustained Combustion of Carbon Monoxide. Combust. Sci. Technol. 2017, 189, 1394–1415. [Google Scholar] [CrossRef]
- Hlaváček, V.; Votruba, J. Hysteresis and Periodic Activity Behavior in Catalytic Chemical Reaction Systems. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 1979; Volume 27, pp. 59–96. [Google Scholar] [CrossRef]
- Casapu, M.; Fischer, A.; Gänzler, A.M.; Popescu, R.; Crone, M.; Gerthsen, D.; Türk, M.; Grunwaldt, J.-D. Origin of the Normal and Inverse Hysteresis Behavior during CO Oxidation over Pt/Al2O3. ACS Catal. 2016, 7, 343–355. [Google Scholar] [CrossRef]
- Mousa, M.S.; Hammoudeh, A.; Loboda-Cackovic, J.; Block, J.H. The CO-oxidation on Pd-rich surfaces of PdCu(110): Hysteresis in reaction rates. J. Mol. Catal. A Chem. 1995, 96, 271–276. [Google Scholar] [CrossRef]
- Fernandes, V.R.; Bossche, M.V.d.; Knudsen, J.; Farstad, M.H.; Gustafson, J.; Venvik, H.J.; Grönbeck, H.; Borg, A. Reversed Hysteresis during CO Oxidation over Pd75Ag25(100). ACS Catal. 2016, 6, 4154–4161. [Google Scholar] [CrossRef]
- Deng, W.; Jesus, J.D.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Low-content gold-ceria catalysts for the water-gas shift and preferential CO oxidation reactions. Appl. Catal. A Gen. 2005, 291, 126–135. [Google Scholar] [CrossRef]
- Beusch, H.; Fieguth, P.; Wicke, E. Thermisch und kinetisch verursachte Instabilitäten im Reaktionsverhalten einzelner Katalysatorkörner. Chem. Ing. Tech. 1972, 44, 445–451. [Google Scholar] [CrossRef]
- Roberts, G.W.; Satterfield, C.N. Effectiveness Factor for Porous Catalysts. Langmuir-Hinshelwood Kinetic Expressions for Bimolecular Surface Reactions. Ind. Eng. Chem. Fundam. 1966, 5, 317–325. [Google Scholar] [CrossRef]
- Schneider, P.; Mitschka, P. Effect of internal diffusion on catalytic reactions. IV. Reversible second-order reaction with Langmuir-Hinshelwood type of rate equation. Collect. Czechoslov. Chem. Commun. 1966, 31, 3677–3701. [Google Scholar] [CrossRef]
- Luss, D.; Lee, J.C.M. Stability of an isothermal catalytic reaction with complex rate expression. Chem. Eng. Sci. 1971, 26, 1433–1443. [Google Scholar] [CrossRef]
- Padberg, G.; Wicke, E. Stabiles und instabiles Verhalten eines adiabatischen Rohrreaktors am Beispiel der katalytischen CO-Oxydation. Chem. Eng. Sci. 1967, 22, 1035–1051. [Google Scholar] [CrossRef]
- Cutlip, M.B.; Kenney, C.N. Limit Cycle Phenomena during Catalytic Oxidation Reactions over a Supported Platinum Catalyst. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1978; pp. 475–486. [Google Scholar] [CrossRef]
- Ekerdt, J.G. Catalytic Hydrocarbon Reactions over Supported Metals. Progress Report, February 1, 1992--March 31, 1993; No. DOE/ER/13604-22; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 1993. [CrossRef]
- Lashina, E.A.; Slavinskaya, E.M.; Chumakova, N.A.; Stonkus, O.A.; Gulyaev, R.V.; Stadnichenko, A.I.; Chumakov, G.A.; Boronin, A.I.; Demidenko, G.V. Self-sustained oscillations in CO oxidation reaction on PdO/Al2O3 catalyst. Chem. Eng. Sci. 2012, 83, 149–158. [Google Scholar] [CrossRef]
- HlavÁČEk, V.; Votruba, J. Experimental Study of Multiple Steady States in Adiabatic Catalytic Systems. In Chemical Reaction Engineering—II; American Chemical Society: Washington, DC, USA, 1975; Volume 133, pp. 545–558. [Google Scholar]
- Saoud, K.; Al Soubaihi, R.; Dutta, J. Self-sustaining and hysteresis behavior of low-temperature CO oxidation on mesoporous Pd/SiO2 aerogel s catalyst under dynamics conditions. In Proceedings of the 256th National Meeting and Exposition of the American-Chemical-Society (ACS)—Nanoscience, Nanotechnology and Beyond, Boston, MA, USA, 19–23 August 2018. [Google Scholar]
- Al Soubaihi, R.M. Thermal Hysteresis of Palladium encaged inside Mesoporous Silica catalyst for Low Temperature CO oxidation. Qatar Found. Annu Res. Conf. Proc. 2018, 2018, EEPD363. [Google Scholar] [CrossRef]
- Eigenberger, G. Kinetic instabilities in heterogeneously catalyzed reactions—I. Chem. Eng. Sci. 1978, 33, 1255–1261. [Google Scholar] [CrossRef]
- Rathouský, J.; Kíra, E.; Hlaváček, V. Experimental observations of complex dynamic behavior in the catalytic oxidation of CO on Pt/alumina catalyst. Chem. Eng. Sci. 1981, 36, 781–782. [Google Scholar] [CrossRef]
- Dauchot, J.P.; Cakenberghe, J.V. Oscillations during Catalytic Oxidation of Carbon Monoxide on Platinum. Nat. Phys. Sci. 1973, 246, 61–63. [Google Scholar] [CrossRef]
- Hugo, P.; Jakubith, M. Dynamisches Verhalten und Kinetik der Kohlenmonoxid-Oxidation am Platin-Katalysator. Chem. Ing. Tech. 1972, 44, 383–387. [Google Scholar] [CrossRef]
- Zahradnik, J.; McCarthy, E.F.; Kuczynski, G.C.; Carberry, J.J. Effect of Ambient Atmosphere on Sintering of αA12O3 Supported Pt Catalysts. In Sintering and Catalysis; Springer: Berlin, Germany, 1975; pp. 199–209. [Google Scholar] [CrossRef]
- Boubnov, A.; Gänzler, A.; Conrad, S.; Casapu, M.; Grunwaldt, J.-D. Oscillatory CO Oxidation Over Pt/Al2O3 Catalysts Studied by In situ XAS and DRIFTS. Top. Catal. 2013, 56, 333–338. [Google Scholar] [CrossRef]
- Gänzler, A.M.; Casapu, M.; Boubnov, A.; Müller, O.; Conrad, S.; Lichtenberg, H.; Frahm, R.; Grunwaldt, J.-D. Operando spatially and time-resolved X-ray absorption spectroscopy and infrared thermography during oscillatory CO oxidation. J. Catal. 2015, 328, 216–224. [Google Scholar] [CrossRef]
- Barth, J. Transport of adsorbates at metal surfaces: From thermal migration to hot precursors. Surf. Sci. Rep. 2000, 40, 75–149. [Google Scholar] [CrossRef]
- Subbotin, A.N.; Gudkov, B.S.; Yakerson, V.I.; Chertkova, S.V.; Golosman, E.Z.; Kozyreva, G.V. Temperature-Hysteresis Effects in CO Oxidation on Cement Catalysts with Various CuO Content. Russ. J. Appl. Chem. 2001, 74, 1506–1508. [Google Scholar] [CrossRef]
- Frank-Kamenetskii, D.A. Diffusion and Heat Exchange in Chemical Kinetics; Princeton University Press: Princeton, NJ, USA, 1955. [Google Scholar] [CrossRef]
- Subbotin, A.N.; Subbotina, I.R.; Golosman, E.Z. Hysteresis phenomena in heterogeneous exothermal catalytic reactions and methods for decreasing the overheating of catalyst nanoclusters. Mendeleev Commun. 2015, 25, 216–218. [Google Scholar] [CrossRef]
- Yuranov, I.; Kiwi-Minsker, L.; Slin’ko, M.; Kurkina, E.; Tolstunova, E.D.; Renken, A. Oscillatory behavior during CO oxidation over Pd supported on glass fibers: Experimental study and mathematical modeling. Chem. Eng. Sci. 2000, 55, 2827–2833. [Google Scholar] [CrossRef]
- Ishchenko, E.V.; Yatsimirskii, V.K.; Gaidai, S.V. Temperature Hysteresis in Oxidation of CO on Complex Oxide Catalysts. Theor. Exp. Chem. 2005, 41, 340–345. [Google Scholar] [CrossRef]
- Kipnis, M. Gold in CO oxidation and PROX: The role of reaction exothermicity and nanometer-scale particle size. Appl. Catal. B Environ. 2014, 152–153, 38–45. [Google Scholar] [CrossRef]
- Kipnis, M.; Volnina, E. New approaches to preferential CO oxidation over noble metals. Appl. Catal. B Environ. 2010, 98, 193–203. [Google Scholar] [CrossRef]
- Rozovskii, A.Y.; Kipnis, M.A.; Volnina, E.A.; Lin, G.I.; Samokhin, P.V. Selective oxidation of CO under conditions of catalyst surface ignition. Kinet. Catal. 2007, 48, 701–710. [Google Scholar] [CrossRef]
- Lindstrom, T.H.; Tsotsis, T.T. Reaction rate oscillations during CO oxidation over Pt/γ-Al2O3; experimental observations and mechanistic causes. Surf. Sci. 1985, 150, 487–502. [Google Scholar] [CrossRef]
- Yablonskii, G.S.; Lazman, M.Z. New correlations to analyze isothermal critical phenomena in heterogeneous catalysis reactions (“Critical simplification”, “hysteresis thermodynamics”). React. Kinet. Catal. Lett. 1996, 59, 145–150. [Google Scholar] [CrossRef]
- Newton, M. Time Resolved Operando X-ray Techniques in Catalysis, a Case Study: CO Oxidation by O2 over Pt Surfaces and Alumina Supported Pt Catalysts. Catalysts 2017, 7, 58. [Google Scholar] [CrossRef]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E. Overview of the Fundamental Reactions and Degradation Mechanisms of NOx Storage/Reduction Catalysts. Catal. Rev. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Heck, R.M. Catalytic converters: State of the art and perspectives. Catal. Today 1999, 51, 351–360. [Google Scholar] [CrossRef]
- Havenith, C.; Verbeek, R.P. Transient Performance of a Urea deNOx Catalyst for Low Emissions Heavy-Duty Diesel Engines. In Proceedings of the SAE Technical Paper Series; SAE: Warrendale, PA, USA, 1997. [Google Scholar] [CrossRef]
- Held, W.; König, A.; Richter, T.; Puppe, L. Catalytic NOx Reduction in Net Oxidizing Exhaust Gas. I. SAE Trans. 1990, 99, 209–216. [Google Scholar]
- Jung, J.; Song, S.; Chun, K.M. Characterization of Catalyzed Soot Oxidation with NO2, NO and O2 using a Lab-Scale Flow Reactor System. In Proceedings of the SAE Technical Paper Series; SAE: Warrendale, PA, USA, 2008. [Google Scholar] [CrossRef]
- Salasc, S.; Skoglundh, M.; Fridell, E. A comparison between Pt and Pd in NOx storage catalysts. Appl. Catal. B Environ. 2002, 36, 145–160. [Google Scholar] [CrossRef]
- Tronci, S.; Baratti, R.; Gavriilidis, A. Catalytic converter design for minimisation of cold-start emissions. Chem. Eng. Commun. 1999, 173, 53–77. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, J.; Fan, Y.; Zhan, Y.; Chen, C.; Li, D.; Jiang, L. Mg-Al hydrotalcite-supported Pd catalyst for low-temperature CO oxidation: Effect of Pdn+ species and surface hydroxyl groups. Dalton Trans. 2018. [Google Scholar] [CrossRef] [PubMed]
- Berzins, A.R.; Vong, M.S.W.L.; Sermon, P.A.; Wurie, A.T. Isothermal Chemisorption upon Oxide-Supported Platinum. Adsorpt. Sci. Technol. 1984, 1, 51–76. [Google Scholar] [CrossRef]
- Kiwi-Minsker, L.; Yuranov, I.; Siebenhaar, B.; Renken, A. Glass fiber catalysts for total oxidation of CO and hydrocarbons in waste gases. Catal. Today 1999, 54, 39–46. [Google Scholar] [CrossRef]
- Miller, B.K.; Crozier, P.A. Operando TEM of Ru\RuO2 Catalyst Performing CO Oxidation. Microsc. Microanal. 2014, 20, 1564–1565. [Google Scholar] [CrossRef]
- Schalow, T.; Brandt, B.; Laurin, M.; Schauermann, S.; Libuda, J.; Freund, H. CO oxidation on partially oxidized Pd nanoparticles. J. Catal. 2006, 242, 58–70. [Google Scholar] [CrossRef]
- Hauptmann, W.; Votsmeier, M.; Vogel, H.; Vlachos, D.G. Modeling the simultaneous oxidation of CO and H2 on Pt—Promoting effect of H2 on the CO-light-off. Appl. Catal. A Gen. 2011, 397, 174–182. [Google Scholar] [CrossRef]
- Koutoufaris, I.; Koltsakis, G. Heat- and mass-transfer induced hysteresis effects during catalyst light-off testing. Can. J. Chem. Eng. 2014, 92, 1561–1569. [Google Scholar] [CrossRef]
- Mobini, S.; Meshkani, F.; Rezaei, M. Surfactant-assisted hydrothermal synthesis of CuCr2O4 spinel catalyst and its application in CO oxidation process. J. Environ. Chem. Eng. 2017, 5, 4906–4916. [Google Scholar] [CrossRef]
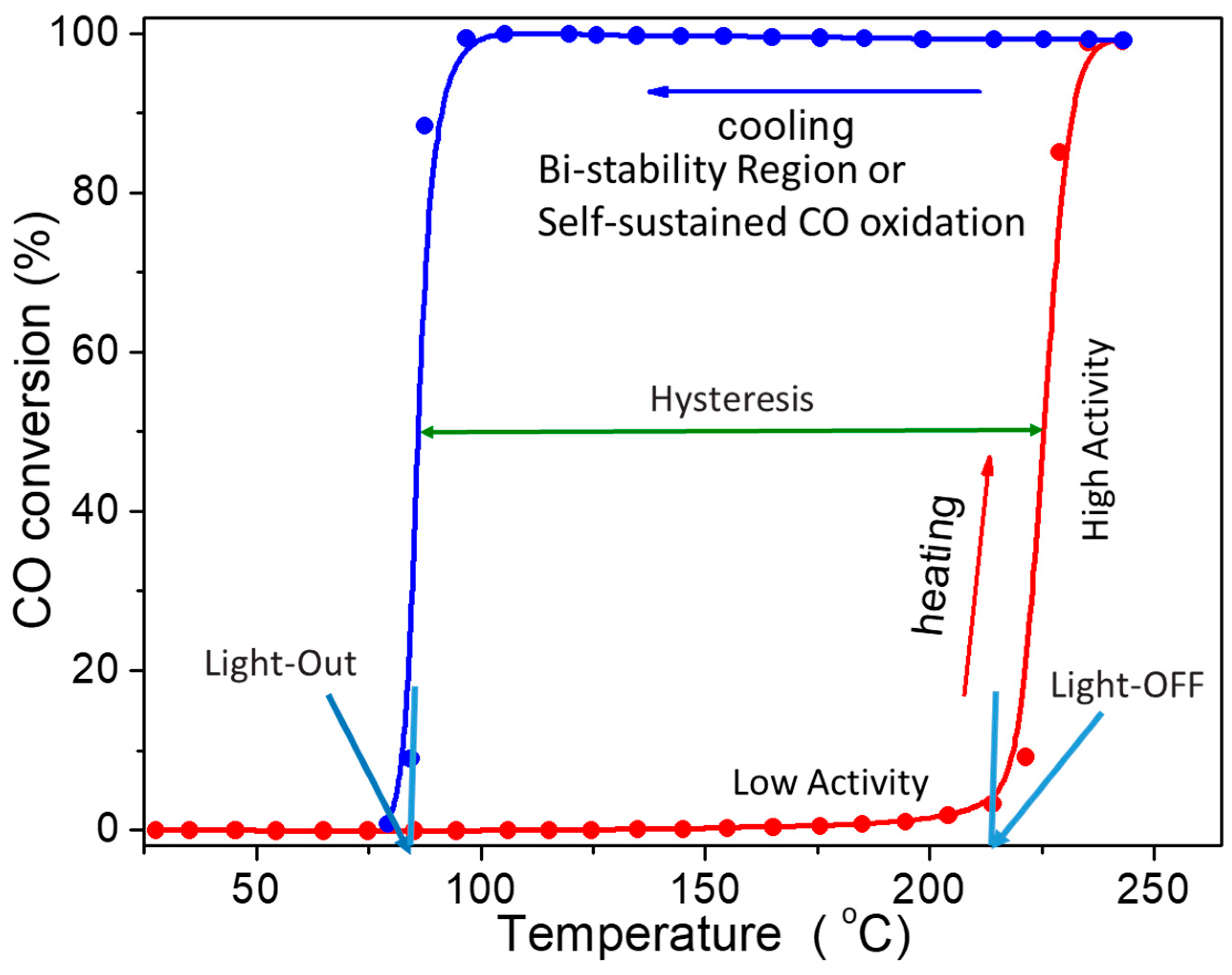
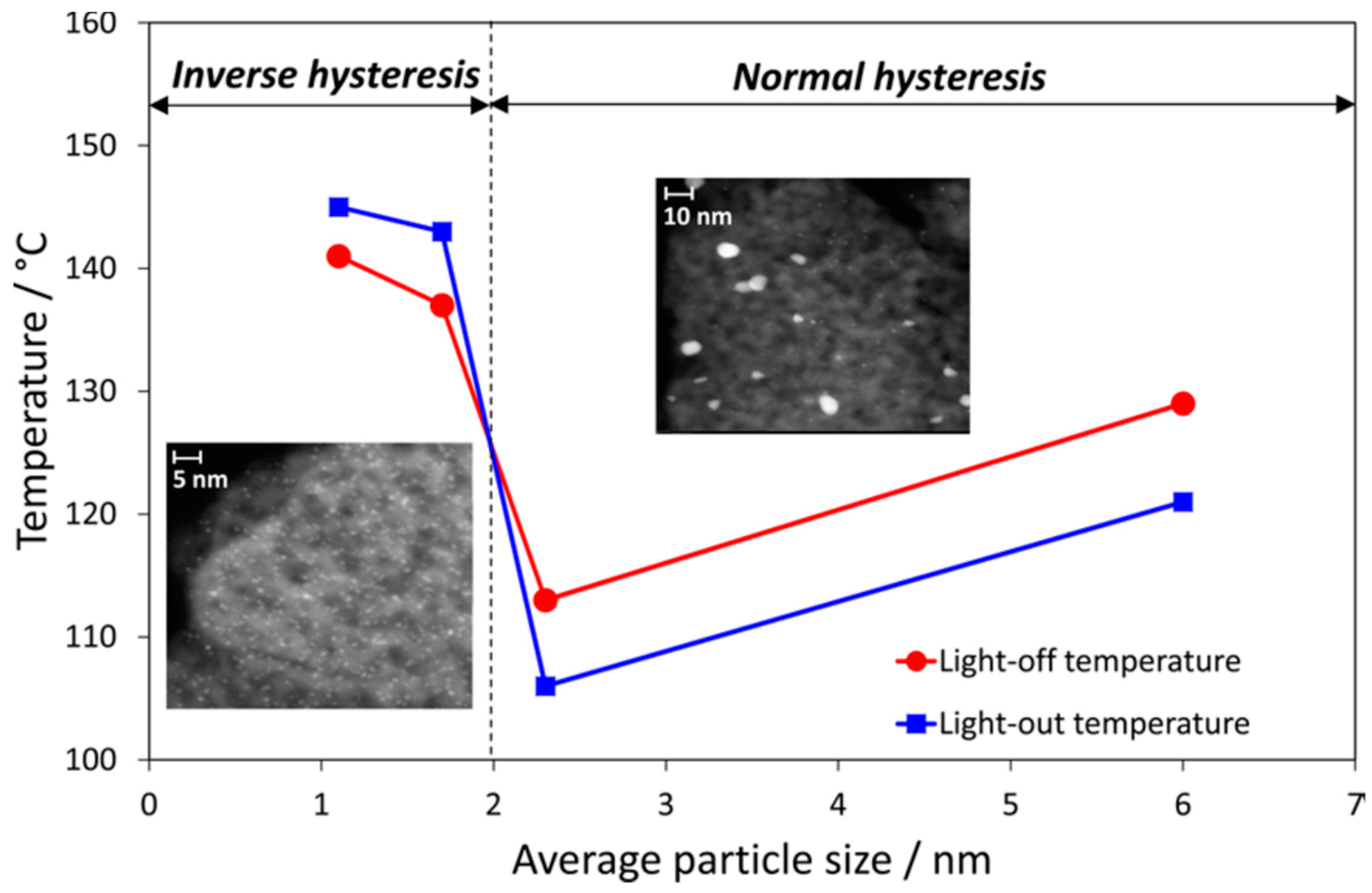
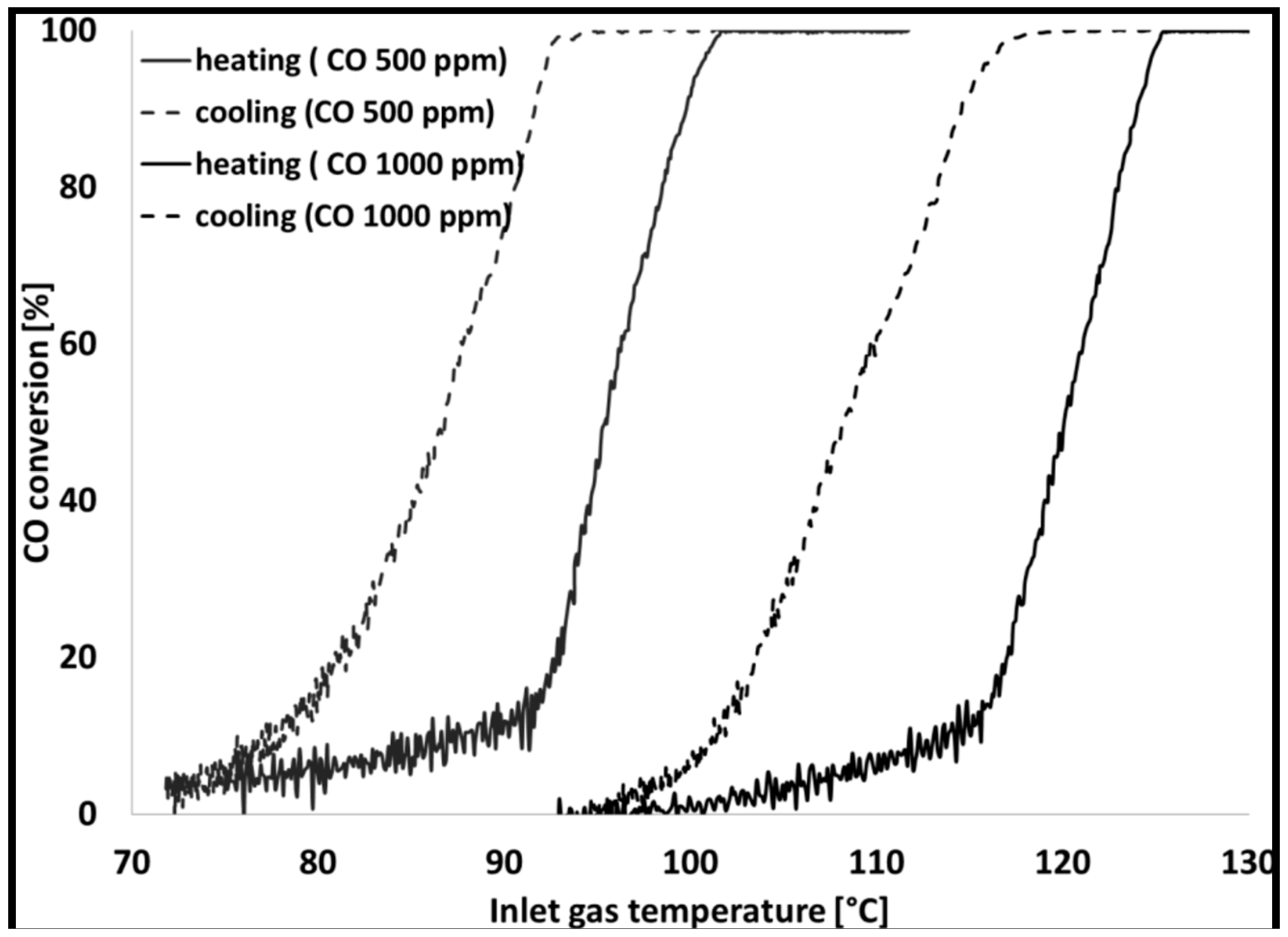
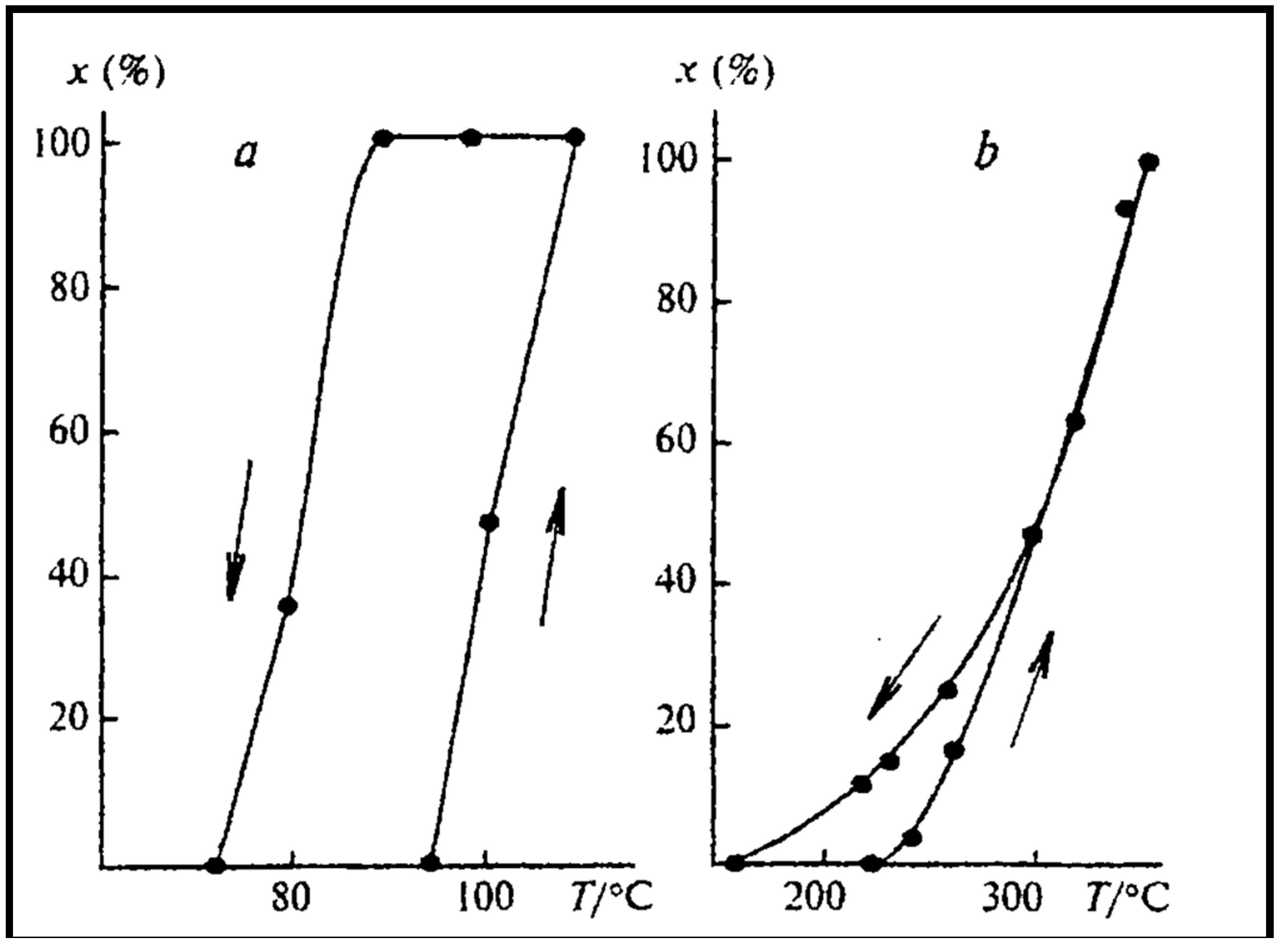
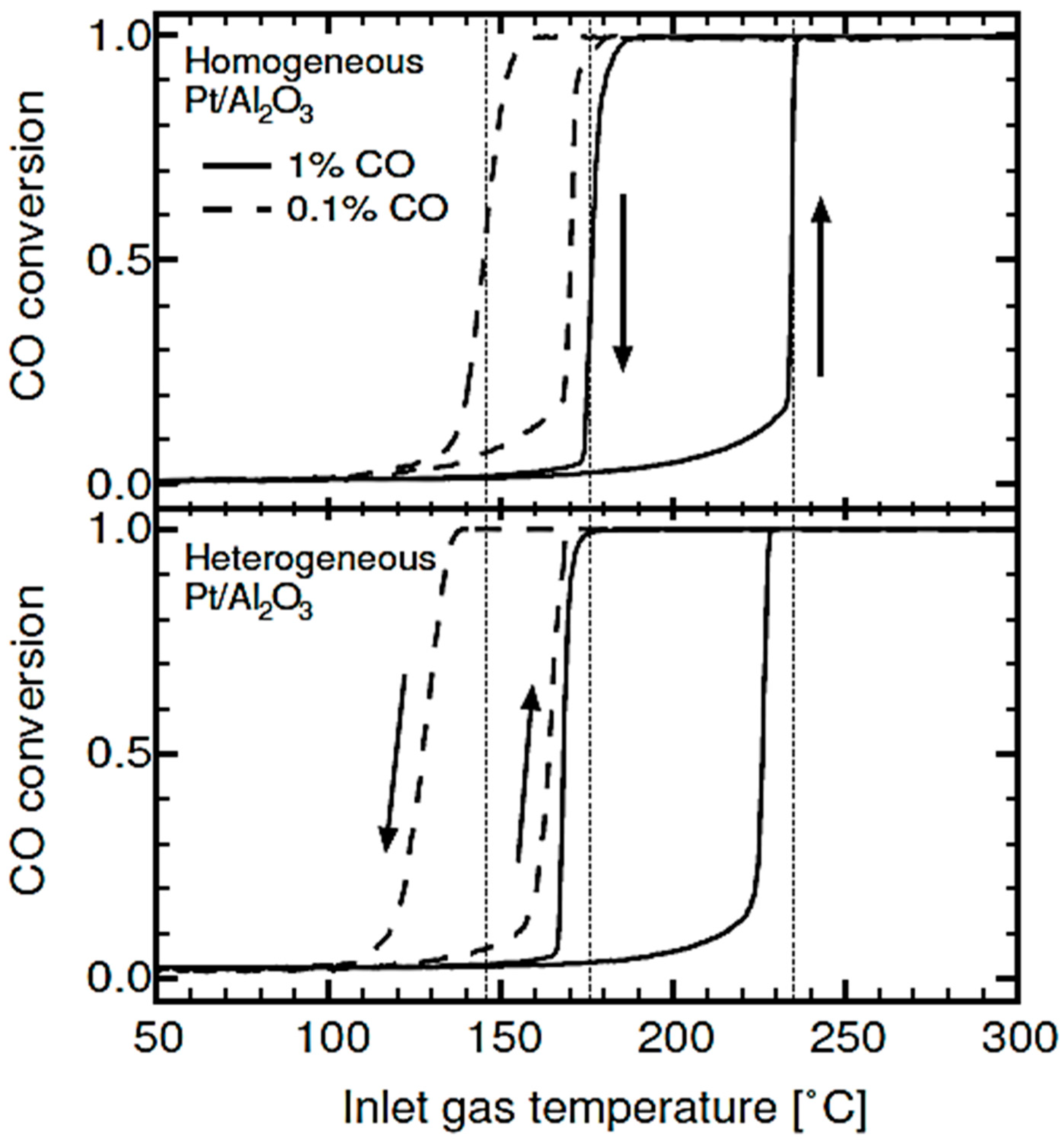
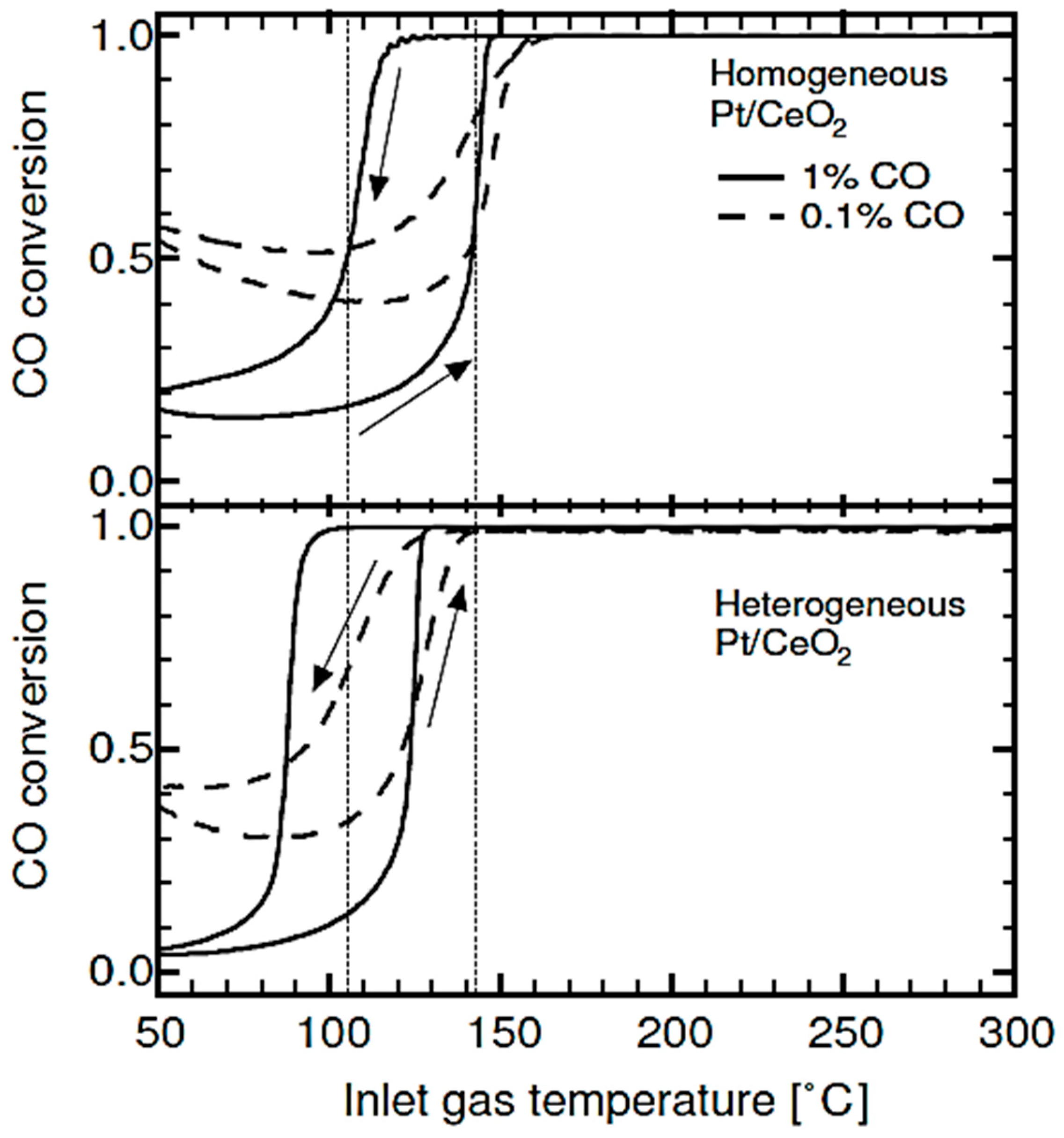
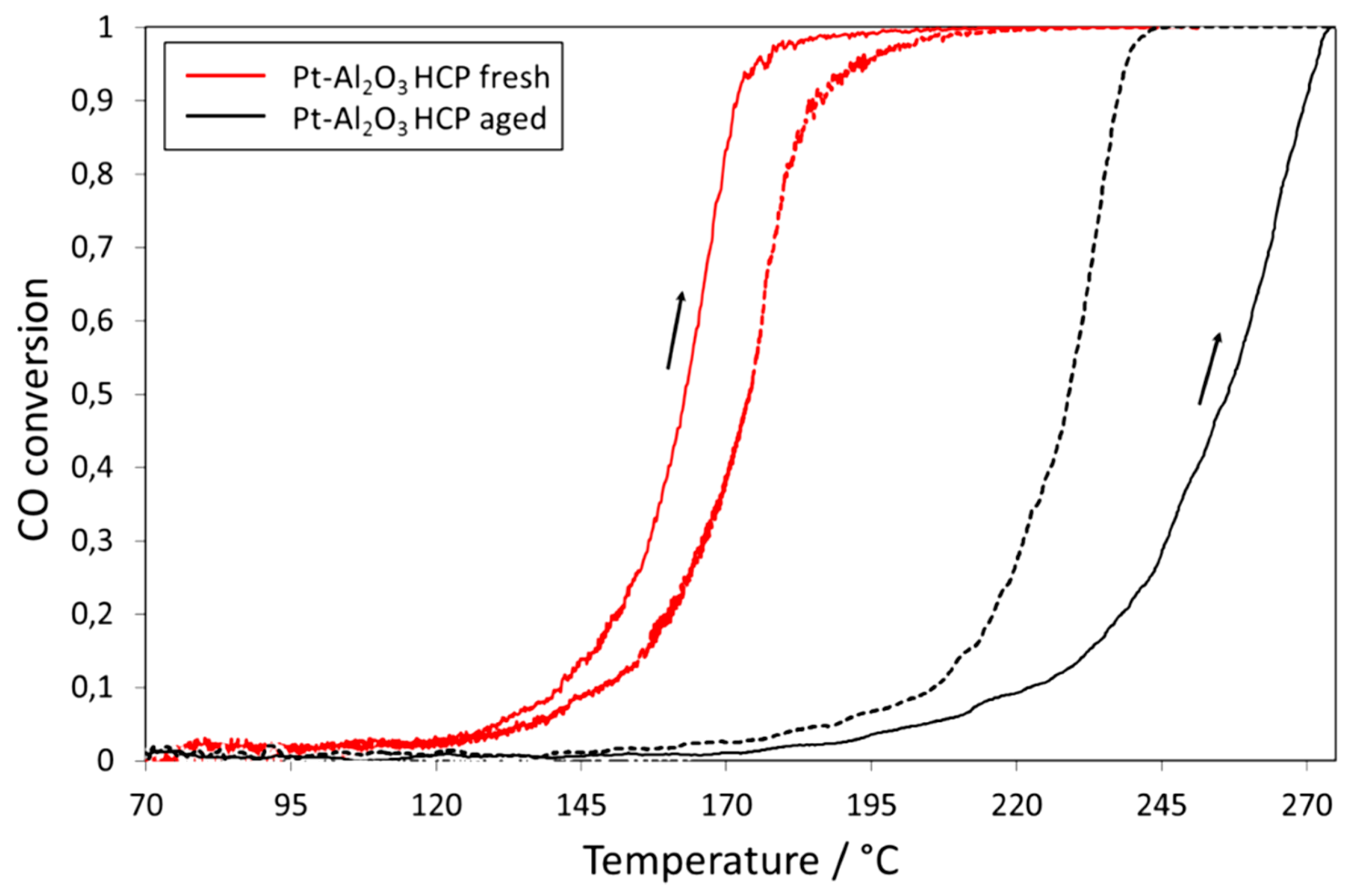
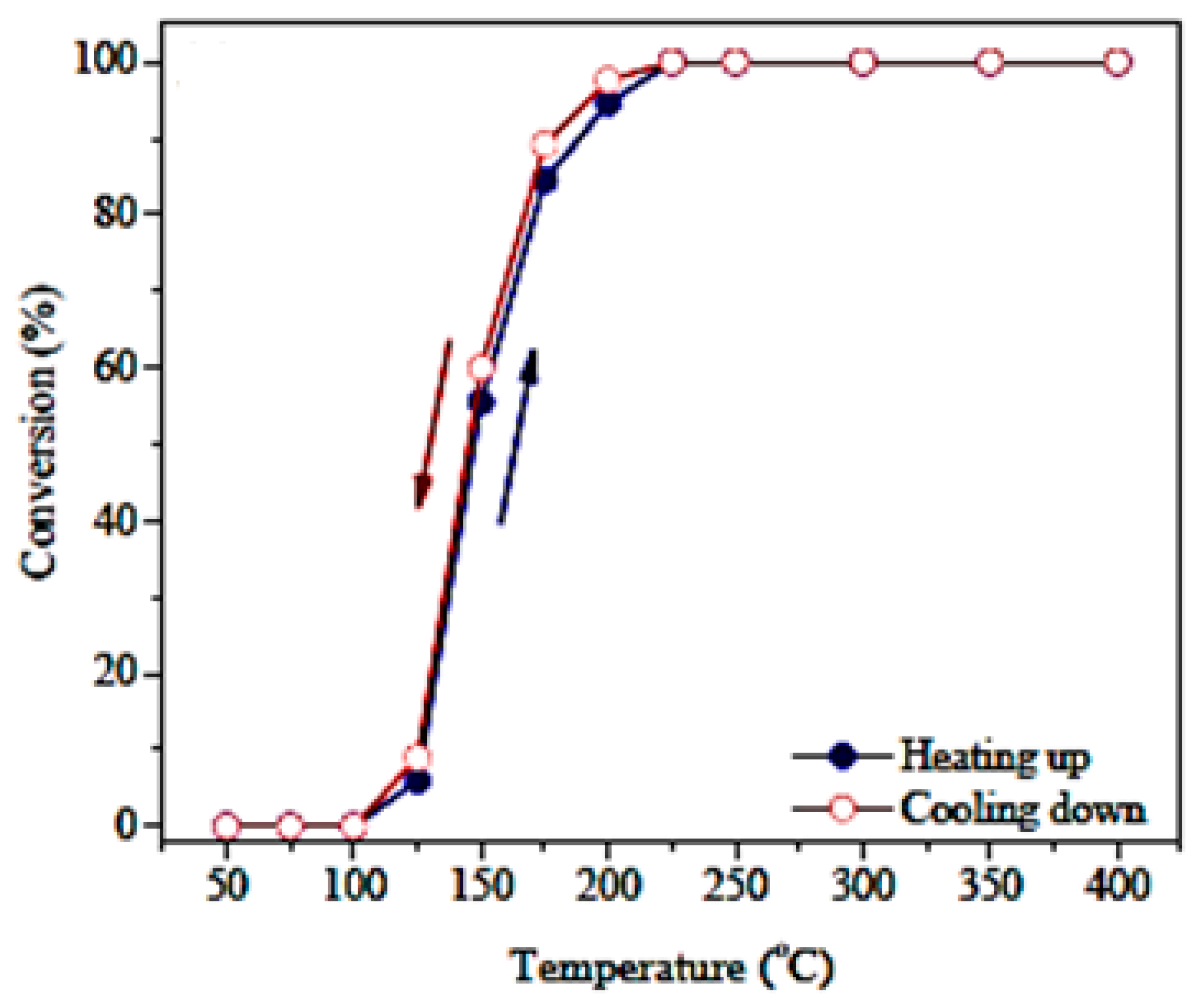
| Gas Used | Catalyst | Hysteresis Type | Reference |
|---|---|---|---|
| CO | Pt/Al2O3 Pt/TiO2, Pt/SiO2 | Normal Normal | [5,30,85,94,114] [93,136,137] |
| CO and CH4 | Pt/Al2O3 and Pt/CeO2 | Inverse | [5,77,81] |
| CO | PdO/Al2O3, Pd/Al(OH)3, Pd/Mg(OH)2 | Normal | [105,135] |
| CO | Cu-Ce/ZSM-5 or CuCe1–x ZrxOy/ZSM-5 | Normal | [72,92] |
| CO | Unsupported Platinum wire, Platinum foil | Normal | [63,75] |
| CO, NO and C3H6 | Unsupported Platinum | Inverse | [5,49,54,67,91,111] |
| CO | Platinum and Palladium on alumina | Normal | [67,77,81,82,83] |
| CO | CuO | Normal | [63,117] |
| CO | Gold nanoparticles | Normal | [122,123,124] |
| CO | Ceria-supported catalyst with 0.28 wt% gold (Au), Germanium (Ge) and Gadolinium (Gd) dopants | Normal | [97,120,125] |
| CO | Pd/Ag, PdCu(110) alloys | Inverse | [95,96] |
| CO | Ru/RuO2 | Normal | [138] |
| Parameter | Effect on Hysteresis Curve | Reference |
|---|---|---|
| Inlet CO concentration | Increasing CO concentration in inlet gas causes a higher light-off temperature or ignition point, the hysteresis loop increases upon increasing the CO concentrations | [52,56,74,77,87,126,142] |
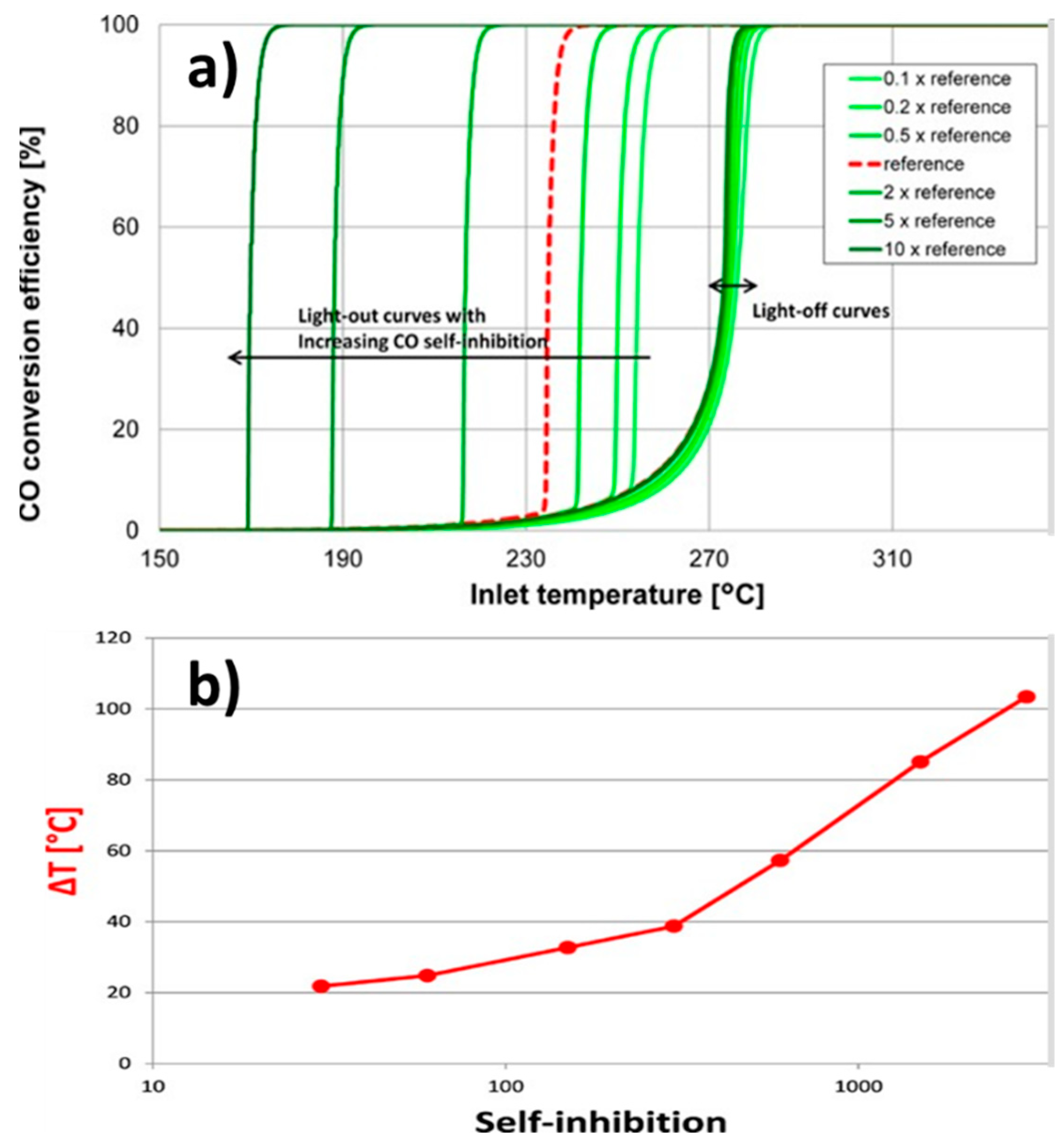
| Inlet Temperature | Ignition and extinction shifted to higher temperatures with rapid increase in CO conversion | [77,128,129,130,131,132,133,134] |
| Surface Contamination and Aging | Increase in the hysteresis width due to the removal of contamination that blocks active sites | [75,94,141] [55,94,140] |
| Rate of temperature ramp-up | Increase in the temperature ramp-up result in shifting the ignition curve to higher temperatures | [72,92] |
| Inhibition parameters | Different inhibiting species accumulation on catalyst surface can change the shape of hysteresis curves and increase the width of the hysteresis. | [141,142]. |
| Particle size | When the size decrease (less than 2 nm), the hysteresis changes from normal to inverse. | [68,94,124] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Soubaihi, R.M.; Saoud, K.M.; Dutta, J. Critical Review of Low-Temperature CO Oxidation and Hysteresis Phenomenon on Heterogeneous Catalysts. Catalysts 2018, 8, 660. https://doi.org/10.3390/catal8120660
Al Soubaihi RM, Saoud KM, Dutta J. Critical Review of Low-Temperature CO Oxidation and Hysteresis Phenomenon on Heterogeneous Catalysts. Catalysts. 2018; 8(12):660. https://doi.org/10.3390/catal8120660
Chicago/Turabian StyleAl Soubaihi, Rola Mohammad, Khaled Mohammad Saoud, and Joydeep Dutta. 2018. "Critical Review of Low-Temperature CO Oxidation and Hysteresis Phenomenon on Heterogeneous Catalysts" Catalysts 8, no. 12: 660. https://doi.org/10.3390/catal8120660
APA StyleAl Soubaihi, R. M., Saoud, K. M., & Dutta, J. (2018). Critical Review of Low-Temperature CO Oxidation and Hysteresis Phenomenon on Heterogeneous Catalysts. Catalysts, 8(12), 660. https://doi.org/10.3390/catal8120660







