Abstract
Hydrogen storage materials have been a subject of intensive research during the last 4 decades. Several developments have been achieved in regard of finding suitable materials as per the US-DOE targets. While the lightweight metal hydrides and complex hydrides meet the targeted hydrogen capacity, these possess difficulties of hard thermodynamics and sluggish kinetics of hydrogen sorption. A number of methods have been explored to tune the thermodynamic and kinetic properties of these materials. The thermodynamic constraints could be resolved using an intermediate step of alloying or by making reactive composites with other hydrogen storage materials, whereas the sluggish kinetics could be improved using several approaches such as downsizing and the use of catalysts. The catalyst addition reduces the activation barrier and enhances the sorption rate of hydrogen absorption/desorption. In this review, the catalytic modifications of lightweight hydrogen storage materials are reported and the mechanism towards the improvement is discussed.
| Outline |
| 1. Introduction |
| 2. The mechanism of Hydrogen Absorption/Desorption and the Need for a Catalyst |
| 3. Catalysts for MgH2 |
| 3.1 Transition Metal Catalysts |
| 3.2 Carbon and Other Elements as Additive |
| 3.3 Metal Oxide Catalysts |
| 3.4 Metal Halide Catalysts |
| 3.5 Hydride, Hydride Forming Alloys and Sulfide as Catalyst |
| 4. Catalysts for Complex Hydrides |
| 4.1 Catalysts for Alanates |
| 4.2 Catalysts for Borohydrides |
| 4.3 Catalysts for Amides |
| 4.4 Catalysts for Silanides |
| 5. Concluding Remarks and Future Prospective |
| References |
1. Introduction
Hydrogen is considered as an alternative fuel which has the capability to replace current fossil fuel based energy infrastructure. It is important to note that hydrogen is not a primary energy source; it can store, transport, and deliver the energy. In contrast to fossil fuels such as gasoline, diesel, etc. which emit greenhouse gases, it emits only water as byproduct when converted to electrical energy. The gravimetric energy content of hydrogen is 2–3 times higher than conventional fuels, however, the volumetric energy density is quite low in comparison to conventional fuels [1]. It needs more than twice the space in liquid state as compared to the space required by gasoline for a car to run 300 miles. The amount is increased to 3–5 times for the compressed state with 10,000 psi and 5000 psi tanks. Moreover, the need for bulky and expensive tanks, low temperature of liquefaction, and the safety issues with both of the above options renders them impractical for commercial application. Thus, the third method of hydrogen storage through solid state materials has attracted the attention of the scientific community as it reduces the required storage pressure as well as increases the gravimetric and volumetric capacity significantly. Thus, hydrogen storage in solid state through the physically or chemically bounded form in the material has been a subject of various studies in last few decades [2,3,4,5,6]. Several materials/families have been developed in search of suitable material having a target operating temperature of −40 °C–85 °C and storage capacity of >8 wt% respectively as per US-DOE [7], which are summarized in Table 1 [8,9,10,11,12,13,14,15,16].

Table 1.
Hydrogen storage families with their storage capacities and operating temperatures.
It is clear that the sorbent systems are not capable of achieving the targeted capacity, moreover, they have very low operating temperature too. The conventional metal hydrides such as LaNi5, FeTi, V–Ti–Cr etc., have been studied extensively. These could be employed for stationary applications such as hydrogen compressors [17], however, these cannot be employed for onboard applications due to their low gravimetric storage capacity. The chemical hydrides have high capacity, but these are irreversible, thus, only suitable for a single use of hydrogen supply. Among all these, light hydrides such as LiH, MgH2, and complex hydrides fit with high capacity of 7–18.5 wt%. Although these light element based hydrides and complex hydrides have very high hydrogen capacity, they possess two serious issues at the same time. The first is the thermodynamic issue, which is caused by the presence of strong covalent/ionic bonds, thus making these hydrides very stable and requiring a high operating temperature (>200 °C). The thermodynamic destabilization can be achieved by two methods: one of which is downsizing the particles, however, it can be achieved only if the size is reduced to several nanometers, which is neither easy to achieve nor to maintain. Thus, the second approach of introducing an intermediate state (Figure 1) can solve this issue [18]. However, this is not in the scope of this article. Herein, we will review the second issue of slow kinetics of hydrogen sorption in light element hydrides/complex hydrides. The challenge of kinetics arises due to the highly directional nature of bonding present in these hydrides, which creates a large diffusion barrier leading to a prohibitively slow reaction rate for hydrogen charging–discharging. Several other factors also contribute to the kinetics problem, e.g., the dissociation of H2 molecule to atomic H, etc. In the next section, we will explain the mechanism of hydrogen absorption and desorption in terms of its kinetics.
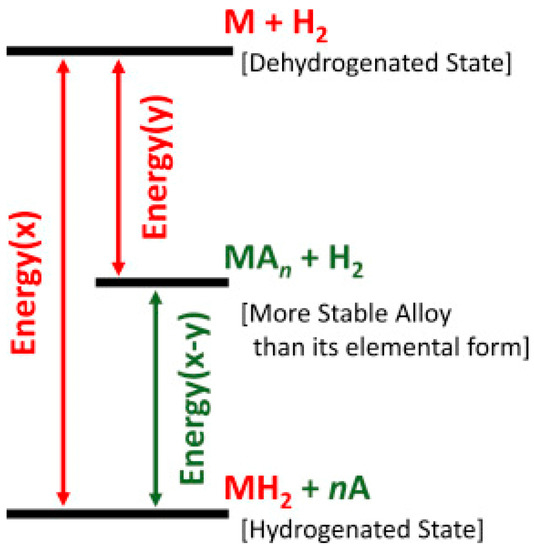
Figure 1.
Schematic of destabilization process of a hydride MH using third element A. Adapted with permission from [18], copyright Elsevier, 2016.
2. The Mechanism of Hydrogen Absorption/Desorption and the Need of Catalyst
The kinetics of hydrogen sorption is defined as the rate of hydrogen absorption and desorption by a particular metal or alloy, and is equally as important and decisive a factor as thermodynamics. While the thermodynamics is represented by enthalpy of formation (∆H) and entropy (∆S) of hydride, the kinetics is usually represented by activation energy (E) of the reaction. The activation energy of sorption reaction can be calculated by Arrhenius Equation (1) and Kissinger Equation (2) as follows:
where Ea is activation energy, k is rate constant, A is frequency factor, R is gas constant, T is temperature, β is heating rate, and Tp is peak temperature.
K = A exp[−Ea/RT]
ln (β/Tp2) = ln (AR/Ea) − Ea/(RTp)
The kinetics of hydrogen sorption is influenced by several factors such as the diffusion coefficient of H2, the occurrence of phase transition, the heat of solution, and the intrinsic rate of H2 transfer through solid–gas interface, etc. [19]. This can be understood on the basis of a hydrogen absorption–desorption mechanism as mentioned by Martin et al. [20]. The hydrogen is stored in the metal/alloys through the following 5 processes as shown in Figure 2:
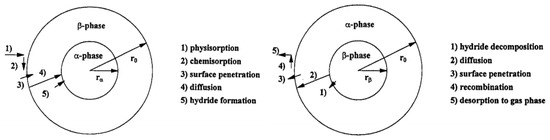
Figure 2.
Reaction partial steps for the absorption (left) and desorption (right) of hydrogen by a spherical metal/hydride powder particle. Adapted with the permission from [20], copyright Elsevier, 2016.
- Physisorption of H2 molecule;
- Chemisorption of H atoms;
- Surface penetration of H atoms;
- Diffusion of hydrogen atoms;
- Hydride formation at metal/hydride interface.
One of these steps is considered as a rate limiting step for a particular reaction and the other steps are in equilibrium. Since the physisorption of H2 molecule need almost no activation energy, it is not a rate limiting step. The concentration of H2 molecules impinged on the surface of metal is directly proportional to the applied pressure. All other steps can be rate-limiting steps and affect the kinetics of hydrogen sorption. The desorption reaction follows the same steps but in a reverse direction as shown in Figure 2.
Several methods have been proposed for reducing the activation barrier and enhancing the kinetics of hydrogen sorption. These include nano-scaling and use of catalysts [21,22,23,24]. Some researchers also include alloying as a tool of altering the kinetics [24], however, in our opinion alloying mainly affects the thermodynamics of a system. In fact, alloying changes the entire path of hydrogen sorption of a metal/alloy. By forming thermodynamically more stable alloys (Figure 1), the operating temperature can be reduced effectively, but it is not really a kinetic alteration. Thus, only downsizing and use of catalysts should be considered as kinetics enhancement tools, where only the activation barrier is altered without affecting the thermodynamics as shown in Figure 3.
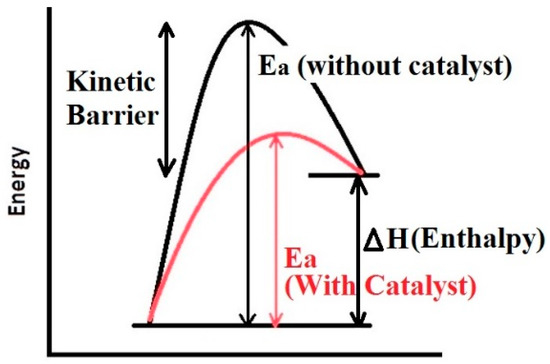
Figure 3.
Representation of the kinetic barrier of the reaction and lowering the activation Energy (Ea) using catalyst.
Downsizing to nano-scale has been considered as an effective method to improve the sorption kinetics. As mentioned above, the kinetics depends on the activation barrier involved with the chemisorption of H atoms on the metal surface, diffusion of hydrogen atoms, or nucleation of the hydride phase, thus, nano-scaling can accelerate the kinetics through the creation of fresh surface for chemisorption, decreased diffusion distance for H atoms, and increased surface to volume ratio, thus providing more nucleation sites for hydride formation [25,26,27]. For example, it has been shown that MgH2 nanowires show a much lower energy barrier (~33.5–38.8 kJ mol−1) than bulk MgH2 (120–142 kJ mol−1) [28]. It is noteworthy that the nanosizing down to <50 nm can enhance the kinetics, whereas the size <5 nm can alter the thermodynamics of MgH2 as well [25]. In spite of the outstanding performance of the nano-sizing, it cannot be used for practical applications, especially because of two issues: (1) The preparation of such small sized structure is not easy on commercial/industry level, (2) The cyclic stability of these nano-structures is not good as these agglomerates during the sorption cycles and they lose their benefits of nano-size. The approach of using a catalyst to enhance the kinetics has a benefit over nano-sizing as it is easy to prepare in comparison to nano-sizing and shows even better performance over it. A catalyst is defined as a material which enhances the sorption rate of a metal/alloy without participating into the chemical reaction and reducing the activation barrier only. In some cases, a catalyst can also participate in the chemical reaction, but remains intact at the end of reaction. Until now, several catalysts have been developed for different hydrogen storage materials, which will be reviewed in the next section in accordance with respective hydrogen storage materials.
3. Catalysts for MgH2
Magnesium hydride (MgH2) has been an attractive contender for hydrogen storage due to its high hydrogen content (7.6 wt%). It can be formed directly through the reaction of magnesium with hydrogen under mild conditions thermodynamically (<1 bar and ~50 °C), however, the sluggish kinetics allow the hydrogenation to occur only at 350–400 °C and high pressure, i.e., 3 MPa [29,30,31]. There are several factors which affect the kinetics of magnesium hydride formation, including the surface oxidation [32], low dissociation rate of H2 molecules on metal surface [33], and slow diffusion of dissociated H2 atoms within the hydride [34]. Even if the surface oxide layer is broken the through activation process, it takes several hours to form MgH2 at >350 °C. And, even if the initial hydrogenation is somehow fast enough due to high pressure, the formation of a hydride layer on the surface blocks further penetration of hydrogen [35,36]. It is reported that a 30–50μm thick hydride layer can stop further hydrogenation abruptly [32,37]. Thus bulk MgH2 cannot be used practically due to the above mentioned reasons and needs to be modified for its sorption kinetics using catalysts. The present section will describe different types of additives, used to improve the sorption kinetics of MgH2.
3.1. Transition Metal Catalysts
Since, the dissociation of H2 molecules and diffusion of hydrogen atoms through the metal are two rate limiting steps for H2 absorption in Mg, several theoretical studies have been conducted to analyze the hydrogen magnesium interaction in respect to the transition metal catalysts [38,39,40,41,42]. Kecik et al. [38] used the first principle molecular dynamics method for the calculation of adsorption energies of Mo, Nb, Mn, Cr, Co, Fe, V, Pd, and Ni on a magnesium surface and suggested that the transition metals including and to the left of the VI-A column tend to be adsorbed on a substitutional site while other transition elements have a tendency to be adsorbed on a bridge site. In addition to this, they also found that the transition metals having a higher chance of adsorption are also capable of causing dissociation of the hydrogen molecule. According to this, all 3d and 4d transition metals should work as effective catalysts, however, it is not always the case with experiments. Slow kinetics is still a problem with many of the transition metals, which can be understood on the basis of DFT calculations carried by Pozzo et al. [39]. They suggested that all the transition metals show two opposite catalytic activities at the same time; the metals effective for the dissociation do not have any effect for the diffusion and vice versa. It can be well understood from Figure 4. The activation energy barrier for H2 dissociation and diffusion strongly correlates with the d-band center. It is clear that except for Ag, Cu, and Pd, all other metals have a very low dissociation barrier, however, at the same time they have a large diffusion barrier. On the other hand, Ag, Cu, and Pd do not bind the H atoms too strongly and, therefore, have almost no diffusion barrier, but at the same time they do not have any effect on the dissociation of H2 molecule. This suggests that Ni, Fe, Rh, and Pd have the best possible catalytic activity with a good balance in overcoming the diffusion barrier and dissociation barrier at the same time. The above theoretical findings are well supported by several experimental works, which were carried out during the 70 s to the 90 s of the last century, however, the operating temperature was still more than 330 or 350 °C for the H2 sorption by magnesium with sufficient rate and capacity. Zaluska et al. [43] were the first ones who prepared nano-crystalline MgH2 decorated with nano-particles of Pd, as well as other metals such as Fe, V, and Zr, and reduced the absorption temperature to less than 200 °C and desorption temperature to less than 280 °C. In a contemporary report, Liang et al. [44] ball milled a number of transition metals, i.e., Ti, V, Mn, Fe, and Ni and found the rapid desorption for MgH2–V followed by Ti, Fe, Ni, and Mn at low temperatures, whereas Ti addition showed rapidest absorption followed by V, Fe, Mn, and Ni. In a later study [45] on MgH2 + 5 at%V, they suggested that hydrogen desorption at high temperature is controlled by the interface motion. However, at low temperatures, where the driving force is small, the hydrogen desorption is first controlled by nucleation and growth followed by long range hydrogen diffusion. Reactive mechanical milling under H2 atmosphere has been found to be an effective method to enhance the H2 sorption properties of Mg. Bobet et al. [46] demonstrated that RMA for even a short time (2 h) with Co, Ni, and Fe improved the sorption properties of magnesium. A further improvement in the surface properties of MgH2 was achieved by the use of nano-sized metal catalysts such as Pd, Pt, Ru [47], Fe, Co, Ni, Cu [48]. Hanada et al. [48] showed that the activation energy of desorption was reduced to 94 ± 3 kJ mol−1 by the addition of 2 mol% nano-Ni in comparison with the 323 ± 40 kJ mol−1 for pure MgH2. The MgH2–2%nano-Ni system desorbs a large amount of H2 (0.5 wt%) in the temperature range of 150–200 °C under He flow (Figure 5).
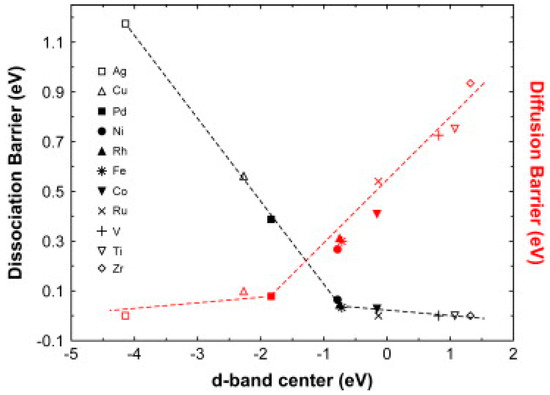
Figure 4.
Activation energy barrier for hydrogen dissociation (black) and diffusion (red) of hydrogen on pure Mg and metal-doped Mg surfaces as a function of d-band center positions. Adapted with the permission from [39], copyright Elsevier, 2009.
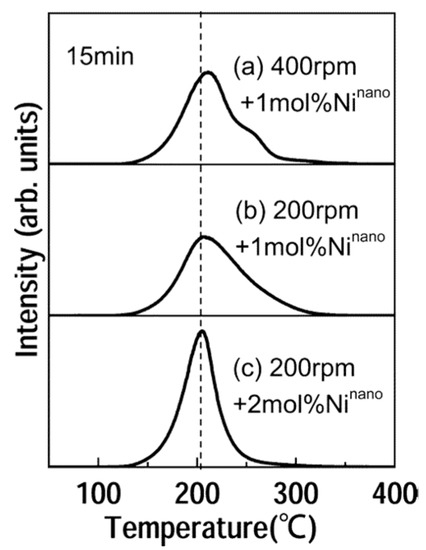
Figure 5.
Thermal desorption mass spectra of hydrogen for the MgH2 composites milled for 15 min at (a) 400 rpm with 1 mol % Ninano, (b) 200 rpm with 1 mol % Ninano, and (c) 200 rpm with 2 mol % Ninano, respectively. Adapted with the permission from [48], copyright ACS, 2005.
Following the above reports, several efforts have been devoted to improve the sorption properties of MgH2 using different nano-transition metals in different amount, prepared by different routes etc. [49,50], however, nano-Ni has always shown superior performance among them [51,52,53,54]. Recently Chen et al. [53] prepared a composite of porous Ni nano-fibers via electrospinning technique and prepared a composite of these with MgH2 using ball milling. The resulting composite has shown an activation energy of 81.5 kJ mol−1 with the onset desorption temperature of 143 °C. Lu et al. [55] prepared a novel core-shell structured Mg–TM (TM = Co, V) composite through a combined arc plasma and electroless plating method. The ternary Mg–Co–V composite has shown a much lower activation energy (Ea = 67.66 kJ mol−1) in comparison with other binary composites or pure MgH2.
3.2. Carbon and Other Elements as Additive
Besides transition metals as catalysts, several other elements have also been studied to modify the kinetics of magnesium hydride. Carbon structures are one of the most studied systems as composite materials with MgH2. Imamura et al. [56], in 2003, focused on the composite of graphite and magnesium prepared by mechanical milling in the presence of organic additives. According to them, the dangling bonds of carbon, produced by high energy milling, act as hydrogen accommodating sites. The hydrogen uptake by the Mg increased in the order of additive benzene, cyclohexene, and cyclohexane. It was observed that the addition of crystalline graphite had very little effect on the desorption properties, but lead to a rapid hydrogen absorption in comparison to pure MgH2 [57]. In addition, it formed a protective layer and inhibited the formation of oxide layer on Mg. Different species of carbon including graphite, activated carbon, multi-walled carbon nano-tubes, and carbon nano-fibers have shown great influence on the sorption properties of MgH2 in terms of lower desorption temperature and fast sorption kinetics [58,59,60,61,62]. Reactive ball milling under hydrogen atmosphere further enhances the effect of graphite/carbon addition [63,64]. Recently Lototsky et al. [64], using time resolved studies, showed that carbon acts as a carrier of activated hydrogen through spill-over mechanism. They suggested that high energy reactive ball milling destructs the original carbon structure and forms graphene layers, which encapsulate MgH2 nanoparticles and prevents the grain growth. This helps to keep the sorption cycling stability much better. Besides carbon material, rare earth metals have also shown positive effects on the sorption properties of MgH2 [65]. It is known that rare earth metals work as oxygen getters, so their presence reduces the possibility of the formation of surface oxide layer of Mg, thus improving the sorption properties. Mainly La, Y and Ce metals (either in metallic form or hydride form) have been used as catalyst for MgH2 [66,67,68]. Shang et al. [66] showed that Ce addition improves desorption kinetics of MgH2, much better than the Y addition. It occured due to CeO2 formation, which produced surface defects on MgH2 benefiting the desorption kinetics. Recently, other rare earth elements such as Nd, Gd and Er were also investigated as catalysts for MgH2 [69]. Zou et al. [69] prepared the Mg–RE nano composite through the arc plasma method with a special metal oxide type core-shell structure. The ultrafine Mg–RE particles covered by nano-sized MgO and Re2O3 showed greatly enhanced kinetics as well as anti-oxidation properties of MgH2.
3.3. Metal Oxide Catalysts
In addition to metal based catalysts, several oxides such as TiO2, V2O5, Cr2O3, Mn2O3, Fe3O4, CuO, Al2O3, SiO2, Sc2O3, CeO2, Nb2O5, ZnO, etc., [70,71,72,73,74] have shown enormous catalytic acceleration of hydrogen sorption properties of MgH2. Oelrich et al. [70], in a comparative study, depicted the comparable effects of TiO2, V2O5, Cr2O3, Mn2O3, Fe3O4, and CuO on the absorption kinetics, whereas Fe3O4 lead V2O5, Mn2O3, Cr2O3, and TiO2 to improve the desorption kinetics. Later, in a systematic study on several high performance catalysts, Barkhordarian et al. [73] suggested that four different physico-thermodynamic properties of these catalysts influence the catalytic activity, namely, (1) a high number of structural defects, (2) the low stability of compound, (3) the high valence state of the transitional metal ion, (4) and high affinity of transition metal ion to hydrogen. On the basis of these factors, they summarized the catalysts according to their catalytic activity as shown in Figure 6. It is clear from the figure that Nb2O5 has the highest catalytic activity for the sorption properties of MgH2, which makes it one of the most studied catalyst in the hydrogen storage community [75,76,77,78,79,80,81,82,83,84,85,86]. In earlier studies, Barkhordarian et al. studied the effect of Nb2O5 content, milling time etc., on the sorption properties [75,76,77] and deduced that the rate limiting step is greatly influenced by the catalyst content. At lower temperatures, i.e., 250 °C and catalyst content up to 0.1 mol%, the reaction is 3-dimensional growth controlled due to slower diffusion (because of low temperature) as well as slow hydrogen draining and a longer diffusion path (because of lack of catalyst). With the same content of catalyst but at a higher temperature, i.e., 300 °C, the reaction becomes surface controlled as the diffusion of hydrogen is easier at 300 °C and the reaction rate is only controlled by a slow gas–solid reaction due to low content of catalyst. When the catalyst amount is increased to 0.2 mol% or more, the reaction is interface controlled because the recombination rate of hydrogen atoms is no longer a rate limiting step. The absorption is diffusion controlled, whereas, the rate limiting step for desorption changes from chemisorption to interface growth with the increase of milling time or Nb2O5 content [77] as shown in Figure 7.
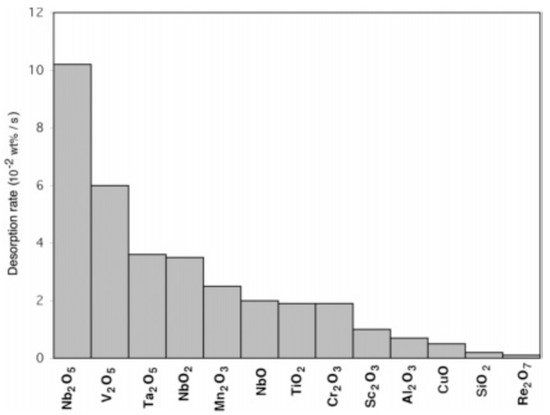
Figure 6.
Various transition-metal oxides and their catalytic effect on the hydrogen desorption reaction rate of magnesium hydride at 300 C. Reaction rates were calculated between 20% and 80% of the respective maximum capacity. Adapted with the permission from [73], copyright ACS, 2006.
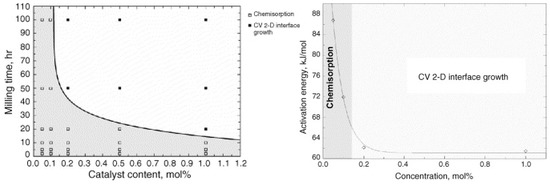
Figure 7.
Kinetic rate-limiting step for desorption as a function of milling time and Nb2O5 concentration (left); Decrease of activation energy for the hydrogen desorption of magnesium hydride with different Nb2O5 catalyst contents milled for 100 h (right). Adapted with permission from [77], copyright Elsevier, 2006.
While other attempts were focused on the sorption behavior at more than 200 °C, the group of Ichikawa presented the room temperature absorption behavior of Nb2O5 catalyzed Mg [78,79,80,81]. They showed that the composite with 1 mol% Nb2O5 absorbs 4.5 wt% H2 at 1 MPa H2 pressure within 15s even at room temperature. A further improvement was achieved by the same group by using mesoporous Nb2O5 as a catalyst, where the absorption occurred at even −50 °C with an activation energy of 70 kJ/mol H2 and 38 kJ/mol H2 for desorption and absorption respectively [80]. It was suggested that Nb2O5 was reduced to the oxides with lower oxidation state of Nb, i.e., NbO, which is responsible for the decrease in activation energy [81]. Another possible mechanism has been suggested by Friedrich et al. [82,83], where they proposed the formation of MgNb2O3.67 phase in addition to Nb and MgO during the first cycling of sorption. They suggested all these phases contribute to the kinetic enhancement of MgH2. Ma et al. [86] also confirmed the existence of above phases using TEM analysis and proposed the Nb-gateway model where Nb facilitated the hydrogen transportation from MgH2 to outside. Inspired from the above study, Pukazhselvan et al. [87,88,89] prepared rock salt structured MgxNb1−xO nano-particles separately and suggested that it can catalyze MgH2 much better than in-situ formed MgxNb1−xO in 2 wt% Nb2O5 catalyzed MgH2.
Several other transition metal oxides also have been subject of intensive research, especially V-oxide [90,91,92], Cr oxide [93,94,95,96], Ti oxide [97,98,99], ZrO2 [100] have shown promising effects in improving the kinetics of MgH2. Recently, rare earth metal oxides [101,102,103,104] have also attracted attention as catalysts for MgH2. In a recent study by Liu et al. [104], CeO2 was shown to have a dramatic catalysis capability toward MgH2 when mixed with CeH2.73 in 1:1 ratio and the hydrogen desorption could be started at temperature as low as 210 °C. Recently, a new class of catalysts emerged with potential catalytic effect, which are termed as complex metal oxides. These complex metal oxides are composed of two metals and oxygen such as CoFe2O4 [105], NiFe2O4 [106], Co2NiO [107], SrFe12O19 [108], Li2TiO3 [109], MnFe2O4 [110], Na2Ti3O7 [111], BiVO4 [112] etc. Some of these could reduce the desorption activation energy of MgH2 down to 70 kJ/mol H2 [111].
Recently, a different mechanism for catalytic behavior of oxides has been proposed [113], which is claimed to have more generalized coverage of all the oxides. The earlier hypothesis given by Klassen et al. [70,73] was more focused on transition metal oxides, which have defective oxide sites and high valence state metals capable of high electron exchange rate, thus are suitable for the sorption kinetics improvement. However, this model fails to describe the effects of MgO and other non-transition metal oxides. A tribological effect model has been proposed to explain the high catalytic activity of MgO, almost comparable to that of Nb2O5, the best known catalyst. According to this model, a correlation between the desorption temperature and electronegativity of the oxide addition has been established and is shown in Figure 8. This correlation has been explained on the basis of a lower friction coefficient at the interface of solid oxides having higher electronegativity, which allows effective grinding process. This enhances the stabilization of small particles which can have fast sorption rate due to shorter diffusion path.
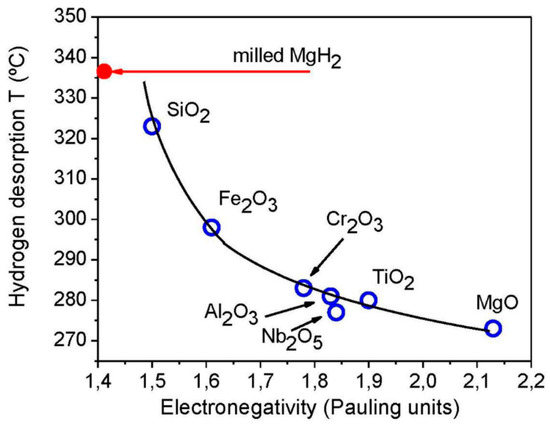
Figure 8.
Correlation between the desorption temperature of hydrogen from MgH2 achieved upon oxide addition during milling and the electronegativity of the oxide additives. Adapted with the permission from [113], copyright MDPI, 2012.
3.4. Metal Halide Catalysts
Metal halides having chloride and fluoride ion have been considered effective catalysts, even better than the metal or metal oxide. In a comparative study, Bhat et al. [114] suggested that NbCl5 addition shows much better catalytic effect than the well-known Nb2O5. Thus, they suggested that neither the oxide ion nor transition metal/transition metal cation are crucial, but it is the chemical nature/iconicity of catalyst which acts as a deciding factor. Malka et al. [115] studied 19 different chlorides and fluorides of different metals and concluded that fluorides are better catalysts than chlorides. In addition, they also suggested that halides with higher oxidation state are more effective in reducing the desorption temperature. As a conclusion of this work, it was stated that the halides of group IV and V, namely ZrF4, TaF5, NbF5, VCl3, and TiCl3 were more effective catalysts than other halides. In a later study, it was suggested that fluorides have higher catalytic activity because of the MgF2 formation in addition to transition metal, which itself acts as a catalyst and further improves the sorption kinetics [116,117]. Several chlorides i.e., CrCl3 [118], NiCl2, CoCl2 [119], TiCl3 [120], FeCl3 [121], LaCl3 [122], CeCl3 [123], ZrCl4 [124] etc., have been extensively studied, however, most of them could reduce the desorption activation energy only to more than 100 kJ/mol H2. A remarkable improvement was observed for ZrCl4 addition, where the reduction of ZrCl4 to ZrCl3 and Zr metal showed good catalytic effect and the desorption activation energy could be lowered to 92 kJ/mol H2. The catalyzed sample could be rehydrogenated even at 0 °C under moderate hydrogen pressure. The results of superior catalytic effect of fluorides on sorption kinetics of MgH2, lead to several comparison studies, mainly focused on the comparison between TiF3 and TiCl3 [125,126,127]. The XPS experiments [125,126] suggested the mechanism of superior catalytic effect of TiF3 over TiCl3. According to this, both samples react with MgH2 and form TiH2 as well as dead magnesium halides. However, F ions participate to generate Mg–Ti–F metastable species in addition to the formation of MgF2. This additional Mg–Ti–F species was found responsible for better catalytic activity of TiF3. Since in-situ formed MgF2 has been suggested as one of the responsible component for catalytic enhancement, Jain et al. [128] reported the effect of direct addition of MgF2 and found that it is beneficial for kinetic enhancement as well as cyclic stability, with low sensitivity to atmospheric conditions and easy handling. Inspired by the merits of F ions, several researchers have developed different fluoride materials such as CeF4, NbF5, ZrF4, TiF3, TiF4 etc. [129,130,131,132,133]. An assumption has been proposed on the basis of intermediate Hδ−–Nbδ+ bond formation, which is favored by large electronic delocalization of the Nb–F bond. This leads to the weakening of surface Mgδ+–Hδ− bonds. It also justifies the higher activity of NbF5 in comparison with Nb2O5, as the electronegativity of fluorine is greater than that of oxygen, which in turn produces more pronounced electronic delocalization for F than O, thus increasing the weakening of Mgδ+–Hδ− bonds. Although all the fluorides have shown promising effects, they all were limited to bringing the activation energy down to 90 kJ/mol H2. A drastic improvement was achieved recently by the addition of TiF4 [132,133], where the activation energy could be reduced down to 70 kJ/mol H2. The onset desorption temperature could be reduced down to 150 °C in comparison to 300 and 400 °C for ball milled and as received MgH2, respectively, as shown in Figure 9 [132]. The XPS study [133] agrees well with the results of Ma et al. [125,126], which suggested that Ti4+ state is reduced to lower oxidation states i.e., Ti3+/Ti2+ and formed TiH2 in addition to Ti–Mg–F species. Inspired by the successful implementation of single metal fluorides, recently several binary metal complex fluorides namely K2TiF6 [134], K2ZrF6 [135], K2NiF6 [136], Na3FeF6 [137] etc. have been tested. The best performance was observed with the addition of 10% Na3FeF6, which reduced the activation energy down to 75 kJ/mol H2. It was suggested that in-situ formation of NaMgF3, NaF and Fe play catalytic roles to improve the sorption kinetics of MgH2. A similar mechanism was also proposed for other complex fluorides.
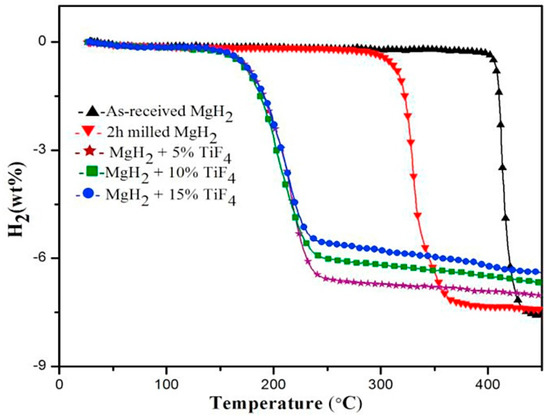
Figure 9.
TG curve for dehydrogenation of the as-received MgH2, 2 h milled MgH2, and MgH2−x wt% TiF4 (x = 5, 10, 15) samples. Adapted with the permission from [132], copyright Elsevier, 2016.
3.5. Hydride, Hydride Forming Alloys and Sulfide as Catalyst
The use of hydride materials as catalyst for MgH2 is led by TiH2 [138,139,140,141,142,143,144]. The mixture of MgH2 and TiH2 in 7:1 molar ratio, prepared through high energy high pressure mechanical milling, showed drastic improvement of H2 desorption kinetics and reduction in desorption temperature. The desorption onset temperature was reduced down to 126 °C, much lower than 381 °C for MgH2 alone [138]. The activation energy was reduced down to 71 kJ/mol H2. In a later report, Lu et al. [139] prepared a nanosized MgH2–0.1TiH2 system with a grain size of 5–10 nm. The transmission electron microscopy (TEM) study suggested uniform distribution of TiH2 among MgH2 particles, which, in addition to nano size, was an important factor for such a drastic improvement. They also reported a reduced ∆H value (−68 kJ/mol H2) for this system, which is significantly lower than that of pure MgH2. It was also observed that this system can reabsorb hydrogen at room temperature with a significant rate and very stable cyclability [139,140]. Three different possibilities have been proposed for this catalytic property of TiH2 by considering TiH2 as (1) grain growth inhibitor (prevents coarsening of MgH2 particles); (2) nucleation and growth center (TiH2 is non-stoichiometric compound and allows faster diffusion); (3) alloying or solid solution forming compound with MgH2 (since ∆H and ∆S values are different from those of MgH2). A theoretical and microscopic investigation on MgH2–TiH2 system [144] suggested TiH2 as a stable component during H2 absorption and desorption which acts as dynamic dopant, i.e., TiH2 particles are located on the top surface of MgH2, which then migrates to the subsurface during dehydrogenation as shown in Figure 10 [144]. Other hydrides for the catalysis of MgH2 sorption are NbH and AlH3 [145,146], however, they don’t show any impressive improvement like TiH2.
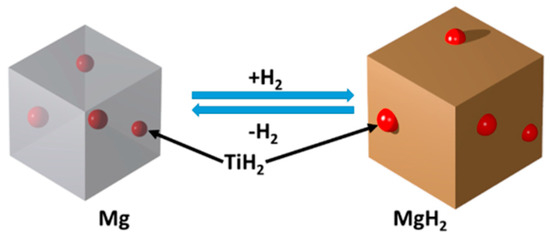
Figure 10.
The catalytic mechanism of TiH2 as dynamic dopant. Adapted with the permission from [144], copyright ACS, 2018.
In addition to metal hydrides, several complex hydrides also have been tried out as catalysts for MgH2 [147,148,149,150,151,152,153]. However, in our opinion, these systems work on the basis of chemical destabilization of MgH2 and thus alter the thermodynamics rather than altering kinetics. The next category of catalyst material is not very different from the hydride materials, the only difference being their use in an unhydrided state in contrast with TiH2, NbH, or other complex hydrides. Several studies have focused on various alloys such as LaNi5 [154], FeTi [155,156], Ti–V–Cr alloys [157,158,159,160], and Zr based alloys [161,162,163,164,165,166,167,168,169,170,171]. Vijay et al. [155] studied the effect of FeTi and FeTiMn alloys in different proportions (5–40 wt%). The lowest absorption and desorption temperature was found as 80 and 240 °C respectively for Mg-40 wt% FeTiMn composite. A remarkable decrease in activation energy down to 71 kJ/mol H2 was achieved using Ti–Cr–Mn–V BCC alloy [157]. A recent study on several V-based hydride forming materials suggested that better kinetic activity can be achieved with the less stable V-based hydrides [159]. A number of Ti-based alloys have been tried out to enhance the sorption kinetics of MgH2 by Zhou et al. [160]. They suggested that a TiAl compound reduced the desorption activation energy of MgH2 to 65.08 kJ/mol H2, which makes it one of the best known catalysts in terms of activation energy, whereas, TiMn2 addition improves the hydrogenation kinetics at room temperature with the activation energy of 20.59 kJ/mol H2. The addition of Zr based AB2 type alloys also attracted attention due to their interesting catalytic activity for MgH2 sorption properties. The addition of ZrCrCu alloy [161] produced Mg2Cu phase at the grain boundaries of Mg and alloy phase during the cycling, which provided diffusion paths and nucleation sites for the easy formation of hydride and enhanced the kinetics. The above reaction was not observed between Mg and other alloy ZrCrX (X = Ni, Fe, Mn, Co) composites [163,164,165,166,167,168,169].
Sulfide materials having transition metal as cation and S as anion have attracted attention as catalysts very recently [172,173,174,175,176]. Jia et al. [172] prepared MgH2–MoS2 composite and suggested the reduced activation energy of 87 kJ/mol H2 for desorption. This improvement was found to be associated with the in-situ formation of MgS and Mo. Zhang et al. [173,176] studied the effect of iron-based sulfides, i.e., Fe3S4 and FeS2, and suggested an almost similar mechanism as found for MoS2 except for the formation of Mg2FeH6 phase in addition to MgS and Fe. This reduced the activation energy down to much lower value, i.e., 68.94 kJ/mol H2, in comparison with MoS2 addition. Even a much lower value of 64.71 kJ/mol H2 could be achieved by the addition of flower-like NiS [175]. The mechanism of sorption process for this nanocomposite system is shown in Figure 11. The prepared nanocomposite comprises of Mg/NiS core/shell structure. The first sorption cycle decomposes NiS shell into Ni, MgS, and Mg2Ni phases, which are decorated on the surface of Mg nanoparticles. So this multi component composite (Mg–MgS–Mg2Ni–Ni) system shows high sorption rate of hydrogen at lower temperature.
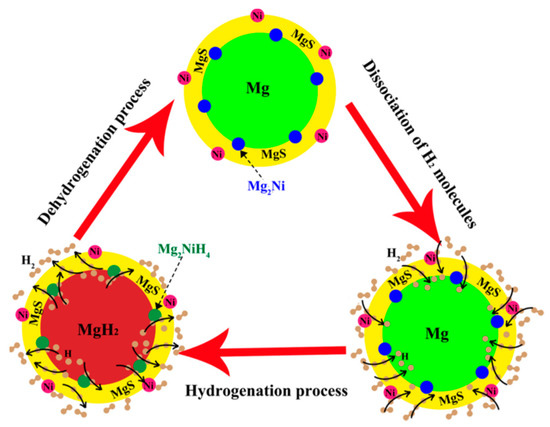
Figure 11.
Schematic diagram of the catalytic mechanism of Ni, Mg2Ni, and MgS catalysts during the hydrogenation/dehydrogenation processes of the nanocomposite. Adapted with the permission from [175], copyright ACS, 2017.
4. Catalysts for Complex hydrides
Complex hydrides have attracted attention as hydrogen storage materials during the last two decades [5] owing to their high hydrogen capacity. While complex hydrides have high hydrogen content compared to metal hydrides, i.e., MgH2, they also possess a complex multistep sorption process due to the presence of an H atom covalently bonded in a tetrahedron AlH4 and BH4 or NH2 anion, which is bonded with the alkali or alkaline metal cations, thus forming alanate, borohydride, and amide families [21,22,23,24,177,178]. The presence of strong covalent bonds, i.e., Al–H, B–H, and N–H, makes them hard to decompose and rehydrogenate. The discovery by Bogdanovic et al. [179] in 1997 of using TiCl3 as catalyst, opened a new era for these complex hydrides by attaining the reversibility of NaAlH4 with sufficiently high kinetics. In this section, we will describe different catalysts for alanate, borohydride, and amides, respectively, in order.
4.1. Catalysts for Alanates
The DECOMPOSITION of alanates undergoes through several steps as follows:
Alkali metals i.e., Li, Na, K
MAlH4 → M3AlH6 + 2Al + 3H2
M3AlH6 + 2Al + 3H2 → 3MH + 3Al + 9/2H2
3MH → 3M + 3/2H2
Magnesium Alanate
Mg(AlH4)2 → MgH2 + 2Al + 3H2
MgH2 → Mg + H2
And Calcium Alanate
Ca(AlH4)2 → CaAlH5 + Al + 3/2H2
CaAlH5 → CaH2 + Al + 3/2H2
Following the discovery of reversibility in complex hydride by Bogdanovic et al. [179], several methodologies have been adopted such as, nanoscaling, alloying, adding catalysts, etc. However, this review focuses only on the use of catalysts to enhance the kinetics, so will include only this approach and explain the mechanism of catalysis here.
The breakthrough invention of Bogdanovic et al. [179] and the following studies [180,181,182] suggested that the activation energy for the two steps of NaAlH4 decomposition could be reduced down to 73 and 97 kJ/mol H2, respectively, by the addition of only 0.9 mol% TiCl3 in comparison to 118 and 124 kJ/mol H2, respectively, for pure NaAlH4. These preliminary studies have been followed by a number of studies on TiCl3 based catalysts in order to understand the mechanism of catalysis, the effect of preparation method of catalyst etc. [183,184,185,186]. The studies were not only limited to TiCl3, several other halides and salts of Ti were also employed to enhance the sorption kinetics of NaAlH4 such as Ti–Al [187,188], TiB2 [189], TiC [190,191], TiF3 [192], TiF4 [193], TiN [194], Ti-oxides [195,196] etc. The use of Ti–Al compounds such as Ti3Al, TiAl3 was motivated from the mechanism that the in-situ formed Ti–Al alloys were found responsible for the catalytic enhancement of TiCl3 or TiCl4 added NaAlH4. However, the catalytic performance of Ti–Al is controversial as some researchers have shown that it has no effect on the decomposition of NaAlH4 [187], whereas some groups found significant improvement in desorption of NaAlH4 [188]. Lee et al. [188] prepared Ti–Al powders through different mechanochemical reactions and achieved a good catalytic effect, depending on the particle size of prepared Ti–Al alloys. Not only Ti–halide, but also TiO2 possesses a good catalytic behavior. The addition of nano-crystalline TiO2 supported on nano-porous carbon decreased the desorption temperature of NaAlH4 and enhanced the reaction kinetics. A systematic XPS and TEM study [196] suggested that Ti undergoes a reduction process of Ti4+ to Ti0 during the milling and/or heating process and forms either TiH2 or Ti–Al compounds. They also suggested that the catalytic effect of Ti based species is in the order of Ti–Al species > TiH2 > TiO2. Inspired by the performance of TiO2, Pukazhselvan et al. [197] studied a number of other metal oxide nano-particles including TiO2, CeO2, La2O3, Pr2O3, Nd2O3, Sm2O3, Eu2O3, and Gd2O3, and observed that TiO2 possessed the best catalytic effect among all the studied oxides. It was also pointed out that the catalytic activity was not very good when Ti powder was directly mixed with NaAlH4, even if it has an advantage of being free of inactive byproducts and unwanted gas impurities [198,199]. In another study, Zidan et al. [200] reported that Zr mixing is inferior to titanium for NaAlH4 to Na3AlH6 reaction, but superior for Na3AlH6 to NaH reaction, thus using a combination of Ti and Zr mixing together from their precursors Ti(OBun)4 and Zr(OPr)4, enhanced the overall kinetics of NaAlH4 and showed a stable reversible capacity of more than 4 wt%. Wang et al. [201] reported the synergetic effect of ternary combination of TiCl3, ZrCl4, and FeCl3 up to a total content of catalyst limit to 4 mol% and observed activation energy as low as 76 kJ/mol for decomposition of NaAlH4, however, the effect for the second step was not as pronounced as for the first step decomposition. TiCl3 was considered the best catalyst until the discovery of ScCl3, CeCl3, and PrCl3 by Bogdanovic et al. in 2006 [202,203]. They found that ScCl3 had a nice effect towards improving the storage capacity, whereas CeCl3 and PrCl3 can improve the cyclic stability with a reduced hydrogenation time by a factor of 2 at high pressure and by a factor of 10 at low pressure. Later, Rongeat et al. [204] pointed out that the efficiency of the above dopants is different for absorption and desorption, which suggested that different reaction mechanism and rate limiting steps took place during both steps. Similar to a TiCl3 doped NaAlH4 system, a CeCl3 doped system exhibited the formation of Ce–Al species, which are considered as catalytically active for the decomposition of NaAlH4 [205]. Thus, CeAl2 and CeAl4 alloys were directly mixed to NaAlH4 and were found to be quite effective in reducing the activation energy of both steps, which were found in the range of 72–90 and 93–104 kJ/mol H2 for first and second step decomposition [206,207]. Similar to NaAlH4 system, several catalysts were developed for solving the kinetic problem of LiAlH4 decomposition, which included Ti based halides [208,209,210,211], other metal halides [212,213,214,215,216,217,218], nano-sized oxides [219,220,221], carbon [222,223,224], etc. Recently a new class of complex precursors for metal doping are being used, such as K2TiF6 [225,226], MnFe2O4 [227,228], NiFe2O4 [229], NiCo2O4 [230], LiTi2O4 [231]. It was suggested that K2TiF6 reacted with NaAlH4/LiAlH4 and thus in-situ formed TiH2, Al3Ti, LiF, and NaH/KH worked together as an active species for the synergetic catalysis [225,226]. The addition of MnFe2O4 nanoparticles reduced the activation energy of both the steps of NaAlH4 decomposition down to 57 kJ and 75 kJ/mol H2, respectively [227], whereas it was reduced to 67 and 76 kJ/mol H2 respectively for both the steps of LiAlH4 decomposition [228]. All of these complex precursors acted as catalyst by following more or less similar mechanism.
In summary, several catalysts have been developed in last two decades, however, the first discovered Ti catalyst is still leading the field in terms of its overall performance. Several efforts have been devoted to understanding the mechanism of Ti induced catalysis in alanates, which were summarized by Terry J. Frankcombe [232]. The earlier mechanism was based on the assumption that the presence of Ti on or in the surface can break and form H–H bonds with very low activation energy as compared to other sites, which enhanced the dehydrogenation/hydrogenation kinetics faster [233]. However, this “hydrogen pump”/spillover behavior could not explain the inactive nature of Pd or Pt, which also has the ability of barrier less adsorption of H2 on their surface. Another argument for slow kinetics was given on the basis of nucleation and growth of compact product phases mainly proposed by Fichtner and group [234,235,236]. Ti particles were suggested to be the centers for nucleation and growth of the decomposed phase [237,238]. However, this mechanism also failed to justify the reabsorption process. On the basis of elemental kinetic theory, which suggests the dependence of decomposition temperature of alanates on the Al–H bond strength, it was proposed that the Ti catalyst destabilized the Al–H bonds [239,240]. Several researchers, on the basis of experimental as well as DFT calculations [241,242,243,244,245], supported the above-mentioned model. Another model based on the existence of Al–H mobile species during decomposition, suggests that the mobility of Al–H species is enhanced due to the hydrogen attached to Ti–Al clusters [246,247,248,249,250]. Araujo et al. [251] proposed a Na vacancy mediated model, according to which Ti catalyst promotes the formation and migration of Na vacancies within alanate crystal. A totally different model based on AlH3 and NaH mobile species vacancies has been proposed by Gunaydin et al. [251], which was also supported by Borgschulte et al. [252] using the H–D exchange experiment. They suggested that Ti in the alanate system acts as shuttle at the Al–NaAlH4 interface. Peles and Vande Walle [253] suggested that the charged hydrogen defect formation energy can be decreased through the alteration of Fermi level through Ti doping. This decrease in defect formation energy increases the diffusion rate of these charged defects. This model easily explains the better catalytic activity of Ti as compared to other metals. For example, Zr doping alters the fermi level of alanate by 0.07 eV, which is much smaller than 0.44 eV for Ti doping. Thus, the defect formation energy (counts same as the alternation of fermi level) by Ti dopant would be decreased by almost 6 orders more than that for Zr dopant and enhanced diffusion rate in a much better way. A “zipper model” was recently proposed by Marashdeh et al. [254], where Ti species on the surface of NaAlH4 worked as a slider of a zip, ejecting Na ions from the subsurface by opening the well-ordered crystal, where they can react quickly. However, this model works only for decomposition reaction like many other models. None of the mechanisms could explain the catalytic effect of Ti for both decomposition and reabsorption. Recently, Atakali et al. [255] proposed a bidirectional mechanism based on the thermodynamic data of all possible hydrides involved in the reaction, which is valid for absorption and desorption. According to this atomistic model, the catalyst transfer of M+ and H− occurs from AlH4− to the AlH63−, thus acting as a bridge for first step. Again in the second step, Ti acts as bridge to form isolated MH and leaves AlH3 behind, which decomposes to Al and H2 spontaneously, as shown in Figure 12.
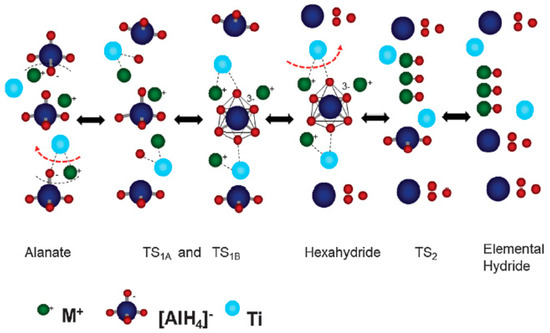
Figure 12.
The function of Ti is to bridge H− and M+ (M+ ··· Ti ··· H−) in order to remove the ion couple from MAlH4 or M3AlH6 without the need to transfer individual M+ and H− ions. Adapted with the permission from [255], copyright RSC, 2015.
4.2. Catalysts for Borohydrides
Borohydrides are considered as favorable materials for hydrogen storage with a high capacity ranging from 10~18 wt%. All the borohydrides generally decompose through following thermolysis reactions:
MBH4 → MH + B + 3/2H2
MH → M + 1/2H2
Some intermediate products were also reported for some of the borohydrides e.g., formation of Li2B12H12 during decomposition of LiBH4 [256]. The emission of diborane in addition to H2 was also reported [257] for some of the borohydrides. It was found that the charge transfer from Mn+ to [BH4]− is a key feature for the stability of M(BH4)n and is directly related to the Pauling electronegativity χp as shown in Figure 13. The charge transfer becomes smaller with the increasing value of χp, which makes ionic bonds weaker, thus reducing the decomposition temperature. It was also noticed that the borohydride contains M with χp ≥ 1.5 desorb diborane in addition to H2, thus making borohydrides with cation of χp ≤ 1.5 suitable as hydrogen storage material. It is clear from Figure 13 that the highly stable borohydride has high temperature of hydrogen desorption, so the catalysis has been considered widely and several catalysts have been investigated to reduce the activation barrier and enhance the kinetics of hydrogen release and uptake.
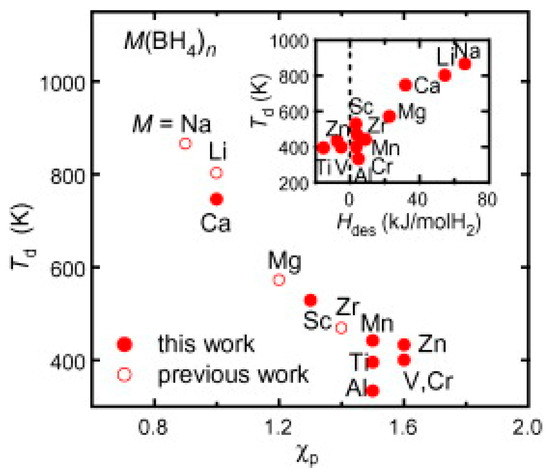
Figure 13.
The desorption temperature Td as a function of the Pauling electronegativity χp. Adapted with the permission from [257], copyright Elsevier, 2012.
Carbon materials were found to be very effective as an additive. It was suggested that increased curvature of carbon structure reduces the hydrogen removal energy [258]. Various forms of carbon have been employed to improve the kinetics and lower the desorption temperature [259,260,261,262,263,264,265]. Yu et al. [259] prepared a mixture of LiBH4 and carbon nanotube mixture by ball milling and showed a lower onset temperature, i.e., 250 °C, of hydrogen desorption. They suggested that the in-situ formed Li2C2 can be reversed to LiH, which contributed to almost 1/4th of total capacity. Carbon nanotubes were also found useful for the kinetic enhancement of Mg(BH4)2 recently [260], where the onset temperature of hydrogen desorption was decreased to 120 °C with an addition of only 5 wt% of CNT with an activation energy of 130 kJ/mol H2, much lower than the pure Mg(BH4)2. Fang et al. [261] compared the performance of various carbon additives and concluded that single walled carbon nanotubes (SWNT) and activated carbon (AC) exhibited much better performance than the normal graphite. The use of fluorinated graphene/graphite [263,264] was also found to be quite effective in reducing the dehydrogenation temperature of LiBH4, which alters the thermodynamics as well as kinetics of LiBH4 decomposition. It was suggested that F− substitutes H anion in LiBH4 or LiH partially, which produces the thermodynamic modification. The activation energy was also found to be reduced down to 131 kJ/mol H2 from 182 kJ/mol H2 for pure LiBH4. Another approach to reduce the activation energy of borohydrides is their nanocaging into carbon scaffold [265]. The onset desorption temperature for nano-caged LiBH4 was found 180 °C lower than that of pure LiBH4 with a reduced activation energy of 113 kJ/mol H2. The in-situ formed Li3BO3 works as an efficient catalyst for the reversible hydrogenation properties.
Not only the carbon materials, but also carbon/graphene supported metal nanoparticles were used as catalysts for the improvement of sorption properties of borohydrides [266,267,268]. The use of nano-Ni particles effectively reduced the activation energy of LiBH4 and Mg(BH4)2. The values were found as 88 kJ/mol H2 [267] and 21.3 kJ/mol H2, respectively, which were almost half of bare borohydrides. Xia et al. [269] showed that addition of Ni powder does not alter the thermodynamics but enhances the sorption rate and could be helpful to achieve the partial reversibility at 600 °C and 10 MPa H2 pressure. Several other pure metals and non-metals were also examined as catalysts in order to reduce the activation barrier and desorption temperature e.g., Mg, Al, Ti, Sc, V, Cr, B etc [270,271,272]. However, it was observed that the use of high amounts of these metals can alter the whole reaction pathway instead of reducing the kinetic barrier only. The use of oxide materials as additives to alter the sorption temperature of borohydrides came up in a very early report by Zuttel et al. [273], where SiO2 was mixed with LiBH4 and allowed the release of H2 starting at 250 °C through the reaction LiBH4 → LiH + B + H2. Several other oxides namely ZrO2, V2O3, SnO2, TiO2, Fe2O3, V2O5, Nb2O5, Co3O4, MoO3, and ZnO then followed as the target of studies [274,275,276,277,278,279,280,281,282,283]. Some of them were claimed to reduce the activation energy of desorption reaction, however, most of them reacted with LiBH4 through redox reactions and thus modified the entire reaction pathway. The next known category of catalyst is halides, which mainly include chloride and fluorides, which were also successfully employed to enhance sorption kinetics of magnesium hydride as well as alanates. With the hope of having similar benefits, several halides were employed as additive with borohydrides [284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303]. It was suggested [284] that transition metal halides reduce the decomposition temperature of LiBH4, however, at the same time the formation of unstable transition metal borohydrides lead to the release of diborane gas due to immediate decomposition, which causes the loss of reversibility. Thus, retaining boron was suggested as a key factor for the reversibility of borohydrides. The addition of TiF3 and ScCl3 to Mg(BH4)2 in a small amount of 5 mol% could significantly improve the desorption rate [286]. It was suggested that the presence of these additives promote the formation of MgB12H12 intermediate during rehydrogenation. Similarly TiF3 was also found to be a promising catalyst for NaBH4 decomposition and rehydrogenation process [288]. The dehydrogenation process was suggested to occur in two steps: (i) NaBH4 partially reacted with TiF3 and forms NaF, TiB2, and B, then (ii) these Ti and F species catalyzed the remaining NaBH4. A partial reversibility could be achieved through the formation of intermediate amorphous Na2B12H12 phase as confirmed by fourier transform infra red (FTIR) spectroscopy. Rare earth fluorides have also shown impressive effects on the reversibility of borohydrides through the formation of metal borides such as PrB4, NdB6 etc. [293,294,295]. Recently ZrCl4 has shown promising effects on the decomposition of NaBH4 and Mg(BH4)2 [300,301]. The activation energy could be lowered down to 180 kJ/mol H2 from 275 kJ/mol H2 through the addition of ZrCl4 to NaBH4. It was suggested that ZrCl4 reduced to ZrCl2 and/or metallic Zr, which acted as catalyst and lowered the dehydrogenation temperature.
In summary, it can be easily seen that the additives can modify the decomposition temperature of borohydrides, however, it is difficult to distinguish the difference between thermodynamic alteration and kinetic modification. Until now, only partial reversibility could be achieved for all the borohydride systems. The key point for the reversibility of borohydride has been decided as “boron retention” during the decomposition rather than the diborane release.
4.3. Catalysts for Amides
The discovery of hydrogen absorption by nitride material in 2002 opened up a new class of hydrogen storage materials, named as amide-imide system [304]. The reaction proceeds through the following two reactions:
Li3N + H2 ↔ Li2NH + LiH
Li2NH + H2 ↔ LiNH2 + LiH
While the reaction (12) proceeds at high temperature because of high enthalpy value (−165 kJ/mol H2), the reaction (13) has a smaller enthalpy of −44 kJ/mol H2 and thus, easily occurs at lower temperatures. So the LiNH2 + LiH system became one of the most studied systems in the last two decades. It was reported that the desorption reaction of LiNH2 + LiH is quite slow even at temperatures higher than 200 °C, thus leading to a search for a catalyst to enhance the sorption rate. TiCl3 was the first catalyst, tested with LiNH2 + LiH system [305,306,307]. It was observed that the activation energy for pristine LiNH2–LiH system was smaller (54 kJ/mol H2) than the TiCl3 doped LiNH2–LiH system (110 kJ/mol H2), however the sorption rate of the TiCl3 doped LiNH2–LiH system was found to be much better. Thus it was concluded that the catalyzed and non-catalyzed systems undergo different rate limiting steps. The positive effect of TiCl3 addition on the sorption kinetics accelerated several studies using different halides for the improvement of the LiNH2–LiH system [308,309,310,311,312,313]. Although the addition of AlCl3 was found effective in enhancing the sorption rate and lowering desorption temperature [308], it was found to occur due to the thermodynamic alteration by the formation of the Li–Al–N–H system. No actual kinetic alteration could be observed in the AlCl3 modified system. A similar effect was observed for MgCl2 addition, where the formation of the Li–Mg–N–H system drastically improved the properties of the Li–N–H system [311]. Recently, the sorption kinetics of the LiNH2–LiH system could be enhanced by the addition of Ce based additives, i.e., CeO2, CeF3, CeF4 [313]. Especially, CeF4 addition showed a significant catalytic effect in reducing the desorption temperature and NH3 suppression by forming CeFx species in-situ. The addition of BN and TiN was also found effective in improving the sorption properties of the Li–N–H system, however they possess different catalytic roles [314]. It was suggested that the catalytic activity originated from the improvement of surface reactivity and diffusion enhancement for TiN and BN respectively. This work also suggested a similar possibility for TiCl3, which can in-situ transform to TiN and follow the same reactivity for the Li–N–H system.
Apart from the LiNH–LiH system, the LiNH2/MgH2 or Mg(NH2)2/LiH system has shown much better properties with much lower enthalpy of 34 kJ/mol H2 with reasonably high hydrogen capacity of 4.5 wt% through following reaction:
2LiNH2 + MgH2 ↔ Li2Mg(NH)2 + 2H2
Several other composition ratios of Mg(NH2)2 and LiH, i.e., 3:8 or 3:12, were also attempted and a higher capacity was observed for these but the desorption temperature also increased at the same time [315,316,317]. The lower enthalpy value of this system suggests near ambient temperature desorption, however, it can occur only at more than 150 °C practically, which must be caused by the kinetic constraints. Thus, in order to improve the sorption kinetics, several additives were attempted with this system [318,319,320,321,322,323,324,325,326]. Shahi et al. [319] studied the effect of various V-based additives and found VCl3 as the best catalyst among them. The kinetics of Mg(NH2)2/2LiH could be enhanced up to 38% followed by 20% and 10% enhancement for V and V2O5 respectively. Hu et al. [321] and Ulmer et al. [322] reported the catalytic effect of ZrCoH3 separately and together with LiBH4. The presence of LiBH4 and ZrCoH3 together showed better catalytic activity, compared to their individual presence. Another high performing catalyst is RbF, the addition of which could significantly enhance the sorption kinetics and reduce the temperature. The Mg(NH2)2–2LiH–0.08 RbF system could store up to 4.76 wt% hydrogen reversibly with an onset temperature of 80 °C and 55 °C for dehydrogenation and rehydrogenation respectively [32]. The addition of carbon has also been found suitable for kinetic enhancement [324,325]. Ru doped SWNT addition to Mg(NH2)2–LiH could effectively suppress the NH3 emission as well as enhance the sorption kinetics. Bill et al. [313] studied calcium halides and suggested that both CaCl2 and CaBr2 reduced the onset temperature by 30 and 45 °C more than the undoped Li–Mg–N–H system. The activation energies were calculated as 91.8 and 78.8 kJ/mol H2 for CaCl2 and CaBr2 doped samples, respectively, in comparison to 104.2 kJ/mol H2 for undoped Li–Mg–N–H system.
Despite several efforts, the dehydrogenation temperature of Li–Mg–N–H was still higher than the theoretical value of 90 °C at 1 bar H2 pressure until the discovery of KH catalyst by Wang et al. in 2009 [326]. The hydrogen desorption peak was shifted to 132 °C with KH doping from 186 °C for undoped Mg(NH2)2/LiH system as shown in Figure 14. The addition of only 3 mol% KH could drastically enhance the kinetics of system and allowed the rehydrogenation by the system at 107 °C. Following the discovery of above enhancement, several attempts were made to understand the mechanism of this improvement and to optimize other parameters [327,328,329,330]. Teng et al. [327] suggested that this improvement can be understood on the basis of NH3 mediated process, where KH reacts with NH3, emitted from Mg(NH2)2, and forms KNH2. This KNH2 immediately reacts with LiH to regenerate KH. These two processes continue as follows and enhance the decomposition of Mg(NH2)2/LiH system via the pseudo-catalytic effect of KH:
Mg(NH2)2 ↔ MgNH + NH3
KH + NH3 ↔ KNH2 + H2
KNH2 + LiH ↔ LiNH2 + KH
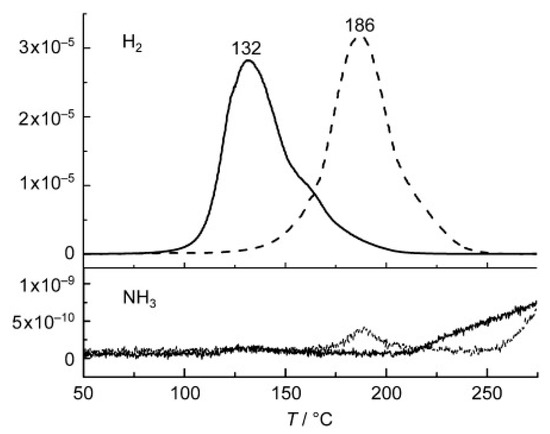
Figure 14.
Temperature dependences of H2 and NH3 release from the potassium-modified (—) and the pristine samples (- - - -). Adapted with the permission from [326], copyright Wiley, 2009.
The activation energy of this reaction was observed to be reduced down to 87 kJ/mol H2 from the 119 kJ/mol H2 for undoped system [328]. Luo et al. [329] has shown through the thermodynamic observation by PCT studies that the ∆H value remained unchanged for the K-doped and undoped Mg(NH2)2/LiH system, which suggested the true catalytic nature of KH instead of thermodynamic alteration. Recently another mechanism has been reported for the abovementioned improvement [330], according to which KH reacts with Mg(NH2)2 and forms stable K2Mg(NH2)4. This kinetically stable K2Mg(NH2)4 phase weakens the N–H bonds and reacts with LiH immediately to produce KH in a metathesis process. These reactions proceed as follows with enhanced kinetics:
2KH + 3Mg(NH2)2 ↔ K2Mg(NH2)4 + 2MgNH + 2H2
2K2Mg(NH2)4 + 3LiH ↔ KLi3(NH2)4 + 2Mg(NH2)4 + 3KH
The above improvement from KH addition ignited the studies using several other hydrides such as RbH, CsH, and CaH2 as catalysts [331,332,333,334,335]. Durojaiye et al. [334] showed the catalytic effect in the order of RbH > KH > CsH > uncatalyzed 2LiNH2/MgH2 system. The higher catalytic role is of Rb, which expands the lattice of Li2Mg(NH)2 by replacing Li by Rb and facilitates the diffusion process. Torre et al. showed the superiority of CaH2 as catalyst, where they found the decomposition started at 78 °C, much lower than 125 °C for pristine Mg(NH2)2 + 2LiH system [335] with a reduced activation energy of 105 kJ/mol H2 in comparison to 133.8 kJ/mol H2 for the undoped Mg(NH2)2/LiH system. Inspired by the catalytic effect of KH, Liu et al. [336] studied the effect of potassium halides on the Mg(NH2)2–2LiH system and suggested that only KF addition was beneficial. The KF added sample showed a desorption onset temperature of 80 °C with a reversible capacity of 5 wt% via two stage reaction. The reaction of KF with LiH, converting into KH and LiF, acts as a catalyst similar to the system with directly KH added [337,338]. In the recent reports [339,340], the addition of KOH was found even better than the KH addition. It was found that KOH reacts with Mg(NH2)2 and LiH to convert into Li2K(NH2)2, KH and MgO, which enhanced the kinetics and started to decompose at 75 °C with a peak appearing at 120 °C.
4.4. Catalysts for Silanides
This is the most recent category of hydrogen storage material, which contains the ternary compound of alkali metal, silicon, and hydrogen. This category attracted the attention of hydrogen community with the detailed investigation of hydrogen absorption properties of KSi by a French group in 2011 [341]. The cubic structured KSi with a space group P43n transforms to KSiH3 upon hydrogen absorption, which is also crystallized in cubic structure with space group Fm3m. The short Si–H bonds (dSi−H = 1.47 Å), similar to that of silane gas (dSi−H = 1.40 Å), makes this compound an interesting candidate for hydrogen storage with a very nice ΔH value of −28 kJ mol−1 H2. Several other zintl phase alloys with other alkali metals (Li, Na, Rb, Cs) were also investigated for their reversible hydrogenation properties [342,343,344,345,346,347,348]. It is very difficult to prepare LiSi single phase by conventional melting method and it needs extremely high temperature (600 °C) and pressure (4 GPa) conditions, however, recently it could be prepared by the ball milling of elemental Li and Si under Ar [343]. Both of the LiSi and NaSi systems undergo disproportionation during the hydrogenation/dehydrogenation reaction, thus losing the reversibility. Thus, the reversibility was observed only in KSiH3, RbSiH3, and CsSiH3 with a total hydrogen capacity of 4.3, 2.6, and 1.85 wt% respectively. In spite of having salient features, it is difficult to use KSiH3 or other similar systems for practical applications due to their extremely slow kinetics. The thermodynamics suggest the possibility of room temperature sorption of KSi/KSiH3 system, however, due to high activation barrier it can absorb hydrogen only at 100 °C at 5 MPa H2 pressure and desorb the hydrogen at around 200 °C. Even at such high temperatures, it needs ~5 h to ab/desorb its total hydrogen content. In the earlier attempts, carbon was added to reduce the activation barrier and enhance the sorption kinetics of KSi/KSiH3 system [341]. Although it enhances the sorption kinetics, it disproportionates the system into KH, Si, and K–Si intermetallic at the same time, which causes the irreversibility. Later, our group investigated the effect of several known catalysts, i.e., TiO2, TiCl3, Nb2O5 [13], and nano metals (Ni, Co, Nb) [349]. We achieved drastic improvement by the addition of 5 mol% of mesoporous Nb2O5, which reduced the activation energy from 142 kJ mol−1 to 63 kJ mol−1 [13]. The XPS study suggested that Nb2O5 was reduced to NbO phase during milling, which acts as a catalyst for the H2 sorption. In addition, it was also pointed out that the heat management during the exothermic reaction of KSi and hydrogen is an important factor and affected the reversibility of above reaction. It was suggested by Chotard et al. [341] that the excess local heat disproportionates the KSiH3 system, which was avoided by allowing hydrogenation of KSi in controlled way. This was achieved by filling H2 at room temperature followed by the increase of temperature up to the desired value [13]. Using a similar methodology, Janot et al. [350] has shown good performance of NbF5 addition with a low value of activation energy, i.e., 61–66 kJ mol−1, in comparison to 121 kJ mol−1 for the non-catalyzed sample [350].
5. Concluding Remark & Future Prospective
We have reviewed the kinetic modifications of hydrogen storage materials. It is clear that there has been tremendous growth in this field in last few decades. While the heavy metals have quite nice thermodynamics as well as kinetics, these have low gravimetric capacities. On the other hand, the light weight hydrides and complex hydrides can deliver high hydrogen amount, but they suffer from poor thermodynamics as well as sluggish kinetics. While the thermodynamic destabilization can be achieved by alloying and/or inserting an intermediate state, the kinetics can be tuned using several approaches such as nano-sizing, use of catalyst etc. The catalytic modification has several advantages over nano-sizing and several catalysts have been developed so far. In particular, Nb2O5 has remained the best catalyst for MgH2 over number of years. Also, the newly developed ZrCl4, TiF4, and some complex oxides have shown similar performance to that of Nb2O5. Ti-based catalysts have shown promising effects for the alanates, whereas no real kinetic alteration could be achieved for borohydrides. The breakthrough discovery of KH as a catalyst for Mg(NH2)2/LiH system opened a new family of catalysts for amide systems and several potassium based catalysts have been explored so far. In summary, a lot of developments have been achieved in the field of catalysts for hydrogen storage materials with a scope of further enhancement, as the activation barrier still remains as a challenge for several potential candidates. The fundamental mechanism of this catalytic modification is also an important aspect, which still needs to be elaborated in order to establish suitable catalysts. As a concluding remark, we can say that the use of catalysts will be an unavoidable part of hydrogen economy establishment.
Author Contributions
A.J. and T.I. prepared the structure of the manuscript. A.J. and Shivani Agarwal collected all the references and contributed to the writing. T.I. contributed to the revision of the review.
Funding
This research received no external funding.
Acknowledgments
Authors are thankful to Bulbul Jha for the English check of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Available online: https://webbook.nist.gov/chemistry/ (accessed on 12 October 2018).
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Tolj, I.; Pickering, L.; Sita, C.; Barbir, F.; Yartys, V. The use of metal hydrides in fuel cell applications. Prog. Nat. Sci. Mater. Int. 2017, 27, 3–20. [Google Scholar] [CrossRef]
- Sreedhar, I.; Kamani, K.M.; Kamani, B.M.; Reddy, B.M.; Venugopal, A. A Bird’s Eye view on process and engineering aspects of hydrogen storage. Renew. Sustain. Energy Rev. 2018, 91, 838–860. [Google Scholar] [CrossRef]
- Rusman, N.A.A.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 12 October 2018).
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High capacity hydrogen storage materials: Attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Jain, A.; Kawasako, E.; Miyaoka, H.; Ma, T.; Isobe, S.; Ichikawa, T.; Kojima, Y. Destabilization of LiH by Li Insertion into Ge. J. Phys. Chem. C 2013, 117, 5650–5657. [Google Scholar] [CrossRef]
- Jain, I.P.; Jain, P.; Jain, A. Novel hydrogen storage materials: A review of lightweight complex hydrides. J. Alloy. Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Jain, A.; Ichikawa, T.; Yamaguchi, S.; Miyaoka, H.; Kojima, Y. Catalytic modification in dehydrogenation properties of KSiH3. Phys. Chem. Chem. Phys. 2014, 16, 26163–26167. [Google Scholar] [CrossRef] [PubMed]
- Klerke, A.; Christensen, C.H.; Norskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Hu, M.G.; Geanangel, R.A.; Wendlandt, W.W. The thermal decomposition of ammonia borane. Thermochim. Acta 1978, 23, 249–255. [Google Scholar] [CrossRef]
- Alhumaidan, F.; Cresswell, D.; Garforth, A. Hydrogen Storage in Liquid Organic Hydride: Producing Hydrogen Catalytically from Methylcyclohexane. Energy Fuels 2011, 25, 4217–4234. [Google Scholar] [CrossRef]
- Selvaraj, S.; Jain, A.; Kumar, S.; Zhang, T.; Isobe, S.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. Study of cyclic performance of V-Ti-Cr alloys employed for hydrogen compressor. Int. J. Hydrogen Energy 2018, 43, 2881–2889. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T. Destabilization of lithium hydride by the substitution of group 14 elements: A review. Int. J. Hydrogen Energy 2016, 41, 5969–5978. [Google Scholar] [CrossRef]
- Pick, M.A. The kinetics of hydrogen absorption-desorption by Metals. In Metal Hydrides; Bambakidis, G., Ed.; NATO Advanced Study Institute Series; Springer: Boston, MA, USA, 1981; Volume 76. [Google Scholar]
- Martin, M.; Gommel, C.; Borkhart, C.; Fromm, E. Absorption and desorption kinetics of hydrogen storage alloys. J. Alloy. Compd. 1996, 238, 193–201. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.J.; Cai, W.T.; Ouyang, L.Z.; Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems—A review of recent progress. J. Alloy. Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Shao, H.; Li, W.; Lin, H. Catalysis and Downsizing in Mg-Based Hydrogen Storage Materials. Catalysts 2018, 8, 89. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Gao, M.; Pan, H. Tailoring Thermodynamics and Kinetics for Hydrogen Storage in Complex Hydrides towards Applications. Chem. Rec. 2016, 16, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Khafidz, N.Z.A.K.; Yaakob, Z.; Lim, K.L.; Timmiati, S.N. The kinetics of lightweight solid-state hydrogen storage materials: A review. Int. J. Hydrogen Energy 2016, 41, 13131–13151. [Google Scholar] [CrossRef]
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Baldé, C.P.; Hereijgers, B.P.C.; Bitter, J.H.; de Jong, K.P. Sodium Alanate Nanoparticles—Linking Size to Hydrogen Storage Properties. J. Am. Chem. Soc. 2008, 130, 6761–6765. [Google Scholar] [CrossRef] [PubMed]
- Aguey-Zinsou, K.-F.; Ares-Fernández, J.-R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Ma, H.; Chen, J. Magnesium Nanowires: Enhanced Kinetics for Hydrogen Absorption and Desorption. J. Am. Chem. Soc. 2007, 129, 6710–6711. [Google Scholar] [CrossRef] [PubMed]
- Mushnikov, N.V.; Ermakov, A.E.; Uimin, M.A.; Gaviko, V.S.; Terent’ev, P.B.; Skripov, A.V.; Tankeev, A.P.; Soloninin, A.V.; Buzlukov, A.L. Kinetics of interaction of Mg-based mechanically activated alloys with hydrogen. Phys. Met. Metall. 2006, 102, 421–431. [Google Scholar] [CrossRef]
- Stampfer, J.F., Jr.; Holley, C.E., Jr.; Suttle, J.F. The Magnesium-Hydrogen System. J. Am. Chem. Soc. 1960, 82, 3504–3508. [Google Scholar] [CrossRef]
- Stander, C.M. Kinetics of decomposition of magnesium hydride. J. Inorg. Nucl. Chem. 1977, 39, 221–223. [Google Scholar] [CrossRef]
- Grant, D. Magnesium Hydride for Hydrogen Storage. In Solid State Hydrogen Storage; Gavin, W., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 357–380. [Google Scholar]
- Schlapbach, L.; Shaltiel, D.; Oelhafen, P. Catalytic effect in the hydrogenation of Mg and Mg compounds: Surface analysis of Mg–Mg2Ni and Mg2Ni. Mater. Res. Bull. 1979, 14, 1235–1246. [Google Scholar] [CrossRef]
- Stioui, M.; Grayevski, A.; Resnik, A.; Shaltiel, D.; Kaplan, N. Macroscopic and microscopic kinetics of hydrogen in magnesium-rich compounds. J. Less-Common Met. 1986, 123, 9–24. [Google Scholar] [CrossRef]
- Krozer, A.; Kasemo, B. Eqilibrium hydrogen uptake and associated kinetics for the Mg–H2 system at low pressures. J. Phys. Condens. Matter 1989, 1, 1533–1538. [Google Scholar] [CrossRef]
- Luz, Z.; Genossar, J.; Rudman, P.S. Identification of the diffusing atom in MgH2. J. Less-Common Met. 1980, 73, 113–118. [Google Scholar] [CrossRef]
- Vigeholm, B.; Kjoller, J.; Larsen, B.; Pedersen, A.S. Formation and decomposition of magnesium hydride. J. Less-Common Met. 1983, 89, 135–144. [Google Scholar] [CrossRef]
- Kecik, D.; Aydinol, M.K. Density functional and dynamics study of the dissociative adsorption of hydrogen on Mg (0001) surface. Surf. Sci. 2009, 603, 304–310. [Google Scholar] [CrossRef]
- Pozzo, M.; Alfè, D. Hydrogen dissociation and diffusion on transition metal (=Ti, Zr, V, Fe, Ru, Co, Rh, Ni, Pd, Cu, Ag)-doped Mg(0001) surfaces. Int. J. Hydrogen Energy 2009, 34, 1922–1930. [Google Scholar] [CrossRef]
- Mamula, B.P.; Novaković, J.G.; Radisavljević, I.; Ivanović, N.; Novaković, N. Electronic structure and charge distribution topology of MgH2 doped with 3d transition metals. Int. J. Hydrogen Energy 2014, 39, 5874–5887. [Google Scholar] [CrossRef]
- German, E.; Gebauer, R. Improvement of Hydrogen Vacancy Diffusion Kinetics in MgH2 by Niobium- and Zirconium-Doping for Hydrogen Storage Applications. J. Phys. Chem. C 2016, 120, 4806–4812. [Google Scholar] [CrossRef]
- Sun, G.; Li, Y.; Zhao, X.; Mi, Y.; Wang, L. First-Principles Investigation of Energetics and Electronic Structures of Ni and Sc Co-Doped MgH2. Am. J. Anal. Chem. 2016, 7, 34–42. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Strom-Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloy. Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Neste, A.V.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–Tm (Tm = Ti, V, Mn, Fe and Ni) systems. J. Alloy. Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Schulz, R. Hydrogen desorption kinetics of a mechanically milled MgH2 + 5at.%V nanocomposite. J. Alloy. Compd. 2000, 305, 239–245. [Google Scholar] [CrossRef]
- Bobet, J.-L.; Akiba, E.; Darriet, B. Study of Mg-M (M = Co, Ni and Fe) mixture elaborated by reactive mechanical alloying: Hydrogen sorption properties. Int. J. Hydrogen Energy 2001, 26, 493–501. [Google Scholar] [CrossRef]
- Xu, X.; Song, C. Improving hydrogen storage/release properties of magnesium with nano-sized metal catalysts as measured by tapered element oscillating microbalance. Appl. Catal. A Gen. 2006, 300, 130–138. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic Effect of Nanoparticle 3d-Transition Metals on Hydrogen Storage Properties in Magnesium Hydride MgH2 Prepared by Mechanical Milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef] [PubMed]
- Denis, A.; Sellier, E.; Aymonier, C.; Bobet, J.-L. Hydrogen sorption properties of magnesium particles decorated with metallic nanoparticles as catalyst. J. Alloy. Compd. 2009, 476, 152–159. [Google Scholar] [CrossRef]
- Yu, H.; Bennici, S.; Auroux, A. Hydrogen storage and release: Kinetic and thermodynamic studies of MgH2 activated by transition metal nanoparticles. Int. J. Hydrogen Energy 2014, 39, 11633–11641. [Google Scholar] [CrossRef]
- Xie, L.; Liu, Y.; Zhang, X.; Qu, J.; Wang, Y.; Li, X. Catalytic effect of Ni nanoparticles on the desorption kinetics of MgH2 nanoparticles. J. Alloy. Compd. 2009, 482, 388–392. [Google Scholar] [CrossRef]
- Zou, J.; Long, S.; Chen, X.; Zeng, X.; Ding, W. Preparation and hydrogen sorption properties of a Ni decorated Mg based Mg@Ni nano-composite. Int. J. Hydrogen Energy 2015, 40, 1820–1828. [Google Scholar] [CrossRef]
- Chen, J.; Xia, G.; Guo, Z.; Huang, Z.; Liu, H.; Yu, X. Porous Ni nanofibers with enhanced catalytic effect on the hydrogen storage performance of MgH2. J. Mater. Chem. A 2015, 3, 15843–15848. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Ali, N.; Aldakheel, F.; Alkandary, A. In-situ catalyzation approach for enhancing the hydrogenation/dehydrogenation kinetics of MgH2 powders with Ni particles. Sci. Rep. 2016, 6, 37335. [Google Scholar] [CrossRef]
- Lu, C.; Zou, J.; Zeng, X.; Ding, W. Hydrogen storage properties of core-shell structured Mg@TM (TM = Co, V) composites. Int. J. Hydrogen Energy 2017, 42, 15246–15255. [Google Scholar] [CrossRef]
- Imamura, H.; Kusuhara, M.; Minami, S.; Matsumoto, M.; Masanari, K.; Sakata, Y.; Keiji, I.; Toshiharu, F. Carbon nanocomposites synthesized by high-energy mechanical milling of graphite and magnesium for hydrogen storage. Acta Mater. 2003, 51, 6407–6414. [Google Scholar] [CrossRef]
- Shang, C.X.; Guo, Z.X. Effect of carbon on hydrogen desorption and absorption of mechanically milled MgH2. J. Power Sources 2004, 129, 73–80. [Google Scholar] [CrossRef]
- Wu, C.Z.; Wang, P.; Yao, X.; Liu, C.; Chen, D.M.; Lu, G.Q.; Cheng, H.M. Effect of carbon/noncarbon addition on hydrogen storage behaviors of magnesium hydride. J. Alloy. Compd. 2006, 414, 259–264. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Guo, Z.X.; Aguey-Zinsou, K.F.; Cazorla-Amorós, D.; Linares-Solano, A. Effects of different carbon materials on MgH2 decomposition. Carbon 2008, 46, 126–137. [Google Scholar] [CrossRef]
- Jia, Y.; Guo, Y.; Zou, J.; Yao, X. Hydrogenation/dehydrogenation in MgH2-activated carbon composites prepared by ball milling. Int. J. Hydrogen Energy 2012, 37, 7579–7585. [Google Scholar] [CrossRef]
- Popilevsky, L.; Skripnyuk, V.M.; Beregovsky, M.; Sezen, M.; Amouyal, Y.; Rabkin, E. Hydrogen storage and thermal transport properties of pelletized porous Mg-2 wt.% multiwall carbon nanotubes and Mg-2 wt.% graphite composites. Int. J. Hydrogen Energy 2016, 41, 14461–14474. [Google Scholar] [CrossRef]
- Liu, W.; Setijadi, E.; Crema, L.; Bartali, R.; Laidani, N.; Aguey-Zinsou, K.F.; Speranza, G. Carbon nanostructures/Mg hybrid materials for hydrogen storage. Diam. Relat. Mater. 2018, 82, 19–24. [Google Scholar] [CrossRef]
- Fuster, V.; Castro, F.J.; Troiani, H.; Urretavizcaya, G. Characterization of graphite catalytic effect in reactively ball-milled MgH2–C and Mg–C composites. Int. J. Hydrogen Energy 2011, 36, 9051–9061. [Google Scholar] [CrossRef]
- Lototskyy, M.; Sibanyoni, J.M.; Denys, R.V.; Williams, M.; Pollet, B.G.; Yartys, V.A. Magnesium–carbon hydrogen storage hybrid materials produced by reactive ball milling in hydrogen. Carbon 2013, 57, 146–160. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Ström-Olsen, J.O. Nanocrystalline metal hydrides. J. Alloy. Compd. 1997, 253–254, 70–79. [Google Scholar] [CrossRef]
- Shang, C.X.; Guo, Z.X. Structural and desorption characterisations of milled (MgH2 + Y,Ce) powder mixtures for hydrogen storage. Int. J. Hydrogen Energy 2007, 32, 2920–2925. [Google Scholar] [CrossRef]
- Zhu, X.; Pei, L.; Zhao, Z.; Liu, B.; Han, S.; Wang, R. The catalysis mechanism of La hydrides on hydrogen storage properties of MgH2 in MgH2 + xwt.% LaH3 (x = 0,10,20, and 30) composites. J. Alloy. Compd. 2013, 577, 64–69. [Google Scholar] [CrossRef]
- Song, J.; Zhao, Z.; Zhao, X.; Fu, R.; Han, S. Hydrogen storage properties of MgH2 co-catalyzed by LaH3 and NbH. Int. J. Miner. Metall. Mater. 2017, 24, 1183–1191. [Google Scholar] [CrossRef]
- Zou, J.; Zeng, X.; Ying, Y.; Chen, X.; Guo, H.; Zhou, S.; Ding, W. Study on the hydrogen storage properties of core–shell structured Mg–RE (RE = Nd, Gd, Er) nano-composites synthesized through arc plasma method. Int. J. Hydrogen Energy 2013, 38, 2337–2346. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J. Alloy. Compd. 2001, 315, 237–242. [Google Scholar] [CrossRef]
- Song, M.Y.; Bobet, J.-L.; Darriet, B. Improvement in hydrogen sorption properties of Mg by reactive mechanical grinding with Cr2O3, Al2O3 and CeO2. J. Alloy. Compd. 2002, 340, 256–262. [Google Scholar] [CrossRef]
- Jung, K.S.; Lee, E.Y.; Lee, K.S. Catalytic effects of metal oxide on hydrogen absorption of magnesium metal hydride. J. Alloy. Compd. 2006, 421, 179–184. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Catalytic Mechanism of Transition-Metal Compounds on Mg Hydrogen Sorption Reaction. J. Phys. Chem. B 2006, 110, 11020–11024. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J. Comparative studies of the influence of different nano-sized metal oxides on the hydrogen sorption properties of magnesium hydride. J. Alloy. Compd. 2009, 486, 697–701. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scr. Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg. J. Alloy. Compd. 2004, 364, 242–246. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Kinetic investigation of the effect of milling time on the hydrogen sorption reaction of magnesium catalyzed with different Nb2O5 contents. J. Alloy. Compd. 2006, 407, 249–255. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of Ni nano-particle and Nb oxide on H-desorption properties in MgH2 prepared by ball milling. J. Alloy. Compd. 2005, 404–406, 716–719. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Hino, S.; Fujii, H. Remarkable improvement of hydrogen sorption kinetics in magnesium catalyzed with Nb2O5. J. Alloy. Compd. 2006, 420, 46–49. [Google Scholar] [CrossRef]
- Kimura, T.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Hydrogen absorption of catalyzed magnesium below room temperature. Int. J. Hydrogen Energy 2013, 38, 13728–13733. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Isobe, S.; Nakagawa, T.; Tokoyoda, K.; Honma, T.; Fujii, H.; Kojima, Y. X-ray Absorption Spectroscopic Study on Valence State and Local Atomic Structure of Transition Metal Oxides Doped in MgH2. J. Phys. Chem. C 2009, 113, 13450–13455. [Google Scholar] [CrossRef]
- Friedrichs, O.; Aguey-Zinsou, F.K.; Fernández, J.R.A.; Sánchez-López, J.C.; Justo, J.; Klasssen, T.; Bormann, R.; Fernández, A. MgH2 with Nb2O5 as additive, for hydrogen storage: Chemical, structural and kinetic behavior with heating. Acta Mater. 2006, 54, 105–110. [Google Scholar] [CrossRef]
- Friedrichs, O.; Klasssen, T.; Sánchez-López, J.C.; Bormann, R.; Fernández, A. Hydrogen sorption improvement of nanocrystalline MgH2 by Nb2O5 nanoparticles. Scr. Mater. 2006, 54, 1293–1297. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Fernandez, J.R.A.; Klassen, T.; Bormann, R. Effect of Nb2O5 on MgH2 properties during mechanical milling. Int. J. Hydrogen Energy 2007, 42, 2400–2407. [Google Scholar] [CrossRef]
- Conceição, M.O.T.; Brum, M.C.; Santos, D.S.; Dias, M.L. Hydrogen sorption enhancement by Nb2O5 and Nb catalysts combined with MgH2. J. Alloy. Compd. 2013, 550, 179–184. [Google Scholar] [CrossRef]
- Ma, T.; Isobe, S.; Wang, Y.; Hashimoto, N.; Ohnuki, S. Nb-Gateway for Hydrogen Desorption in Nb2O5 Catalyzed MgH2Nanocomposite. J. Phys. Chem. C 2013, 117, 10302–10307. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Antunes, I.; Russo, S.L.; Perez, J.; Fagg, D.P. Synthesis of catalytically active rock salt structured MgxNb1−xO nanoparticles for MgH2 system. Int. J. Hydrogen Energy 2014, 39, 18984–18988. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Otero-Irurueta, G.; Pérez, J.; Singh, B.; Fagg, D.P. Crystal structure, phase stoichiometry and chemical environment of MgxNbyOx+y nanoparticles and their impact on hydrogen storage in MgH2. Int. J. Hydrogen Energy 2016, 41, 11709–11715. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Perez, J.; Nasani, N.; Bdikin, I.; Kovalevsky, A.V.; Fagg, D.P. Formation of MgxNbyOx+y through the Mechanochemical Reaction of MgH2 and Nb2O5, and Its Effect on the Hydrogen-Storage Behavior of MgH2. ChemPhysChem 2016, 17, 178–183. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Comparison of the catalytic effects of V, V2O5, VN, and VC on the hydrogen sorption of nanocrystalline Mg. J. Alloy. Compd. 2001, 322, L5–L9. [Google Scholar] [CrossRef]
- Milošević, S.; Rašković-Lovre, Ž.; Kurko, S.; Vujasin, R.; Cvjetićanin, N.; Matović, L.; Novaković, J.G. Influence of VO2 nanostructured ceramics on hydrogen desorption properties from magnesium hydride. Ceram. Int. 2013, 39, 51–56. [Google Scholar] [CrossRef]
- Milošević, S.; Kurko, S.; Pasquini, L.; Matović, L.; Vujasin, R.; Novaković, N.; Novaković, J.G. Fast hydrogen sorption from MgH2–VO2(B) composite materials. J. Power Sources 2016, 307, 481–488. [Google Scholar] [CrossRef]
- Dehouche, Z.; Klassen, T.; Oelerich, W.; Goyette, J.; Bose, T.K.; Schulz, R. Cycling and thermal stability of nanostructured MgH2–Cr2O3 composite for hydrogen storage. J. Alloy. Compd. 2002, 347, 319–323. [Google Scholar] [CrossRef]
- Vijay, R.; Sundaresan, R.; Maiya, M.P.; Murthy, S.S. Hydrogen storage properties of Mg–Cr2O3 nanocomposites: The role of catalyst distribution and grain size. J. Alloy. Compd. 2006, 424, 289–293. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Plocinski, T. The effect of milling conditions on microstructure and hydrogen absorption/desorption properties of magnesium hydride (MgH2) without and with Cr2O3 nanoparticles. Int. J. Hydrogen Energy 2008, 33, 1859–1867. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Varin, R.A.; Plocinski, T.; Pisarek, M. The effect of chromium (III) oxide (Cr2O3) nanopowder on the microstructure and cyclic hydrogen storage behavior of magnesium hydride (MgH2). J. Alloy. Compd. 2011, 509, 2386–2391. [Google Scholar] [CrossRef]
- Wang, P.; Wang, A.M.; Zhang, H.F.; Ding, B.Z.; Hu, Z.Q. Hydrogenation characteristics of Mg–TiO2 (rutile) composite. J. Alloy. Compd. 2000, 313, 218–223. [Google Scholar] [CrossRef]
- Jung, K.S.; Kim, D.H.; Lee, E.Y.; Lee, K.S. Hydrogen sorption of magnesium hydride doped with nano-sized TiO2. Catal. Today 2007, 120, 270–275. [Google Scholar] [CrossRef]
- Jardim, P.M.; Conceição, M.O.T.; Brum, M.C.; dos Santos, D.S. Hydrogen sorption kinetics of ball-milled MgH2–TiO2 based 1D nanomaterials with different morphologies. Int. J. Hydrogen Energy 2015, 40, 17110–17117. [Google Scholar] [CrossRef]
- Chen, B.-H.; Chuang, Y.-S.; Chen, C.-K. Improving the hydrogenation properties of MgH2 at room temperature by doping with nano-size ZrO2 catalyst. J. Alloy. Compd. 2016, 655, 21–27. [Google Scholar] [CrossRef]
- Gupta, R.; Agresti, F.; Russo, S.L.; Maddalena, A.; Palade, P.; Principi, G. Structure and hydrogen storage properties of MgH2 catalysed with La2O3. J. Alloy. Compd. 2008, 450, 310–313. [Google Scholar] [CrossRef]
- Singh, R.K.; Sadhasivam, T.; Sheeja, G.I.; Singh, P.; Srivastava, O.N. Effect of different sized CeO2 nano particles on decomposition and hydrogen absorption kinetics of magnesium hydride. Int. J. Hydrogen Energy 2013, 38, 6221–6225. [Google Scholar] [CrossRef]
- Lin, H.-J.; Tang, J.-J.; Yu, Q.; Wang, H.; Ouyang, L.-Z.; Zhao, Y.-J.; Liu, J.-W.; Wang, W.-H.; Zhu, M. Symbiotic CeH2.73/CeO2 catalyst: A novel hydrogen pump. Nano Energy 2014, 9, 80–87. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismail, M. Hydrogen sorption improvement of MgH2 catalyzed by CeO2 nanopowder. J. Alloy. Compd. 2017, 695, 2532–2538. [Google Scholar] [CrossRef]
- Shan, J.; Li, P.; Wan, Q.; Zhai, F.; Zhang, J.; Li, Z.; Liu, Z.; Volinsky, A.A.; Qu, X. Significantly improved dehydrogenation of ball-milled MgH2 doped with CoFe2O4 nanoparticles. J. Power Sources 2014, 268, 778–786. [Google Scholar] [CrossRef]
- Wan, Q.; Li, P.; Shan, J.; Zhai, F.; Li, Z.; Qu, X. Superior Catalytic Effect of Nickel Ferrite Nanoparticles in Improving Hydrogen Storage Properties of MgH2. J. Phys. Chem. C 2015, 119, 2925–2934. [Google Scholar] [CrossRef]
- Juahir, N.; Mustafa, N.S.; Sinin, A.M.; Ismail, M. Improved hydrogen storage properties of MgH2 by addition of Co2NiO nanoparticles. RSC Adv. 2015, 5, 60983–60989. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Sulaiman, N.N.; Ismail, M. Effect of SrFe12O19 nanopowder on the hydrogen sorption properties of MgH2. RSC Adv. 2016, 6, 110004–110010. [Google Scholar] [CrossRef]
- Zhang, T.; Isobe, S.; Jain, A.; Wang, Y.; Yamaguchi, S.; Miyaoka, H.; Ichikawa, T.; Kojima, Y.; Hashimoto, N. Enhancement of hydrogen desorption kinetics in magnesium hydride by doping with lithium metatitanate. J. Alloy. Compd. 2017, 711, 400–405. [Google Scholar] [CrossRef]
- Idris, N.H.; Mustafa, N.S.; Ismail, M. MnFe2O4 nanopowder synthesised via a simple hydrothermal method for promoting hydrogen sorption from MgH2. Int. J. Hydrogen Energy 2017, 42, 21114–21120. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Fan, X.; Xiao, X.; Zheng, J.; Huang, X. Enhanced hydrogen storage properties of MgH2 with numerous hydrogen diffusion channels provided by Na2Ti3O7 nanotubes. J. Mater. Chem. A 2017, 5, 6178–6185. [Google Scholar] [CrossRef]
- Xu, G.; Shen, N.; Chen, L.; Chen, Y.; Zhang, W. Effect of BiVO4 additive on the hydrogen storage properties of MgH2. Mater. Res. Bull. 2017, 89, 197–203. [Google Scholar] [CrossRef]
- Ares-Fernández, J.-R.; Aguey-Zinsou, K.-F. Superior MgH2 Kinetics with MgO Addition: A Tribological Effect. Catalysts 2012, 2, 330–343. [Google Scholar] [CrossRef]
- Bhat, V.V.; Rougier, A.; Aymard, L.; Darok, X.; Nazri, G.; Tarascon, J.M. Catalytic activity of oxides and halides on hydrogen storage of MgH2. J. Power Sources 2006, 159, 107–110. [Google Scholar] [CrossRef]
- Malka, I.E.; Czujko, T.; Bystrzycki, J. Catalytic effect of halide additives ball milled with magnesium hydride. Int. J. Hydrogen Energy 2010, 35, 1706–1712. [Google Scholar] [CrossRef]
- Malka, I.E.; Pisarek, M.; Czujko, T.; Bystrzycki, J. A study of the ZrF4, NbF5, TaF5, and TiCl3 influences on the MgH2 sorption properties. Int. J. Hydrogen Energy 2011, 36, 12909–12917. [Google Scholar] [CrossRef]
- Jin, S.-A.; Shim, J.-H.; Cho, Y.W.; Yi, K.-W. Dehydrogenation and hydrogenation characteristics of MgH2 with transition metal fluorides. J. Power Sources 2007, 172, 859–862. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, Y.; Erde, W. Hydrogen storage properties of the Mg–Ni–CrCl3 nanocomposite. J. Alloy. Compd. 2002, 333, 207–214. [Google Scholar]
- Mao, J.; Guo, Z.; Yu, X.; Liu, H.; Wu, Z.; Ni, J. Enhanced hydrogen sorption properties of Ni and Co-catalyzed MgH2. Int. J. Hydrogen Energy 2010, 35, 4569–4575. [Google Scholar] [CrossRef]
- Cui, J.; Wang, H.; Liu, J.; Ouyang, L.; Zhang, Q.; Sun, D.; Yao, X.; Zhu, M. Remarkable enhancement in dehydrogenation of MgH2 by a nano-coating of multi-valence Ti-based catalysts. J. Mater. Chem. A 2013, 1, 5603–5611. [Google Scholar] [CrossRef]
- Ismail, M. Influence of different amounts of FeCl3 on decomposition and hydrogen sorption kinetics of MgH2. Int. J. Hydrogen Energy 2014, 39, 2567–2574. [Google Scholar] [CrossRef]
- Ismail, M. Effect of LaCl3 addition on the hydrogen storage properties of MgH2. Energy 2015, 79, 177–182. [Google Scholar] [CrossRef]
- Ismail, M.; Mustafa, N.S.; Juahir, N.; Yap, F.A.H. Catalytic effect of CeCl3 on the hydrogen storage properties of MgH2. Mater. Chem. Phys. 2016, 170, 77–82. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Yamaguchi, S.; Miyaoka, H.; Ichikawa, T.; Mukherjee, A.; Dey, G.K.; Kojima, Y. Surface modification of MgH2 by ZrCl4 to tailor the reversible hydrogen storage performance. Int. J. Hydrogen Energy 2017, 42, 6152–6159. [Google Scholar] [CrossRef]
- Ma, L.-P.; Kang, X.-D.; Dai, H.-B.; Liang, Y.; Fang, Z.-Z.; Wang, P.-J.; Wang, P.; Cheng, H.-M. Superior catalytic effect of TiF3 over TiCl3 in improving the hydrogen sorption kinetics of MgH2: Catalytic role of fluorine anion. Acta Mater. 2009, 57, 2250–2258. [Google Scholar] [CrossRef]
- Ma, L.-P.; Wang, P.; Cheng, H.-M. Hydrogen sorption kinetics of MgH2 catalyzed with titanium compounds. Int. J. Hydrogen Energy 2010, 35, 3046–3050. [Google Scholar] [CrossRef]
- Wang, J.; Du, Y.; Sun, L.; Li, X. Effects of F and Cl on the stability of MgH2. Int. J. Hydrogen Energy 2014, 39, 877–883. [Google Scholar] [CrossRef]
- Jain, P.; Dixit, V.; Jain, A.; Srivastava, O.N.; Huot, J. Effect of Magnesium Fluoride on Hydrogenation Properties of Magnesium Hydride. Energies 2015, 8, 12546–12556. [Google Scholar] [CrossRef]
- Lin, H.-J.; Matsuda, J.; Li, H.-W.; Zhu, M.; Akiba, E. Enhanced hydrogen desorption property of MgH2 with the addition of cerium fluorides. J. Alloy. Compd. 2015, 645, S392–S396. [Google Scholar] [CrossRef]
- Recham, N.; Bhat, V.V.; Kandavel, M.; Aymard, L.; Rougier, A. Reduction of hydrogen desorption temperature of ball-milled MgH2 by NbF5 addition. J. Alloy. Compd. 2008, 464, 377–382. [Google Scholar] [CrossRef]
- Danaie, M.; Mitlin, D. TEM analysis of the microstructure in TiF3-catalyzed and pure MgH2 during the hydrogen storage cycling. Acta Mater. 2012, 60, 6441–6456. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Yamaguchi, S.; Ichikawa, T.; Lal, C.; Jain, I.P. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 14178–14183. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Kumar, S.; Yamaguchi, S.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. How does TiF4 affect the decomposition of MgH2 and its complex variants?—An XPS investigation. J. Mater. Chem. A 2017, 5, 15543–15551. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismail, M. Influence of K2TiF6 additive on the hydrogen sorption properties of MgH2. Int. J. Hydrogen Energy 2014, 39, 15563–15569. [Google Scholar] [CrossRef]
- Yap, F.A.H.; Mustafa, N.S.; Ismail, M. A study on the effects of K2ZrF6 as an additive on the microstructure and hydrogen storage properties of MgH2. RSC Adv. 2015, 5, 9255–9260. [Google Scholar]
- Sulaiman, N.N.; Juahir, N.; Mustafa, N.S.; Yap, F.A.H.; Ismail, M. Improved hydrogen storage properties of MgH2 catalyzed with K2NiF6. J. Energy Chem. 2016, 25, 832–839. [Google Scholar] [CrossRef]
- Sulaiman, N.N.; Mustafa, N.S.; Ismail, M. Effect of Na3FeF6 catalyst on the hydrogen storage properties of MgH2. Dalton Trans. 2016, 45, 7085–7093. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lu, J.; Sohn, H.Y.; Fang, Z.Z. Hydrogen storage properties of the Mg–Ti–H system prepared by high-energy–high-pressure reactive milling. J. Power Sources 2008, 180, 491–497. [Google Scholar] [CrossRef]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y.; Rönnebro, E. Hydrogen Storage Properties of Nanosized MgH2−0.1TiH2 Prepared by Ultrahigh-Energy−High-Pressure Milling. J. Am. Chem. Soc. 2009, 131, 15843–15852. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y.; Rönnebro, E. Hydrogenation of Nanocrystalline Mg at Room Temperature in the Presence of TiH2. J. Am. Chem. Soc. 2010, 132, 6616–6617. [Google Scholar] [CrossRef]
- Sabitu, S.T.; Gallo, G.; Goudy, A.J. Effect of TiH2 and Mg2Ni additives on the hydrogen storage properties of magnesium hydride. J. Alloy. Compd. 2010, 499, 35–38. [Google Scholar] [CrossRef]
- Cuevas, F.; Korablova, D.; Latroche, M. Synthesis, structural and hydrogenation properties of Mg-rich MgH2–TiH2 nanocomposites prepared by reactive ball milling under hydrogen gas. Phys. Chem. Chem. Phys. 2012, 14, 1200–1211. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Agarwal, S.; Zhang, T.; Kumar, S.; Selvaraj, S.; Ichikawa, T.; Jain, I.P. The enhanced de/re-hydrogenation performance of MgH2 with TiH2 additive. Int. J. Energy Res. 2018, 42, 1139–1147. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Johnson, J.K.; Shaz, M.A.; Srivastava, O.N. TiH2 as a Dynamic Additive for Improving the De/Rehydrogenation Properties of MgH2: A Combined Experimental and Theoretical Mechanistic Investigation. J. Phys. Chem. C 2018, 122, 21248–21261. [Google Scholar] [CrossRef]
- Yavari, A.R.; de Castro, J.F.R.; Vaughan, G.; Heunen, G. Structural evolution and metastable phase detection in MgH2–5%NbH nanocomposite during in-situ H-desorption in a synchrotron beam. J. Alloy. Compd. 2003, 353, 246–251. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Liu, Y.; Dong, Z.; Ge, H.; Li, S.; Yan, M. Hydrogen Desorption Properties of the MgH2–AlH3 Composites. J. Phys. Chem. C 2014, 118, 37–45. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismail, M. Enhanced hydrogen storage properties of 4MgH2 + LiAlH4 composite system by doping with Fe2O3 nanopowder. Int. J. Hydrogen Energy 2014, 39, 7834–7841. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Mao, J.F.; Dou, S.X. The hydrogen storage properties and reaction mechanism of the MgH2–NaAlH4 composite system. Int. J. Hydrogen Energy 2011, 36, 9045–9050. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Meethom, S.; Utke, R. Dehydrogenation kinetics, reversibility, and reaction mechanisms of reversible hydrogen storage material based on nanoconfined MgH2−NaAlH4. J. Phys. Chem. Solids 2015, 87, 16–22. [Google Scholar] [CrossRef]
- Johnson, S.R.; Anderson, P.A.; Edwards, P.P.; Gameson, I.; Prendergast, J.W.; Al-Mamouri, M.; Book, D.; Harris, I.R.; Speight, J.D.; Walton, A. Chemical activation of MgH2; a new route to superior hydrogen storage materials. Chem. Commun. 2005, 22, 2823–2825. [Google Scholar] [CrossRef] [PubMed]
- Bösenberg, U.; Doppiu, S.; Mosegaard, L.; Barkhordarian, G.; Eigen, N.; Borgschulte, A.; Torben, R.J.; Yngve, C.; Oliver, G.; Thomas, K.; et al. Hydrogen sorption properties of MgH2–LiBH4 composites. Acta Mater. 2007, 55, 3951–3958. [Google Scholar] [CrossRef]
- Pan, Y.; Leng, H.; Wei, J.; Li, Q. Effect of LiBH4 on hydrogen storage property of MgH2. Int. J. Hydrogen Energy 2013, 38, 10461–10469. [Google Scholar] [CrossRef]
- Czujko, T.; Varin, R.A.; Wronski, Z.; Zaranski, Z.; Durejko, T. Synthesis and hydrogen desorption properties of nanocomposite magnesium hydride with sodium borohydride (MgH2 + NaBH4). J. Alloy. Compd. 2007, 427, 291–299. [Google Scholar] [CrossRef]
- Pan, Y.-B.; Wu, Y.-F.; Li, Q. Modeling and analyzing the hydriding kinetics of Mg–LaNi5 composites by Chou model. Int. J. Hydrogen Energy 2011, 36, 12892–12901. [Google Scholar] [CrossRef]
- Vijay, R.; Sundaresan, R.; Maiya, M.P.; Murthy, S.S.; Fu, Y.; Klein, H.-P.; Groll, M. Characterisation of Mg–x wt.% FeTi (x = 5–30) and Mg–40wt.% FeTiMn hydrogen absorbing materials prepared by mechanical alloying. J. Alloy. Compd. 2004, 384, 283–295. [Google Scholar] [CrossRef]
- Amirkhiz, B.S.; Zahiri, B.; Kalisvaart, P.; Mitlin, D. Synergy of elemental Fe and Ti promoting low temperature hydrogen sorption cycling of magnesium. Int. J. Hydrogen Energy 2011, 36, 6711–6722. [Google Scholar] [CrossRef]
- Yu, X.B.; Yang, Z.X.; Liu, H.K.; Grant, D.M.; Walker, G.S. The effect of a Ti-V-based BCC alloy as a catalyst on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2010, 35, 6338–6344. [Google Scholar] [CrossRef]
- Laversenne, L.; Andrieux, J.; Plante, D.; Lyard, L.; Miraglia, S. In operando study of TiVCr additive in MgH2 composites. Int. J. Hydrogen Energy 2013, 38, 11937–11945. [Google Scholar] [CrossRef]
- Ren, C.; Fang, Z.Z.; Zhou, C.; Lu, J.; Ren, Y.; Zhang, X. Hydrogen Storage Properties of Magnesium Hydride with V-Based Additives. J. Phys. Chem. C 2014, 118, 21778–21784. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Ren, C.; Li, J.; Lu, J. Effect of Ti Intermetallic Catalysts on Hydrogen Storage Properties of Magnesium Hydride. J. Phys. Chem. C 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- Agarwal, S.; Jain, A.; Jain, P.; Jangir, M.; Jain, I.P. Kinetic Enhancement in the Sorption Properties by Forming Mg–x wt % ZrCrCu Composites. J. Phys. Chem. C 2013, 117, 11953–11959. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-H.; Hwang, K.-T.; Kang, Y.-M. Hydrogen storage in magnesium based-composite hydride through hydriding combustion synthesis. Int. J. Hydrogen Energy 2010, 35, 9641–9645. [Google Scholar] [CrossRef]
- Agarwal, S.; Aurora, A.; Jain, A.; Jain, I.P.; Montone, A. Catalytic effect of ZrCrNi alloy on hydriding properties of MgH2. Int. J. Hydrogen Energy 2009, 34, 9157–9162. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.F.; Ding, B.Z.; Hu, Z.Q. Direct hydrogenation of Mg and decomposition behavior of the hydride formed. J. Alloy. Compd. 2000, 313, 209–213. [Google Scholar] [CrossRef]
- Wang, P.; Wang, A.; Zhang, H.; Ding, B.; Hu, Z. Hydriding properties of a mechanically milled Mg–50 wt.% ZrFe1.4Cr0.6 composite. J. Alloy. Compd. 2000, 297, 240–245. [Google Scholar] [CrossRef]
- Agarwal, S.; Aurora, A.; Jain, A.; Montone, A. Structural and H2 sorption properties of MgH2–10 wt%ZrCrM (M = Cu, Ni) nano-composites. J. Nanopart. Res. 2011, 13, 5719–5726. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Jain, P.; Gislon, P.; Prosini, P.P.; Jain, I.P. Hydriding behavior of Mg-50 wt% ZrCrFe composite Prepared by high energy ball milling. Int. J. Hydrogen Energy 2012, 37, 3665–3670. [Google Scholar] [CrossRef]
- Jain, A.; Jain, P.; Agarwal, S.; Gislon, P.; Prosini, P.P.; Jain, I.P. Structural and Hydrogen Storage Properties Of Mg-x Wt% ZrCrMn Composites. Adv. Mater. Lett. 2014, 5, 692–698. [Google Scholar] [CrossRef]
- Agarwal, S.; Jain, A.; Jain, P.; Jangir, M.; Vyas, D.; Jain, I.P. Effect of ZrCrCo alloy on hydrogen storage properties of Mg. J. Alloy. Compd. 2015, 645, S518–S523. [Google Scholar] [CrossRef]
- Molinas, B.; Ghilarducci, A.A.; Melnichuk, M.; Corso, H.L.; Peretti, H.A.; Agresti, F.; Bianchin, A.; Russo, S.L.; Maddalena, A.; Principi, G. Scaled-up production of a promising Mg-based hydride for hydrogen storage. Int. J. Hydrogen Energy 2009, 34, 4597–4601. [Google Scholar] [CrossRef]
- Pighin, S.A.; Capurso, G.; Russo, S.L.; Peretti, H.A. Hydrogen sorption kinetics of magnesium hydride enhanced by the addition of Zr8Ni21 alloy. J. Alloy. Compd. 2012, 530, 111–115. [Google Scholar] [CrossRef]
- Jia, Y.; Han, S.; Zhang, W.; Zhao, X.; Wang, J. Hydrogen absorption and desorption kinetics of MgH2 catalyzed by MoS2 and MoO2. Int. J. Hydrogen Energy 2013, 38, 2352–2356. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, Y.; Han, D.; Han, S. The hydrogen storage properties of MgH2–Fe3S4 composites. Energy 2015, 93, 625–630. [Google Scholar] [CrossRef]
- Xie, X.; Chen, M.; Liu, P.; Shang, J.; Liu, T. High hydrogen desorption properties of Mg-based nanocomposite at moderate temperatures: The effects of multiple catalysts in situ formed by adding nickel sulfides/graphene. J. Power Sources 2017, 371, 112–118. [Google Scholar] [CrossRef]
- Xie, X.; Ma, X.; Liu, P.; Shang, J.; Li, X.; Liu, T. Formation of Multiple-Phase Catalysts for the Hydrogen Storage of Mg Nanoparticles by Adding Flowerlike NiS. ACS Appl. Mater. Interfaces 2017, 9, 5937–5946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, G.; Cheng, Y.; Chen, L.; Huo, Q.; Liu, S. Improved hydrogen storage properties of MgH2 by the addition of FeS2 micro-spheres. Dalton Trans. 2018, 47, 5217–5225. [Google Scholar] [CrossRef] [PubMed]
- Milanese, C.; Garroni, S.; Gennari, F.; Marini, A.; Klassen, T.; Dornheim, M.; Pistidda, C. Solid State Hydrogen Storage in Alanates and Alanate-Based Compounds: A Review. Metals 2018, 8, 567. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Z.; Zhang, X.; Jian, N.; Yang, Y.; Gao, M.; Pan, H. Development of Catalyst-Enhanced Sodium Alanate as an Advanced Hydrogen-Storage Material for Mobile Applications. Energy Technol. 2018, 6, 487–500. [Google Scholar] [CrossRef]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloy. Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Bogdanović, B.; Sandrock, G. Catalyzed Complex Metal Hydrides. MRS Bull. 2002, 27, 712–716. [Google Scholar] [CrossRef]
- Leon, A.; Kircher, O.; Rothe, J.; Fichtner, M. Chemical state and local structure around titanium atoms in NaAlH4 doped with TiCl3 using X-ray absorption spectroscopy. J. Phys. Chem. B 2004, 108, 16372–16376. [Google Scholar] [CrossRef]
- Luo, W.; Gross, K.J. A kinetics model of hydrogen absorption and desorption in Ti-doped NaAlH4. J. Alloy. Compd. 2004, 385, 224–231. [Google Scholar] [CrossRef]
- Gross, K.J.; Majzoub, E.H.; Spangler, S.W. The effects of titanium precursors on hydriding properties of alanates. J. Alloy. Compd. 2003, 356–357, 423–428. [Google Scholar] [CrossRef]
- von Colbe, J.M.B.; Felderhoff, M.; Bogdanovic, B.; Schuth, F.; Weidenthaler, C. One-step direct synthesis of a Ti-doped sodium alanate hydrogen storage material. Chem. Commun. 2005, 37, 4732–4734. [Google Scholar] [CrossRef]
- Onkawa, M.; Zhang, S.; Takeshita, H.T.; Kuriyama, N.; Kiyobayashi, T. Dehydrogenation kinetics of Ti-doped NaAlH4—Influence of Ti precursors and preparation methods. Int. J. Hydrogen Energy 2008, 33, 718–721. [Google Scholar] [CrossRef]
- Paskevicius, M.; Filsø, U.; Karimi, F.; Puszkiel, J.; Pranjas, P.K.; Pistidda, C.; Armin, H.; Edmund, W.; Andreas, S.; Thomas, K.; et al. Cyclic stability and structure of nanoconfined Ti-doped NaAlH4. Int. J. Hydrogen Energy 2016, 41, 4159–4167. [Google Scholar] [CrossRef]
- Resan, M.; Hampton, M.D.; Lomness, J.K.; Slattery, D.K. Effect of TixAly catalysts on hydrogen storage properties of LiAlH4 and NaAlH4. Int. J. Hydrogen Energy 2005, 30, 1417–1421. [Google Scholar] [CrossRef]
- Lee, G.-J.; Kim, J.W.; Shim, J.-H.; Cho, Y.W.; Lee, K.S. Synthesis of ultrafine titanium aluminide powders and their catalytic enhancement in dehydrogenation kinetics of NaAlH4. Scr. Mater. 2007, 56, 125–128. [Google Scholar] [CrossRef]
- Li, L.; Qiu, F.; Wang, Y.; Liu, G.; Yan, C.; An, C.; Xu, Y.; Wang, Y.; Song, D.; Jiao, L.; et al. Improved dehydrogenation performances of TiB2-doped sodium alanate. Mater. Chem. Phys. 2012, 134, 1197–1202. [Google Scholar] [CrossRef]
- Chen, L.-X.; Fan, X.-L.; Xiao, X.-Z.; Xue, J.-W.; Li, S.-Q.; Ge, H.-W.; Chen, C.-P. Influence of TiC catalyst on absorption/desorption behaviors and microstructures of sodium aluminum hydride. Trans. Nonferr. Met. Soc. China 2011, 21, 1297–1302. [Google Scholar] [CrossRef]
- Wu, R.; Du, H.; Wang, Z.; Gao, M.; Pan, H.; Liu, Y. Remarkably improved hydrogen storage properties of NaAlH4 doped with 2D titanium carbide. J. Power Sources 2016, 327, 519–525. [Google Scholar] [CrossRef]
- Xiong, R.; Sang, G.; Zhang, G.; Yan, X.; Li, P.; Yao, Y.; Luo, D.; Chen, C.; Tang, T. Evolution of the active species and catalytic mechanism of Ti doped NaAlH4 for hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 6088–6095. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Cao, Y.; Gao, M.; Pan, H.; Wang, Q. Mechanisms for the enhanced hydrogen desorption performance of the TiF4-catalyzed Na2LiAlH6 used for hydrogen storage. Energy Environ. Sci. 2010, 3, 645–653. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Qiu, F.; Wang, Y.; Xu, Y.; An, C.; Jiao, L.; Yuan, H. Reversible hydrogen storage properties of NaAlH4 enhanced with TiN catalyst. J. Alloy. Compd. 2003, 356–357, 423–428. [Google Scholar] [CrossRef]
- Lee, G.-J.; Shim, J.-H.; Cho, Y.W.; Lee, K.S. Improvement in desorption kinetics of NaAlH4 catalyzed with TiO2 nanopowder. Int. J. Hydrogen Energy 2008, 33, 3748–3753. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Wang, K.; Gao, M.; Pan, H. Remarkably improved hydrogen storage properties of nanocrystalline TiO2-modified NaAlH4 and evolution of Ti-containing species during dehydrogenation/hydrogenation. Nano Res. 2015, 8, 533–545. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Sterlin, M.; Hudson, L.; Sinha, A.S.K.; Srivastava, O.N. Studies on metal oxide nanoparticles catalyzed sodium aluminum hydride. Energy 2010, 35, 5037–5042. [Google Scholar] [CrossRef]
- Wang, P.; Jensen, C.M. Method for preparing Ti-doped NaAlH4 using Ti powder: Observation of an unusual reversible dehydrogenation behavior. J. Alloy. Compd. 2004, 379, 99–102. [Google Scholar] [CrossRef]
- Wang, P.; Jensen, C.M. Preparation of Ti-Doped Sodium Aluminum Hydride from Mechanical Milling of NaH/Al with Off-the-Shelf Ti Powder. J. Phys. Chem. B 2004, 108, 15827–15829. [Google Scholar] [CrossRef]
- Zidan, R.A.; Takara, S.; Hee, A.G.; Jensen, C.M. Hydrogen cycling behavior of zirconium and titanium–zirconium-doped sodium aluminum hydride. J. Alloy. Compd. 1999, 285, 119–122. [Google Scholar] [CrossRef]
- Wang, J.; Ebner, A.D.; Zidan, R.; Ritter, J.A. Synergistic effects of co-dopants on the dehydrogenation kinetics of sodium aluminum hydride. J. Alloy. Compd. 2005, 391, 245–255. [Google Scholar] [CrossRef]
- Bogdanović, B.; Felderhoff, M.; Pommerin, A.; Schüth, F.; Spielkamp, N. Advanced Hydrogen-Storage Materials Based on Sc-, Ce-, and Pr-Doped NaAlH4. Adv. Mater. 2006, 18, 1198–1201. [Google Scholar] [CrossRef]
- Bogdanović, B.; Felderhoff, M.; Pommerin, A.; Schüth, F.; Spielkamp, N.; Stark, A. Cycling properties of Sc- and Ce-doped NaAlH4 hydrogen storage materials prepared by the one-step direct synthesis method. J. Alloy. Compd. 2005, 391, 245–255. [Google Scholar] [CrossRef]
- Rongeat, C.; Scheerbaum, N.; Schultz, L.; Gutfleisch, O. Catalysis of H2 sorption in NaAlH4: General description and new insights. Acta Mater. 2011, 59, 1725–1733. [Google Scholar] [CrossRef]
- Fan, X.; Xiao, X.; Chen, L.; Yu, K.; Wu, Z.; Li, S.; Wang, Q. Active species of CeAl4 in the CeCl3-doped sodium aluminium hydride and its enhancement on reversible hydrogen storage performance. Chem. Commun. 2009, 44, 6857–6859. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xiao, X.; Chen, L.; Li, S.; Ge, H.; Wang, Q. Enhanced Hydriding−Dehydriding Performance of CeAl2-Doped NaAlH4 and the Evolvement of Ce-Containing Species in the Cycling. J. Phys. Chem. C 2011, 115, 2537–2543. [Google Scholar] [CrossRef]
- Fan, X.; Xiao, X.; Chen, L.; Li, S.; Ge, H.; Wang, Q. Thermodynamics, Kinetics, and Modeling Investigation on the Dehydrogenation of CeAl4-Doped NaAlH4 Hydrogen Storage Material. J. Phys. Chem. C 2011, 115, 22680–22687. [Google Scholar] [CrossRef]
- Balema, V.P.; Wiench, J.W.; Dennis, K.W.; Pruski, M.; Pecharsky, V.K. Titanium catalyzed solid-state transformations in LiAlH4 during high-energy ball-milling. J. Alloy. Compd. 2001, 329, 108–114. [Google Scholar] [CrossRef]
- Liu, X.; McGrady, G.S.; Langmi, H.W.; Jensen, C.M. Facile cycling of Ti-doped LiALH4 for high performance hydrogen storage. J. Am. Chem. Soc. 2009, 131, 5032–5033. [Google Scholar] [CrossRef]
- Blanchard, D.; Brinks, H.W.; Hauback, B.C.; Norby, P. Desorption of LiAlH4 with Ti- and V-based additives. Mater. Sci. Eng. B 2004, 108, 54–59. [Google Scholar] [CrossRef]
- Zang, L.; Cai, J.; Zhao, L.; Gao, W.; Liu, J.; Wang, Y. Improved hydrogen storage properties of LiAlH4 by mechanical milling with TiF3. J. Alloy. Compd. 2015, 647, 756–762. [Google Scholar] [CrossRef]
- Resan, M.; Hampton, M.D.; Lomness, J.K.; Slattery, D.K. Effects of various catalysts on hydrogen release and uptake characteristics of LiAlH4. Int. J. Hydrogen Energy 2008, 33, 3748–3753. [Google Scholar]
- Fernandez, J.R.A.; Aguey-Zinsou, F.; Elsaesser, M.; Ma, X.Z.; Bormann, R. Mechanical and thermal decomposition of LiAlH4 with metal halides. Int. J. Hydrogen Energy 2007, 32, 1033–1040. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y.; Matsumoto, M.; Haga, T. Hydrogen release of catalyzed lithium aluminum hydride by a mechanochemical reaction. J. Alloy. Compd. 2008, 462, 275–278. [Google Scholar] [CrossRef]
- Cai, J.; Zang, L.; Zhao, L.; Liu, J.; Wang, Y. Dehydrogenation characteristics of LiAlH4 improved by in-situ formed catalysts. J. Energy Chem. 2016, 25, 868–873. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, X.; Wang, H.; Ouyang, L. Catalytic effect of ScCl3 on the dehydrogenation properties of LiAlH4. J. Alloy. Compd. 2018, 762, 73–79. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Dou, S.X. Effects of NbF5 addition on the hydrogen storage properties of LiAlH4. Int. J. Hydrogen Energy 2010, 35, 2361–2367. [Google Scholar] [CrossRef]
- Sun, T.; Huang, C.K.; Wang, H.; Sun, L.X.; Zhu, M. The effect of doping NiCl2 on the dehydrogenation properties of LiAlH4. Int. J. Hydrogen Energy 2008, 33, 6216–6221. [Google Scholar] [CrossRef]
- Ismail, M.; Sinin, A.M.; Sheng, C.K.; Nik, W.B.W. Desorption Behaviours of Lithium Alanate with Metal Oxide Nanopowder Additives. Int. J. Electrochem. Sci. 2014, 9, 4959–4973. [Google Scholar]
- Qu, X.; Li, P.; Zhang, L.; Ahmad, M. Hydrogen Sorption Improvement of LiAlH4 Catalyzed by Nb2O5 and Cr2O3 Nanoparticles. J. Phys. Chem. C 2011, 115, 13088–13099. [Google Scholar]
- Liu, S.; Ma, Q.; Zheng, X.; Fang, X.; Guo, X.; Zheng, X. Influences of Y2O3 Doping on Hydrogen Release Property of LiAlH4. Rare Metal Mater. Eng. 2014, 43, 0287–0290. [Google Scholar]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Ranjbar, A.; Dou, S.X. Improved hydrogen desorption in lithium alanate by addition of SWCNT–metallic catalyst composite. Int. J. Hydrogen Energy 2011, 36, 3593–3599. [Google Scholar] [CrossRef]
- Hsu, W.-C.; Yang, C.-H.; Tsai, W.-T. Catalytic effect of MWCNTs on the dehydrogenation behavior of LiAlH4. Int. J. Hydrogen Energy 2014, 39, 927–933. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Nanoconfined lithium aluminium hydride (LiAlH4) and hydrogen reversibility. Int. J. Hydrogen Energy 2017, 42, 14144–14153. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, C.; Zhou, H.; Gao, M.; Pan, H.; Wang, Q. A novel catalyst precursor K2TiF6 with remarkable synergetic effects of K, Ti and F together on reversible hydrogen storage of NaAlH4. Chem. Commun. 2011, 47, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, S.; Si, X.; Zhang, J.; Jiao, C.; Wang, S. Significantly improved dehydrogenation of LiAlH4 destabilized by K2TiF6. Int. J. Hydrogen Energy 2012, 37, 3261–3267. [Google Scholar] [CrossRef]
- Wan, Q.; Li, P.; Li, Z.; Zhao, K.; Liu, Z.; Wang, L. NaAlH4 dehydrogenation properties enhanced by MnFe2O4 nanoparticles. J. Power Sources 2014, 248, 388–395. [Google Scholar] [CrossRef]
- Zhai, F.; Li, P.; Sun, A.; Wu, S.; Wan, Q.; Zhang, W.; Li, Y.; Cui, L.; Qu, X. Significantly Improved Dehydrogenation of LiAlH4 Destabilized by MnFe2O4 Nanoparticles. J. Phys. Chem. C 2012, 116, 11939–11945. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Wan, Q.; Zhang, J.; Li, Y.; Li, R.; Dong, X.; Qu, X. Improved dehydrogenation performance of NaAlH4 using NiFe2O4 nanoparticles. J. Alloy. Compd. 2017, 709, 850–856. [Google Scholar] [CrossRef]
- Li, L.; An, C.; Wang, Y.; Xu, Y.; Qiu, F.; Wang, Y.; Jiao, L.; Yuan, H. Enhancement of the H2 desorption properties of LiAlH4 doping with NiCo2O4 nanorods. Int. J. Hydrogen Energy 2014, 39, 4414–4420. [Google Scholar] [CrossRef]
- Zhang, T.; Isobe, S.; Wang, Y.; Oka, H.; Hashimoto, N.; Ohnuki, S. A metal-oxide catalyst enhanced the desorption properties in complex metal hydrides. J. Mater. Chem. A 2014, 2, 4361–4365. [Google Scholar] [CrossRef]
- Frankcombe, T.J. Proposed Mechanisms for the Catalytic Activity of Ti in NaAlH4. Chem. Rev. 2012, 112, 2164–2178. [Google Scholar] [CrossRef]
- Ivanov, E.; Konstanchuk, I.; Stepanov, A.; Boldyrev, V. Magnesium mechanical alloys for hydrogen storage. J. Less Common Met. 1987, 131, 25–29. [Google Scholar] [CrossRef]
- Fichtner, M.; Fuhr, O.; Kircher, O.; Rothe, J. Small Ti clusters for catalysis of hydrogen exchange in NaAlH4. Nanotechnology 2003, 14, 778. [Google Scholar] [CrossRef]
- Fichtner, M.; Engel, J.; Fuhr, O.; Kircher, O.; Rubner, O. Nanocrystalline aluminium hydrides for hydrogen storage. Mater. Sci. Eng. B 2004, 108, 42–47. [Google Scholar] [CrossRef]
- Kircher, O.; Fichtner, M. Hydrogen exchange kinetics in NaAlH4 catalyzed in different decomposition states. J. Appl. Phys. 2014, 95, 7748. [Google Scholar] [CrossRef]
- Sun, D.; Srinivasan, S.S.; Chen, G.; Jensen, C.M. Rehydrogenation and cycling studies of dehydrogenated NaAlH4. J. Alloy. Compd. 2004, 373, 265–269. [Google Scholar] [CrossRef]
- Singh, S.; Eijt, S.W.H.; Huot, J.; Kockelmann, W.A.; Wagemaker, M.; Mulder, F.M. The TiCl3 catalyst in NaAlH4 for hydrogen storage induces grain refinement and impacts on hydrogen vacancy formation. Acta Mater. 2007, 55, 5549–5557. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Xu, Q.; Takeshita, H.T.; Sakai, T. Reversible Hydrogen Storage via Titanium-Catalyzed LiAlH4 and Li3AlH6. J. Phys. Chem. B 2001, 105, 11214–11220. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Takeshita, H.T.; Sakai, T. Nanocrystalline Ti-doped Li3AlH6 as a Reversible Hydrogen Storage Material. Adv. Eng. Mater. 2001, 3, 695–698. [Google Scholar] [CrossRef]
- Sandrock, G.; Gross, K.; Thomas, G. Effect of Ti-catalyst content on the reversible hydrogen storage properties of the sodium alanates. J. Alloy. Compd. 2002, 339, 299–308. [Google Scholar] [CrossRef]
- Wang, P.; Kang, X.-D.; Cheng, H.-M. Exploration of the Nature of Active Ti Species in Metallic Ti-Doped NaAlH4. J. Phys. Chem. B 2005, 109, 20131–20136. [Google Scholar] [CrossRef]
- Blomqvist, A.; Araújo, C.M.; Jena, P.; Ahuja, R. Dehydrogenation from 3d-transition-metal-doped NaAlH4: Prediction of catalysts. Appl. Phys. Lett. 2007, 90, 141904. [Google Scholar] [CrossRef]
- Bai, K.; Wu, P. Role of Ti in the reversible dehydrogenation of Ti-doped sodium alanate. Appl. Phys. Lett. 2006, 89, 201904. [Google Scholar] [CrossRef]
- Araújo, C.M.; Ahuja, R.; Guillén, J.M.O. Role of titanium in hydrogen desorption in crystalline sodium alanate. Appl. Phys. Lett. 2005, 86, 251913. [Google Scholar] [CrossRef]
- Gross, K.J.; Guthrie, S.; Takara, S.; Thomas, G. In-situ X-ray diffraction study of the decomposition of NaAlH4. J. Alloy. Compd. 2000, 297, 270–281. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, J.; Chen, G.; Sun, D.; He, B.; Wei, Z.; Wei, S. Nature and Role of Ti Species in the Hydrogenation of a NaH/Al Mixture. J. Phys. Chem. C 2007, 111, 3476–3479. [Google Scholar]
- Fu, Q.J.; Ramirez-Cuesta, A.J.; Tsang, S.C. Molecular Aluminum Hydrides Identified by Inelastic Neutron Scattering during H2 Regeneration of Catalyst-Doped NaAlH4. J. Phys. Chem. B 2006, 110, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.T.; Scogin, J.H. A reversible hydrogen storage mechanism for sodium alanate: The role of alanes and the catalytic effect of the dopant. J. Alloy. Compd. 2004, 379, 135–142. [Google Scholar] [CrossRef]
- Ivancic, T.M.; Hwang, S.-J.; Bowman, R.C., Jr.; Birkmire, D.S.; Jensen, C.M.; Udovic, T.J.; Conradi, M.S. Discovery of A New Al Species in Hydrogen Reactions of NaAlH4. J. Phys. Chem. Lett. 2010, 1, 2412–2416. [Google Scholar] [CrossRef]
- Gunaydin, H.; Houk, K.N.; Ozoliņš, V. Vacancy-mediated dehydrogenation of sodium alanate. Proc. Natl. Acad. Sci. USA 2008, 105, 3673–3677. [Google Scholar] [CrossRef] [PubMed]
- Borgschulte, A.; Züttel, A.; Hug, P.; Barkhordarian, G.; Eigen, N.; Dornheim, M.; Bormann, R.; Ramirez-Cuesta, A.J. Hydrogen–deuterium exchange experiments to probe the decomposition reaction of sodium alanate. Phys. Chem. Chem. Phys. 2008, 10, 4045–4055. [Google Scholar] [CrossRef] [PubMed]
- Peles, A.; Van de Walle, C.G. Role of charged defects and impurities in kinetics of hydrogen storage materials: A first-principles study. Phys. Rev. B 2007, 76, 214101. [Google Scholar] [CrossRef]
- Marashdeh, A.; Olsen, R.A.; Løvvik, O.M.; Kroes, G.-J. Density Functional Theory Study of the TiH2 Interaction with a NaAlH4 Cluster. J. Phys. Chem. C 2008, 112, 15759–15764. [Google Scholar] [CrossRef]
- Atakli, Z.Ö.K.; Callini, E.; Kato, S.; Mauron, P.; Orimo, S.-I.; Züttel, A. The catalyzed hydrogen sorption mechanism in alkali alanates. Phys. Chem. Chem. Phys. 2015, 17, 20932–20940. [Google Scholar] [CrossRef] [PubMed]
- Ohba, N.; Miwa, K.; Aoki, M.; Noritake, T.; Towata, S.; Nakamori, Y.; Orimo, S.; Züttel, A. First-principles study on the stability of intermediate compounds of LiBH4. Phys. Rev. B 2006, 74, 075110. [Google Scholar] [CrossRef]
- Nakamori, Y.; Li, H.-W.; Kikuchi, K.; Aoki, M.; Miwa, K.; Towata, S.; Orimo, S. Thermodynamical stabilities of metal-borohydrides. J. Alloy. Compd. 2007, 446–447, 296–300. [Google Scholar] [CrossRef]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Martin, D.; Flemming, B.; Torben, R.J. Metal borohydrides and derivatives—Synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.B.; Wu, Z.; Chen, Q.R.; Li, Z.L.; Weng, B.C.; Huang, T.S. Improved hydrogen storage properties of LiBH4 destabilized by carbon. Appl. Phys. Lett. 2007, 90, 034106. [Google Scholar] [CrossRef]
- Jiang, Z.; Yuan, J.; Han, H.; Wu, Y. Effect of carbon nanotubes on the microstructural evolution and hydrogen storage properties of Mg(BH4)2. J. Alloy. Compd. 2018, 743, 11–16. [Google Scholar] [CrossRef]
- Fang, Z.-Z.; Kang, X.-D.; Wang, P. Improved hydrogen storage properties of LiBH4 by mechanical milling with various carbon additives. Int. J. Hydrogen Energy 2010, 35, 8247–8252. [Google Scholar] [CrossRef]
- Xu, J.; Meng, R.; Cao, J.; Gu, X.; Qi, Z.; Wang, W.; Chen, Z. Enhanced dehydrogenation and rehydrogenation properties of LiBH4 catalyzed by graphene. Int. J. Hydrogen Energy 2013, 38, 2796–2803. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, X.; Fan, X.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Fast hydrogen release under moderate conditions from NaBH4 destabilized by fluorographite. RSC Adv. 2014, 4, 2550–2556. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, G.; Chen, L.; Pan, S.; Jing, X.; Wang, J.; Han, S. Enhanced hydrogen storage performances of LiBH4 modified with three-dimensional porous fluorinated graphene. Int. J. Hydrogen Energy 2017, 42, 15262–15270. [Google Scholar] [CrossRef]
- Guo, L.; Li, Y.; Ma, Y.; Liu, Y.; Peng, D.; Zhang, L.; Han, S. Enhanced hydrogen storage capacity and reversibility of LiBH4 encapsulated in carbon nanocages. Int. J. Hydrogen Energy 2017, 42, 2215–2222. [Google Scholar] [CrossRef]
- Xua, J.; Meng, R.; Cao, J.; Gu, X.; Song, W.-L.; Qi, Z.; Wang, W.; Chen, Z. Graphene-supported Pd catalysts for reversible hydrogen storage in LiBH4. J. Alloy. Compd. 2013, 564, 84–90. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, F.; Tang, R.; Wang, Y.; Dong, H.; Li, Z. Hydrogen storage performance of nano Ni decorated LiBH4 on activated carbon prepared through organic solvent. J. Alloy. Compd. 2014, 612, 287–292. [Google Scholar] [CrossRef]
- Wahab, M.A.; Young, D.J.; Karim, A.; Fawzia, S.; Beltramini, J.N. Low-temperature hydrogen desorption from Mg(BH4)2 catalysed by ultrafine Ni nanoparticles in a mesoporous carbon matrix. Int. J. Hydrogen Energy 2016, 41, 20573–20582. [Google Scholar] [CrossRef]
- Xia, G.L.; Guo, Y.H.; Wu, Z.; Yu, X.B. Enhanced hydrogen storage performance of LiBH4–Ni composite. J. Alloy. Compd. 2009, 479, 545–548. [Google Scholar] [CrossRef]
- Au, M.; Jurgensen, A.; Zeigler, K. Modified Lithium Borohydrides for Reversible Hydrogen Storage (2). J. Phys. Chem. B 2006, 110, 26482–26487. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sudik, A.; Wolverton, C. Destabilizing LiBH4 with a Metal (M = Mg, Al, Ti, V, Cr, or Sc) or Metal Hydride (MH2 = MgH2, TiH2, or CaH2). J. Phys. Chem. C 2007, 111, 19134–19140. [Google Scholar] [CrossRef]
- Pendolino, F.; Mauron, P.; Borgschulte, A.; Züttel, A. Effect of Boron on the Activation Energy of the Decomposition of LiBH4. J. Phys. Chem. C 2009, 113, 17231–17234. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloy. Compd. 2003, 356–357, 515–520. [Google Scholar]
- Au, M.; Jurgensen, A. Modified Lithium Borohydrides for Reversible Hydrogen Storage. J. Phys. Chem. B 2006, 110, 7062–7067. [Google Scholar] [CrossRef]
- Yu, X.B.; Grant, D.M.; Walker, G.S. Low-Temperature Dehydrogenation of LiBH4 through Destabilization with TiO2. J. Phys. Chem. C 2008, 112, 11059–11062. [Google Scholar] [CrossRef]
- Au, M.; Spencer, W.; Jurgensen, A.; Zeigler, C. Hydrogen storage properties of modified lithium borohydrides. J. Alloy. Compd. 2008, 462, 303–309. [Google Scholar] [CrossRef]
- Mosegaard, L.; Møller, B.; Jørgensen, J.-E.; Filinchuk, Y.; Cerenius, Y.; Hanson, J.C.; Dimasi, E.; Besenbacher, F.; Jensen, T.R. Reactivity of LiBH4: In Situ Synchrotron Radiation Powder X-ray Diffraction Study. J. Phys. Chem. C 2008, 112, 1299–1303. [Google Scholar] [CrossRef]
- Yu, X.B.; Grant, D.M.; Walker, G.S. Dehydrogenation of LiBH4 Destabilized with Various Oxides. J. Phys. Chem. C 2009, 113, 17945–17949. [Google Scholar] [CrossRef]
- Ploysuksai, W.; Rangsunvigit, P.; Kulprathipanja, S. Effects of TiO2 and Nb2O5 on Hydrogen desorption of Mg(BH4)2. Int. J. Mater. Metall. Eng. 2012, 6, 311–315. [Google Scholar]
- Zavorotynska, O.; Saldan, I.; Hino, S.; Humphries, T.D.; Deledda, S.; Hauback, B.C. Hydrogen cycling in γ-Mg(BH4)2 with cobalt-based additives. J. Mater. Chem. A 2015, 3, 6592–6602. [Google Scholar] [CrossRef]
- Saldan, I.; Frommen, C.; Llamas-Jansa, I.; Kalantzopoulos, G.N.; Hino, S.; Arstad, B.; Heyn, R.H.; Zavorotynska, O.; Deledda, S.; Sørby, M.H. Hydrogen storage properties of γ–Mg(BH4)2 modified by MoO3 and TiO2. Int. J. Hydrogen Energy 2015, 40, 12286–12293. [Google Scholar] [CrossRef]
- Xu, X.; Zang, L.; Zhao, Y.; Zhao, Y.; Wang, Y.; Jiao, L. Hydrogen storage behavior of LiBH4 improved by the confinement of hierarchical porous ZnO/ZnCo2O4 nanoparticles. J. Power Sources 2017, 359, 134–141. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Liu, Y.; Wang, Y.; Yuan, H.; Jiao, L. Synergistic effects of destabilization, catalysis and nanoconfinement on dehydrogenation of LiBH4. Int. J. Hydrogen Energy 2017, 42, 1354–1360. [Google Scholar] [CrossRef]
- Au, M.; Walters, R.T. Reversibility aspect of lithium borohydrides. Int. J. Hydrogen Energy 2010, 35, 10311–10316. [Google Scholar] [CrossRef]
- Zhang, B.J.; Liu, B.H. Hydrogen desorption from LiBH4 destabilized by chlorides of transition metal Fe, Co, and Ni. Int. J. Hydrogen Energy 2010, 35, 7288–7294. [Google Scholar] [CrossRef]
- Newhouse, R.J.; Stavila, V.; Hwang, S.-J.; Klebanoff, L.E.; Zhang, J.Z. Reversibility and Improved Hydrogen Release of Magnesium Borohydride. J. Phys. Chem. C 2010, 114, 5224–5232. [Google Scholar] [CrossRef]
- Bardají, E.G.; Hanada, N.; Zabara, O.; Fichtner, M. Effect of several metal chlorides on the thermal decomposition behaviour of α-Mg(BH4)2. Int. J. Hydrogen Energy 2011, 36, 12313–12318. [Google Scholar] [CrossRef]
- Mao, J.; Guo, Z.; Nevirkovets, I.P.; Liu, H.K.; Dou, S.X. Hydrogen De-/Absorption Improvement of NaBH4 Catalyzed by Titanium-Based Additives. J. Phys. Chem. C 2012, 116, 1596–1604. [Google Scholar] [CrossRef]
- Al-Kukhun, A.; Hwang, H.T.; Varma, A. NbF5 additive improves hydrogen release from magnesium borohydride. Int. J. Hydrogen Energy 2012, 37, 17671–17677. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Wang, H.; Liu, J.W.; Zhu, M. Thermal decomposition behaviors of magnesium borohydride doped with metal fluoride additives. Thermochim. Acta 2013, 560, 82–88. [Google Scholar] [CrossRef]
- Paduani, C.; Jena, P. Role of Ti-based catalysts in the dehydrogenation mechanism of magnesium borohydride: A cluster approach. Int. J. Hydrogen Energy 2013, 38, 2357–2362. [Google Scholar] [CrossRef]
- Humphries, T.D.; Kalantzopoulos, G.N.; Llamas-Jansa, I.; Olsen, J.E.; Hauback, B.C. Reversible Hydrogenation Studies of NaBH4 Milled with Ni-Containing Additives. J. Phys. Chem. C 2013, 117, 6060–6065. [Google Scholar] [CrossRef]
- Chong, L.; Zou, J.; Zeng, X.; Ding, W. Mechanisms of reversible hydrogen storage in NaBH4 through NdF3 addition. J. Mater. Chem. A 2013, 1, 3983–3991. [Google Scholar] [CrossRef]
- Chong, L.; Zou, J.; Zeng, X.; Ding, W. Reversible hydrogen sorption in NaBH4 at lower temperatures. J. Mater. Chem. A 2013, 1, 13510–13523. [Google Scholar] [CrossRef]
- Zou, J.; Li, L.; Zeng, X.; Ding, W. Reversible hydrogen storage in a 3NaBH4/YF3 composite. Int. J. Hydrogen Energy 2012, 37, 17118–17125. [Google Scholar] [CrossRef]
- Saldan, I.; Hino, S.; Humphries, T.D.; Zavorotynska, O.; Chong, M.; Jensen, C.M.; Deledda, S.; Hauback, B.C. Structural Changes Observed during the Reversible Hydrogenation of Mg(BH4)2 with Ni-Based Additives. J. Phys. Chem. C 2014, 118, 23376–23384. [Google Scholar] [CrossRef]
- Chong, L.; Zou, J.; Zeng, X.; Ding, W. Study on reversible hydrogen sorption behaviors of a 3NaBH4/HoF3 composite. Int. J. Hydrogen Energy 2014, 39, 14275–14281. [Google Scholar] [CrossRef]
- Tu, G.; Xiao, X.; Jiang, Y.; Qin, T.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Composite cooperative enhancement on the hydrogen desorption kinetics of LiBH4 by co-doping with NbCl5 and hexagonal BN. Int. J. Hydrogen Energy 2015, 33, 10527–10535. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, L.; Gao, S.; Liu, H.; Xu, L.; Wang, X.; Yan, M. Hydrogen storage properties of activated carbon confined LiBH4 doped with CeF3 as catalyst. Int. J. Hydrogen Energy 2017, 42, 23010–23017. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Nakajima, K.; Jain, A.; Miyaoka, H.; Ichikawa, T.; Dey, G.K.; Kojima, Y. Improved hydrogen release from magnesium borohydride by ZrCl4 additive. Int. J. Hydrogen Energy 2017, 42, 22342–22347. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Study on the thermal decomposition of NaBH4 catalyzed by ZrCl4. Int. J. Hydrogen Energy 2017, 42, 22432–22437. [Google Scholar] [CrossRef]
- Zhao, S.X.; Wang, C.Y.; Liu, D.M.; Tan, Q.J.; Li, Y.T.; Si, T.Z. Destabilization of LiBH4 by SrF2 for reversible hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 5098–5103. [Google Scholar] [CrossRef]
- Zhao, N.; Zou, J.; Zeng, X.; Ding, W. Mechanisms of partial hydrogen sorption reversibility in a 3NaBH4/ScF3 composite. RSC Adv. 2018, 8, 9211–9217. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Isobe, S.; Hanada, N.; Fujii, H. Lithium nitride for reversible hydrogen storage. J. Alloy. Compd. 2004, 365, 271–276. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hanada, N.; Isobe, S.; Leng, H.Y.; Fujii, H. Hydrogen storage properties in Ti catalyzed Li–N–H system. J. Alloy. Compd. 2005, 404–406, 435–438. [Google Scholar] [CrossRef]
- Matsumoto, M.; Haga, T.; Kawai, Y.; Kojima, Y. Hydrogen desorption reactions of Li–N–H hydrogen storage system: Estimation of activation free energy. J. Alloy. Compd. 2007, 439, 358–362. [Google Scholar] [CrossRef]
- Albanesi, L.F.; Larochette, P.A.; Gennari, F.C. Destabilization of the LiNH2–LiH hydrogen storage system by aluminum incorporation. Int. J. Hydrogen Energy 2013, 38, 12325–12334. [Google Scholar] [CrossRef]
- Albanesia, L.F.; Garroni, S.; Larochette, P.A.; Nolis, P.; Mulas, G.; Enzo, S.; Baró, M.D.; Gennari, F.C. Role of aluminum chloride on the reversible hydrogen storage properties of the Li–N–H system. Int. J. Hydrogen Energy 2015, 39, 13506–13517. [Google Scholar] [CrossRef]
- Lin, H.-J.; Li, H.-W.; Murakami, H.; Akiba, E. Remarkably improved hydrogen storage properties of LiNH2-LiH composite via the addition of CeF4. J. Alloy. Compd. 2018, 735, 1017–1022. [Google Scholar] [CrossRef]
- Leng, H.; Wu, Z.; Duan, W.; Xia, G.; Li, Z. Effect of MgCl2 additives on the H-desorption properties of Li–N–H system. Int. J. Hydrogen Energy 2012, 37, 903–907. [Google Scholar] [CrossRef]
- Price, C.; Gray, J.; Lascola, R.; Anton, D.L. The effects of halide modifiers on the sorption kinetics of the Li-Mg-N-H System. Int. J. Hydrogen Energy 2012, 37, 2742–2749. [Google Scholar] [CrossRef]
- Bill, R.F.; Reed, D.; Book, D.; Anderson, P.A. Effect of the calcium halides, CaCl2 and CaBr2, on hydrogen desorption in the Li–Mg–N–H system. J. Alloy. Compd. 2015, 645, S96–S99. [Google Scholar] [CrossRef]
- Nayebossadri, S.; Aguey-Zinsou, K.F.; Guo, Z.X. Effect of nitride additives on Li–N–H hydrogen storage system. Int. J. Hydrogen Energy 2011, 36, 7920–7926. [Google Scholar] [CrossRef]
- Xiong, Z.; Wu, G.; Hu, J.; Chen, P. Ternary Imides for Hydrogen Storage. Adv. Mater. 2004, 16, 1522–1525. [Google Scholar] [CrossRef]
- Leng, H.Y.; Ichikawa, T.; Hino, S.; Hanada, N.; Isobe, S.; Fujii, H. New Metal−N−H System Composed of Mg(NH2)2 and LiH for Hydrogen Storage. J. Phys. Chem. B 2004, 108, 8763–8765. [Google Scholar] [CrossRef]
- Nakamori, Y.; Kitahara, G.; Orimo, S. Synthesis and dehydriding studies of Mg–N–H systems. J. Power Sources 2004, 138, 309–312. [Google Scholar] [CrossRef]
- Ma, L.-P.; Dai, H.-B.; Liang, Y.; Kang, X.-D.; Fang, Z.-Z.; Wang, P.-J.; Wang, P.; Cheng, H.-M. Catalytically Enhanced Hydrogen Storage Properties of Mg(NH2)2 + 2LiH Material by Graphite-Supported Ru Nanoparticles. J. Phys. Chem. C 2008, 112, 18280–18285. [Google Scholar] [CrossRef]
- Shahi, R.R.; Yadav, T.P.; Shaz, M.A.; Srivastva, O.N. Studies on dehydrogenation characteristic of Mg(NH2)2/LiH mixture admixed with vanadium and vanadium based catalysts (V, V2O5 and VCl3). Int. J. Hydrogen Energy 2010, 35, 238–246. [Google Scholar] [CrossRef]
- Anton, D.L.; Price, C.J.; Gray, J. Affects of Mechanical Milling and Metal Oxide Additives on Sorption Kinetics of 1:1 LiNH2/MgH2 Mixture. Energies 2011, 4, 826–844. [Google Scholar] [CrossRef]
- Hu, J.; Poh, A.; Wang, S.; Rothe, J.; Fichtner, M. Additive Effects of LiBH4 and ZrCoH3 on the Hydrogen Sorption of the Li-Mg-N-H Hydrogen Storage System. J. Phys. Chem. C 2012, 116, 20246–20253. [Google Scholar] [CrossRef]
- Ulmer, U.; Hu, J.; Franzreb, M.; Fichtner, M. Preparation, scale-up and testing of nanoscale, doped amide systems for hydrogen storage. Int. J. Hydrogen Energy 2013, 38, 1439–1449. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Gu, Y.; Gao, M.; Pan, H. Improved Hydrogen-Storage Thermodynamics and Kinetics for an RbF-Doped Mg(NH2)2–2 LiH System. Chem. Asian J. 2013, 8, 2136–2143. [Google Scholar] [CrossRef]
- Demirocak, D.E.; Srinivasan, S.S.; Ram, M.K.; Kuhn, J.N.; Muralidharan, R.; Li, X.; Goswami, D.Y.; Stefanakos, E.K. Reversible hydrogen storage in the Li–Mg–N–H system—The effects of Ru doped single walled carbon nanotubes on NH3 emission and kinetics. Int. J. Hydrogen Energy 2013, 38, 10039–10049. [Google Scholar] [CrossRef]
- Yan, M.-y.; Sun, F.; Liu, X.-p.; Ye, J.-h. Effects of compaction pressure and graphite content on hydrogen storage properties of Mg(NH2)2–2LiH hydride. Int. J. Hydrogen Energy 2014, 39, 19656–19661. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Wu, G.; Li, W.; Liu, Y.; Araújo, C.M. Potassium—Modified Mg(NH2)2/2 LiH System for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 5828–5832. [Google Scholar] [CrossRef]
- Teng, Y.-L.; Ichikawa, T.; Miyaoka, H.; Kojima, Y. Improvement of hydrogen desorption kinetics in the LiH–NH3 system by addition of KH. Chem. Commun. 2011, 47, 12227–12229. [Google Scholar] [CrossRef] [PubMed]
- Durojaiye, T.; Goudy, A. Desorption kinetics of lithium amide/magnesium hydride systems at constant pressure thermodynamic driving forces. Int. J. Hydrogen Energy 2012, 37, 3298–3304. [Google Scholar] [CrossRef]
- Luo, W.; Stavila, V.; Klebanoff, L.E. New insights into the mechanism of activation and hydrogen absorption of (2LiNH2–MgH2). Int. J. Hydrogen Energy 2012, 37, 6646–6652. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Pan, H.; Xiong, Z.; Gao, M.; Wu, G.; Liang, C.; Li, C.; Li, B.; Wang, J. Solid–Solid Heterogeneous Catalysis: The Role of Potassium in Promoting the Dehydrogenation of the Mg(NH2)2/2 LiH Composite. ChemSusChem 2013, 6, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Ma, R.; Zhang, X.; Li, Y.; Gao, M.; Pan, H. Superior Dehydrogenation/Hydrogenation Kinetics and Long-Term Cycling Performance of K and Rb Cocatalyzed Mg(NH2)2-2LiH system. ACS Appl. Mater. Interfaces 2014, 6, 17024–17033. [Google Scholar] [CrossRef]
- Durojaiye, T.; Hayes, J.; Goudy, A. Rubidium Hydride: An Exceptional Dehydrogenation Catalyst for the Lithium Amide/Magnesium Hydride System. J. Phys. Chem. C 2013, 117, 6554–6560. [Google Scholar] [CrossRef]
- Hayes, J.; Durojaiye, T.; Goudy, A. Hydriding and dehydriding kinetics of RbH-doped 2LiNH2/MgH2 hydrogen storage system. J. Alloy. Compd. 2015, 645, S496–S499. [Google Scholar] [CrossRef]
- Durojaiye, T.; Hayes, J.; Goudy, A. Potassium, rubidium and cesium hydrides as dehydrogenation catalysts for the lithium amide/magnesium hydride system. Int. J. Hydrogen Energy 2015, 40, 2266–2273. [Google Scholar] [CrossRef]
- Torre, F.; Valentoni, A.; Milanese, C.; Pistidda, C.; Marini, A.; Dornheim, M. Kinetic improvement on the CaH2-catalyzed Mg(NH2)2 + 2LiH system. J. Alloy. Compd. 2015, 645, S284–S287. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Li, B.; Gao, M.; Pan, H. Metathesis Reaction-Induced Significant Improvement in Hydrogen Storage Properties of the KF-Added Mg(NH2)2–2LiH System. J. Phys. Chem. C 2013, 117, 866–875. [Google Scholar] [CrossRef]
- Sun, F.; Yan, M.; Ye, J.; Liu, X.; Jiang, L. Effect of CO on hydrogen storage performance of KF doped 2LiNH2 + MgH2 material. J. Alloy. Compd. 2014, 616, 47–50. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Yang, Y.; Gao, M.; Pan, H. High-temperature failure behaviour and mechanism of K-based additives in Li–Mg–N–H hydrogen storage systems. J. Mater. Chem. A 2014, 2, 7345–7353. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Gao, M.; Pan, H. Understanding the role of K in the significantly improved hydrogen storage properties of a KOH-doped Li–Mg–N–H system. J. Mater. Chem. A 2013, 1, 5031–5036. [Google Scholar]
- Liu, Y.; Yang, Y.; Zhang, X.; Li, Y.; Gao, M.; Pan, H. Insights into the dehydrogenation reaction process of a K-containing Mg(NH2)2–2LiH system. Dalton Trans. 2015, 44, 18012–18018. [Google Scholar] [CrossRef]
- Chotard, J.-N.; Tang, W.S.; Raybaud, P.; Janot, R. Potassium Silanide (KSiH3): A Reversible Hydrogen Storage Material. Chem. Eur. J. 2011, 17, 12302–12309. [Google Scholar] [CrossRef]
- Tang, W.S.; Chotard, J.-N.; Raybaud, P.; Janot, R. Hydrogenation properties of KSi and NaSi Zintl phases. Phys. Chem. Chem. Phys. 2012, 14, 13319–13324. [Google Scholar] [CrossRef]
- Tang, W.S.; Chotard, J.-N.; Janot, R. Syntheis of single phase LiSi by ball milling: Electrochemical behavior and hydrogenation properties. J. Electrochem. Soc. 2013, 160, A1232–1240. [Google Scholar] [CrossRef]
- Tang, W.S.; Chotard, J.-N.; Raybaud, P.; Janot, R. Enthalpy-entropy compensation effect in hydrogen storage materials: Striking example of alkali silanides MSiH3 (M = K, Rb, Cs). J. Phys. Chem. C 2014, 118, 3409–3419. [Google Scholar] [CrossRef]
- Kranak, V.F.; Lin, Y.C.; Karlsson, M.; Mink, J.; Norberg, S.T.; Haussermann, U. Structural and vibrational properties of Silyl (SiH3−) anion in KSiH3 and RbSiH3: New insight into Si-H interactions. Inorg. Chem. 2015, 54, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.S.; Dimitrievska, M.; Chotard, J.-N.; Zhou, W.; Janot, R.; Skripov, A.V.; Udovic, T.J. Structural and dynamical trends in alkali-metal silanides characterized by neutron-scattering methods. J. Phys. Chem. C 2016, 120, 21218–21227. [Google Scholar] [CrossRef]
- Auer, H.; Kohlmann, H. In situ investigations on the formation and decomposition of KSiH3 and CsSiH3. Z. Anorg. Allg. Chem. 2017, 643, 945–951. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Qu, H.; Yu, X.F.; Peng, P. Alkali metal silanides α-MSiH3: A family of complex hydrides for solid-state hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 12405–12413. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Tailoring the absorption-desorption properties of KSiH3 compound using nano-metals (Ni, Co, Nb) as catalyst. J. Alloy. Compd. 2015, 645, 1441–1447. [Google Scholar] [CrossRef]
- Janot, R.; Tang, W.S.; Clemencon, D.; Chotard, J.-N. Catalyzed KSiH3 as a reversible hydrogen storage material. J. Mater. Chem. A 2016, 4, 19045–19052. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).