Abstract
Bromate () residue in drinking water poses a great health risk. Ultra-fast reduction of , under aerobic conditions, was realized using an ultraviolet (UV)/sulfite process in the presence of iodide (UV/sulfite/iodide). The UV/sulfite/iodide process produced removal efficiency of 100% at about 5 min with complete conversion to bromide, while UV/sulfite induced 13.1% reduction under the same conditions. Hydrated electrons, generated from the photolysis of sulfite and iodide, was confirmed as the main contributor to degradation (77.4% of the total contribution). As the concentration of iodide was kept constant, its presence remarkably enhancing the generation of hydrated electrons led to its consideration as a homogeneous catalyst in the UV/sulfite/iodide system. Sulfite played a role not only as a hydrated electron precursor, but also as a reactive iodine species shielding agent and a regenerant of iodide. Results surrounding the effects on common water quality parameters (pH, bicarbonate, nitrate, natural organic matter, and solution temperature) indicated that preferred degradation of occurred in an environment of alkaline pH, low-content natural organic matter/bicarbonate/nitrate, and high natural temperature.
1. Introduction
Bromate () is considered as a carcinogen, and is produced during bromide-containing water treatment processes including chlorination, ozonation, and advanced oxidation [1]. It is of great importance to remove the formed in drinking water due to its carcinogenic risk. Notably, once is generated in the disinfection unit of a drinking water plant, there are limited steps to prevent the occurrence of in tap water. One solution is to install household water purifiers to remove . The small site occupancy for household water purifiers means that the technologies adopted must remove within a short contact time and at high operational cost. The methods of degrading from drinking water include activated carbon or resin adsorption [2], electron beam irradiation [3], membrane separation [4], biological reduction [5], zero-valent iron (ZVI) reduction [6], photo [7] or photocatalytic reduction [8], layered double hydroxide reduction [9], advanced reduction processes (ARPs) [10], and so on. Among these technologies, hydrated electron ()-based ARPs distinguished themselves in degradation due to the high rate constants between and (~109 M−1·s−1) [10,11]; thus, they show potential for their use in household drinking water purifiers, which are characterized by a small hydraulic retention time.
Hydrated electron is one of the most reducing agents (−2.9 V) [12], and it can be generated from the ionizing irradiation of electron beam [13], ultraviolet (UV) activation of sulfite/iodide ()/nitrilotriacetic acid [14,15], vacuum-UV irradiation [7], and photoexcitation of organic chromophores [16]. From a practical perspective, the key for—based ARPs is generating efficiently under ambient conditions. Hydrated electron generation by UV activation of sulfite (UV/sulfite) is promising due to its oxygen self-cleaning property. However, generation by UV/sulfite was dependent on a high concentration of sulfite and high pH due to the relatively low absorptivity and the protonation of sulfite. Recently, Li etc. [17] proposed a UV/sulfite/ process to generate , and successfully realized efficient degradation of monochloroacetic acid (12.86 μM·min−1). Enhanced production of through such a process was based on two facts: (1) both sulfite and act as precursors; (2) sulfite can reduce the scavenging by reactive iodine species (RIS, e.g., , , and ). Given the similar rate constant of toward monochloroacetic acid (1.0 × 109 M−1·s−1 [18]) and (3.4 × 109 M−1·s−1 [10]), it is reasonable to speculate that the UV/sulfite/ process can also degrade with high efficiency. Yet, to the authors’ knowledge, there exist no other reports using this process to degrade under aerobic conditions. Furthermore, the degradation performance using the UV/sulfite/ process under aerobic conditions, and the possible factors that may affect the degradation efficiency (i.e., pH, anions, and natural organic matter (NOM)) are yet to be investigated.
In this work, we aimed to (1) investigate the reduction efficiency of in prepared water and real water in the UV/sulfite/ process; (2) explore the mechanism of reduction (role of sulfite/ and contributors to reduction); (3) identify the transformation product of ; and (4) evaluate the influence of common water quality parameters (solution temperature, pH, NOM, bicarbonate, and nitrate).
2. Results and Discussion
2.1. Reduction Efficiency of Using UV/Sulfite/
2.1.1. Degradation Efficiency in Prepared Water
Firstly, a comparison test of UV alone, sulfite/, UV/, UV/sulfite, and UV/sulfite/ processes was conducted to explore the efficiency and advantages of the UV/sulfite/ process in degradation. To exclude the interference of the water matrix in real water, these comparison experiments were carried out in prepared water (bromate-containing water prepared with deionized water). As shown Figure 1a, under aerobic conditions ([DO]0 = 7.0 mg·L−1), 10 μM was degraded slowly using the UV/sulfite process or UV/ process, but completely disappeared within 5 min using the UV/sulfite/ process at 20 °C. As a comparison, direct UV photolysis and sulfite/ reduction showed a slow degradation rate, which can be attributed to the weak absorbance at 254 nm for [10] and low reduction potential of sulfite/ (E(/) = 0.535 V, and E(S(VI)/S(IV)) = −0.94 V [19]). Figure 1b presents the degradation curves of at different initial concentration ([]0) using the UV/sulfite/ process. Without exception, concentrations ranging from 0.1–50 μM were completely removed within 10 min. Generally, the degradation rate decreased with increased []0. Notably, 0.1 μM (World Health Organization (WHO) guideline value) could be degraded thoroughly within 3 min. One can expect a complete removal of in seconds if a UV lamp with higher power (such as 100 W) is used.
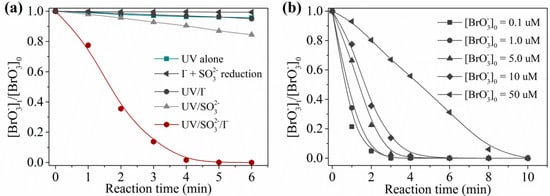
Figure 1.
Efficiency of degradation by ultraviolet (UV)/sulfite/ in prepared water: (a) different processes; (b) different concentrations of . Conditions: []0 = 100 μM, [sulfite]0 = 1.0 mM, [DO]0 = 7.0 mg⋅L−1, pH = 9.2, 20 ± 1 °C.
Table 1 summarizes the degradation methods previously reported. One can find that the UV/sulfite/ process is not inferior to any of the previously reported processes. Although the degradation efficiency is dependent on many factors such as reactor geometry and reaction conditions, the crude comparison still indicates the attractive prospect of the UV/sulfite/ process. Table 2 lists the related reactions in the UV/sulfite/ system. A preliminary judgment can be made that such a superior degradation efficiency may be attributed to the enhanced formation of / (No. (1)–(45) in Table 2).

Table 1.
Comparison of bromate removal efficiency with different processes. UV—ultraviolet; BDD—boron-doped diamond; SCE—saturated calomel electrode; TMP—transmembrane pressure.

Table 2.
Summary of related reaction equations during the degradation.
2.1.2. Validation of UV/Sulfite/ Process with Real Water
To evaluate the potential for practical water treatment, we also investigated the degradation efficiency of with four different tap waters (TP1, TP2, TP3, and TP4) using UV/sulfite/ process (Figure 2). The water quality parameters of four real waters are listed in Table S1 (Supplementary Materials). These four tap waters were collected from four different cities of Eastern China. Their pH values were nearly the same, but the other parameters (dissolved organic carbon (DOC), , , , and ) were quite different. The degradation efficiency of with the four authentic waters suffered some degree of decrease compared to that in pure water. For a UV dosage of 231.38 mJ·cm−2 (typical UV dosage for virus inactivation is 40–100 mJ·cm−2 [40]), the degradation rates of for the four tap waters were 91%, 88%, 85%, and 67%. Such results indicated that the degradation efficiency varied with changes in the water matrix. Over all, the degradation process of in real water using UV/sulfite/ was also satisfactory. These results powerfully confirm that reduction with the UV/sulfite/ process in real water is still efficient.
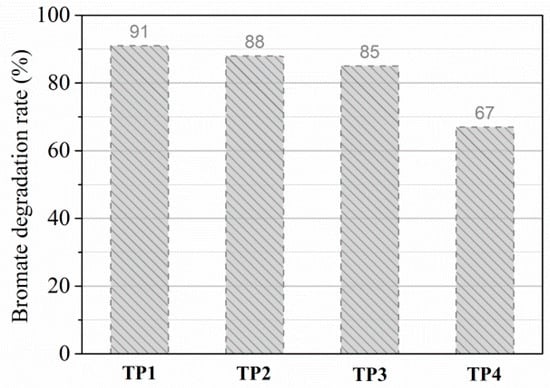
Figure 2.
Efficiencies of degradation using UV/sulfite/ in real water. Conditions: []0 = 10 μM, []0 = 100 μM, [sulfite]0 = 1.0 mM, UV dosage = 231.38 mJ·cm−2.
2.2. Mechanism of Reduction
2.2.1. Main Contributor to Reduction
Hydrated electrons and were certified as the main reductive reactive species in the UV/sulfite/ process [17]. As and are a conjugated acid–base pairs and both of them showed high reactivity toward (k(+) = 3.4 × 109 M−1·s−1 [10]; k(+) = 2.0 × 107 M−1·s−1 [41]), which of the two dominated reduction needed clarification. Monochloroacetic acid (MCAA) and nitrite () were used as radical scavengers to explore the contributions of and (No. (50)–(53) in Table 2). As shown in Figure 3, the presence of (scavenger for and ) yielded a degradation rate of 0.31 μM·min−1, while presence of MCAA (scavenger for ) generated a degradation rate of 0.59 μM·min−1. Based on the rate constant for the case without scavenger addition (2.61 μM·min−1), contributions for and were calculated to be 77.4% and 10.7%, respectively, under current conditions.
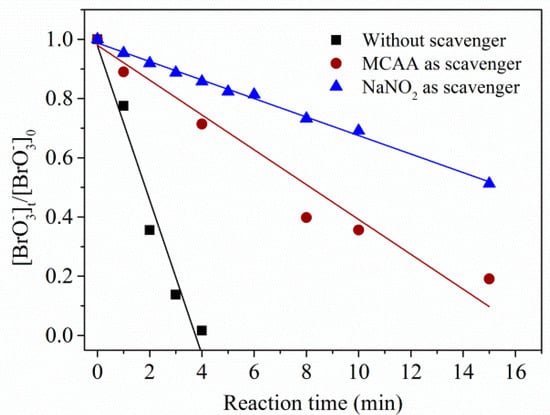
Figure 3.
Degradation of using UV/sulfite/ process in the presence of monochloroacetic acid (MCAA) and . Conditions: []0 = 10 μM, []0 = 100 μM, [sulfite]0 = 1.0 mM, [MCAA]0 = 1.0 mM, []0 = 4.0 mM, [DO]0 = 7.0 mg⋅L−1, pH = 9.2, 20 ± 1 °C.
2.2.2. The Role of and Sulfite
By fitting the degradation curves of using the UV/sulfite/ process, pseudo zero-order kinetics was found to be followed. Figure 4a shows that degradation improved with increased [sulfite]0. Figure 4b reveals three distinct phases for the influence of sulfite dosage. The degradation rate of (r) accelerated slightly from ~0.10 μM·min−1 to 0.12 μM·min−1 (phase I) by 0.5 mM sulfite, and a further increase of sulfite concentration caused a remarkable linear enhancement (phase II). However, much higher sulfite dosage (1.5–2.0 mM) only brought subtle increases of rate constants (phase III), which may be explained by the fact that incident photons were in short supply and obvious self-quenching of radicals occurred.
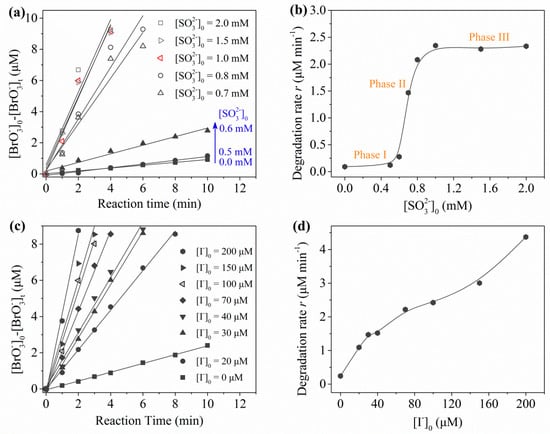
Figure 4.
Degradation of using UV/sulfite/ process: (a) effects of sulfite dosage ([]0 = 100 μM); (b) degradation rate constant (r) vs. [sulfite]0; (c) effects of dosage; (d) degradation rate constant (r) vs. []0. Conditions: []0 = 10 μM, pH = 9.2, [DO]0 = 7.0 mg⋅L−1, 20 ± 1 °C.
Regarding the role of , one can find that a concentration change at the 10 μM level would result in a significant variation in degradation efficiency of (Figure 4c). Comparing the two linear relationships in Figure 4b,d, the degradation efficiency was about 5.6 times (normalized to molar concentration) more dependent on than on sulfite. Thus, may play an important role in the process. Figure 5 presents the simultaneous evolution of sulfite and during the degradation of . Sulfite decreased rapidly within the first 2 min and then slowly depleted, while almost stayed constant as long as sulfite was available. The UV photolysis mechanism of is shown in No. (6)–(12) in Table 2. Iodide was a stronger UV-absorber at 254 nm compared to sulfite and had a higher quantum yield than sulfite [17]. However, the UV/ process failed to show superior reduction efficiency compared to the UV/sulfite process due to the scavenging effect of by the photogenerated RISs and self-consumption of (No. (5)–(14) in Table 2). The nearly constant concentration of indicated that the presence of sulfite may inhibit negative effects of RISs and promote the regeneration of . Based on the above discussion, one can conclude that mainly served as an precursor, while sulfite not only played a role as an precursor, but also as an RIS shielding agent and a regenerant of . Considering the negligible chemical change of during the whole reaction, acted similarly to a catalyst.
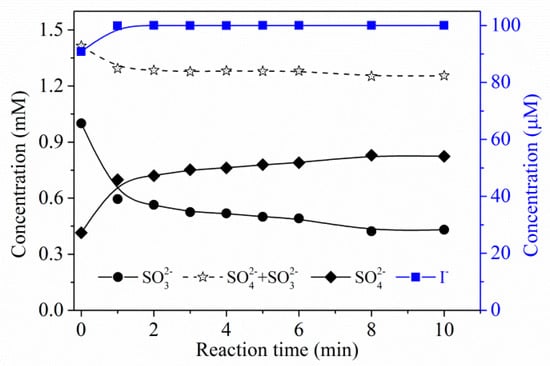
Figure 5.
Mass balance of sulfur and iodine during degradation of using UV/sulfite/ process. Conditions: []0 = 10 μM, []0 = 100 μM, [sulfite]0 = 1.0 mM, [DO]0 = 7.0 mg·L−1, pH = 9.2, 20 ± 1 °C.
2.2.3. Transformation Products of
Transformation products of (10 μM) treated by combining 1 mM sulfite and 100 μM at pH 9.2 under 254-nm UV irradiation were monitored, and the results are shown in Figure 6. For the prepared water, all converted to during degradation without other bromine-containing substances. No other inorganic bromine-containing substances, such as /, were detected. This could be proven by the yield per μM degradation (k0 = 1.0). We also tested the degradation products in four tap waters (Figure 6), and a similar phenomenon was observed. These results declared that -based reduction initiated with the UV/sulfite/ process was an efficient method of removing .
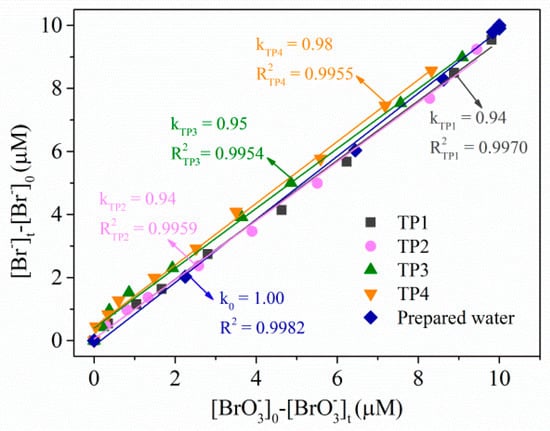
Figure 6.
Transformation products analysis of in prepared water and four tap waters using the UV/sulfite/ process. Conditions: []0 = 10 μM, []0 = 100 μM, [sulfite]0 = 1.0 mM, [DO]0 = 7.0 mg·L−1, pH = 9.2, 20 ± 1 °C.
2.3. Influence of Water Quality Parameters
As stated above, the water matrix presented non-negligible effects on degradation using the UV/sulfite/ process. How the water matrix influences the degradation process is of great concern. Thus, the effects on individual common water quality parameters were investigated one by one.
Taking into consideration the temperature fluctuation of drinking water, the degradation efficiency under different temperatures ranging from 9–38 °C was investigated (Figure 7a). When the solution temperature changed from 8.9 to 38 °C, the degradation rate increased about fivefold. It should be noted that, even at a relatively low temperature (8.9 °C), could still be degraded thoroughly within 10 min. According to van ’t Hoff’s law, a temperature increase of 30 °C will lead to an 8–64-fold increase in reaction rate. Thus, the degradation process was not entirely thermodynamically controlled. In addition, based on the degradation data under different temperatures, the apparent activation energy of in the UV/sulfite/ process was calculated (Ea = 34.55 kJ·mol−1; Figure S1, Supplementary Materials). This low apparent activation energy reasonably explained the efficient degradation of .
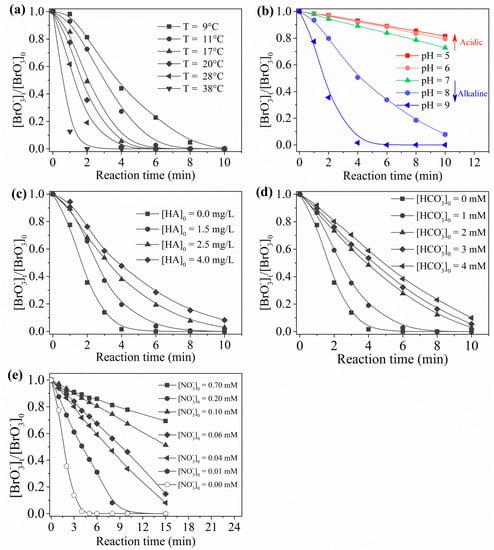
Figure 7.
Influence on water quality parameters with regards to the degradation of using the UV/sulfite/ process: (a) temperature; (b) pH; (c) humic acid (HA); (d) ; (e) . Conditions: []0 = 10 μM, []0 = 100 μM, [sulfite]0 = 1.0 mM, [DO]0 = 7.0 mg·L−1, pH = 9.2, 20 ± 1 °C.
Figure 7b shows the effect of pH on reduction. Increased pH improved the degradation of and the improvement became especially remarkable when the pH was raised above 7. Photolysis of was almost not influenced by pH change [30]. Solution pH plays a significant role in the distribution ratio of and the interconversion between and [14,18]. As regards S(IV) species, is dominant at pH 3–7, while becomes dominant at pH > 7 [42,43]. Given that was mainly produced due to the UV activation of rather than (No. (1) and (2)) and that was the main contributor to , one can easily understand why the alkaline environment benefited the degradation process. In addition, high pH promoted the conversion of to (No. (33) and (34)). Thus, pH exerted its influence through changing the fraction and the interconversion of and .
Humic acid (HA) was used as a representative of NOM herein. A concentration range of 1.5–4.0 mg·L−1 HA was dosed in the reaction process to assess its influence (Figure 7c). It is well known that excitation of HA by UV can produce reactive species such as excited triplet states of HA and hydroxyl radicals [44]. These oxidative reactive species can compete with for , which slowed degradation. HA also competed for incident photons (inner filter effect) with sulfite/, which may have affected the generation of . The results in Figure 7c confirmed these speculations. Overall, the removal rate of decreased with an increase in HA concentration; 4.0 mg·L−1 HA resulted in a removal rate decrease from 98.5% to 41% (4 min reaction time). Thus, HA was confirmed as a strong inhibitor for the degradation of using UV/sulfite/.
Figure 7d displays the influence of bicarbonate () on the degradation process. Bicarbonate at an environmentally relevant concentration level (1–4 mM) caused obvious inhibition effects. Specifically, 1 mM and 2 mM resulted in a degradation rate decrease by about 20% and 50%, respectively. Nevertheless, a further increase in concentration (3–4 mM) only brought about an extra 3–5% inhibition. The rate constant of with (<1.0 × 106 M−1·s−1 [45]) is much lower than that of with (3.0 × 109 M−1·s−1 [10]). Thus, 1.0–4.0 mM produced an scavenging rate of <(1.0–4.0) × 103 s−1, while 10 μM produced an scavenging rate of 3.0 × 104 s−1. From the point of view of competitive kinetics, 1.0–4.0 mM would confer a subtle influence on degradation. On the other hand, can react with to generate oxidative (No. (48)), and the formed will compete for sulfite with RIS (No. (48)). Such a competitive effect undoubtedly interfered with the cycle of . Given the important role of , a deteriorating degradation performance was expected. Hence, unlike the case using the UV/sulfite system, the effect of could not be ignored in the UV/sulfite/ process.
Nitrate (), a common water matrix component, is a strong electron scavenger (No. (52)) and, thus, its influence was explored (Figure 7e). Due to fast reaction kinetics, the degradation of was greatly inhibited by the presence of just 0.01 mM (0.14mg/L as N). Existence of 0.7 mM (United States Environmental Protection Agency (USEPA) guideline value) produced 90% inhibition, suggesting that elimination to an acceptable level was necessary for control using the UV/sulfite/ process.
3. Materials and Methods
3.1. Materials
Sodium sulfite, KI, HA, , , MCAA, atrazine (ATZ), and NaBrO3 were American Chemical Society (ACS) reagent grade and were purchased from Sigma-Aldrich (San Francisco, CA, USA). Sodium bicarbonate, , and were analytic reagent (AR) grade and were obtained from Sinopharm Chemical Reagents (Shanghai, China). Boracic acid (ACS reagent, 99.5%) and (ACS reagent, 99.5%) were purchased from J&K Scientific Ltd, while d-mannitol (AR reagent, 98.0%) was purchased from Macklin (Shanghai, China). Glass-fiber filters (0.45 μm) were ordered from Millipore (London, UK). HA stock solution was prepared in a procedure similar to that described in our previous work [10]. The concentration of the HA stock solution was calibrated using a total organic carbon (TOC)-VCPH analyzer (Shimadzu, Japan). Unless otherwise specified, all fresh stock solutions were prepared with 18.2·MΩ·cm ultrapure water. Four different tap waters were collected from four different cities located in east China and were dechlorinated before use.
3.2. Experimental Procedure
An 800-mL cylindrical glass photoreactor was used to conduct the experiments, and the reaction volume was 500 mL. A Heraeus low-pressure UV lamp (10W, ozone-free, light centered at 254 nm, Hanau, Germany) was used, and d-mannitol (3%) was used to fix sulfite ions before detecting. The pH of the reaction solution was adjusted with 0.2 M borate buffer and/or 0.1 M·. Other than the experiments to evaluate the impact of pH, the solution pH was set at 9.2 to guarantee the valid concentration of sulfite. The constant temperature (10–40 °C) of reaction solution was controlled using a thermostat bath. The UV lamp was preheated for 10 min to ensure achieving a stable output prior to experiment start. Samples were drawn at predetermined time intervals and quenched with before analysis. In addition, all experiments were carried out under aerobic conditions without deoxygenation.
3.3. Analytical Methods
Solution pH was measured using a portable pH meter (Orion 3-Star, Thermo Scientific, MA, USA). Bromate, , ,, , , , and were analyzed using ion chromatography (Dionex ICS-2000, Chameleon 6.8, Sunnyvale, CA, USA) in isocratic elution mode (20 mM and 50 mA suppressor current). The / was analyzed using the diethyl-p-phenylenediamine colorimetric method [46]. Atrazine was detected with a high-performance liquid chromatograph equipped with a XDB-C18 column (5 μm × 4.6 mm × 150 mm). The eluent was 40% water and 60% methanol and run at 1 mL·min−1. Bicarbonate was detected using chemical titration with a standard HCl solution, and cesium chloride was first added to deposit and before titration [47].
3.4. UV Intensity and UV Fluence Calculation
Considering the actual output loss of the lamp, the effective ultraviolet dosage was calibrated with atrazine as an exposure agent [48]. The photon fluence was determined to be 4.5 × 10−7 Einstein·s−1. The effective optical path length (14.77 cm) was determined using as an exposure agent. The UV dosage was calculated to be 0.0964 mW·cm−2.
4. Conclusions
This study examined the potential of an iodide-assisted UV/sulfite process (UV/Sulfite/) for the degradation of in drinking water. The results indicated that UV/Sulfite/ showed much faster reaction kinetics in an aerobic environment than other methods reported. Competitive kinetic experiments demonstrated that degradation was mainly achieved due to reduction (77.4% contribution). With respect to the roles of sulfite and , both acted as precursors, and sulfite was involved in the regeneration of . Given the unvaried concentration of , was considered to play a catalyst-like role. Bromide was the only transformation product of even in the presence of background in tap water. The investigation on the influence of common water quality parameters revealed that UV/Sulfite/ was especially sensitive to nitrate, 0.04 mM of which resulted in ~50% inhibition of reduction. The solution pH exerted its influence through adjusting the valid concentration of sulfite. Bicarbonate and HA at an environment relevant level caused moderate inhibition. Overall, UV/Sulfite/ is promising for practical treatment of -polluted drinking water, and pretreatment to reduce nitrate is suggested prior to application.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4344/8/12/652/s1: Figure S1: Determination of activation energy of degradation in the UV/sulfite/ process; Table S1: Water quality parameters of four tap waters.
Author Contributions
Conceptualization, X.L.; data curation, J.W. and X.L.; formal analysis, T.Z., J.W., D.Y., and L.W.; project administration, T.Z.; writing—original draft, J.W.; writing—review and editing, X.L.; Y.S. contributed reagents/materials/analysis tools.
Acknowledgments
This research was funded by the special S&T project on the treatment and control of water pollution (Grant No. 2017ZX07201-003), the Natural Science Foundation of Zhejiang Province (Grant No. LQ19E080023), the Major International (Regional) Joint Research Program of China (Grant No. 51761145022), and the National Key Research and Development Program of China (No. 2016YFC0400601, 2016YFC0400606). The authors greatly thank Luo, F. for his discussion and suggestion.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Krasner, S.W.; Glaze, W.H.; Weinberg, H.S.; Daniel, P.A.; Najm, I.N. Formation and control of bromate during ozonation of waters containing bromide. Water Res. 1993, 85, 73–81. [Google Scholar] [CrossRef]
- Huang, X.; Gao, N.; Lu, P. Bromate reduction by granular activated carbon. Environ. Sci. 2007, 28, 2264–2269. [Google Scholar] [CrossRef]
- Wang, L.; Batchelor, B.; Pillai, S.D.; Botlaguduru, V.S.V. Electron beam treatment for potable water reuse: Removal of bromate and perfluorooctanoic acid. Chem. Eng. J. 2016, 302, 58–68. [Google Scholar] [CrossRef]
- Merle, T.; Pronk, W.; Gunten, U.V. MEMBRO3X, a novel combination of a membrane contactor with advanced oxidation (O3/H2O2) for simultaneous micropollutant abatement and bromate minimization. Environ. Sci. Technol. Lett. 2017, 4, 180–185. [Google Scholar] [CrossRef]
- Kirisits, M.J.; Snoeyink, V.L.; Inan, H.; Chee-sanford, J.C.; Raskin, L.; Brown, J.C. Water quality factors affecting bromate reduction in biologically active carbon filters. Water Res. 2001, 35, 891–900. [Google Scholar] [CrossRef]
- Lin, K.A.; Lin, C.H. Enhanced reductive removal of bromate using acid-washed zero-valent iron in the presence of oxalic acid. Chem. Eng. J. 2017, 325, 144–150. [Google Scholar] [CrossRef]
- Gonzalez, M.G.; Oliveros, E.; Wörner, M.; Braun, A.M. Vacuum-ultraviolet photolysis of aqueous reaction systems. J. Photoch. Photobiol. C 2004, 5, 225–246. [Google Scholar] [CrossRef]
- Noguchi, H.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Removal of bromate ion from water using TiO2 and alumina-loaded TiO2 photocatalysts. Water Sci. Technol. 2002, 46, 27–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, S.; Liu, H. Reactivity and mechanism of bromate reduction from aqueous solution using Zn–Fe(II)–Al layered double hydroxides. Chem. Eng. J. 2015, 266, 21–27. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Shao, Y. Aqueous bromate reduction by UV activation of sulfite. Clean-Soil Air Water 2015, 42, 1370–1375. [Google Scholar] [CrossRef]
- Xiao, Q.; Yu, S.; Li, L.; Wang, T.; Liao, X.; Ye, Y. An overview of advanced reduction processes for bromate removal from drinking water: Reducing agents, activation methods, applications and mechanisms. J. Hazard. Mater. 2017, 324, 230–240. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Jones, C.G.; Silverman, J.; Al-Sheikhly, M.; Neta, P.; Poster, D.L. Dechlorination of polychlorinated biphenyls in industrial transformer oil by radiolytic and photolytic methods. Environ. Sci. Technol. 2003, 37, 5773–5777. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, T.; Wang, L.; Shao, Y.; Fang, L. Hydrated electron-based degradation of atenolol in aqueous solution. Chem. Eng. J. 2015, 260, 740–748. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, C.; Xing, L.; Zhou, Q.; Dong, W.; Hoffmann, M.R. UV/nitrilotriacetic acid process as a novel strategy for efficient photoreductive degradation of perfluorooctanesulfonate. Environ. Sci. Technol. 2018, 52, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ma, J.; Jiang, J.; Yang, L.; Yang, J.; Zhang, J.; Chi, H.; Song, Y.; Sun, S.; Tian, W.Q. Hydrated electron (eaq−) generation from phenol/UV: Efficiency, influencing factors, and mechanism. Appl. Catal. B. Environ. 2017, 200, 585–593. [Google Scholar] [CrossRef]
- Yu, K.; Li, X.; Chen, L.; Fang, J.; Chen, H.; Li, Q.; Chi, N.; Ma, J. Mechanism and efficiency of contaminant reduction by hydrated electron in the sulfite/iodide/UV process. Water Res. 2017, 129, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, J.; Liu, G.; Fang, J.; Yue, S.; Guan, Y.; Chen, L.; Liu, X. Efficient reductive dechlorination of monochloroacetic acid by sulfite/UV process. Environ. Sci. Technol. 2012, 46, 7342–7349. [Google Scholar] [CrossRef] [PubMed]
- Bard, E.B.; Parsons, R.; Jordan, J. Standard Potentials in Aqueous Solution; Routledge: New York, NY, USA, 1985. [Google Scholar]
- Bensalah, N.; Liu, X.; Abdelwahab, A. Bromate reduction by ultraviolet light irradiation using medium pressure lamp. Int. J. Environ. Stud. 2013, 70, 566–582. [Google Scholar] [CrossRef]
- Jung, B.; Nicola, R.; Batchelor, B.; Abdel-Wahab, A. Effect of low- and medium-pressure Hg UV irradiation on bromate removal in advanced reduction process. Chemosphere 2014, 117, 663–672. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Ng, J.; Pan, J.; Sun, D. Transformation of bromine species in TiO2 photocatalytic system. Environ. Sci. Technol. 2010, 44, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, Y.; Guo, Y.; Shi, W.; Wang, W.; Zhang, B.; Zhang, R.; Bao, X.; Wu, S.; Cui, F. Pd and Pt nanoparticles supported on the mesoporous silica molecular sieve SBA-15 with enhanced activity and stability in catalytic bromate reduction. Chem. Eng. J. 2018, 344, 114–123. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.; Li, A.; Shen, Y.; Qu, J. Bromate removal by electrochemical reduction at boron-doped diamond electrode. Electrochim. Acta 2012, 62, 181–184. [Google Scholar] [CrossRef]
- Assunção, A.; Martins, M.; Silva, G.; Lucas, H.; Coelho, M.R.; Costa, M.C. Bromate removal by anaerobic bacterial community: Mechanism and phylogenetic characterization. J. Hazard. Mater. 2011, 197, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Listiarini, K.; Tor, J.T.; Sun, D.D.; Leckie, J.O. Hybrid coagulation–nanofiltration membrane for removal of bromate and humic acid in water. J. Membr. Sci. 2010, 365, 154–159. [Google Scholar] [CrossRef]
- Fischer, M.; Warneck, P. Photodecomposition and photooxidation of hydrogen sulfite in aqueous solution. J. Phys. Chem. A 1996, 100, 15111–15117. [Google Scholar] [CrossRef]
- Lian, R.; Oulianov, D.A.; And, R.A.C.; Shkrob, I.A.; And, X.C.; Bradforth, S.E. Electron photodetachment from aqueous anions. 3. Dynamics of geminate pairs derived from photoexcitation of mono- vs polyatomic anions. J. Phys. Chem. A 2006, 110, 9071–9078. [Google Scholar] [CrossRef] [PubMed]
- Ichino, T.; Fessenden, R.W. Reactions of hydrated electron with various radicals: Spin factor in diffusion-controlled reactions. J. Phys. Chem. A 2007, 111, 2527–2541. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.C., Jr.; Crowell, R.A.; Shkrob, I.A. Electron photodetachment from aqueous anions. 1. Quantum yields for generation of hydrated electron by 193 and 248 nm laser photoexcitation of miscellaneous inorganic anions. J. Phys. Chem. A 2004, 108, 5490–5502. [Google Scholar] [CrossRef]
- Alfassi, Z.B.; Huie, R.E.; Marguet, S.; Natarajan, E.; Neta, P. Rate constants for reactions of iodine atoms in solution. Int. J. Chem. Kinet. 1995, 27, 181–188. [Google Scholar] [CrossRef]
- Jortner, J.; Levine, R.; Ottolenghi, M.; Stein, G. The photochemistry of the iodide ion in aqueous solution. J. Phys. Chem. 1961, 65, 1232–1238. [Google Scholar] [CrossRef]
- Park, H.; Vecitis, C.D.; Cheng, J.; Dalleska, N.F.; Mader, B.T.; Hoffmann, M.R. Reductive degradation of perfluoroalkyl compounds with aquated electrons generated from iodide photolysis at 254 nm. Photochem. Photobiol. Sci. 2011, 10, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Yiin, B.S.; Margerum, D.W. Nonmetal redox kinetics: Reactions of iodine and triiodide with sulfite and hydrogen sulfite and the hydrolysis of iodosulfate. Inorg. Chem. 1990, 29, 1559–1564. [Google Scholar] [CrossRef]
- Hayon, E.; Treinin, A.; Wilf, J. Electronic spectra, photochemistry, and autoxidation mechanism of the sulfite-bisulfite-pyrosulfite systems SO2−, SO3−, SO4−, and SO5− radicals. J. Am. Chem. Soc. 1972, 94, 47–57. [Google Scholar] [CrossRef]
- Grebel, J.E.; Pignatello, J.J.; Mitch, W.A. Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters. Environ. Sci. Technol. 2010, 44, 6822–6828. [Google Scholar] [CrossRef]
- Ross, A.B.; Neta, P. Rate Constants for Reactions of Inorganic Radicals in Aqueous-Solution; Report NSRDS-NBS65; National Bureau of Standards: Washington, DC, USA, 1979; pp. 5–6.
- Neta, P.; Huie, R.E. Free-radical chemistry of sulfite. Environ. Health Perspect. 1985, 64, 209–217. [Google Scholar] [CrossRef]
- Buchanan, W.; Roddick, F.; Porter, N.; Drikass, M. Fractionation of UV and VUV pretreated natural organic matter from drinking water. Environ. Sci. Technol. 2005, 39, 4647–4654. [Google Scholar] [CrossRef]
- Kishore, K.; Moorthy, P.N.; Rao, K.N. Use of riboflavin as a standard solute for estimating H-atom reaction rate constants by competition kinetic method. Radiat. Phys. Chem. 1984, 23, 495–497. [Google Scholar] [CrossRef]
- Zelinsky, A.G.; Pirogov, B.Y. Electrochemical oxidation of sulfite and sulfur dioxide at a renewable graphite electrode. Electrochim. Acta 2017, 231, 371–378. [Google Scholar] [CrossRef]
- Xiao, Q.; Ren, Y.; Yu, S. Pilot study on bromate reduction from drinking water by UV/sulfite systems: Economic cost comparisons, effects of environmental parameters and mechanisms. Chem. Eng. J. 2017, 330, 1203–1210. [Google Scholar] [CrossRef]
- Wenk, J.; von Gunten, U.; Canonica, S. Effect of dissolved organic matter on the transformation of contaminants induced by excited triplet states and the hydroxyl radical. Environ. Sci. Technol. 2011, 45, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhou, L.; Ferronato, C.; Yang, X.; Salvador, A.; Zeng, C.; Chovelon, J.M. Photocatalytic degradation of atenolol in aqueous titanium dioxide suspensions: Kinetics, intermediates and degradation pathways. J. Photochem. Photobiol. A Chem. 2013, 254, 35–44. [Google Scholar] [CrossRef]
- Fang, J.; Shang, C. Bromate formation from bromide oxidation by the UV/persulfate process. Environ. Sci. Technol. 2012, 46, 8976–8983. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Zheng, B.; Mao, S.; Jin, H. Comparative study on the determination of bicarbonate in bicarbonated ringer s injection by titrimetric method and suppressed ion chromatography. West China J. Pharm. Sci. 2016, 31, 286–288. [Google Scholar] [CrossRef]
- Rosenfeldt, E.J.; Linden, K.G.; Canonica, S.; Gunten, U.V. Comparison of the efficiency of ·OH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2. Water Res. 2006, 40, 3695–3704. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).