Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol
Abstract
1. Introduction
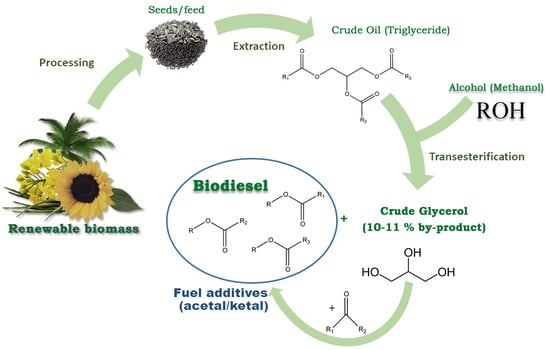
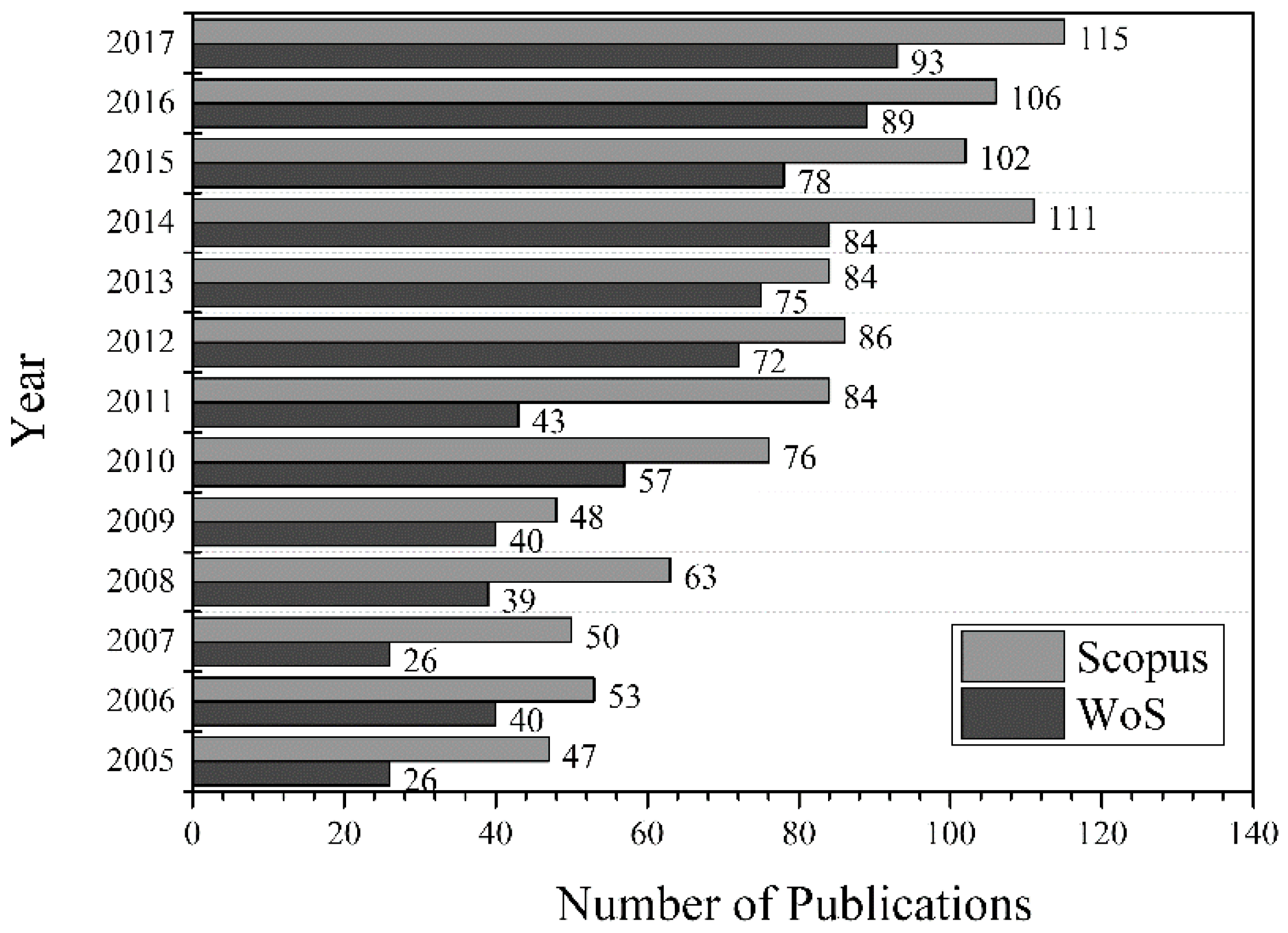
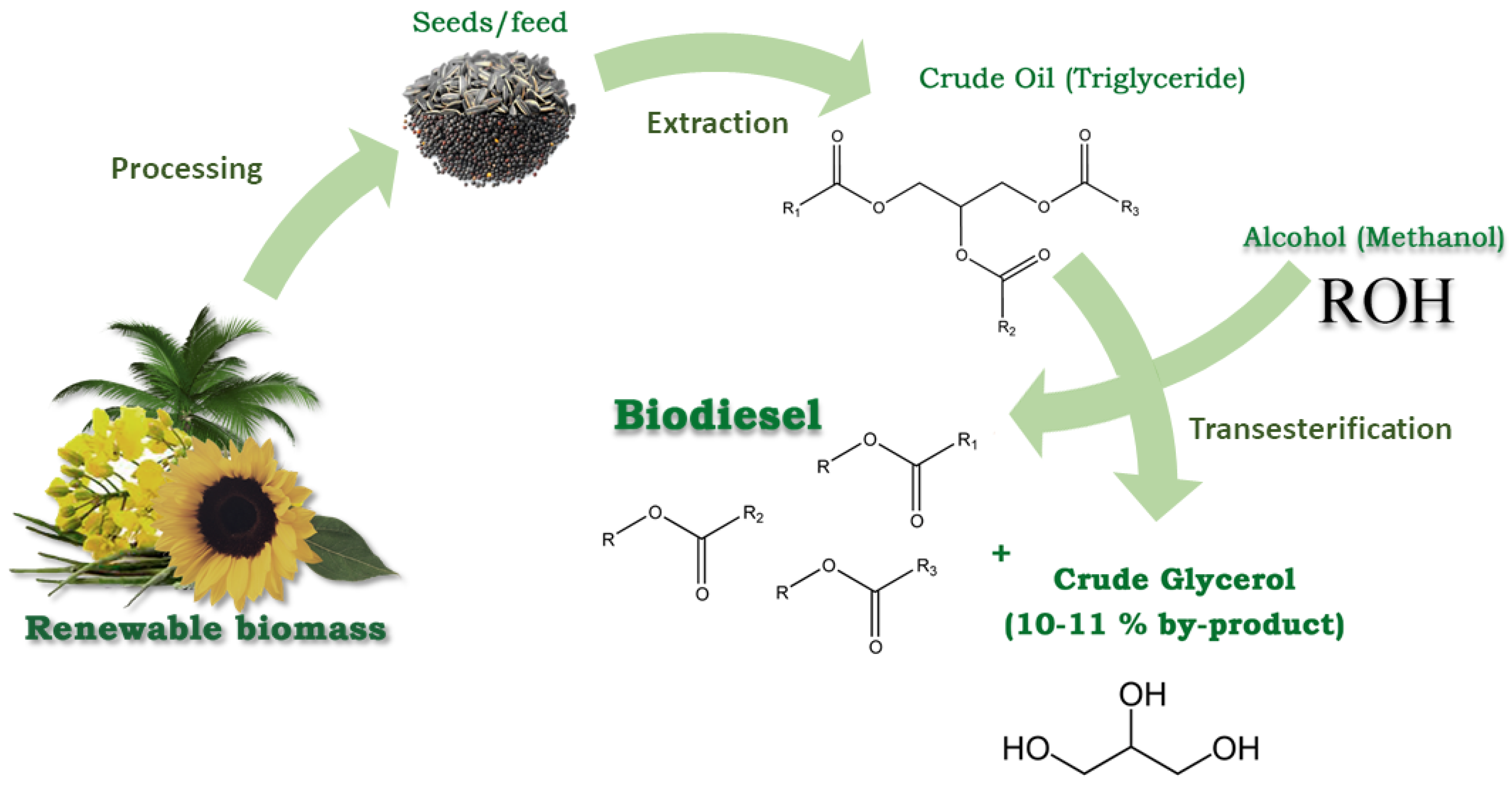
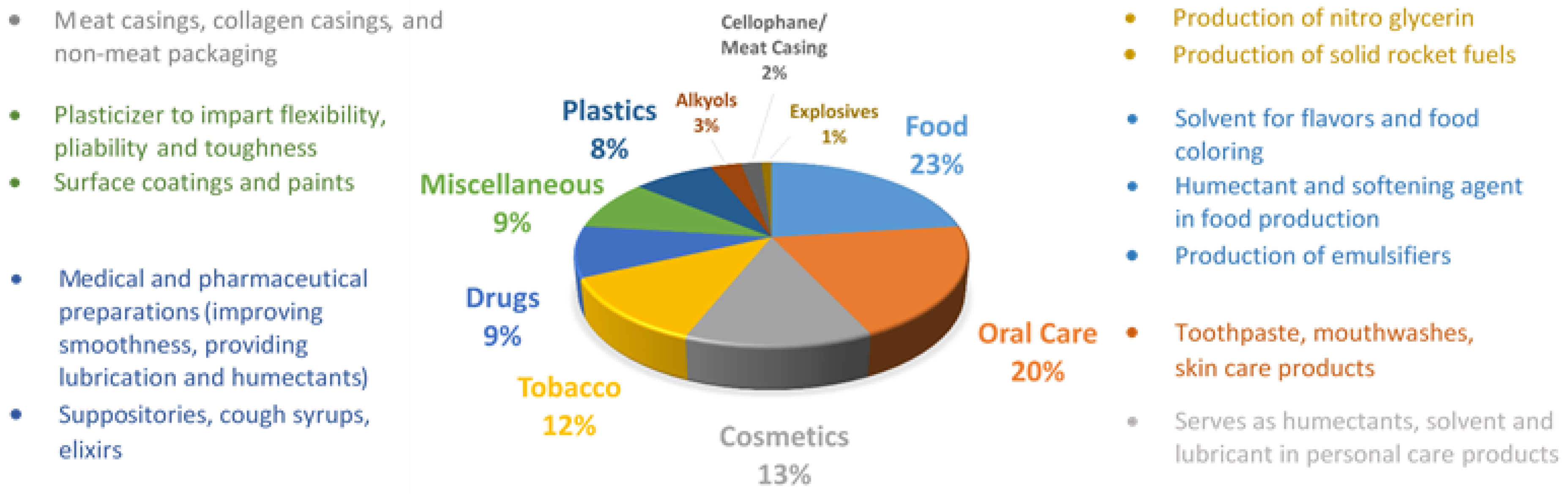
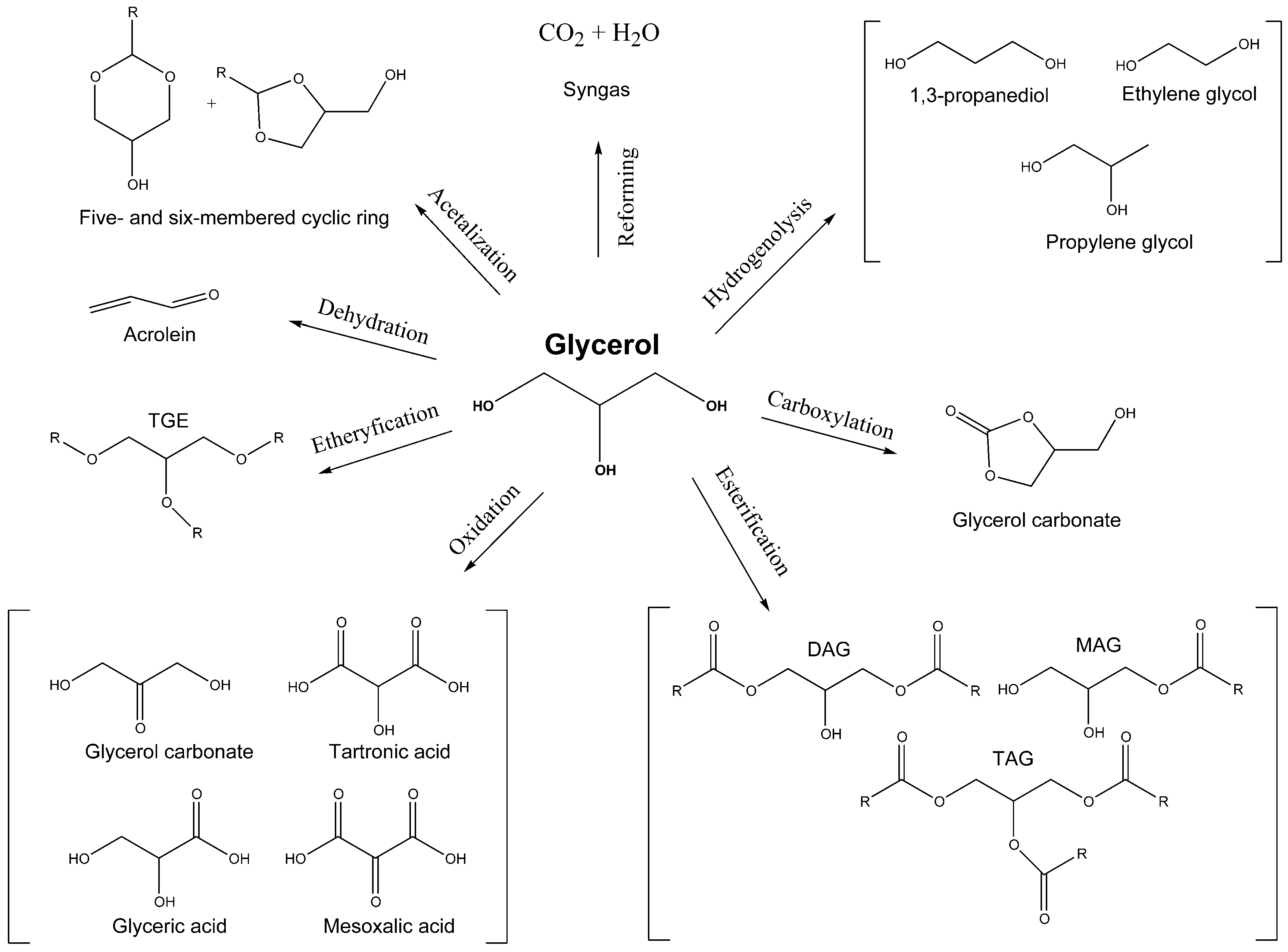
2. Glycerol Production, Consumption and Characterization
3. Acetals Derived from Glycerin as a New Oxygenated Fuel Additives
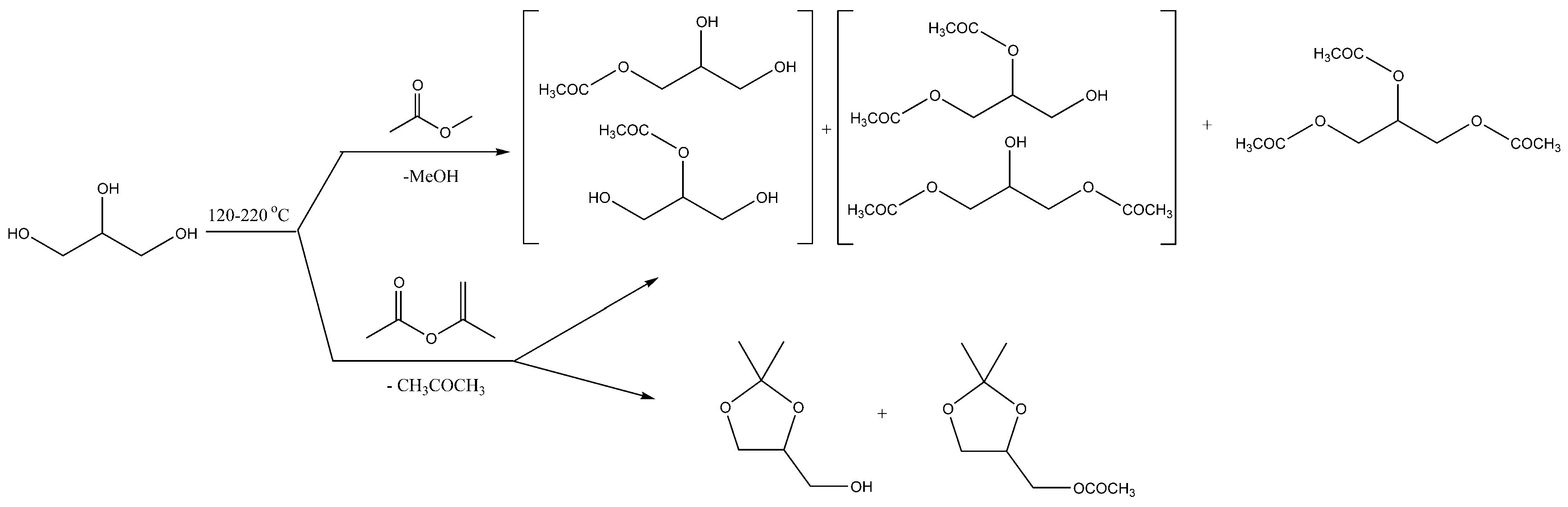
4. Catalyst-Free Acetalization of Glycerol
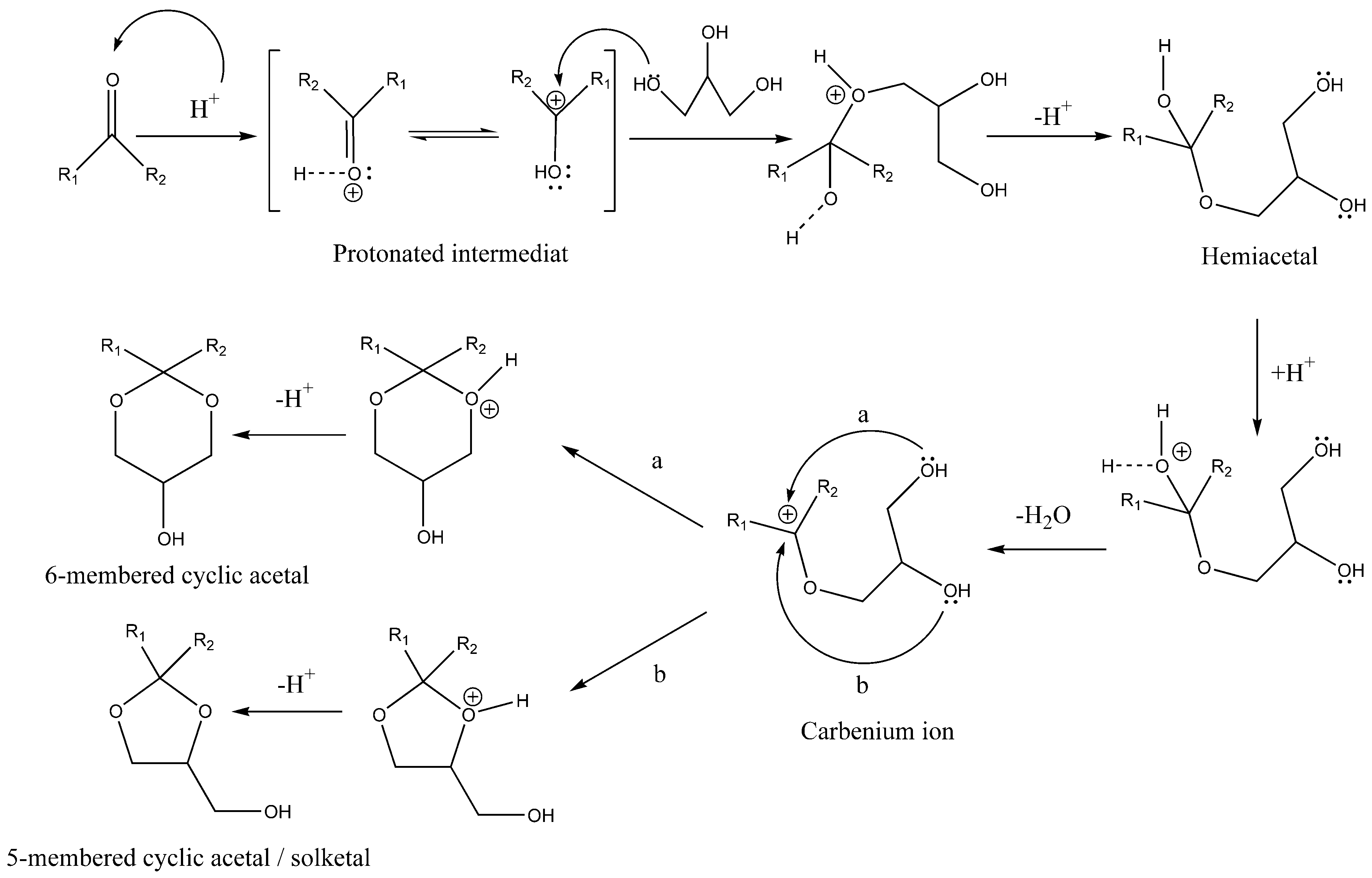
5. Homogeneous Catalysts for Acetalization
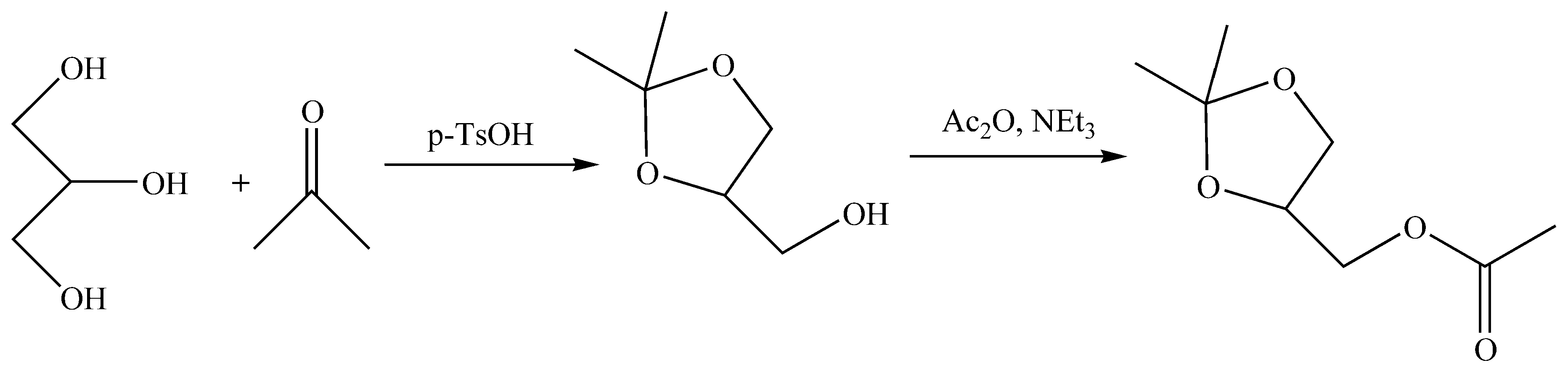
6. Heterogeneous Catalysts for Glycerol Acetalization
7. Conclusions
- -
- The use of microwave-assisted reaction or supercritical conditions makes it possible to obtain high acetal formation rates without using a catalyst.
- -
- The formation of water during acetalization decreases the rate of acetal formation, which requires the development of methods for drying the substrate or the use of heterogeneous catalysts that are resistant to water adsorption on the surface.
- -
- The use of ionic liquids with a complex structure makes it possible to vary the selectivity for the formation of a 5-membered cycle or six-membered cyclic acetals.
- -
- Brønsted acid sites promote the acetalization of glycerol with acetone higher than Lewis acidic sites.
- -
- The acetalization reaction is a reversible process. In order to shift the reaction equilibrium to the formation of acetal or ketal, it is necessary to use an excess of the reagent with respect to glycerol.
- -
- The nature of the reagent and the presence of a solvent can affect the reaction rate. For example, electron donating or electron withdrawing substituent on the molecule and the size of reagent affect the conversion and selectivity. The presence of a solvent can lead to a decrease in the viscosity of the reaction components and increase their diffusion to the active sites.
- -
- For acetalization of glycerol, textural characteristics of the catalyst, such as acidity, pore size, and influence of the promoter, play an important role.
Author Contributions
Funding
Conflicts of Interest
References
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Ibarra-Gonzalez, P.; Rong, B.-G. A review of the current state of biofuels production from lignocellulosic biomass using thermochemical conversion routes. Chin. J. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh-Bandbafha, H.; Tabatabaei, M.; Aghbashlo, M.; Khanali, M.; Demirbas, A. A comprehensive review on the environmental impacts of diesel/biodiesel additives. Energy Convers. Manag. 2018, 174, 579–614. [Google Scholar] [CrossRef]
- Ayhan, V.; Tunca, S. Experimental investigation on using emulsified fuels with different biofuel additives in a DI diesel engine for performance and emissions. Appl. Therm. Eng. 2018, 129, 841–854. [Google Scholar] [CrossRef]
- Suresh, M.; Jawahar, C.P.; Richard, A. A review on biodiesel production, combustion, performance, and emission characteristics of non-edible oils in variable compression ratio diesel engine using biodiesel and its blends. Renew. Sustain. Energy Rev. 2018, 92, 38–49. [Google Scholar] [CrossRef]
- Mohd Noor, C.W.; Noor, M.M.; Mamat, R. Biodiesel as alternative fuel for marine diesel engine applications: A review. Renew. Sustain. Energy Rev. 2018, 94, 127–142. [Google Scholar] [CrossRef]
- Chen, S.; Pan, X.; Miao, C.; Xie, H.; Zhou, G.; Jiao, Z.; Zhang, X. Study of catalytic hydrodeoxygenation performance for the Ni/KIT-6 catalysts. J. Saudi Chem. Soc. 2018, 22, 614–627. [Google Scholar] [CrossRef]
- Hachemi, I.; Jeništová, K.; Mäki-Arvela, P.; Kumar, N.; Eränen, K.; Hemming, J.; Murzin, D.Y. Comparative study of sulfur-free nickel and palladium catalysts in hydrodeoxygenation of different fatty acid feedstocks for production of biofuels. Catal. Sci. Technol. 2016, 6, 1476–1487. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Boi, L.V.; Maeda, Y. Catalytic Technologies for Biodiesel Fuel Production and Utilization of Glycerol: A Review. Catalysts 2012, 2, 191–222. [Google Scholar] [CrossRef]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Li, R.; Song, H.; Chen, J. Propylsulfonic Acid Functionalized SBA-15 Mesoporous Silica as Efficient Catalysts for the Acetalization of Glycerol. Catalysts 2018, 8, 297. [Google Scholar] [CrossRef]
- Sun, S.; He, M.; Dai, Y.; Li, X.; Liu, Z.; Yao, L. Catalytic Acetalization: An Efficient Strategy for High-Value Utilization of Biodiesel-Derived Glycerol. Catalysts 2017, 7, 184. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Shay, E.G. Diesel fuel from vegetable oils: Status and opportunities. Biomass Bioenergy 1993, 4, 227–242. [Google Scholar] [CrossRef]
- Chang, A.-F.; Liu, Y.A. Integrated Process Modeling and Product Design of Biodiesel Manufacturing. Ind. Eng. Chem. Res. 2010, 49, 1197–1213. [Google Scholar] [CrossRef]
- Asal, S.; Marcus, R.; Hilbert, J.A. Opportunities for and obstacles to sustainable biodiesel production in Argentina. Energy Sustain. Dev. 2006, 10, 48–58. [Google Scholar] [CrossRef]
- Da Silva César, A.; Conejero, M.A.; Barros Ribeiro, E.C.; Batalha, M.O. Competitiveness analysis of “social soybeans” in biodiesel production in Brazil. Renew. Energy 2018. [Google Scholar] [CrossRef]
- Shankar, A.A.; Pentapati, P.R.; Prasad, R.K. Biodiesel synthesis from cottonseed oil using homogeneous alkali catalyst and using heterogeneous multi walled carbon nanotubes: Characterization and blending studies. Egypt. J. Pet. 2017, 26, 125–133. [Google Scholar] [CrossRef]
- Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar] [CrossRef]
- Vicente, G.; Martinez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Han, Y.; Wang, H. Magnetic Fe3O4/MCM-41 composite-supported sodium silicate as heterogeneous catalysts for biodiesel production. Renew. Energy 2018, 125, 675–681. [Google Scholar] [CrossRef]
- Singh, V.; Belova, L.; Singh, B.; Sharma, Y.C. Biodiesel production using a novel heterogeneous catalyst, magnesium zirconate (Mg2Zr5O12): Process optimization through response surface methodology (RSM). Energy Convers. Manag. 2018, 174, 198–207. [Google Scholar] [CrossRef]
- Kaur, M.; Malhotra, R.; Ali, A. Tungsten supported Ti/SiO2 nanoflowers as reusable heterogeneous catalyst for biodiesel production. Renew. Energy 2018, 116, 109–119. [Google Scholar] [CrossRef]
- Christopher, L.P.; Hemanathan, K.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Xu, W.; Gao, L.; Wang, S.; Xiao, G. Biodiesel production in a membrane reactor using MCM-41 supported solid acid catalyst. Bioresour. Technol. 2014, 159, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Bautista, L.F.; Iglesias, J.; Morales, G.; Sánchez-Vazquez, R. Production of biodiesel from waste cooking oil in a continuous packed bed reactor with an agglomerated Zr-SBA-15/bentonite catalyst. Appl. Catal. B Environ. 2014, 145, 197–204. [Google Scholar] [CrossRef]
- Benavides, P.T.; Diwekar, U. Studying various optimal control problems in biodiesel production in a batch reactor under uncertainty. Fuel 2013, 103, 585–592. [Google Scholar] [CrossRef]
- Hu, S.; Luo, X.; Wan, C.; Li, Y. Characterization of Crude Glycerol from Biodiesel Plants. J. Agric. Food Chem. 2012, 60, 5915–5921. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.C.; Ooi, T.L.; Dzulkefly, K.; Wan Yunus, W.M.Z.; Hassan, H.A. Refining of crude glycerine recovered from glycerol residue by simple vacuum distillation. J. Oil Palm Res. 2001, 13, 39–44. [Google Scholar]
- Kale, S.; Umbarkar, S.B.; Dongare, M.K.; Eckelt, R.; Armbruster, U.; Martin, A. Selective formation of triacetin by glycerol acetylation using acidic ion-exchange resins as catalyst and toluene as an entrainer. Appl. Catal. A Gen. 2015, 490, 10–16. [Google Scholar] [CrossRef]
- Manosak, R.; Limpattayanate, S.; Hunsom, M. Sequential-refining of crude glycerol derived from waste used-oil methyl ester plant via a combined process of chemical and adsorption. Fuel Process. Technol. 2011, 92, 92–99. [Google Scholar] [CrossRef]
- Díaz-Álvarez, A.E.; Francos, J.; Lastra-Barreira, B.; Crochet, P.; Cadierno, V. Glycerol and derived solvents: New sustainable reaction media for organic synthesis. Chem. Commun. 2011, 47, 6208–6227. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.S.; Aroua, M.K.; Daud, W.M.A.W. Conversion of crude and pure glycerol into derivatives: A feasibility evaluation. Renew. Sustain. Energy Rev. 2016, 63, 533–555. [Google Scholar] [CrossRef]
- Priya, S.S.; Bhattacharya, S. Toward the Sustainable Synthesis of Propanols from Renewable Glycerol over MoO3-Al2O3 Supported Palladium Catalysts. Catalysts 2018, 8, 385. [Google Scholar]
- Pala Rosas, I.; Contreras, J.; Salmones, J.; Tapia, C.; Zeifert, B.; Navarrete, J.; Vázquez, T.; García, D. Catalytic Dehydration of Glycerol to Acrolein over a Catalyst of Pd/LaY Zeolite and Comparison with the Chemical Equilibrium. Catalysts 2017, 7, 73. [Google Scholar] [CrossRef]
- Kumar, G.S.; Wee, Y.; Lee, I.; Sun, H.J.; Zhao, X.; Xia, S.; Kim, S.; Lee, J.; Wang, P.; Kim, J. Stabilized glycerol dehydrogenase for the conversion of glycerol to dihydroxyacetone. Chem. Eng. J. 2015, 276, 283–288. [Google Scholar] [CrossRef]
- Hong, C.S.; Chin, S.Y.; Cheng, C.K.; Sabri, M.M.; Chua, G.K. Enzymatic Conversion of Glycerol to Glyceric Acid with Immobilised Laccase in Na-Alginate Matrix. Procedia Chem. 2015, 16, 632–639. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.; García-Moreno, L.; Vizcaíno, A. Production of Renewable Hydrogen from Glycerol Steam Reforming over Bimetallic Ni-(Cu,Co.,Cr) Catalysts Supported on SBA-15 Silica. Catalysts 2017, 7, 55. [Google Scholar] [CrossRef]
- Delparish, A.; Koc, S.; Caglayan, B.S.; Avci, A.K. Oxidative steam reforming of glycerol to synthesis gas in a microchannel reactor. Catal. Today 2018. [Google Scholar] [CrossRef]
- Ratchadapiban, K.; Praserthdam, P.; Tungasmita, D.; Tangku, C.; Anutrasakda, W. Effect of Surface Modifications of SBA-15 with Aminosilanes and 12-Tungstophosphoric Acid on Catalytic Properties in Environmentally Friendly Esterification of Glycerol with Oleic Acid to Produce Monoolein. Catalysts 2018, 8, 360. [Google Scholar] [CrossRef]
- Tangestanifard, M.; Ghaziaskar, H. Arenesulfonic Acid-Functionalized Bentonite as Catalyst in Glycerol Esterification with Acetic Acid. Catalysts 2017, 7, 211. [Google Scholar] [CrossRef]
- Torbina, V.; Vodyankin, A.; Ten, S.; Mamontov, G.; Salaev, M.; Sobolev, V.; Vodyankina, O. Ag-Based Catalysts in Heterogeneous Selective Oxidation of Alcohols: A Review. Catalysts 2018, 8, 447. [Google Scholar] [CrossRef]
- Xue, W.; Wang, Z.; Liang, Y.; Xu, H.; Liu, L.; Dong, J. Promoting Role of Bismuth on Hydrotalcite-Supported Platinum Catalysts in Aqueous Phase Oxidation of Glycerol to Dihydroxyacetone. Catalysts 2018, 8, 20. [Google Scholar] [CrossRef]
- Nanda, M.; Yuan, Z.; Shui, H.; Xu, C. Selective Hydrogenolysis of Glycerol and Crude Glycerol (a By-Product or Waste Stream from the Biodiesel Industry) to 1,2-Propanediol over B2O3 Promoted Cu/Al2O3 Catalysts. Catalysts 2017, 7, 196. [Google Scholar] [CrossRef]
- Da Silva, G.P.; de Lima, C.J.B.; Contiero, J. Production and productivity of 1,3-propanediol from glycerol by Klebsiella pneumoniae GLC29. Catal. Today 2015, 257, 259–266. [Google Scholar] [CrossRef]
- Yang, X.; Choi, H.S.; Lee, J.H.; Lee, S.K.; Han, S.O.; Park, C.; Kim, S.W. Improved production of 1,3-propanediol from biodiesel-derived crude glycerol by Klebsiella pneumoniae in fed-batch fermentation. Chem. Eng. J. 2018, 349, 25–36. [Google Scholar] [CrossRef]
- Yang, X.; Kim, D.S.; Choi, H.S.; Kim, C.K.; Thapa, L.P.; Park, C.; Kim, S.W. Repeated batch production of 1,3-propanediol from biodiesel derived waste glycerol by Klebsiella pneumoniae. Chem. Eng. J. 2017, 314, 660–669. [Google Scholar] [CrossRef]
- Wang, W. Thermodynamic analysis of glycerol partial oxidation for hydrogen production. Fuel Process. Technol. 2010, 91, 1401–1408. [Google Scholar] [CrossRef]
- Lin, Y.-C. Catalytic valorization of glycerol to hydrogen and syngas. Int. J. Hydrogen Energy 2013, 38, 2678–2700. [Google Scholar] [CrossRef]
- Dauenhauer, P.J.; Salge, J.R.; Schmidt, L.D. Renewable hydrogen by autothermal steam reforming of volatile carbohydrates. J. Catal. 2006, 244, 238–247. [Google Scholar] [CrossRef]
- Qing, Y.; Lu, H.; Liu, Y.; Liu, C.; Liang, B.; Jiang, W. Production of glycerol carbonate using crude glycerol from biodiesel production with DBU as a catalyst. Chin. J. Chem. Eng. 2018, 26, 1912–1919. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhang, J.; He, D. Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO2 preparation methods and reaction parameters. Appl. Catal. A Gen. 2016, 513, 9–18. [Google Scholar] [CrossRef]
- Priya, S.S.; Kumar, V.P.; Kantam, M.L.; Bhargava, S.K.; Periasamy, S.; Chary, K.V.R. Metal-acid bifunctional catalysts for selective hydrogenolysis of glycerol under atmospheric pressure: A highly selective route to produce propanols. Appl. Catal. A Gen. 2015, 498, 88–98. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, W.; Xu, Y.; Yang, B.; Wang, Y. Enzymatic Synthesis of Extremely Pure Triacylglycerols Enriched in Conjugated Linoleic Acids. Molecules 2013, 18, 9704–9716. [Google Scholar] [CrossRef] [PubMed]
- Subroto, E.; Wisamputri, M.F.; Utami, T.; Hidayat, C. Enzymatic and chemical synthesis of high mono- and diacylglycerol from palm stearin and olein blend at different type of reactor stirrers. J. Saudi Soc. Agric. Sci. 2018. [Google Scholar] [CrossRef]
- Reinoso, D.M.; Tonetto, G.M. Bioadditives synthesis from selective glycerol esterification over acidic ion exchange resin as catalyst. J. Environ. Chem. Eng. 2018, 6, 3399–3407. [Google Scholar] [CrossRef]
- Okoye, P.U.; Hameed, B.H. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 53, 558–574. [Google Scholar] [CrossRef]
- Melero, J.A.; van Grieken, R.; Morales, G.; Paniagua, M. Acidic Mesoporous Silica for the Acetylation of Glycerol: Synthesis of Bioadditives to Petrol Fuel. Energy Fuels 2007, 21, 1782–1791. [Google Scholar] [CrossRef]
- Izquierdo, J.F.; Montiel, M.; Palés, I.; Outón, P.R.; Galán, M.; Jutglar, L.; Villarrubia, M.; Izquierdo, M.; Hermo, M.P.; Ariza, X. Fuel additives from glycerol etherification with light olefins: State of the art. Renew. Sustain. Energy Rev. 2012, 16, 6717–6724. [Google Scholar] [CrossRef]
- Hernández, D.; Fernández, J.J.; Mondragón, F.; López, D. Production and utilization performance of a glycerol derived additive for diesel engines. Fuel 2012, 92, 130–136. [Google Scholar] [CrossRef]
- Noureddini, H.; Dailey, W.R.; Hunt, B.A. Production of Ethers of Glycerol from Crude Glycerol—The by-Product of Biodlesel Production; Papers in Biomaterials; University of Nebraska: Lincoln, NE, USA, 1998; p. 18. [Google Scholar]
- Melero, J.A.; Vicente, G.; Morales, G.; Paniagua, M.; Bustamante, J. Oxygenated compounds derived from glycerol for biodiesel formulation: Influence on EN 14214 quality parameters. Fuel 2010, 89, 2011–2018. [Google Scholar] [CrossRef]
- Samoilov, V.O.; Ramazanov, D.N.; Nekhaev, A.I.; Maximov, A.L.; Bagdasarov, L.N. Heterogeneous catalytic conversion of glycerol to oxygenated fuel additives. Fuel 2016, 172, 310–319. [Google Scholar] [CrossRef]
- Dodson Jennifer, R.; Avellar, T.; Athayde, J.; Mota Claudio, J.A. Glycerol acetals with antioxidant properties. Pure Appl. Chem. 2014, 86, 905–911. [Google Scholar] [CrossRef]
- García, E.; Laca, M.; Pérez, E.; Garrido, A.; Peinado, J. New Class of Acetal Derived from Glycerin as a Biodiesel Fuel Component. Energy Fuels 2008, 22, 4274–4280. [Google Scholar] [CrossRef]
- Silva, P.H.R.; Gonçalves, V.L.C.; Mota, C.J.A. Glycerol acetals as anti-freezing additives for biodiesel. Bioresour. Technol. 2010, 101, 6225–6229. [Google Scholar] [CrossRef] [PubMed]
- De Torres, M.; Jiménez-osés, G.; Mayoral, J.A.; Pires, E.; de los Santos, M. Glycerol ketals: Synthesis and profits in biodiesel blends. Fuel 2012, 94, 614–616. [Google Scholar] [CrossRef]
- Oprescu, E.-E.; Dragomir, R.E.; Radu, E.; Radu, A.; Velea, S.; Bolocan, I.; Stepan, E.; Rosca, P. Performance and emission characteristics of diesel engine powered with diesel–glycerol derivatives blends. Fuel Process. Technol. 2014, 126, 460–468. [Google Scholar] [CrossRef]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renew. Sustain. Energy Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- De Carvalho, D.C.; Oliveira, A.C.; Ferreira, O.P.; Filho, J.M.; Tehuacanero-Cuapa, S.; Oliveira, A.C. Titanate nanotubes as acid catalysts for acetalization of glycerol with acetone: Influence of the synthesis time and the role of structure on the catalytic performance. Chem. Eng. J. 2017, 313, 1454–1467. [Google Scholar] [CrossRef]
- Umbarkar, S.B.; Kotbagi, T.V.; Biradar, A.V.; Pasricha, R.; Chanale, J.; Dongare, M.K.; Mamede, A.-S.; Lancelot, C.; Payen, E. Acetalization of glycerol using mesoporous MoO3/SiO2 solid acid catalyst. J. Mol. Catal. A Chem. 2009, 310, 150–158. [Google Scholar] [CrossRef]
- Hasabnis, A.; Mahajani, S. Acetalization of Glycerol with Formaldehyde by Reactive Distillation. Ind. Eng. Chem. Res. 2014, 53, 12279–12287. [Google Scholar] [CrossRef]
- Pawar, R.R.; Jadhav, S.V.; Bajaj, H.C. Microwave-assisted rapid valorization of glycerol towards acetals and ketals. Chem. Eng. J. 2014, 235, 61–66. [Google Scholar] [CrossRef]
- Calmanti, R.; Galvan, M.; Amadio, E.; Perosa, A.; Selva, M. High-Temperature Batch and Continuous-Flow Transesterification of Alkyl and Enol Esters with Glycerol and Its Acetal Derivatives. ACS Sustain. Chem. Eng. 2018, 6, 3964–3973. [Google Scholar] [CrossRef]
- Royon, D.; Locatelli, S.; Gonzo, E.E. Ketalization of glycerol to solketal in supercritical acetone. J. Supercrit. Fluids 2011, 58, 88–92. [Google Scholar] [CrossRef]
- ToddColeman, A.B. Process for the Preparation of Glycerol Formal. U.S. Patent 20,100,094,027, 15 April 2010. [Google Scholar]
- Sato, S.; Araujo, A.S.; Miyano, M. Process for the Production of Glycerol Acetals. U.S. Patent 20,080,207,927, 28 August 2008. [Google Scholar]
- Ch, V.L.; Ravuru, U.; Kotra, V.; Bankupalli, S.; Prasad, R.B.N. Novel Route for Recovery of Glycerol from Aqueous Solutions by Reversible Reactions. Int. J. Chem. React. Eng. 2009, 7, 1–16. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C.C. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel 2014, 117, 470–477. [Google Scholar] [CrossRef]
- Maksimov, A.L.; Nekhaev, A.I.; Ramazanov, D.N.; Arinicheva, Y.A.; Dzyubenko, A.A.; Khadzhiev, S.N. Preparation of high-octane oxygenate fuel components from plant-derived polyols. Pet. Chem. 2011, 51, 61–69. [Google Scholar] [CrossRef]
- Trifoi, A.R.; Agachi, P.Ş.; Pap, T. Glycerol acetals and ketals as possible diesel additives. A review of their synthesis protocols. Renew. Sustain. Energy Rev. 2016, 62, 804–814. [Google Scholar] [CrossRef]
- Calvino-Casilda, V.; Stawicka, K.; Trejda, M.; Ziolek, M.; Bañares, M.A. Real-Time Raman Monitoring and Control of the Catalytic Acetalization of Glycerol with Acetone over Modified Mesoporous Cellular Foams. J. Phys. Chem. C 2014, 118, 10780–10791. [Google Scholar] [CrossRef]
- Serafim, H.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorization of glycerol into fuel additives over zeolites as catalysts. Chem. Eng. J. 2011, 178, 291–296. [Google Scholar] [CrossRef]
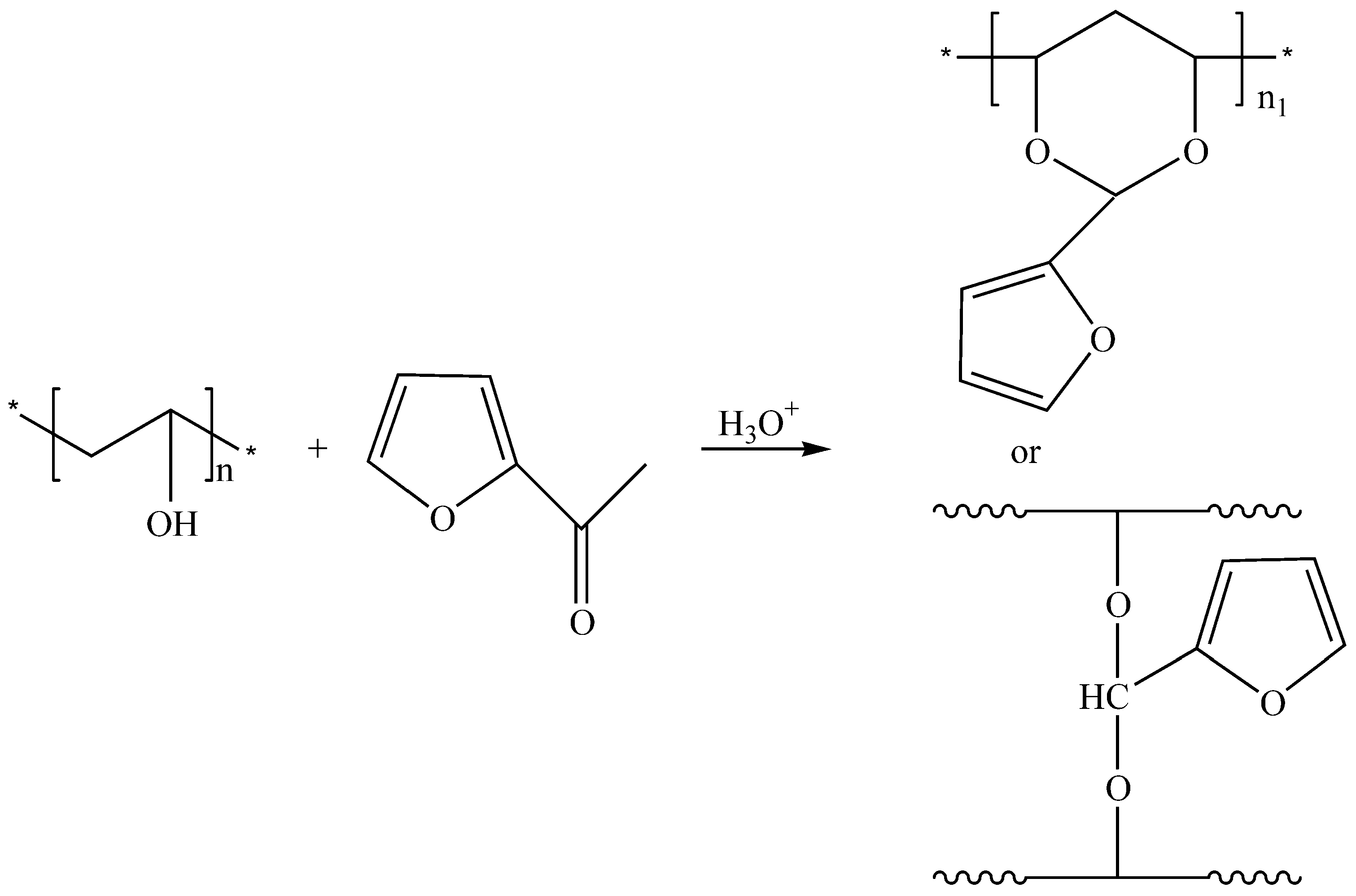
- Gousse, C.; Gandini, A. Acetalization of polyvinyl alcohol with furfural. Eur. Polym. J. 1997, 33, 667–671. [Google Scholar] [CrossRef]
- Li, D.; Shi, F.; Peng, J.; Guo, S.; Deng, Y. Application of Functional Ionic Liquids Possessing Two Adjacent Acid Sites for Acetalization of Aldehydes. J. Org. Chem. 2004, 69, 3582–3585. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, Y.; Sun, J.; Xu, F.; Sun, R. Conversion of platform chemical glycerol to cyclic acetals promoted by acidic ionic liquids. RSC Adv. 2014, 4, 18917–18923. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Hirao, M.; Ito-Akita, K.; Ohno, H. Ion conduction in zwitterionic-type molten salts and their polymers. J. Mater. Chem. 2001, 11, 1057–1062. [Google Scholar] [CrossRef]
- Gomes, I.S.; de Carvalho, D.C.; Oliveira, A.C.; Rodríguez-Castellón, E.; Tehuacanero-Cuapa, S.; Freire, P.T.C.; Filho, J.M.; Saraiva, G.D.; de Sousa, F.F.; Lang, R. On the reasons for deactivation of titanate nanotubes with metals catalysts in the acetalization of glycerol with acetone. Chem. Eng. J. 2018, 334, 1927–1942. [Google Scholar] [CrossRef]
- Ruiz, V.R.; Velty, A.; Santos, L.L.; Leyva-Pérez, A.; Sabater, M.J.; Iborra, S.; Corma, A. Gold catalysts and solid catalysts for biomass transformations: Valorization of glycerol and glycerol–water mixtures through formation of cyclic acetals. J. Catal. 2010, 271, 351–357. [Google Scholar] [CrossRef]
- Kapkowski, M.; Ambrożkiewicz, W.; Siudyga, T.; Sitko, R.; Szade, J.; Klimontko, J.; Balin, K.; Lelątko, J.; Polanski, J. Nano silica and molybdenum supported Re, Rh, Ru or Ir nanoparticles for selective solvent-free glycerol conversion to cyclic acetals with propanone and butanone under mild conditions. Appl. Catal. B Environ. 2017, 202, 335–345. [Google Scholar] [CrossRef]
- Kapkowski, M.; Popiel, J.; Siudyga, T.; Dzida, M.; Zorębski, E.; Musiał, M.; Sitko, R.; Szade, J.; Balin, K.; Klimontko, J.; et al. Mono- and bimetallic nano-Re systems doped Os, Mo, Ru, Ir as nanocatalytic platforms for the acetalization of polyalcohols into cyclic acetals and their applications as fuel additives. Appl. Catal. B Environ. 2018, 239, 154–167. [Google Scholar] [CrossRef]
- Deutsch, J.; Martin, A.; Lieske, H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Nandan, D.; Sreenivasulu, P.; Sivakumar Konathala, L.N.; Kumar, M.; Viswanadham, N. Acid functionalized carbon–silica composite and its application for solketal production. Microporous Mesoporous Mater. 2013, 179, 182–190. [Google Scholar] [CrossRef]
- Faria, R.P.V.; Pereira, C.S.M.; Silva, V.M.T.M.; Loureiro, J.M.; Rodrigues, A.E. Glycerol Valorization as Biofuel: Thermodynamic and Kinetic Study of the Acetalization of Glycerol with Acetaldehyde. Ind. Eng. Chem. Res. 2013, 52, 1538–1547. [Google Scholar] [CrossRef]
- Agirre, I.; Güemez, M.B.; Ugarte, A.; Requies, J.; Barrio, V.L.; Cambra, J.F.; Arias, P.L. Glycerol acetals as diesel additives: Kinetic study of the reaction between glycerol and acetaldehyde. Fuel Process. Technol. 2013, 116, 182–188. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C. Catalytic conversion of glycerol to oxygenated fuel additive in a continuous flow reactor: Process optimization. Fuel 2014, 128, 113–119. [Google Scholar] [CrossRef]
- Shirani, M.; Ghaziaskar, H.S.; Xu, C. Optimization of glycerol ketalization to produce solketal as biodiesel additive in a continuous reactor with subcritical acetone using Purolite® PD206 as catalyst. Fuel Process. Technol. 2014, 124, 206–211. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorisation of glycerol by condensation with acetone over silica-included heteropolyacids. Appl. Catal. B Environ. 2010, 98, 94–99. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Kaliaguine, S. Glycerol acetalization with formaldehyde using water-tolerant solid acids. Appl. Catal. A Gen. 2016, 509, 143–152. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Glycerol acetalization with formaldehyde using heteropolyacid salts supported on mesostructured silica. Appl. Catal. A Gen. 2018, 549, 207–215. [Google Scholar] [CrossRef]
- Fan, C.-N.; Xu, C.-H.; Liu, C.-Q.; Huang, Z.-Y.; Liu, J.-Y.; Ye, Z.-X. Catalytic acetalization of biomass glycerol with acetone over TiO2–SiO2 mixed oxides. React. Kinet. Mech. Catal. 2012, 107, 189–202. [Google Scholar] [CrossRef]
- Arias, K.S.; Garcia-Ortiz, A.; Climent, M.J.; Corma, A.; Iborra, S. Mutual Valorization of 5-Hydroxymethylfurfural and Glycerol into Valuable Diol Monomers with Solid Acid Catalysts. ACS Sustain. Chem. Eng. 2018, 6, 4239–4245. [Google Scholar] [CrossRef]
- Konwar, L.J.; Samikannu, A.; Mäki-Arvela, P.; Boström, D.; Mikkola, J.-P. Lignosulfonate-based macro/mesoporous solid protonic acids for acetalization of glycerol to bio-additives. Appl. Catal. B Environ. 2018, 220, 314–323. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Z.; Ao, Y. Design of highly efficient Zn-, Cu-, Ni- and Co-promoted M-AlPO4 solid acids: The acetalization of glycerol with acetone. Appl. Catal. A Gen. 2015, 496, 32–39. [Google Scholar] [CrossRef]
- Li, Z.; Miao, Z.; Wang, X.; Zhao, J.; Zhou, J.; Si, W.; Zhuo, S. One-pot synthesis of ZrMo-KIT-6 solid acid catalyst for solvent-free conversion of glycerol to solketal. Fuel 2018, 233, 377–387. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Khan, N.A.; Hasan, Z.; Prosvirin, I.P.; Tsybulya, S.V.; Jhung, S.H. Isostructural metal-carboxylates MIL-100(M) and MIL-53(M) (M: V, Al, Fe and Cr) as catalysts for condensation of glycerol with acetone. Appl. Catal. A Gen. 2017, 529, 167–174. [Google Scholar] [CrossRef]
- Li, X.; Zheng, L.; Hou, Z. Acetalization of glycerol with acetone over Co.[II](Co.[III]xAl2−x)O4 derived from layered double hydroxide. Fuel 2018, 233, 565–571. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Hameed, B.H. Solventless acetalization of glycerol with acetone to fuel oxygenates over Ni–Zr supported on mesoporous activated carbon catalyst. Appl. Catal. A Gen. 2013, 464–465, 191–199. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Abbas, A.; Hameed, B.H.; Triwahyono, S.; Jalil, A.A.; Harris, A.T.; Minett, A.I. Selective Acetalization of Glycerol with Acetone Over Nickel Nanoparticles Supported on Multi-Walled Carbon Nanotubes. Catal. Lett. 2014, 144, 1009–1015. [Google Scholar] [CrossRef]
- Priya, S.S.; Selvakannan, P.R.; Chary, K.V.R.; Kantam, M.L.; Bhargava, S.K. Solvent-free microwave-assisted synthesis of solketal from glycerol using transition metal ions promoted mordenite solid acid catalysts. Mol. Catal. 2017, 434, 184–193. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Mallesham, B.; Prasad, A.N.; Reddy, P.S.; Reddy, B.M. Synthesis of bio–additive fuels from acetalization of glycerol with benzaldehyde over molybdenum promoted green solid acid catalysts. Fuel Process. Technol. 2013, 106, 539–545. [Google Scholar] [CrossRef]
- Reddy, P.S.; Sudarsanam, P.; Mallesham, B.; Raju, G.; Reddy, B.M. Acetalisation of glycerol with acetone over zirconia and promoted zirconia catalysts under mild reaction conditions. J. Ind. Eng. Chem. 2011, 17, 377–381. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Raju, G.; Reddy, B.M. Design of highly efficient Mo and W-promoted SnO2 solid acids for heterogeneous catalysis: Acetalization of bio-glycerol. Green Chem. 2013, 15, 478–489. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy, B.M. Eco-friendly synthesis of bio-additive fuels from renewable glycerol using nanocrystalline SnO2-based solid acids. Catal. Sci. Technol. 2014, 4, 803–813. [Google Scholar] [CrossRef]

| Entry | Substrate | Sub/Gly Molar Ratio | Condition | Time (min) | X 1 (%) | Select. 6-M/5-M 2 (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | acetone | 1:1 | 110 °C, 150 rpm | 360 | 0.01 | NA | [75] |
| 2 | benzaldehyde | 0.1:0.11 | 100 °C, toluene | 480 | 20 | 49/51 | [76] |
| 3 | formaldehyde | 1:1 | 75 °С, 1200 rpm | 120 | 21 | NA | [77] |
| 4 | benzaldehyde | 1:2 | 600 W microwave, 140 °C | 15 | 95 | 44/56 | [78] |
| 5 | chlorobenzaldehyde | 1:2 | 600 W microwave, 140 °C | 15 | 84 | 46/54 | [78] |
| 6 | 3,4-dimethoxybenzaldehyde | 1:2 | 600 W microwave, 140 °C | 15 | 96 | 47/53 | [78] |
| 7 | cyclohexanone | 1:2 | 600 W microwave, 140 °C | 15 | 90 | 2/98 | [78] |
| 8 | 2-methylcyclohexanone | 1:2 | 600 W microwave, 140 °C | 15 | 60 | 78/22 | [78] |
| 9 | butanal | 22.8:21.7 | 70 °C, dimethylsulfoxide | 120 | 20 | NA | [71] |
| 10 | hexanal | 22.8:21.7 | 70 °C, dimethylsulfoxide | 120 | 10 | NA | [71] |
| 11 | decanal | 22.8:21.7 | 70 °C, dimethylsulfoxide | 120 | 5 | NA | [71] |
| 12 | isopropenyl acetate | 1:1 | 180 °C, 0.8 MPa | 300 | 73 | 0/44 | [79] |
| 13 | isopropenyl acetate | 5:1 | 180 °C, 0.8 MPa | 300 | 73 | 0/29 | [79] |
| 14 | acetone | 2.1 | 250 °С, 8 MPa | 240 | 13.5 | 0/75 | [80] |
| 15 | acetone | 3.6 | 250 °С, 8 MPa | 240 | 23 | 0/81 | [80] |
| 16 | acetone | 10.8 | 250 °С, 8 MPa | 240 | 28.2 | 0/80 | [80] |
| Entry | Catalyst | Yield 5-M, % | Yield 6-M, % |
|---|---|---|---|
| 1 | No catalyst | 12 | 4 |
| 2 | [BSPy]HSO4 | 26 | 44 |
| 3 | [BSMim]CF3SO3 | 22 | 46 |
| 4 | [BSMim]HSO4 | 26 | 42 |
| 5 | [Bpy]HSO4 | 28 | 47 |
| 6 | [HMim]HSO4 | 29 | 35 |
| 7 | H2SO4 | 21 | 34 |
| 8 | HCl | 20 | 33 |
| 9 | NH2SO3H | 21 | 38 |
| 10 | 4-toluenesulfonic acid | 14 | 17 |
| Entry | Catalyst | Conversion of glycerol, % | Yield of ketals, % | Selectivity, (6-M/5-M) |
|---|---|---|---|---|
| 1 | [MeSO3bmim][MeSO4] | 86 | 84 | 28/69 |
| 2 | [MeSO3Py][MeSO4] | 83 | 80 | 26/70 |
| 3 | [MeSO3bm3N][MeSO4] | 86 | 83 | 26/71 |
| 4 | [EtSO3bmim]EtSO4] | 81 | 78 | 27/70 |
| 5 | [HSO3bmim][HSO4] | 84 | 81 | 24/73 |
| 6 | [bmim][MeSO4] | 80 | 78 | 17/80 |
| 7 | [bmim][EtSO4] | 73 | 71 | 16/82 |
| 8 | [MeSO3bmim][MeSO4] | 85 | 83 | 29/69 |
| 9 | [MeSO3bmim][MeSO4] | 87 | 83 | 28/69 |
| Entry | Catalyst | Substrate | Sub/Gly Molar Ratio | Condition | Time (h) | X 1 (%) | Select. 6-M/5-M 2 (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Pt-TNT | acetone | 1:1 | 50 °С, 0.1 MPa, 150 rpm, mcat = 130 mg. | 6 | 40 | 0/30 | [93] |
| 2 | Pt-TNT | acetone | 4:1 | 50 °С, 0.1 MPa, 150 rpm, mcat = 130 mg. | 6 | 87 | 0/20 | [93] |
| 3 | Pt-TNT | acetone | 8:1 | 50 °С, 0.1 MPa, 150 rpm, mcat = 130 mg. | 6 | 78 | 0/0 | [93] |
| 4 | AuCl3 | trioxane | 1:1 | 80 °С, 0.1 MPa, dioxane as a solvent, 5 mol% of cat. | 4 | NA 3 (93) | NA 3 (59/34) | [94] |
| 5 | AuCl3 | trioxane | 1:1 | 80 °С, 0.1 MPa, dioxane as a solvent, 2 mol% of cat. | 7 | NA 3 (92) | NA 3 (61/31) | [94] |
| 6 | AuCl3 | formaldehyde | 3:1 | 60 °С, 0.1 MPa, dioxane as solvent, 2 mol% of cat. | 3 | NA 3 (50) | NA 3 (37/13) | [94] |
| 7 | AuPPh3NTf2 | n-heptanal | 1:1 | 22 °С, 0.1 MPa, 75 mol.% of acetonitrile, 5 mol% of cat., after 7 times of the use. | 24 | NA 3 (90) | NA 3 (40/50) | [94] |
| 8 | 1.0% Re/SiO2 | acetone | 10:1 | 30 °С, 0.1 MPa, 200 rpm, ultrasounds for 10 min, mcat = 20 mg | 1 | 100 | 2/97 | [95] |
| 9 | 1.0% Re/SiO2 | acetone | 10:1 | 55 °С, 0.1 MPa, 200 rpm, ultrasounds for 10 min, mcat = 50 mg | 3 | 100 | 3/94 | [95] |
| 10 | 1.0% Re/Mo | acetone | 10:1 | 55 °С, 0.1 MPa, 200 rpm, ultrasounds for 10 min, mcat = 50 mg | 3 | 20 | 25/48 | [95] |
| Entry | Catalyst | Acidity (mmol/g) | Substrate | Sub/Gly Molar Ratio | Conditions | Time (h) | X 1 (%) | Select. 6-M/5-M 2 (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | USY1 | 2.68 | butanal | 0.11:0.04 | 70 °С, 0.1 MPa, mcat = 0.3 g. | 4 | 45 | 19/81 | [88] |
| 2 | USY2 | 1.28 | butanal | 0.11:0.04 | 70 °С, 0.1 MPa, mcat = 0.3 g. | 4 | 76 | 23/77 | [88] |
| 3 | USY3 | 0.53 | butanal | 0.11:0.04 | 70 °С, 0.1 MPa, mcat = 0.3 g. | 4 | 84 | 22/78 | [88] |
| 4 | USY4 | 0.2 | butanal | 0.11:0.04 | 70 °С, 0.1 MPa, mcat = 0.3 g. | 4 | 72 | 20/80 | [88] |
| 5 | BEA | 0.41 | butanal | 0.11:0.04 | 70 °С, 0.1 MPa, mcat = 0.3 g. | 4 | 87 | 21/79 | [88] |
| 6 | Amberlyst-15 | 1.5 | butanal | 1:1.05 | 85 °С, 0.1 MPa, dimethylsulfoxide as a solvent, mcat = 0.36 g | 2 | 80 | 21/79 | [71] |
| 7 | Amberlyst-36 | 5.4 | benzaldehyde | 0.1:0.11 | 61,2 °С, 0.1 MPa, refluxing chloroform as a solvent, mcat = 0.1 g | 4 | NA 3 (94) | NA 3 (57/37) | [97] |
| 8 | Nafion-H NR-50 | 0.8 | benzaldehyde | 0.1:0.11 | 61,2 °С, 0.1 MPa, refluxing chloroform as a solvent, mcat = 0.1 g | 4 | NA 3 (94) | NA 3 (49/45) | [97] |
| 9 | Amberlyst-36 | 5.4 | benzaldehyde dimethyl acetal | 0.1:0.11 | 20 °С, 0.1 MPa, dichloromethane and methanol as a solvent, mcat = 0.1 g | 8 | NA 3 (81) | NA 3 (48/33) | [97] |
| 10 | Nafion-H NR-50 (0.8) | 0.8 | benzaldehyde dimethyl acetal | 0.1:0.11 | 20 °С, 0.1 MPa, dichloromethane and methanol as a solvent, mcat = 0.1 g | 8 | NA 3 (7) | NA 3 (-/-) | [97] |
| 11 | SCS1/2 | 1.35 | acetone | 6: 1 | 70 °С, 0.1 MPa, mcat = 0.25 g | 0.5 | 75 | 0/90 | [98] |
| 12 | HSCS1/2 | 2.25 | acetone | 6: 1 | 70 °С, 0.1 MPa, mcat = 0.25 g | 0.5 | 82 | 0/99 | [98] |
| 13 | Amberlyst-15 | 5.5 | acetone | 4:1 | 25 °С, 0.1 MPa, WHSV = 2 h−1, mcat = 0.25 g | - | NA | NA 3 0/94 | [101] |
| 14 | Purolite® PD206 | NA | acetone | 5:1 | 20 °C, 120 bar, 0.1 mL·min−1 mcat = 0.77 g | - | 95 | 0/100 | [102] |
| 15 | TiO2-SiO2 | 0.11 | acetone | 4:1 | 90 °С, 0.1 MPa, mcat = 0.22 g | 3 | 95 | 0/90 | [106] |
| 16 | HBet | NA | 5-hydroxymethylfurfural | 1:2 | 83 °С, 0.1 MPa, 1000 rpm, mcat = 0.025 g | 8 | 60 | 22/71 | [107] |
| 17 | USY | NA | 5-hydroxymethylfurfural | 1:2 | 83 °С, 0.1 MPa, 1000 rpm, mcat = 0.025 g | 8 | 68 | 22/78 | [107] |
| 18 | ITQ-2 | NA | 5-hydroxymethylfurfural | 1:2 | 83 °С, 0.1 MPa, 1000 rpm, mcat = 0.025 g | 3 | 98 | 26/74 | [107] |
| 19 | MCM-41 | NA | 5-hydroxymethylfurfural | 1:2 | 83 °С, 0.1 MPa, 1000 rpm, mcat = 0.025 g | 8 | 94 | 20/80 | [107] |
| 20 | 80LS20PS450H+ | 3.49 | furfural | 4:1 | 100 °С, 0.1 MPa, 300 rpm, mcat = 0.5 wt % | 1 | 100 | 49/51 | [108] |
| Entry | Catalyst | Substrate | Sub/Gly Molar Ratio | Condition | Time (h) | X 1 (%) | Select. 6-M/5-M 2 (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | M-NiAlPO4 | acetone | 8:1 | 80 °С, mcat = 0.2 g | 1 | NA | NA 3 (0/75) | [109] |
| 2 | M-CuAlPO4 | acetone | 8:1 | 80 °С, mcat = 0.2 g. | 1 | NA | NA 3 (0/69) | [109] |
| 3 | M-CoAlPO4 | acetone | 8:1 | 80 °С, mcat = 0.2 g. | 1 | NA | NA 3 (0/67) | [109] |
| 4 | M-ZnAlPO4 | acetone | 8:1 | 80 °С, mcat = 0.2 g | 1 | NA | NA 3 (0/66) | [109] |
| 5 | M-AlPO4 | acetone | 8:1 | 80 °С, mcat = 0.2 g | 1 | NA | NA 3 (0/57) | [109] |
| 6 | ZrMo-KIT-6 | acetone | 8:1 | 50 °С, mcat = 0.05 g | 4 | 86 | 0/98 | [110] |
| 7 | MIL-100(V) | acetone | 10.2:2.17 | 70 °С, acetonitrile as a solvent, mcat = 0.2 g | 1.25 | 85 | 2/98 | [111] |
| 8 | MIL-100(Al) | acetone | 10.2:2.17 | 70 °С, acetonitrile as a solvent, mcat = 0.2 g | 1.25 | 44 | 6/94 | [111] |
| 9 | MIL-100(Fe) | acetone | 10.2:2.17 | 70 °С, acetonitrile as a solvent, mcat = 0.2 g | 1.25 | 19 | 14/86 | [111] |
| 10 | MIL-100(Cr) | acetone | 10.2:2.17 | 70 °С, acetonitrile as a solvent, mcat = 0.2 g. | 1.25 | 4 | 22/78 | [111] |
| 11 | Co.[II](Co.[III]1.25Al0.75)O4 | acetone | 10:1 | 130 °С, mcat = 0.1 g | 3 | 69 | 1/99 | [112] |
| 12 | Co.[II](Co.[III]1.4Al0.6)O4 | acetone | 10:1 | 130 °С, mcat = 0.1 g | 3 | 32 | 3/97 | [112] |
| 13 | 1% Ni/AC | acetone | 8:1 | 45 °С, 530 rpm, mcat = 0.2 g | 3 | 65 | 3/91 | [113] |
| 14 | 5% Ni/AC | acetone | 8:1 | 45 °С, 530 rpm, mcat = 0.2 g | 3 | 98 | 10/86 | [113] |
| 15 | 5% Ni–1%Zr/AC | acetone | 8:1 | 45 °С, 530 rpm, mcat = 0.2 g | 3 | 100 | 26/74 | [113] |
| 16 | Ni(1.8)/MWCNTs | acetone | 6:1 | 40 °С, 530 rpm, mcat = 0.3 g | 3 | 96 | 28/72 | [114] |
| 17 | Cu-mordenite | acetone | 3:1 | 100 °С, 500 W, mcat = 0.3 g | 0.25 | 95 | 0/98 | [115] |
| 18 | 1% MoO3/SiO2 | benzaldehyde | 0.1:0.11 | 100 °С, toluene as a solvent, mcat = 10 wt % | 8 | 37 | 63/37 | [76] |
| 19 | 10% MoO3/SiO2 | benzaldehyde | 0.1:0.11 | 100 °С, toluene as a solvent, mcat = 10 wt % | 8 | 43 | 63/37 | [76] |
| 20 | 20% MoO3/SiO2 | benzaldehyde | 0.1:0.11 | 100 °С, toluene as a solvent, mcat = 10 wt % | 8 | 72 | 60/40 | [76] |
| 21 | 10% MoO3/TiO2-ZrO2 | benzaldehyde | 1:1 | 100 °С, mcat = 5 wt % | 0.5 | 74 | 51/49 | [116] |
| 22 | SnO2 | furfural | 1:1 | 20 °С, mcat = 5 wt % | 0.5 | 51 | 38/62 | [118] |
| 23 | WO3/SnO2 | furfural | 1:1 | 20 °С, mcat = 5 wt % | 0.5 | 67 | 37/63 | [118] |
| 24 | MoO3/SnO2 | furfural | 1:1 | 20 °С, mcat = 5 wt % | 0.5 | 75 | 36/64 | [118] |
| 25 | SO42−/SnO2 | acetone | 1.5:1 | 20 °С, mcat = 5 wt % | 4 | 98 | NA | [119] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnov, A.A.; Selishcheva, S.A.; Yakovlev, V.A. Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts 2018, 8, 595. https://doi.org/10.3390/catal8120595
Smirnov AA, Selishcheva SA, Yakovlev VA. Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts. 2018; 8(12):595. https://doi.org/10.3390/catal8120595
Chicago/Turabian StyleSmirnov, Andrey A., Svetlana A. Selishcheva, and Vadim A. Yakovlev. 2018. "Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol" Catalysts 8, no. 12: 595. https://doi.org/10.3390/catal8120595
APA StyleSmirnov, A. A., Selishcheva, S. A., & Yakovlev, V. A. (2018). Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts, 8(12), 595. https://doi.org/10.3390/catal8120595