Photocatalytic Oxidation of Toluene on Fluorine Doped TiO2/SiO2 Catalyst Under Simulant Sunlight in a Flat Reactor
Abstract
1. Introduction
2. Results and Discussion
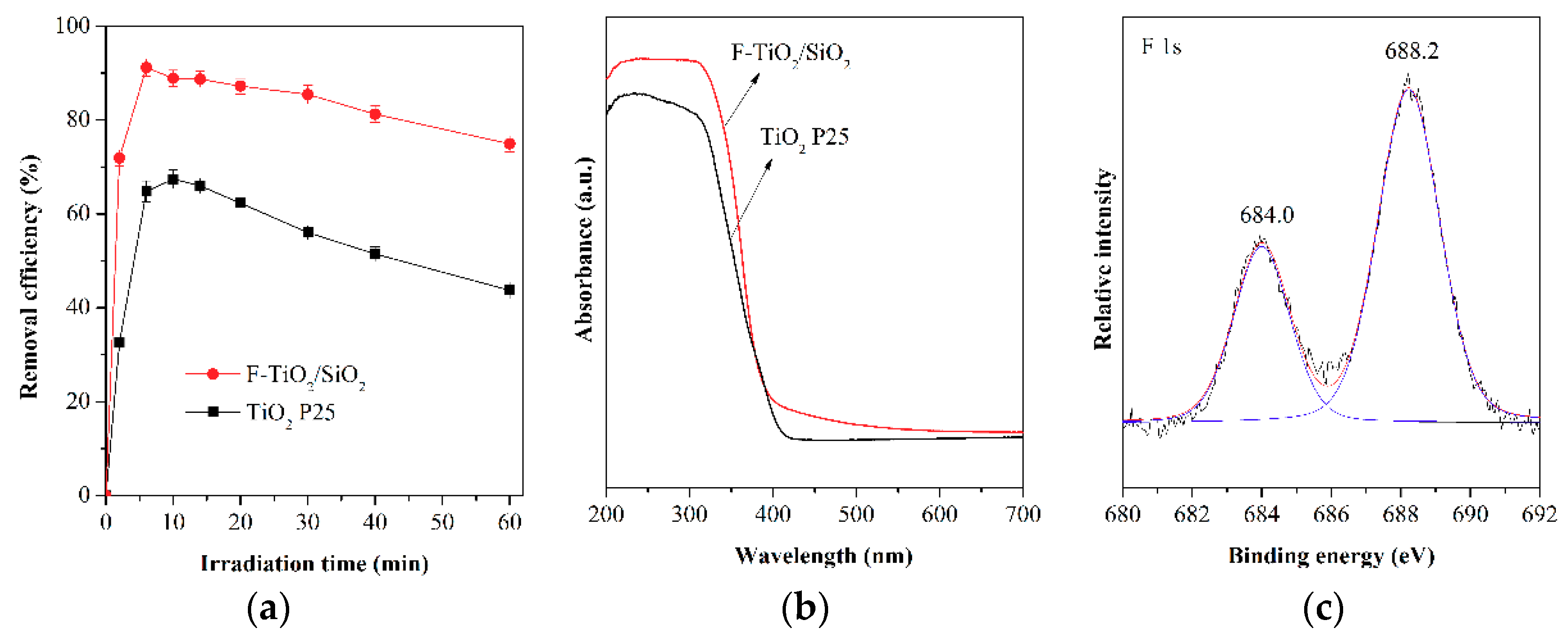
2.1. Photocatalytic Performance of the F-TiO2/SiO2 Catalyst in Toluene Oxidation
2.2. Optimization of the Catalyst Coating and Reactor Conditions
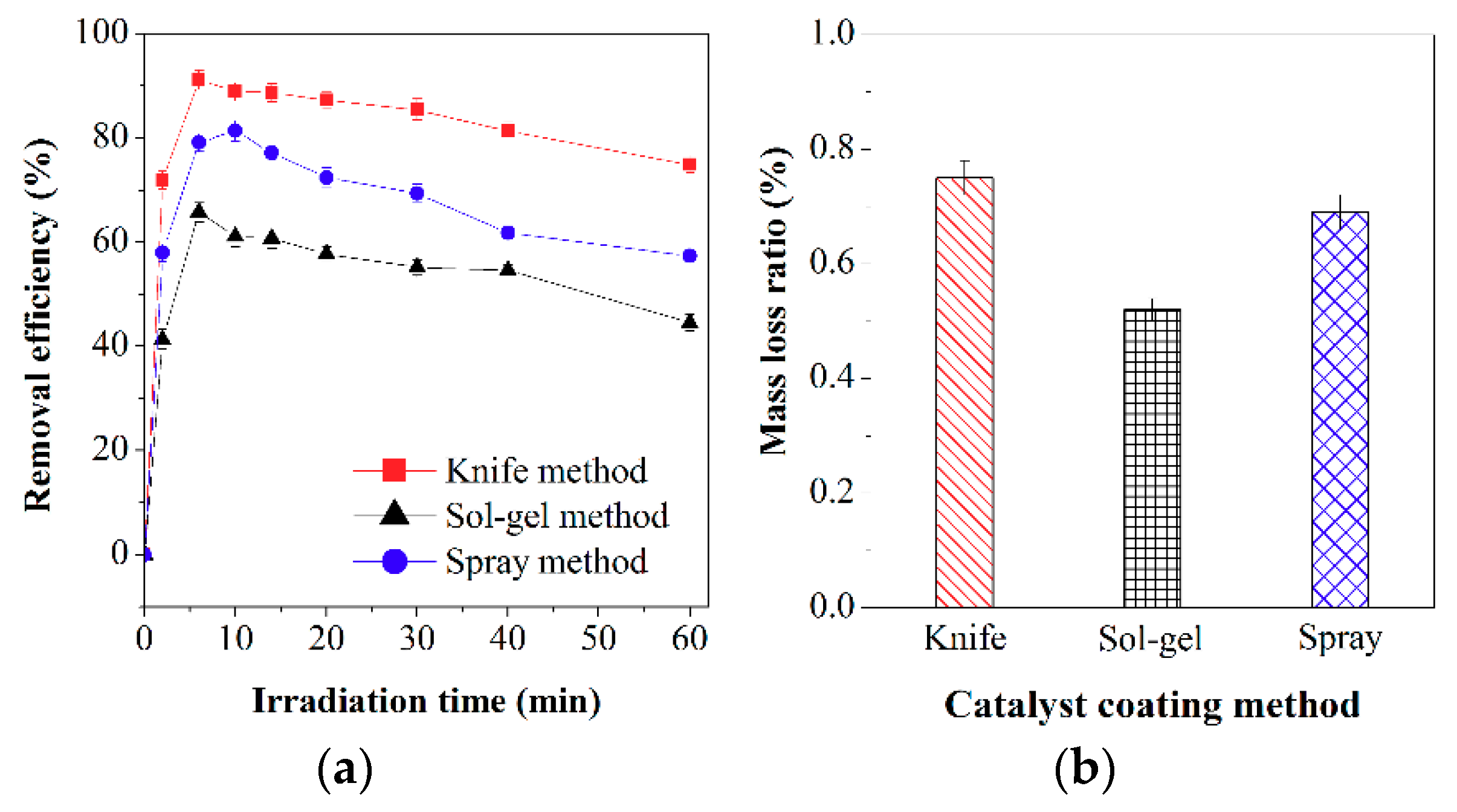
2.2.1. Effect of Coating Method on Activity of F-TiO2/SiO2
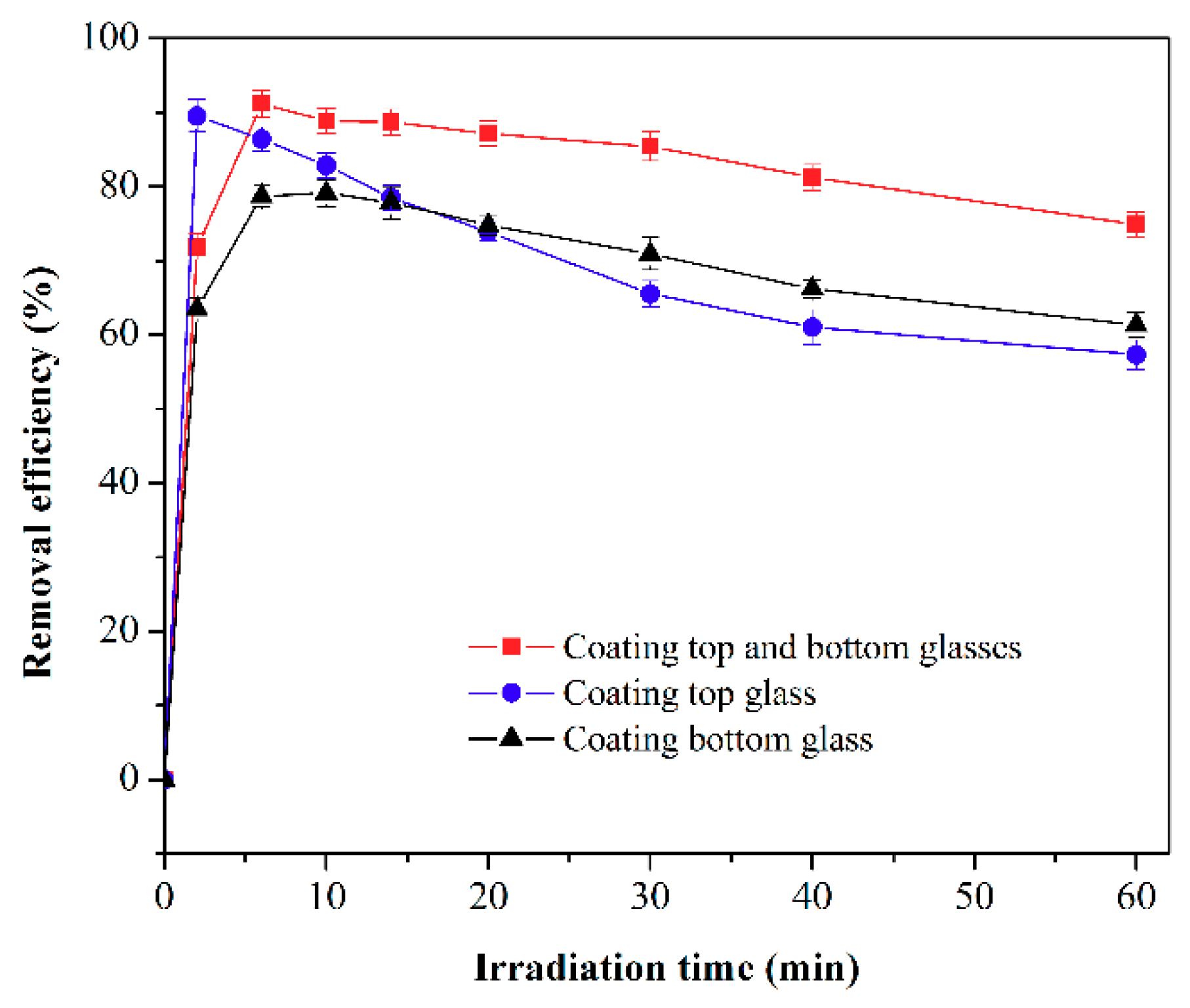
2.2.2. Effect of Coating Location and Layers on Activity of F-TiO2/SiO2
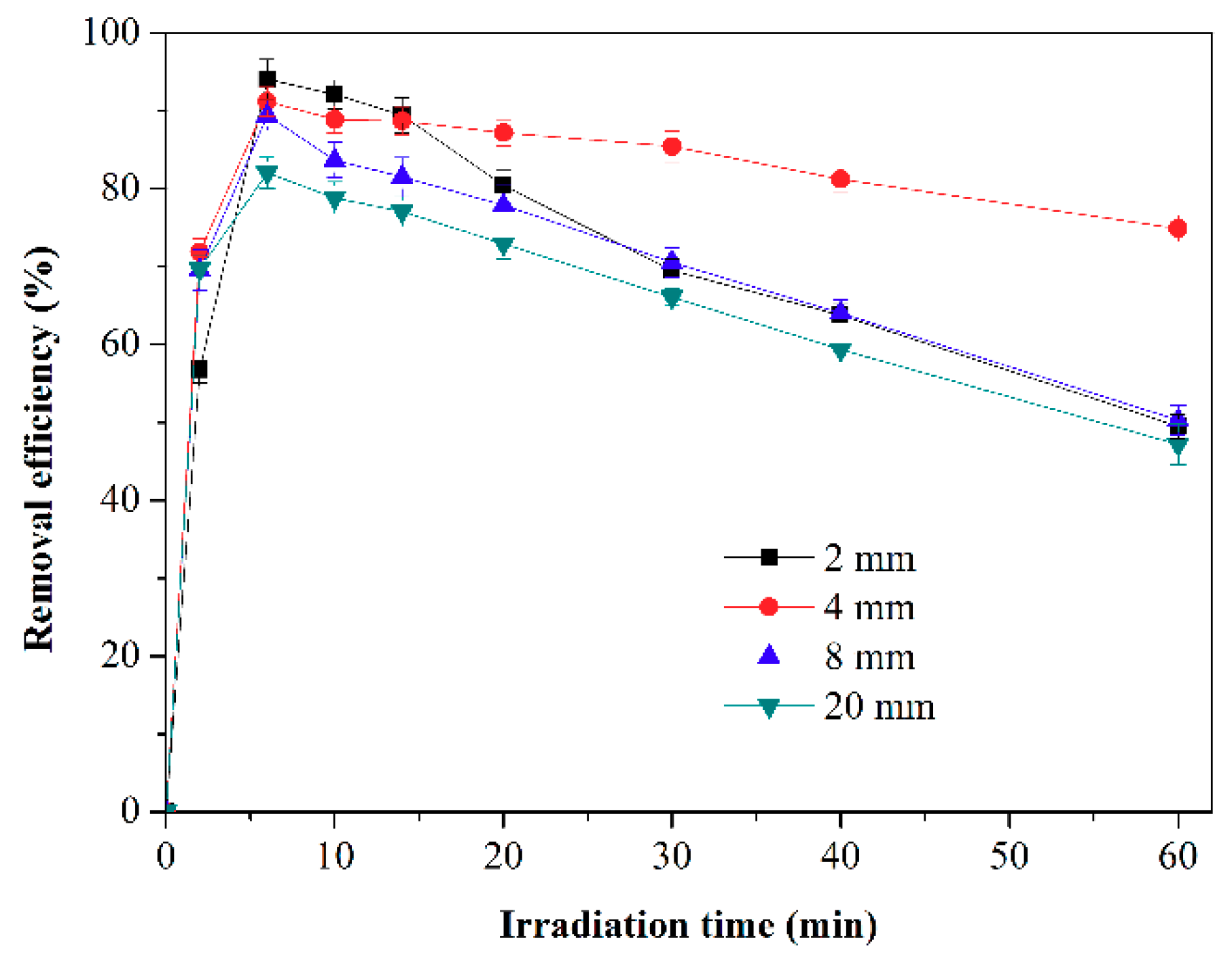
2.2.3. Effect of Reactor Height on Activity of F-TiO2/SiO2
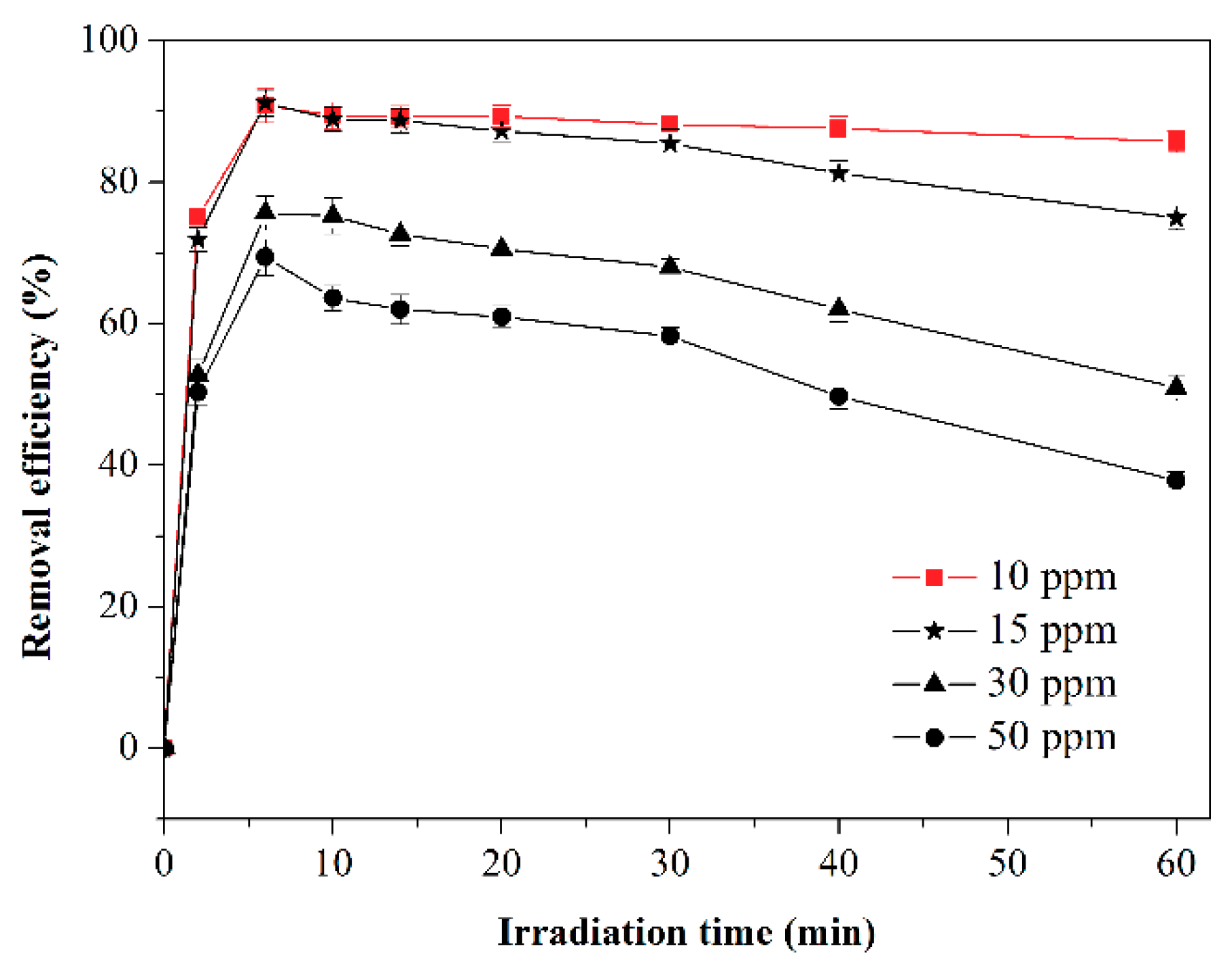
2.3. Effect of Initial Toluene Concentration on Toluene Photodegradation
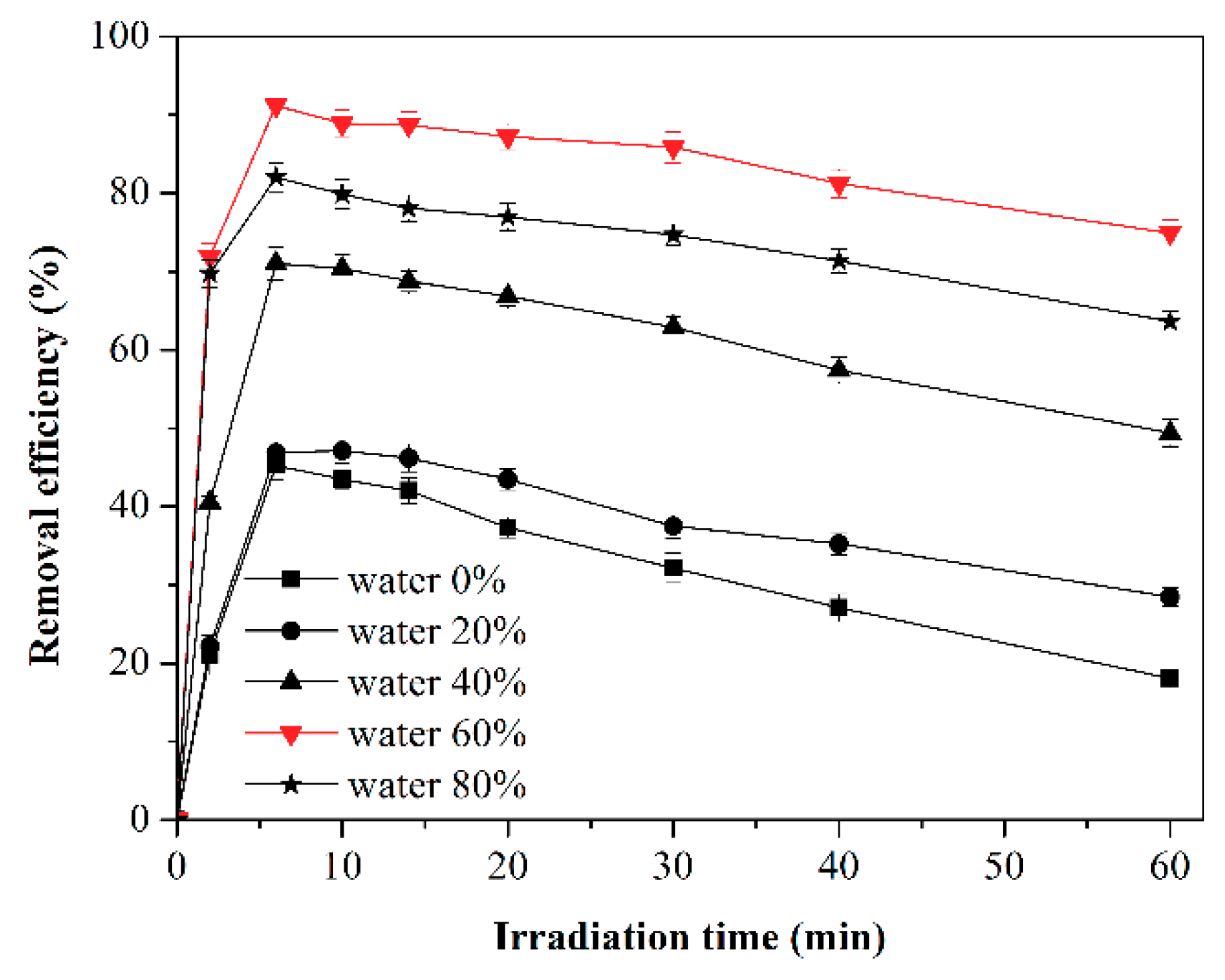
2.4. Effect of Water Vapor on Toluene Photodegradation
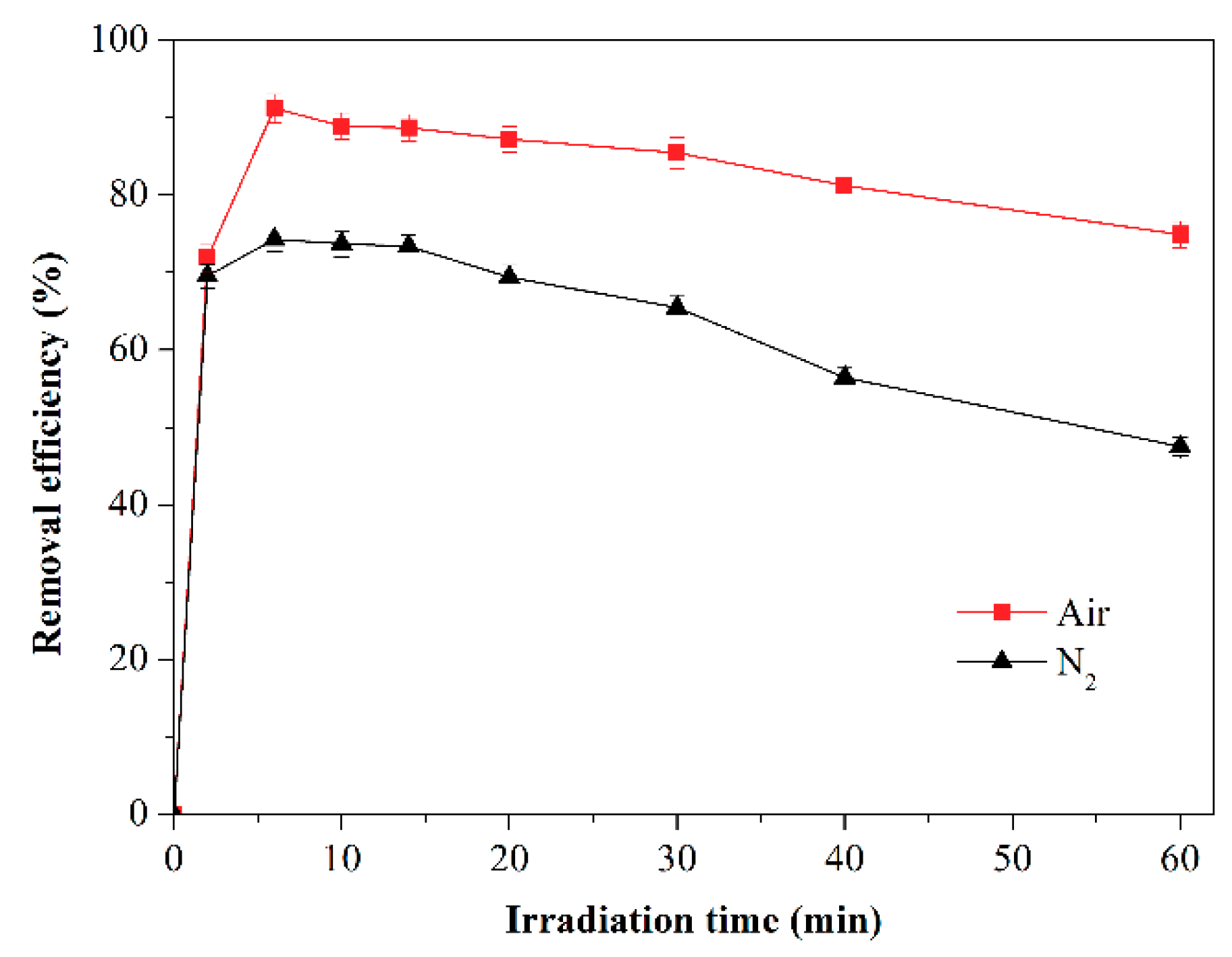
2.5. Effect of O2 on Toluene Photodegradation
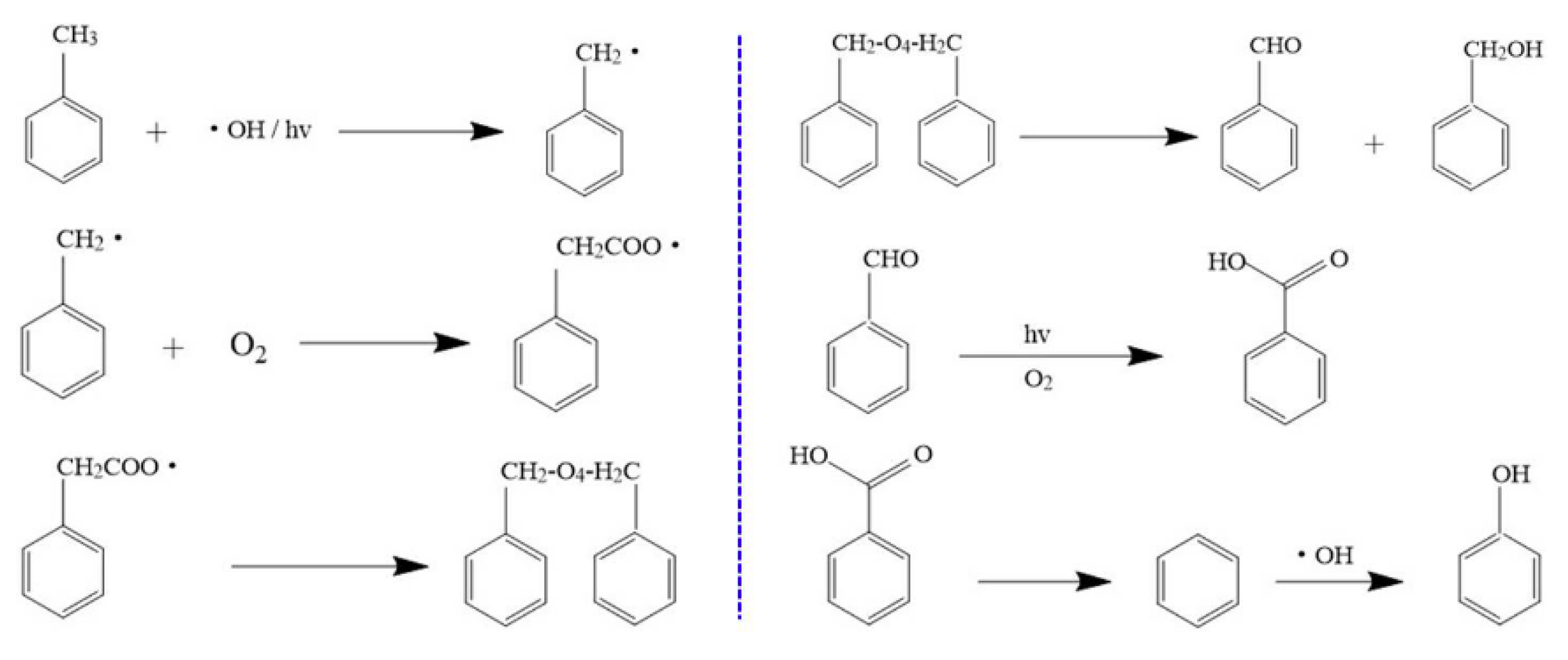
2.6. Catalyst Deactivation
3. Materials and Methods
3.1. Catalyst Preparation
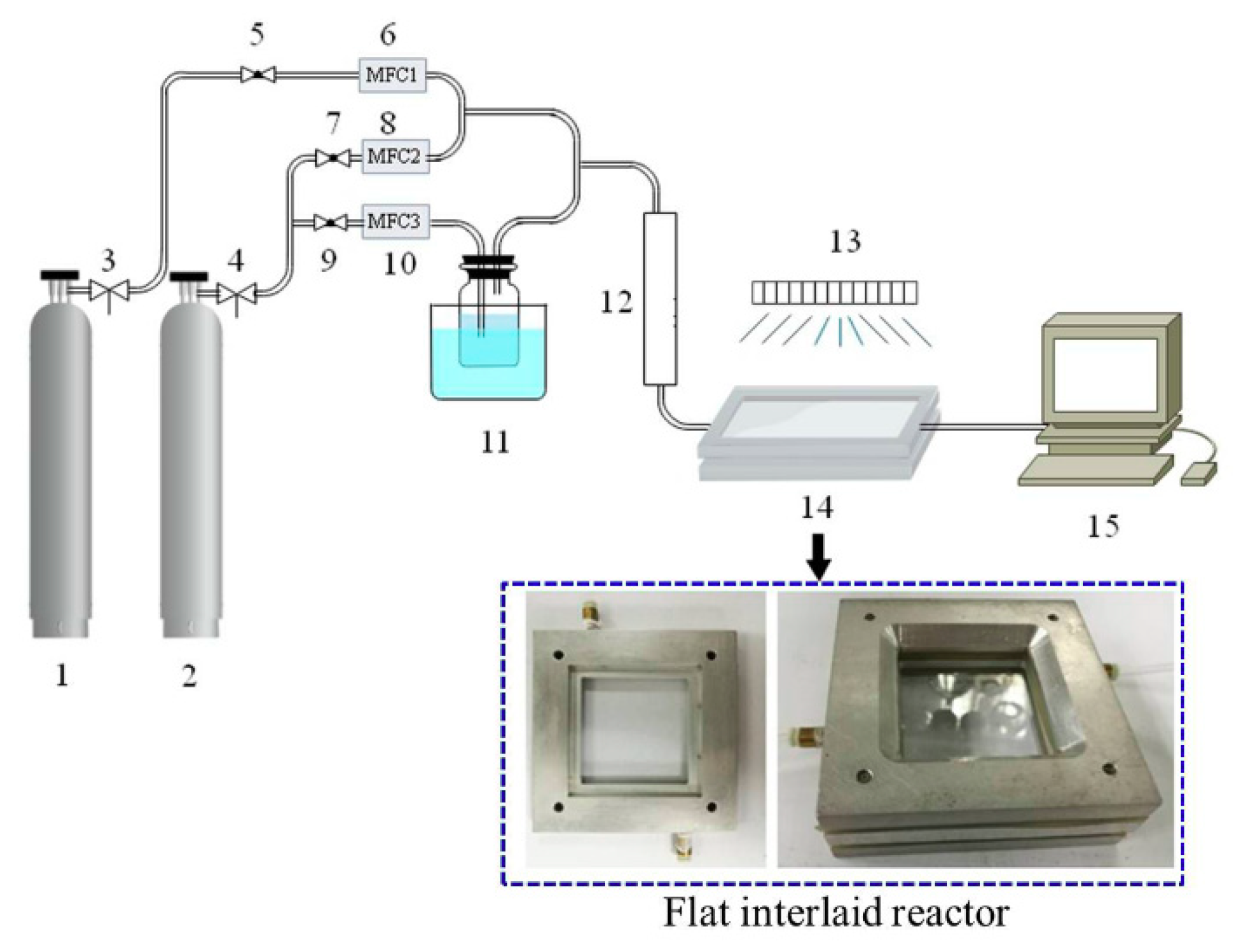
3.2. Catalyst Evaluation
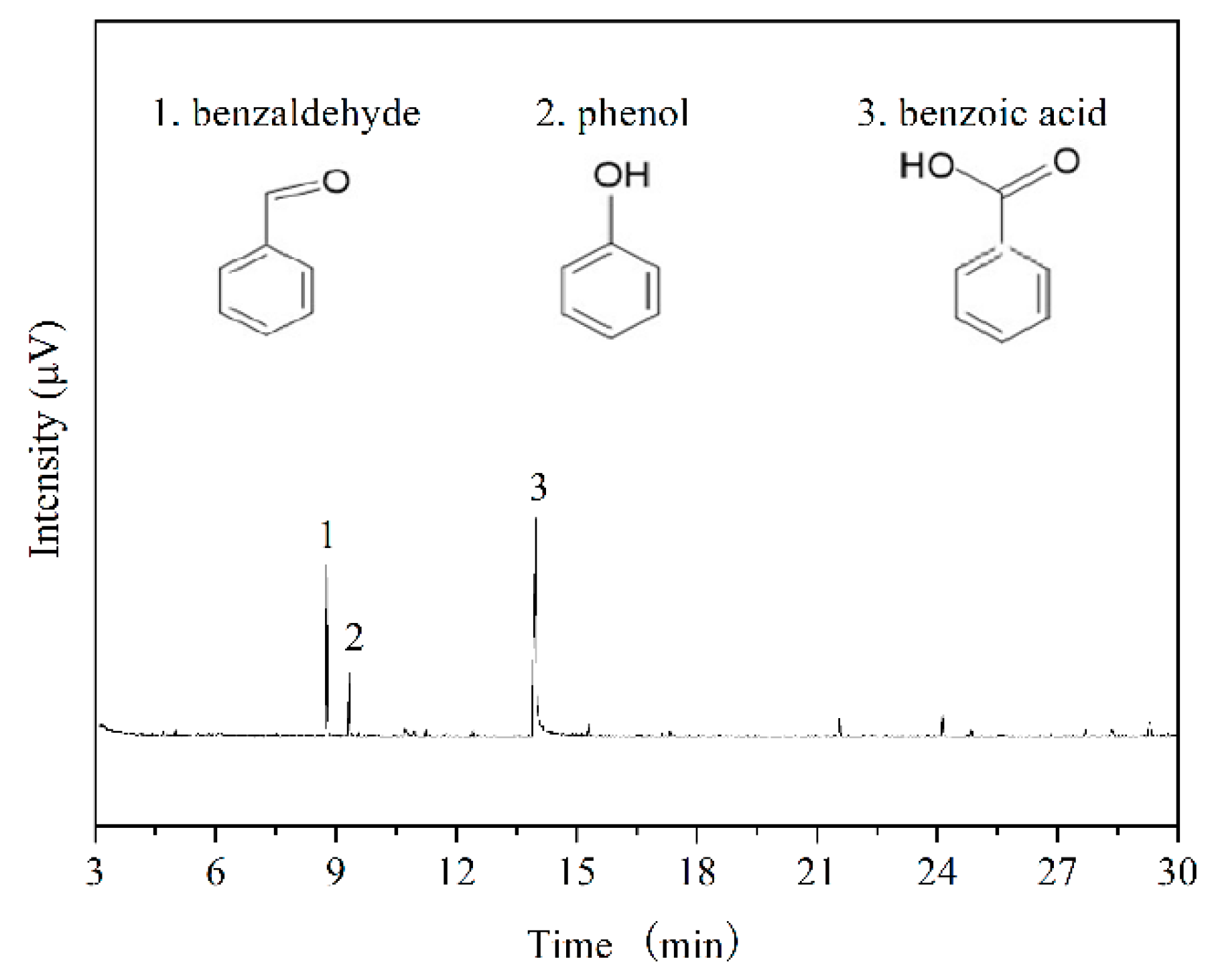
3.3. GC-MS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Korologos, C.A.; Nikolaki, M.D.; Zerva, C.N.; Philippopoulos, C.J.; Poulopoulos, S.G. Photocatalytic oxidation of benzene, toluene, ethylbenzene and m-xylene in the gas-phase over TiO2-based catalysts. J. Photochem. Photobiol. A 2012, 244, 24–31. [Google Scholar] [CrossRef]
- Cao, L.X.; Gao, Z.; Suib, S.L.; Obee, T.N.; Hay, S.O.; Freihaut, J.D. Photocatalytic oxidation of toluene on nanoscale TiO2 catalysts: Studies of deactivation and regeneration. J. Catal. 2000, 196, 253–261. [Google Scholar] [CrossRef]
- Boulamanti, A.K.; Philippopoulos, C.J. Photocatalytic degradation of methyl tert-butyl ether in the gas-phase: A kinetic study. J. Hazard. Mater. 2008, 160, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Deveau, P.A.; Arsac, F.; Thivel, P.X.; Ferronato, C.; Delpech, F.; Chovelon, J.M.; Kaluzny, P.; Monnet, C. Different methods in TiO2 photodegradation mechanism studies: Gaseous and TiO2-adsorbed phases. J. Hazard. Mater. 2007, 144, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ding, J.J.; Bao, J.; Gao, C.; Qi, Z.M.; Yang, X.Y.; He, B.; Li, C.X. Photocatalytic degradation of gaseous toluene on Fe-TiO2 under visible light irradiation: A study on the structure, activity and deactivation mechanism. Appl. Surf. Sci. 2012, 258, 5031–5037. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Anthony Byrne, J.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Labhsetwar, N.K.; Hishita, S.; Ohashi, N. Visible-light-driven photocatalysis on fluorine-doped TiO2 powders by the creation of surface oxygen vacancies. Chem. Phys. Lett. 2005, 401, 579–584. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N.; Labhsetwar, N.K. Fluorine-doped TiO2 powders prepared by spray pyrolysis and their improved photocatalytic activity for decomposition of gas-phase acetaldehyde. J. Fluor. Chem. 2005, 126, 69–77. [Google Scholar] [CrossRef]
- Ho, W.; Yu, J.C.; Lee, S. Synthesis of hierarchical nanoporous F-doped TiO2 spheres with visible light photocatalytic activity. Chem. Commun. 2006, 10, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Zhou, W.Q.; Yang, K.; Rong, G. Hydrothermal synthesis of hemisphere-like F-doped anatase TiO2 with visible light photocatalytic activity. J. Mater. Sci. 2010, 45, 5756–5761. [Google Scholar] [CrossRef]
- Minero, C.; Mariella, G.; Maurino, V.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic anions. 1. Hydroxyl-mediated and direct electron-transfer reactions of phenol on a titanium dioxide-fluoride system. Langmuir 2000, 16, 2632–2641. [Google Scholar] [CrossRef]
- Minero, C.; Mariella, G.; Maurino, V.; Vione, D.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic ions. 2. Competitive reactions of phenol and alcohols on a titanium dioxide-fluoride system. Langmuir 2000, 16, 8964–8972. [Google Scholar] [CrossRef]
- Vohra, M.S.; Kim, S.; Choi, W. Effects of surface fluorination of TiO2 on the photocatalytic degradation of tetramethylammonium. J. Photochem. Photobiol. A 2003, 160, 55–60. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.G.; Ho, W.K.; Jiang, Z.T.; Zhang, L.Z. Effects of F- doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Pang, D.D.; Wang, Y.T.; Ma, X.D.; Ouyang, F. Fluorine promoted and silica supported TiO2 for photocatalytic decomposition of acrylonitrile under simulant solar light irradiation. Chem. Eng. J. 2014, 258, 43–50. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Song, K.Y.; Lee, W.I.; Choi, G.J.; Do, Y.R. Photocatalytic behavior of WO3-loaded TiO2 in an oxidation reaction. J. Catal. 2000, 191, 192–199. [Google Scholar] [CrossRef]
- Keller, V.; Bernhardt, P.; Garin, F. Photocatalytic oxidation of butyl acetate in vapor phase on TiO2, Pt/TiO2 and WO3/TiO2 catalysts. J. Catal. 2003, 215, 129–138. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.; Wang, Y.; Ji, H.; Ma, W.; Zang, L.; Zhao, J. Surface modification of TiO2 by phosphate: Effect on photocatalytic activity and mechanism implication. J. Phys. Chem. C 2008, 112, 5993–6001. [Google Scholar] [CrossRef]
- Yu, J.G.; Zhao, X.J.; Du, J.C.; Chen, W.M. Preparation, Microstructure and photocatalytic activity of the porous TiO2 anatase coating by sol-gel processing. J. Sol-Gel Sci. Technol. 2000, 17, 163–171. [Google Scholar] [CrossRef]
- Yu, J.G.; Zhao, X.J. Effect of substrates on the photocatalytic activity of nanometer TiO2 thin films. Mater. Res. Bull. 2000, 35, 1293–1301. [Google Scholar] [CrossRef]
- Yu, J.G. Photocatalytic activity and characterization of the sol-gel derived Pb-doped TiO2 thin films. J. Sol-Gel Sci. Technol. 2002, 24, 39–48. [Google Scholar] [CrossRef]
- Obee, T.N.; Hay, S.O. Effects of moisture and temperature on the photooxidation of ethylene on titania. Environ. Sci. Technol. 1997, 31, 2034–2038. [Google Scholar] [CrossRef]
- Puddu, V.; Choi, H.; Dionysiou, D.D.; Puma, G.L. TiO2 photocatalyst for indoor air remediation: Influence of crystallinity, crystal phase, and UV radiation intensity on trichloroethylene degradation. Appl. Catal. B Environ. 2010, 94, 211–218. [Google Scholar] [CrossRef]
- Salvadó-Estivill, I.; Brucato, A.; Puma, G.L. Two-dimensional modeling of a flatplate photocatalytic reactor for oxidation of indoor air pollutants. Ind. Eng. Chem. Res. 2007, 46, 7489–7496. [Google Scholar] [CrossRef]
- Blommaerts, N.; Asapu, R.; Claes, N.; Bals, S.; Lenaerts, S.; Verbruggen, S.W. Gas phase photocatalytic spiral reactor for fast and efficient pollutant degradation. Chem. Eng. J. 2017, 316, 850–856. [Google Scholar] [CrossRef]
- Jović, F.; Kosar, V.; Tomašić, V.; Gomzi, Z. Non-ideal flow in an annular photocatalytic reactor. Chem. Eng. Res. Des. 2012, 90, 1297–1306. [Google Scholar] [CrossRef]
- Tomašić, V.; Jović, F.; Gomzi, Z. Photocatalytic oxidation of toluene in the gas phase: Modelling an annular photocatalytic reactor. Catal. Today 2008, 137, 350–356. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Ang, M.; Pareek, V. Light intensity distribution in multi-lamp photocatalytic reactors. Chem. Eng. Sci. 2013, 93, 11–21. [Google Scholar] [CrossRef]
- Wu, Y.M.; Xing, M.Y.; Tian, B.Z.; Zhang, J.L.; Chen, F. Preparation of nitrogen and fluorine co-doped mesoporous TiO2 microsphere and photodegradation of acid orange 7 under visible light. Chem. Eng. J. 2010, 162, 710–717. [Google Scholar] [CrossRef]
- Yang, G.D.; Jiang, Z.; Shi, H.H.; Jones, M.O.; Xiao, T.C.; Edwards, P.P.; Yan, Z.F. Study on the photocatalysis of F-S co-doped TiO2 prepared using solvothermal method. Appl. Catal. B Environ. 2010, 96, 458–465. [Google Scholar] [CrossRef]
- Huang, H.; Huang, H.; Lu, Z.; Peng, H.; Ye, X.; Leung, D.Y.C. Enhanced degradation of gaseous benzene under vacuum ultraviolet (VUV) irradiation over TiO2 modified by transition metals. Chem. Eng. J. 2015, 259, 534–541. [Google Scholar] [CrossRef]
- Korologos, C.A.; Philippopoulos, C.J.; Poulopoulos, S.G. The effect of water presence on the photocatalytic oxidation of benzene, toluene, ethylbenzene and m-xylene in the gas-phase. Atmos. Environ. 2011, 45, 7089–7095. [Google Scholar] [CrossRef]
- Jeong, J.; Sekiguchi, K.; Sakamoto, K. Photochemical and photocatalytic degradation of gaseous toluene using short-wavelength UV irradiation with TiO2 catalyst: Comparison of three UV sources. Chemosphere 2004, 57, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Hwang, H.T.; Hong, S.C. Photocatalytic degradation of volatile organic compounds at the gas–solid interface of a TiO2 photocatalyst. Chemosphere 2002, 48, 437–444. [Google Scholar] [CrossRef]
- Luo, Y.; Ollis, D.F. Heterogeneous photocatalytic oxidation of trichloroethylene and toluene mixtures in air: Kinetic promotion and inhibition, time-dependent catalyst activity. J. Catal. 1996, 163, 1–11. [Google Scholar] [CrossRef]
- D’Hennezel, O.; Pichat, P.; Ollis, D.F. Benzene and toluene gas-phase photocatalytic degradation over H2O and HCL pretreated TiO2: By-products and mechanisms. J. Photochem. Photobiol. A 1998, 118, 197–204. [Google Scholar] [CrossRef]
- Guo, T.; Bai, Z.P.; Wu, C.; Zhu, T. Influence of relative humidity on the photocatalytic oxidation (PCO) of toluene by TiO2 loaded on activated carbon fibers: PCO rate and intermediates accumulation. Appl. Catal. B 2008, 79, 171–178. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Sysmans, W.; Leys, C.; Van Langenhove, H. Abatement and degradation pathways of toluene in indoor air by positive corona discharge. Chemosphere 2007, 68, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.H.; Zhang, Y.P.; Xu, Q.J.; Zhu, Y.F.; Lamson, J.J.; Zhao, R.Y. Determination and risk assessment of by-products resulting from photocatalytic oxidation of toluene. Appl. Catal. B Environ. 2009, 89, 570–576. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Smith, S.C. OH-initiated oxidation of toluene. 1. Quantum chemistry investigation of the reaction path. J. Phys. Chem. A 2007, 111, 3686–3690. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Wang, Y.; Li, H.; Cao, G.; Ouyang, F.; Zhu, R. Photocatalytic Oxidation of Toluene on Fluorine Doped TiO2/SiO2 Catalyst Under Simulant Sunlight in a Flat Reactor. Catalysts 2018, 8, 596. https://doi.org/10.3390/catal8120596
Qiu L, Wang Y, Li H, Cao G, Ouyang F, Zhu R. Photocatalytic Oxidation of Toluene on Fluorine Doped TiO2/SiO2 Catalyst Under Simulant Sunlight in a Flat Reactor. Catalysts. 2018; 8(12):596. https://doi.org/10.3390/catal8120596
Chicago/Turabian StyleQiu, Lu, Yanan Wang, Hanlinag Li, Gang Cao, Feng Ouyang, and Rongshu Zhu. 2018. "Photocatalytic Oxidation of Toluene on Fluorine Doped TiO2/SiO2 Catalyst Under Simulant Sunlight in a Flat Reactor" Catalysts 8, no. 12: 596. https://doi.org/10.3390/catal8120596
APA StyleQiu, L., Wang, Y., Li, H., Cao, G., Ouyang, F., & Zhu, R. (2018). Photocatalytic Oxidation of Toluene on Fluorine Doped TiO2/SiO2 Catalyst Under Simulant Sunlight in a Flat Reactor. Catalysts, 8(12), 596. https://doi.org/10.3390/catal8120596



