Thermal Stability and Potential Cycling Durability of Nitrogen-Doped Graphene Modified by Metal-Organic Framework for Oxygen Reduction Reactions
Abstract
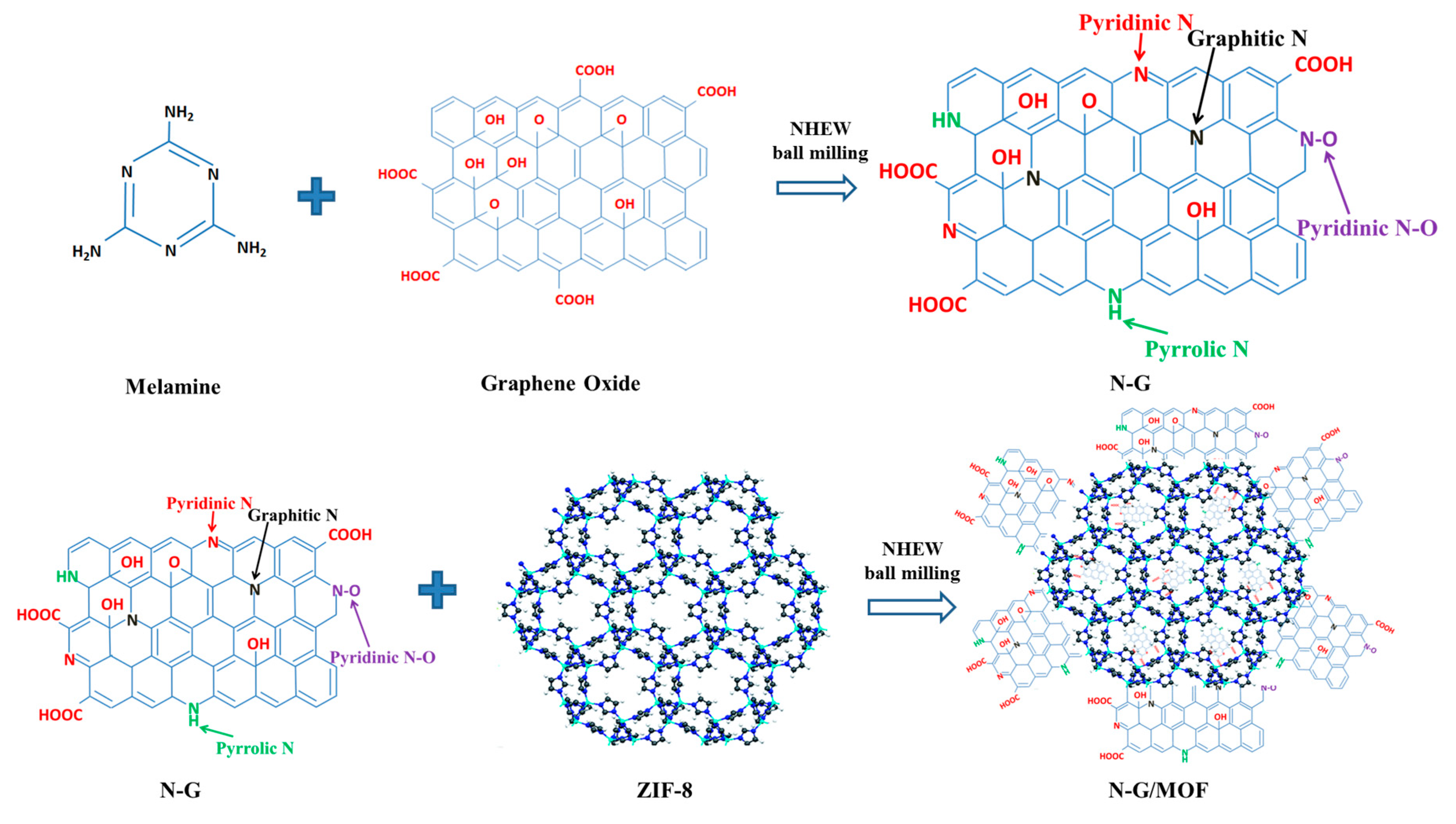
1. Introduction
2. Results and Discussion
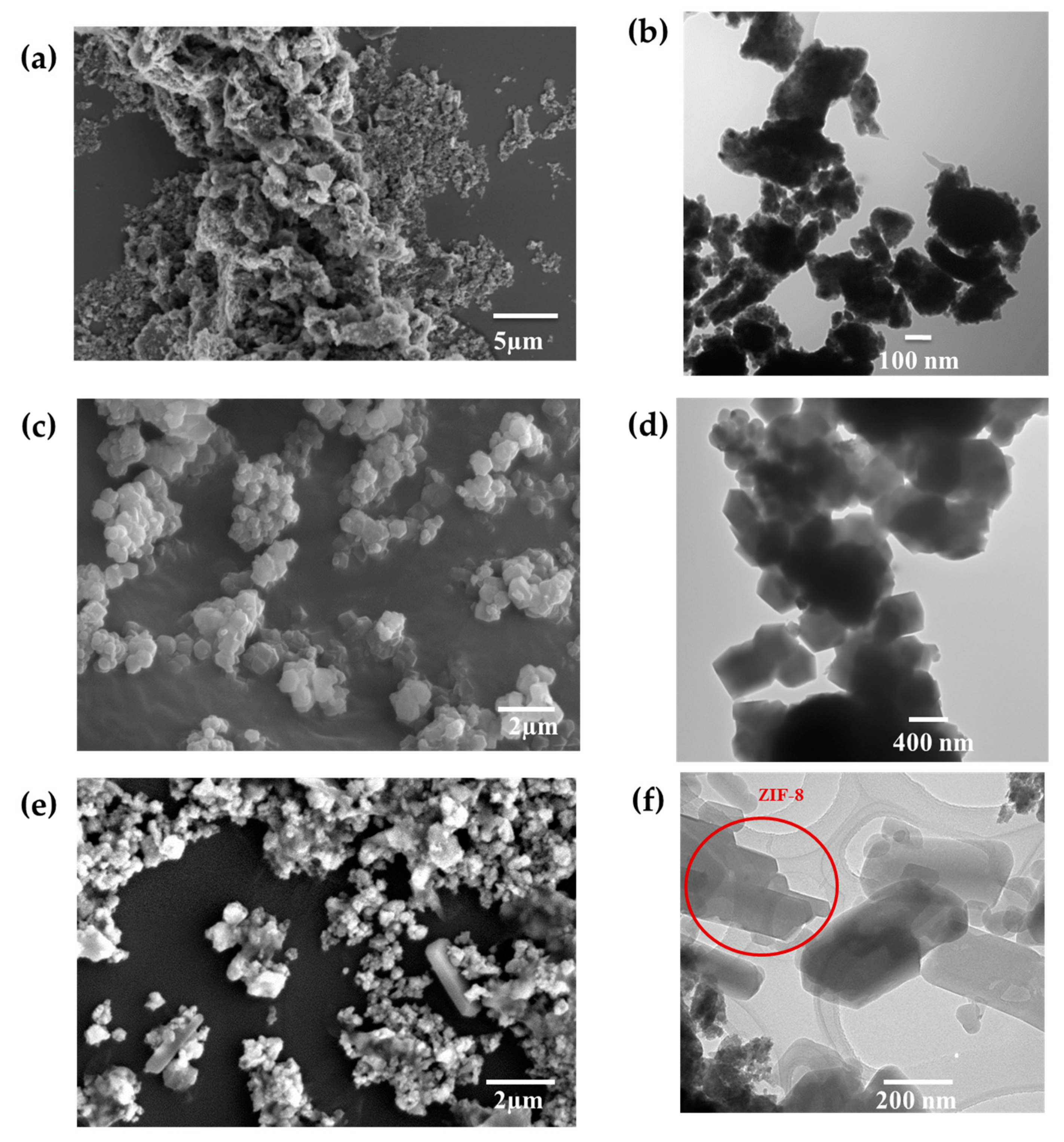
2.1. Morphology
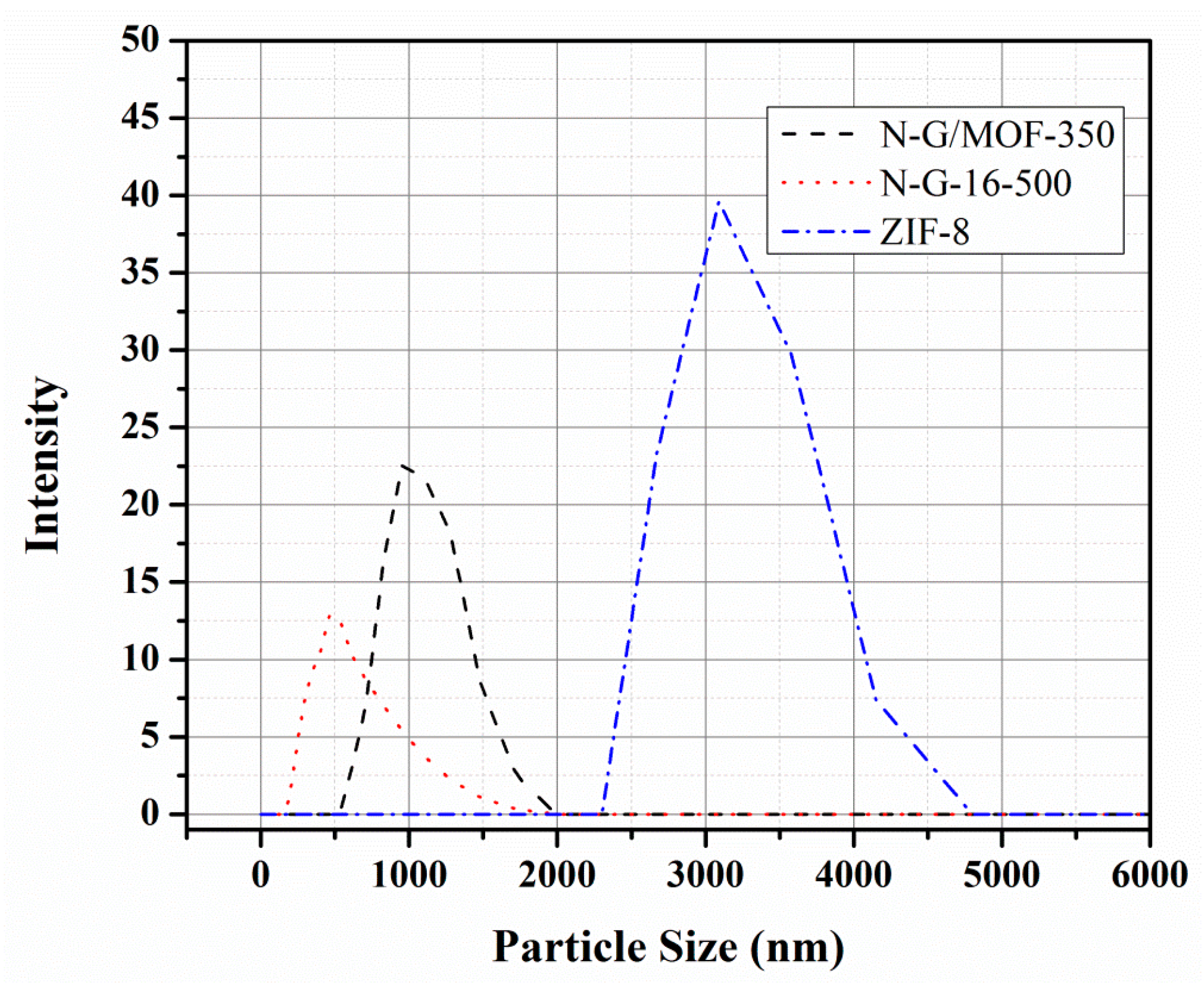
2.2. BET Surface Area
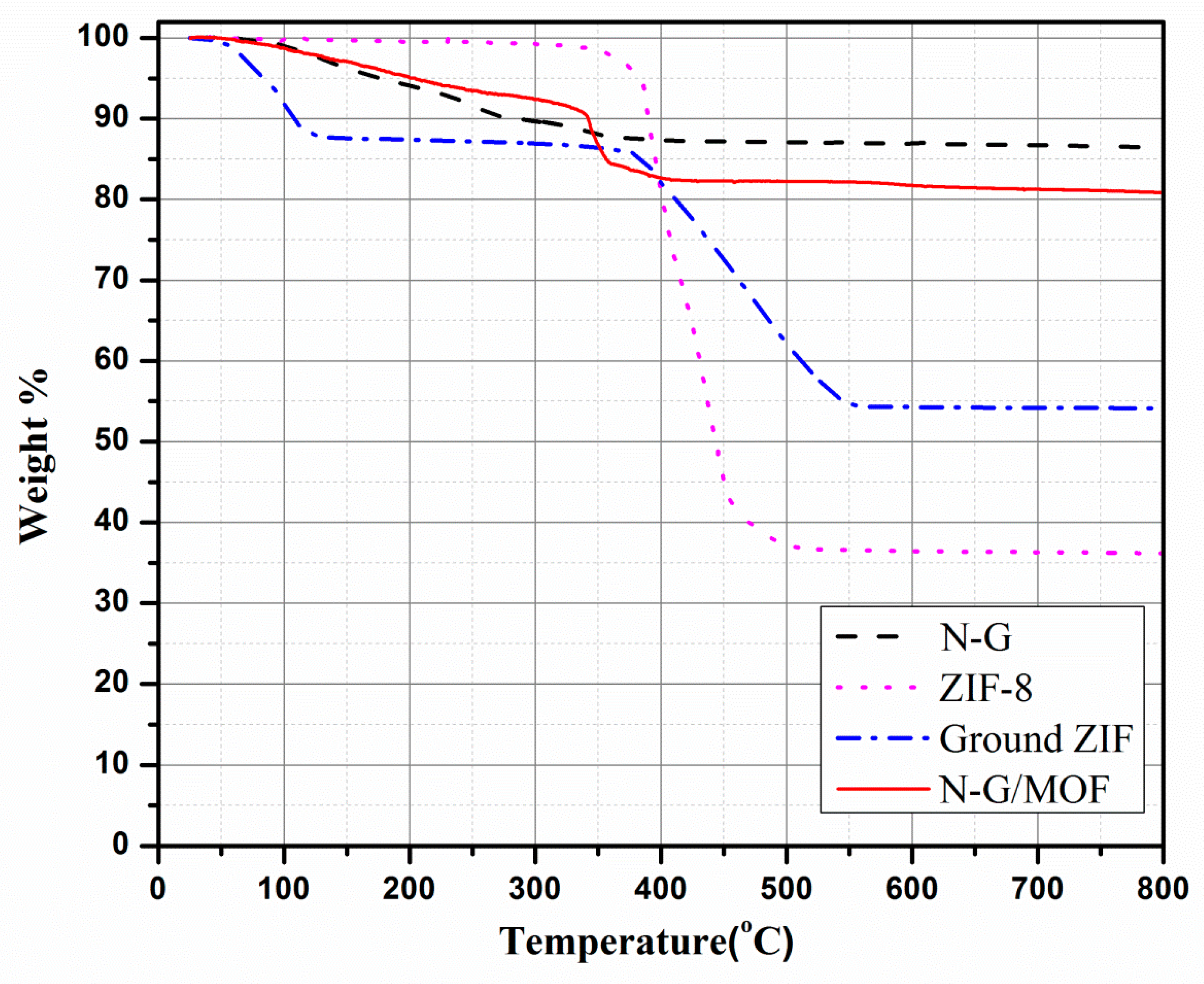
2.3. Thermogravimetric Analysis
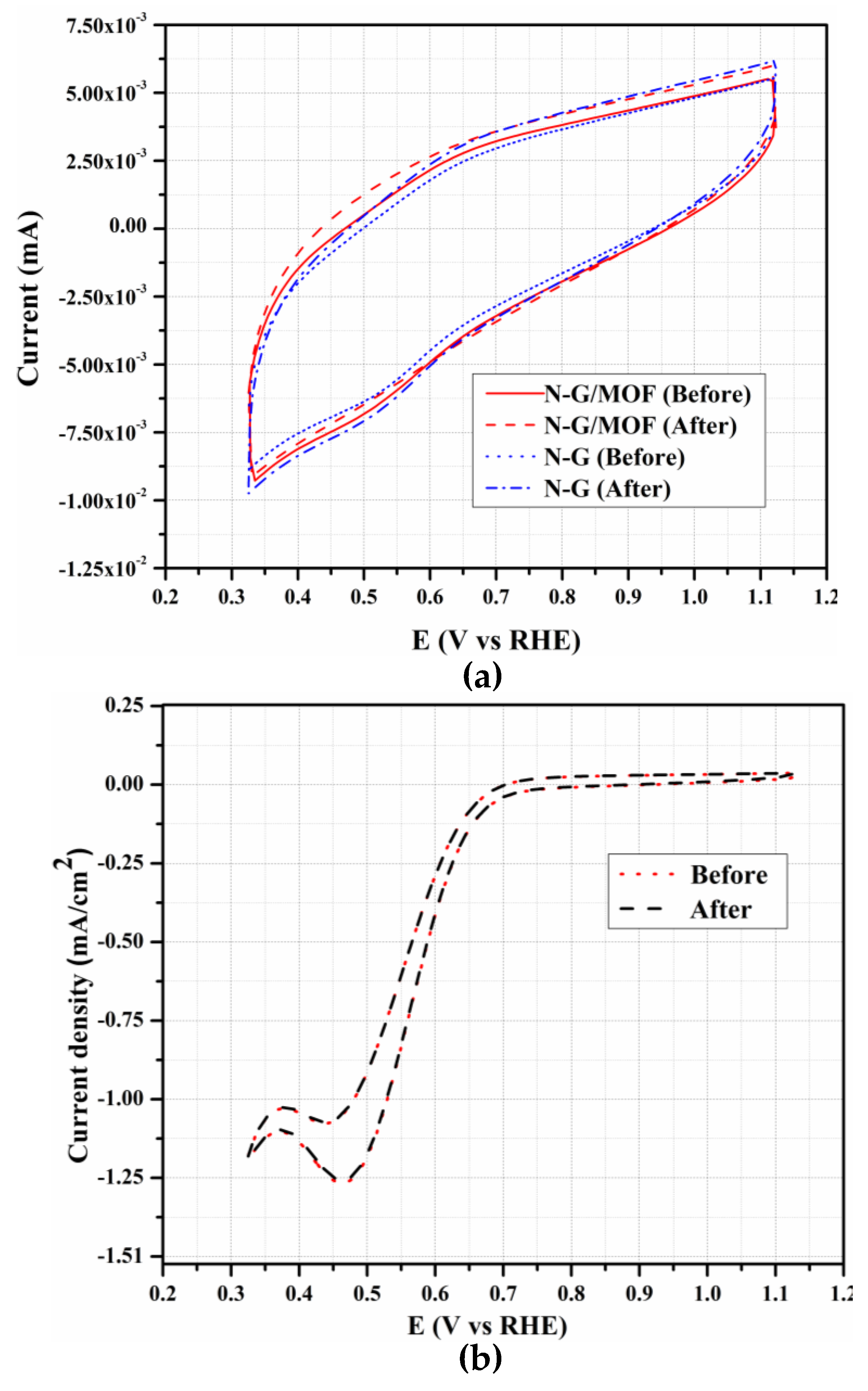
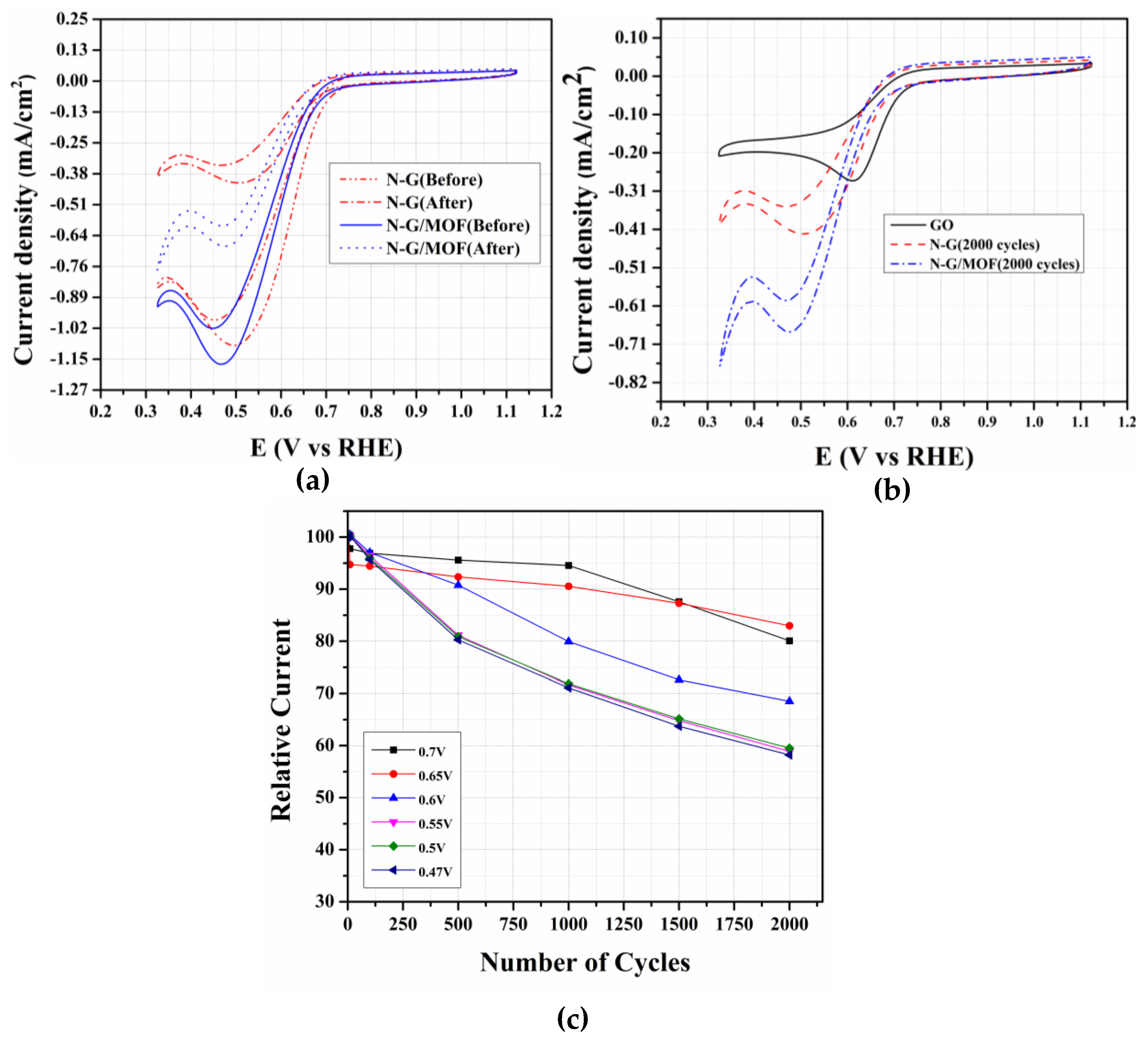
2.4. Durability Study of N-G/MOF and N-G
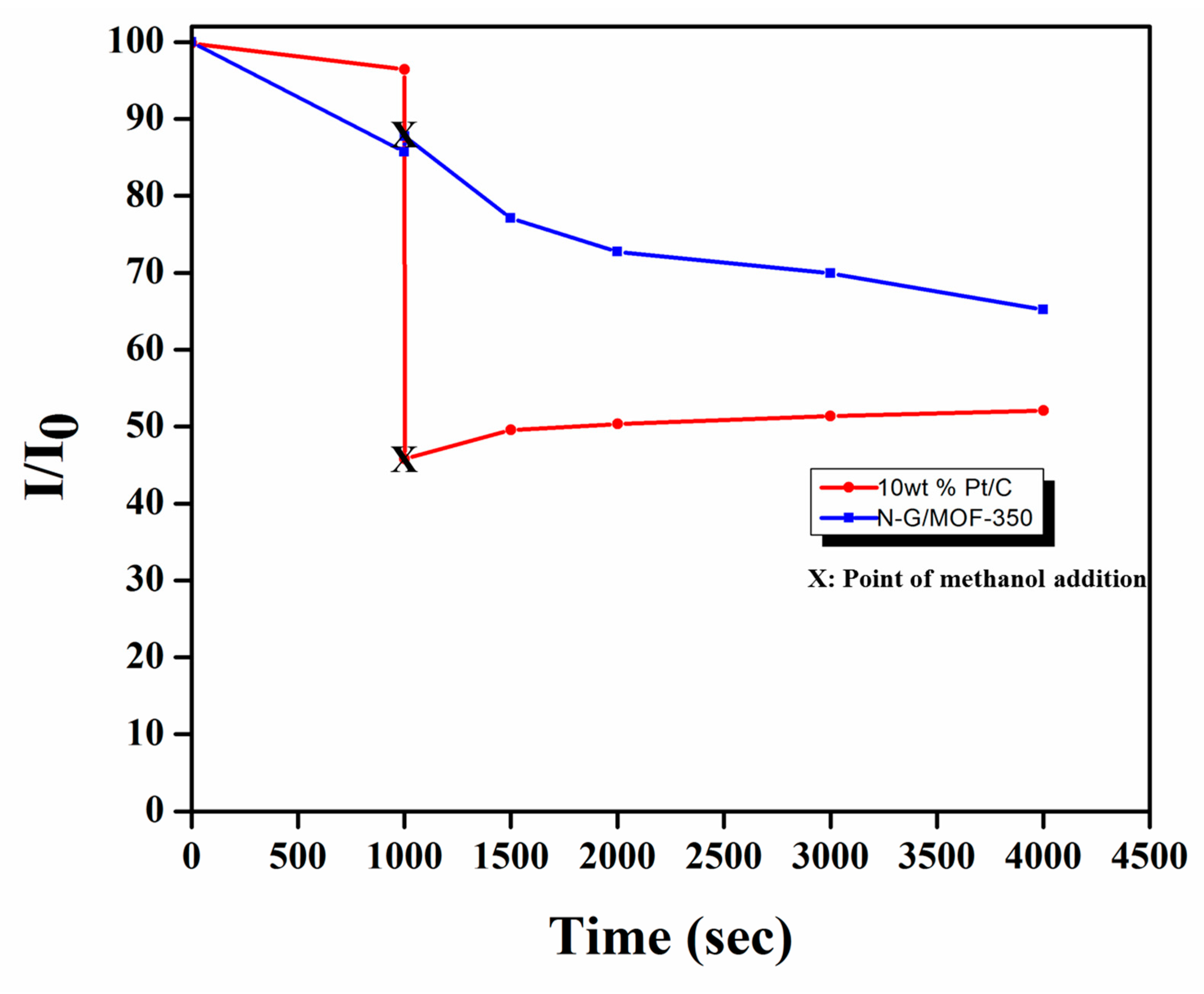
2.5. Effect of Methanol Crossover
3. Experimental Methods
3.1. Materials
3.2. Synthesis
3.3. Catalyst Ink Preparation
3.4. Electrochemical Characterizations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, W.; Tao, Y.; Lv, W.; Su, F.Y.; Ke, L.; Li, J.; Wang, D.W.; Li, B.; Kang, F.; Yang, Q.H. Unusual high oxygen reduction performance in all-carbon electrocatalysts. Sci. Rep. 2014, 4, 6289. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Serov, A.; Robson, M.H.; Halevi, B.; Artyushkova, K.; Atanassov, P. Highly active and durable templated non-PGM cathode catalysts derived from iron and aminoantipyrine. Electrochem. Commun. 2012, 22, 53–56. [Google Scholar] [CrossRef]
- Zhong, H.X.; Wang, J.; Zhang, Y.W.; Xu, W.L.; Xing, W.; Xu, D.; Zhang, Y.F.; Zhang, X.B. ZIF-8 Derived Graphene-Based Nitrogen-Doped Porous Carbon Sheets as Highly Efficient and Durable Oxygen Reduction Electrocatalysts. Angew. Chem. Int. Ed. 2014, 53, 14235–14239. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically active graphene–porphyrin MOF composite for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Song, M.K.; Ding, Y.; Liu, Y.; Liu, M.; Wong, C.P. Facile preparation of nitrogen-doped graphene as a metal-free catalyst for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2012, 14, 3381–3387. [Google Scholar] [CrossRef] [PubMed]
- Strickland, K.; Miner, E.; Jia, Q.; Tylus, U.; Ramaswamy, N.; Liang, W.; Sougrati, M.T.; Jaouen, F.; Mukerjee, S. Highly active oxygen reduction non-platinum group metal electrocatalyst without direct metal–nitrogen coordination. Nat. Commun. 2015, 6, 7343. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.I.; Kishimoto, T.; Imashiro, Y. A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Wang, B. Recent development of non-platinum catalysts for oxygen reduction reaction. J. Power Sources 2005, 152, 1–15. [Google Scholar] [CrossRef]
- Deng, D.; Yu, L.; Chen, X.; Wang, G.; Jin, L.; Pan, X.; Deng, J.; Sun, G.; Bao, X. Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction. Angew. Chem. 2013, 125, 389–393. [Google Scholar] [CrossRef]
- Schulenburg, H.; Stankov, S.; Schünemann, V.; Radnik, J.; Dorbandt, I.; Fiechter, S.; Bogdanoff, P.; Tributsch, H. Catalysts for the oxygen reduction from heat-treated iron (III) tetramethoxyphenylporphyrin chloride: Structure and stability of active sites. J. Phys. Chem. B 2003, 107, 9034–9041. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Popov, B. Stability study of nitrogen-modified carbon composite catalysts for oxygen reduction reaction in polymer electrolyte membrane fuel cells. ECS Trans. 2009, 25, 1251–1259. [Google Scholar]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Mo, Z.; Liao, S.; Liang, H.; Yang, L.; Luo, F.; Song, H.; Zhong, Y.; Zhang, B. High performance Fe-and N-doped carbon catalyst with graphene structure for oxygen reduction. Sci. Rep. 2013, 3, 1765. [Google Scholar] [CrossRef]
- Bashyam, R.; Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Manzoli, M.; Boccuzzi, F. Characterisation of Co-based electrocatalytic materials for O2 reduction in fuel cells. J. Power Sources 2005, 145, 161–168. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications, 10.1021/cs200652. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Xu, H.; Ma, L.; Jin, Z. Nitrogen-doped graphene: Synthesis, characterizations and energy applications. J. Energy Chem. 2017, 27, 146–160. [Google Scholar] [CrossRef]
- Zhuang, S.; Lee, E.S.; Lei, L.; Nunna, B.B.; Kuang, L.; Zhang, W. Synthesis of nitrogen-doped graphene catalyst by high energy wet ball milling for electrochemical systems. Int. J. Energy Res. 2016, 40, 2136–2149. [Google Scholar] [CrossRef]
- Zhuang, S.; Nunna, B.B.; Boscoboinik, J.A.; Lee, E.S. Nitrogen-doped graphene catalysts: High energy wet ball milling synthesis and characterizations of functional groups and particle size variation with time and speed. Int. J. Energy Res. 2017, 41, 2535–2554. [Google Scholar] [CrossRef]
- Zhuang, S.; Nunna, B.B.; Lee, E.S. Metal organic framework-modified nitrogen-doped graphene oxygen reduction reaction catalyst synthesized by nanoscale high-energy wet ball-milling structural and electrochemical characterization. MRS Commun. 2018, 8, 40–48. [Google Scholar] [CrossRef]
- Singh, H.; Zhuang, S.; Nunna, B.B.; Lee, E.S. Morphology and Chemical Structure of Modified Nitrogen-Doped Graphene for Highly Active Oxygen Reduction Reactions. In Proceedings of the 48th Power Source Conference, Denver, CO, USA, 14 June 2018. [Google Scholar]
- Zhuang, S.; Singh, H.; Nunna, B.B.; Mandal, D.; Boscoboinik, J.A.; Lee, E.S. Nitrogen-doped graphene-based catalyst with metal-reduced organic framework: Chemical analysis and structure control. Carbon 2018, 139, 933–944. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Tylus, U.; Jia, Q.; Mukerjee, S. Activity descriptor identification for oxygen reduction on nonprecious electrocatalysts: Linking surface science to coordination chemistry. J. Am. Chem. Soc. 2013, 135, 15443–15449. [Google Scholar] [CrossRef]
- Campos-Roldán, C.A.; González-Huerta, R.G.; Alonso-Vante, N. Experimental Protocol for HOR and ORR in Alkaline Electrochemical Measurements. J. Electrochem. Soc. 2018, 165, J3001–J3007. [Google Scholar] [CrossRef]
| Characteristics/Materials | Graphene Oxide | N-G-16-500 | ZIF-8 | N-G/MOF-350 |
|---|---|---|---|---|
| BET Surface Area (m2/g) | 347.23 | 77.81 | 1775.4 | 1039.79 |
| Pore Volume (cc/g) | 0.4 | 0.08 | 0.62 | 0.42 |
| Avg. Pore Size (nm) | 2.76 | 1.4 | 1.1 | 1.08 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, H.; Zhuang, S.; Nunna, B.B.; Lee, E.S. Thermal Stability and Potential Cycling Durability of Nitrogen-Doped Graphene Modified by Metal-Organic Framework for Oxygen Reduction Reactions. Catalysts 2018, 8, 607. https://doi.org/10.3390/catal8120607
Singh H, Zhuang S, Nunna BB, Lee ES. Thermal Stability and Potential Cycling Durability of Nitrogen-Doped Graphene Modified by Metal-Organic Framework for Oxygen Reduction Reactions. Catalysts. 2018; 8(12):607. https://doi.org/10.3390/catal8120607
Chicago/Turabian StyleSingh, Harsimranjit, Shiqiang Zhuang, Bharath Babu Nunna, and Eon Soo Lee. 2018. "Thermal Stability and Potential Cycling Durability of Nitrogen-Doped Graphene Modified by Metal-Organic Framework for Oxygen Reduction Reactions" Catalysts 8, no. 12: 607. https://doi.org/10.3390/catal8120607
APA StyleSingh, H., Zhuang, S., Nunna, B. B., & Lee, E. S. (2018). Thermal Stability and Potential Cycling Durability of Nitrogen-Doped Graphene Modified by Metal-Organic Framework for Oxygen Reduction Reactions. Catalysts, 8(12), 607. https://doi.org/10.3390/catal8120607






