Metal Micro-Monoliths for the Kinetic Study and the Intensification of the Water Gas Shift Reaction
Abstract
1. Introduction
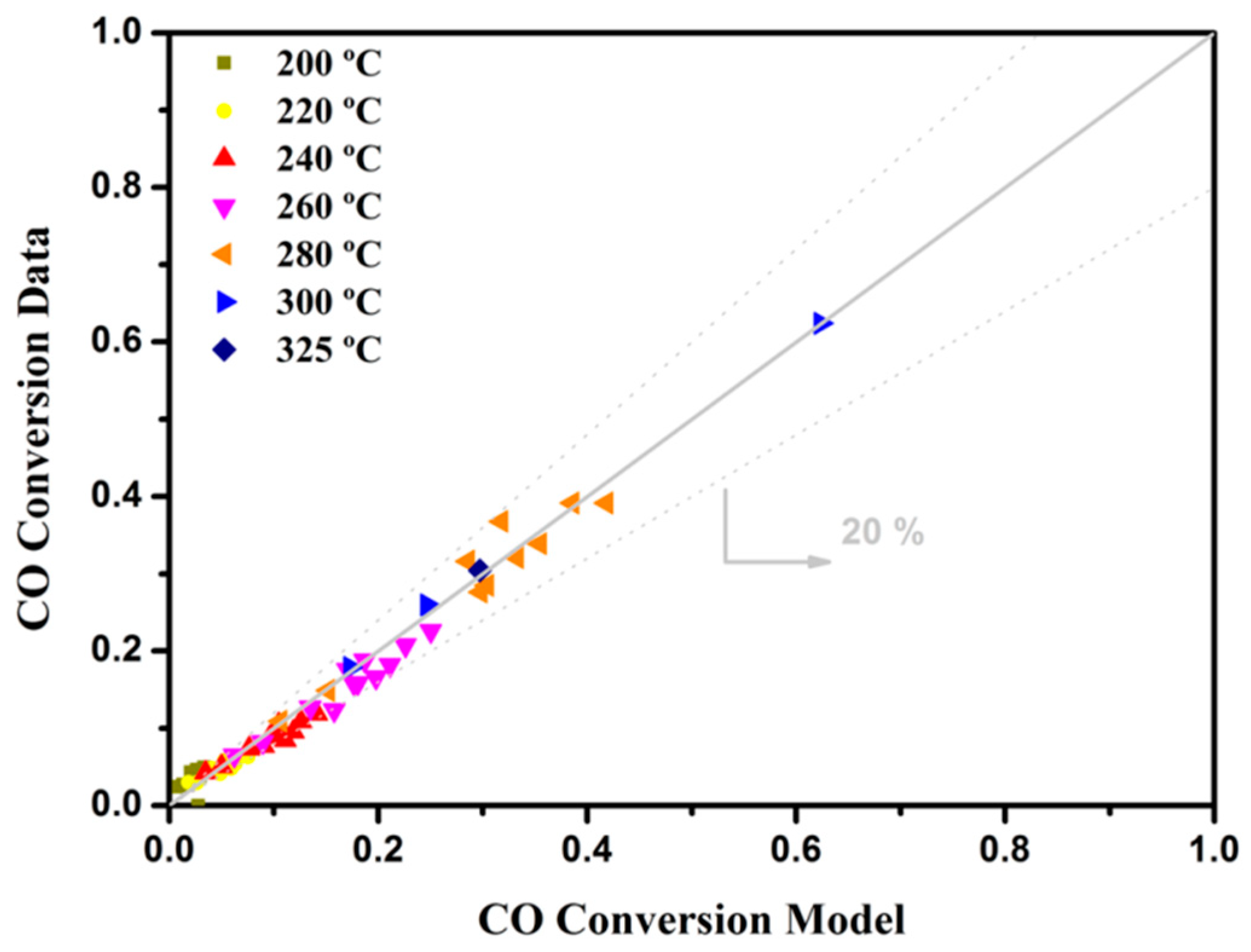
2. Kinetic Analysis Methodology
3. Results
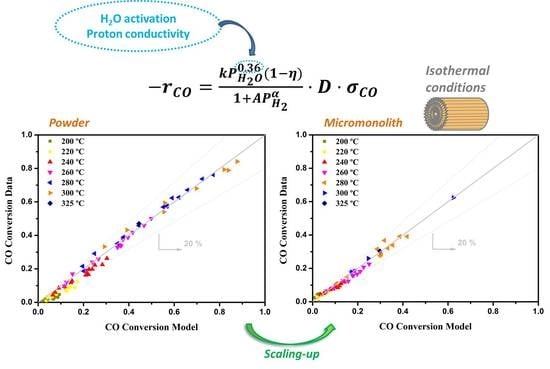
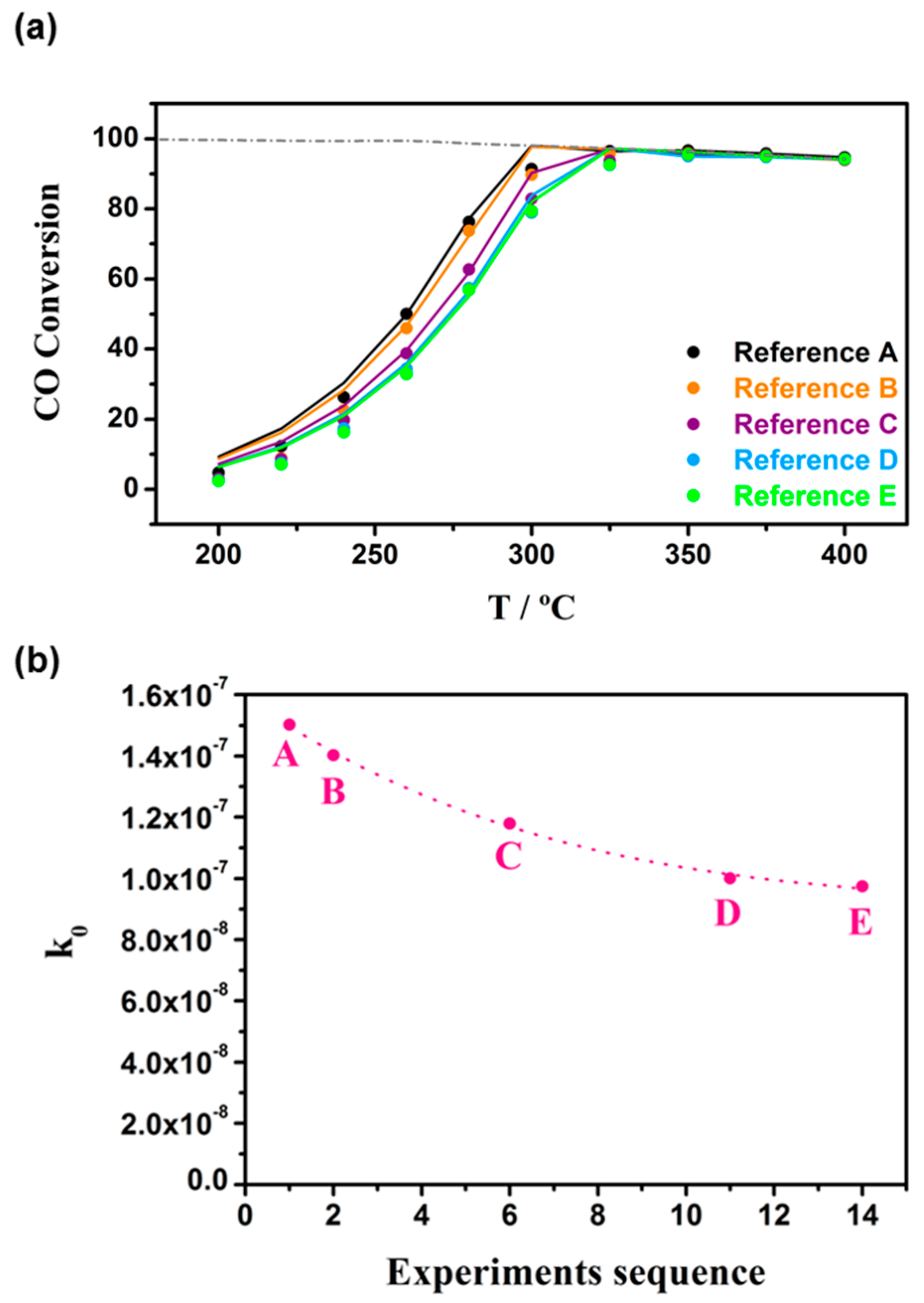
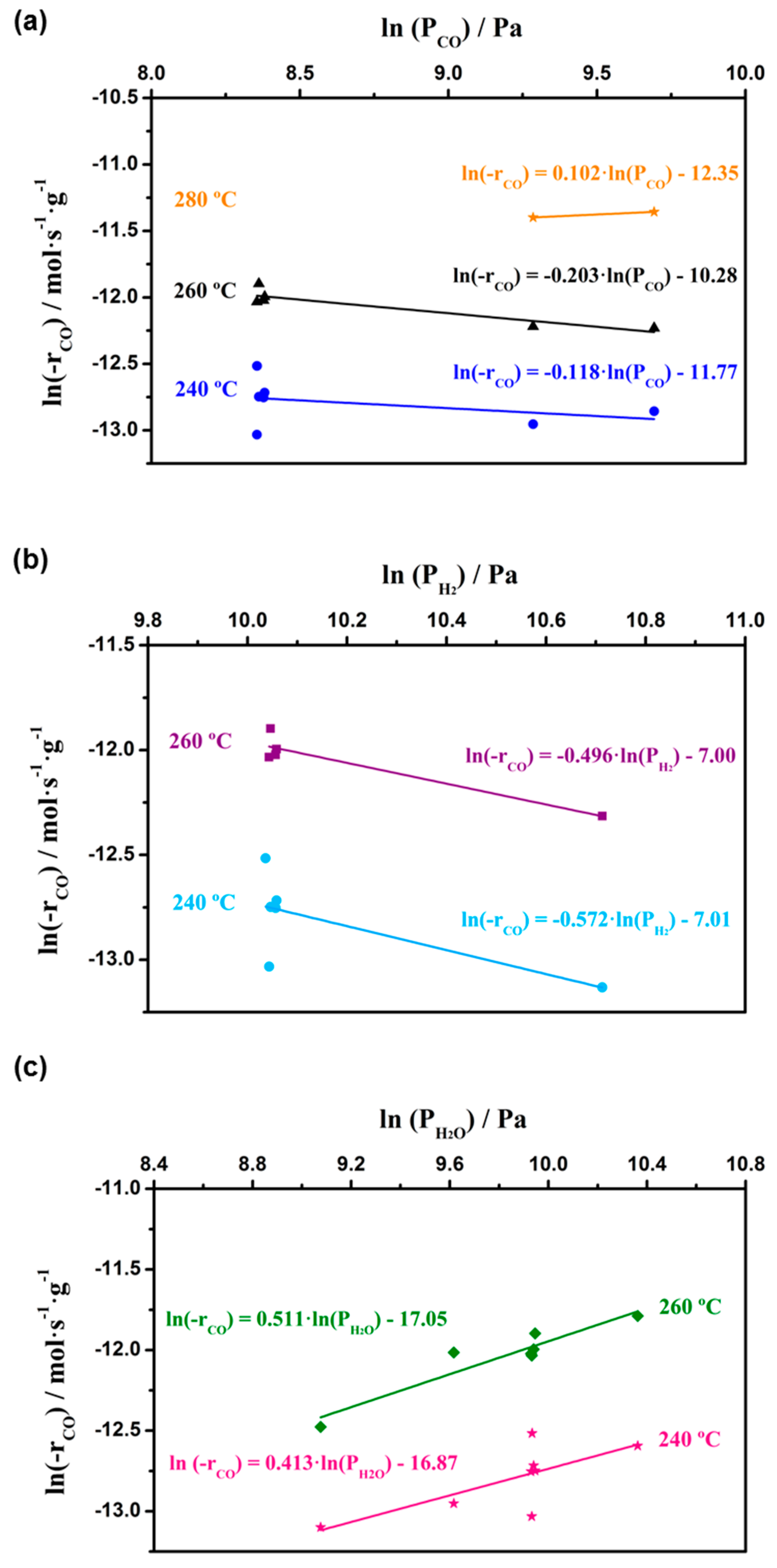
3.1. Kinetic Study on Powdered Catalyst
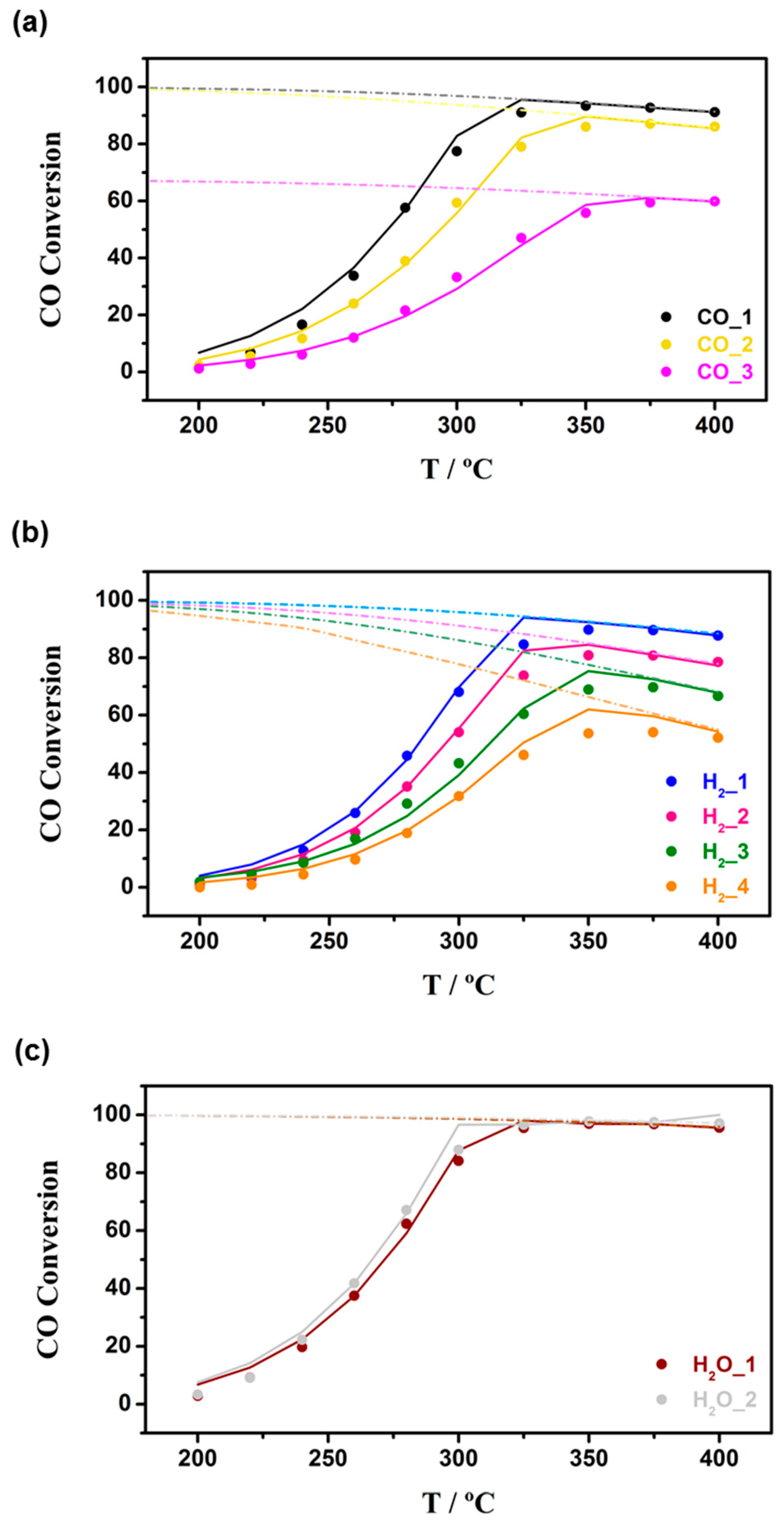
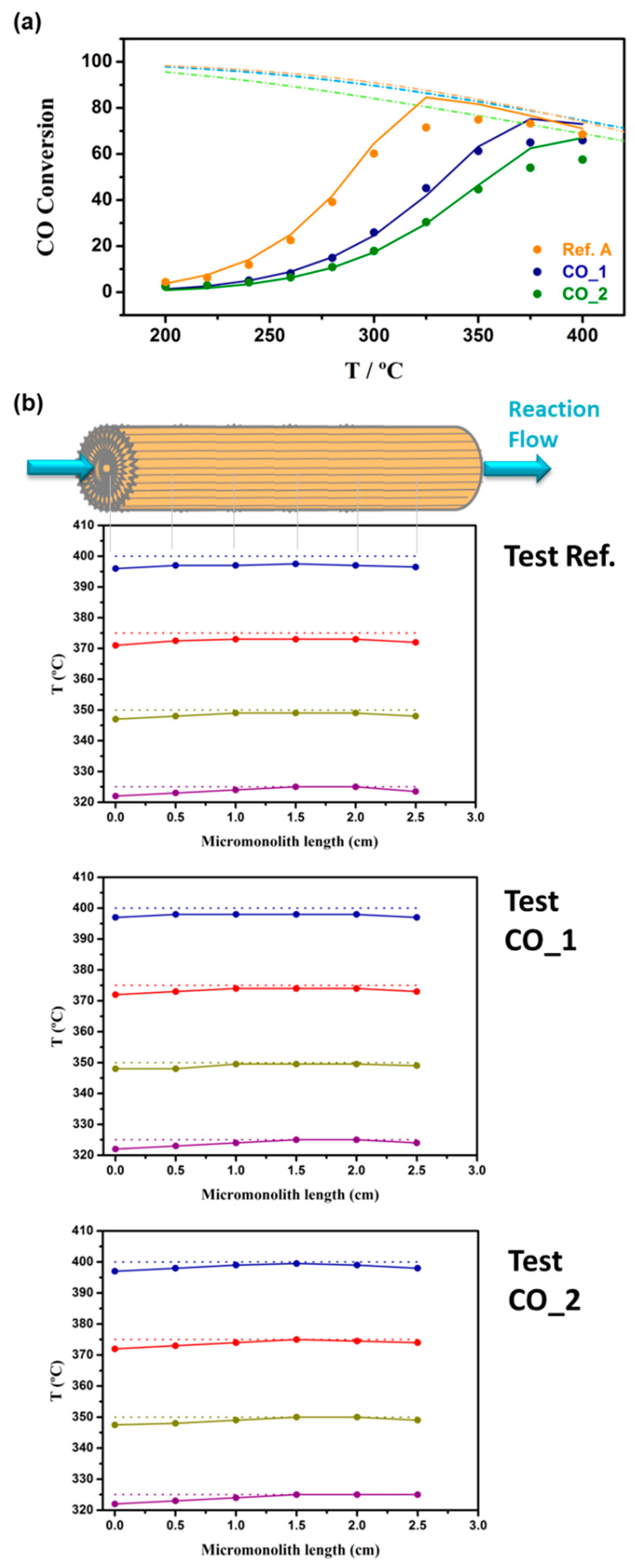
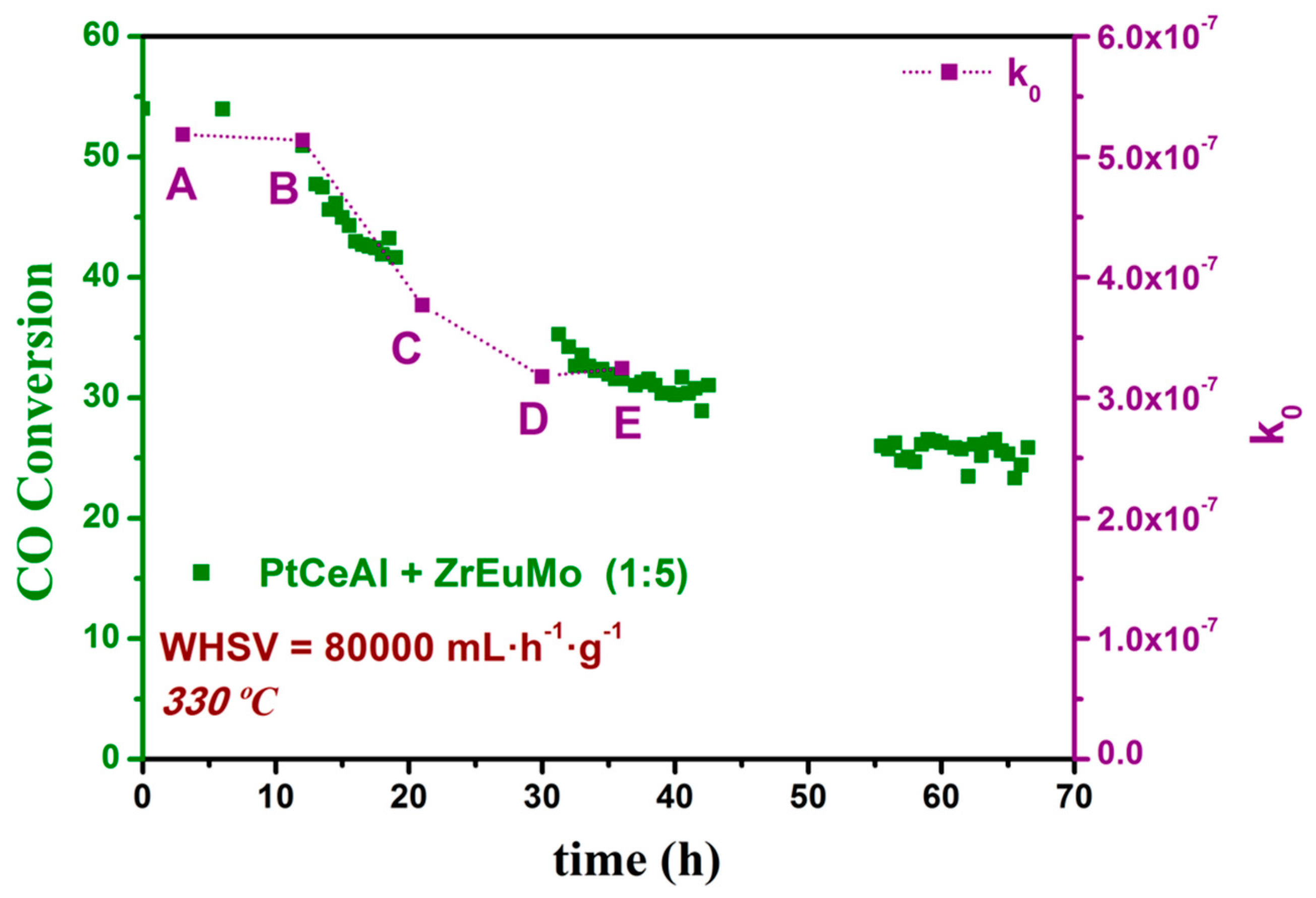
3.2. Kinetics Study on Structured Catalyst
4. Materials and Methods
4.1. Catalyst
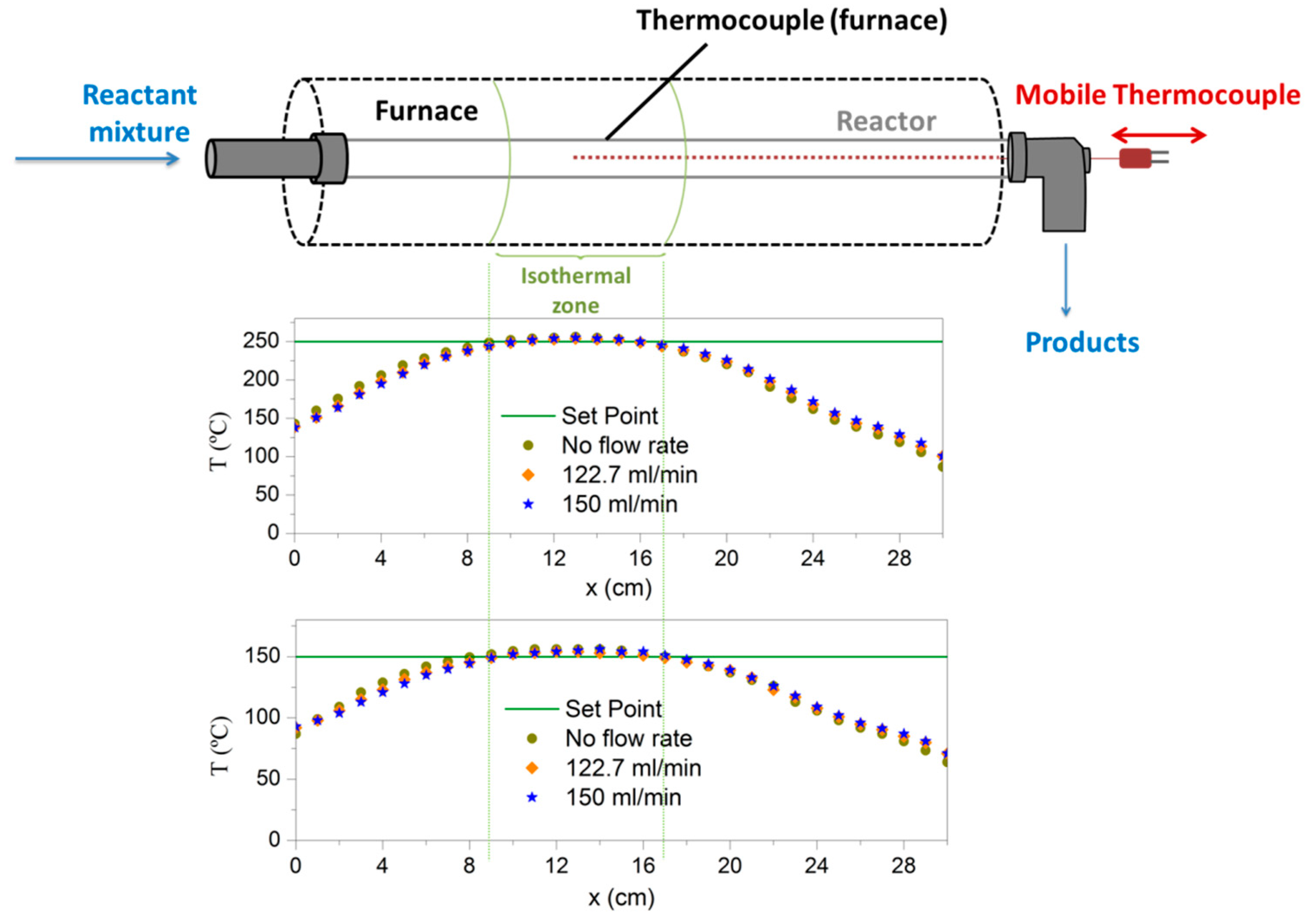
4.2. Experimental Setup for Powdered Catalyst
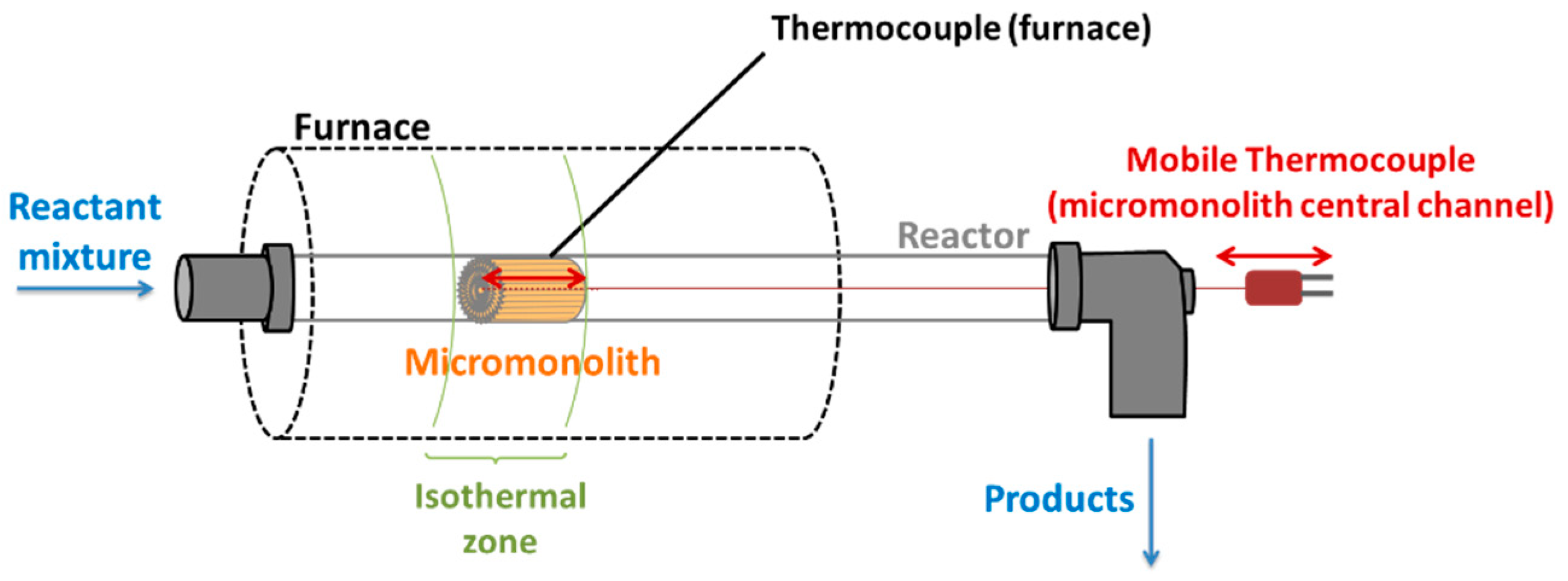
4.3. Experimental Setup for Structured Catalyst
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Germani, G.; Schuurman, Y. Water-Gas Shift Reaction Kinetics over µ-Structured Pt/CeO2/Al2O3 Catalysts. AIChE J. 2006, 52, 1806–1813. [Google Scholar] [CrossRef]
- Grabow, L.C.; Gokhale, A.A.; Evans, S.T.; Dumesic, J.A.; Mavrikakis, M. Mechanism of the Water Gas Shift Reaction on Pt: First Principles, Experiments, and Microkinetic Modeling. J. Phys. Chem. C 2008, 112, 4608–4617. [Google Scholar] [CrossRef]
- Grenoble, D.C.; Estadt, M.M.; Ollis, D.F. The Chemistry and Catalysis of the Water Gas Shift Reaction: 1. The Kinetics over Supported Metal Catalysts. J. Catal. 1981, 67, 90–102. [Google Scholar] [CrossRef]
- Phatak, A.A.; Koryabkina, N.; Rai, S.; Ratts, J.L.; Ruettinger, W.; Farrauto, R.J.; Blau, G.E.; Delgass, W.N.; Ribeiro, F.H. Kinetics of the water-gas shift reaction on Pt catalysts supported on alumina and ceria. Catal. Today 2007, 123, 224–234. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Dionysiou, D.D.; Efstathiou, A.M. Mechanistic Studies of the Water–Gas Shift Reaction over Pt/CexZr1–xO2 Catalysts: The Effect of Pt Particle Size and Zr Dopant. ACS Catal. 2012, 2, 2729–2742. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Panagiotopoulou, P.; Kondarides, D.I.; Efstathiou, A.M. Kinetic and mechanistic studies of the water-gas shift reaction on Pt/TiO2 catalyst. J. Catal. 2009, 264, 117–129. [Google Scholar] [CrossRef]
- Clay, J.P.; Greeley, J.P.; Ribeiro, F.H.; Delgass, W.N.; Schneider, W.F. DFT comparison of intrinsic WGS kinetics over Pd and Pt. J. Catal. 2014, 320, 106–117. [Google Scholar] [CrossRef]
- Huang, S.-C.; Lin, C.-H.; Wang, J.-H. Trends of Water Gas Shift Reaction on Close-Packed Transition Metal Surfaces. J. Phys. Chem. C 2010, 114, 9826–9834. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A. Water-gas-shift reaction on metal nanoparticles and surfaces. J. Chem. Phys. 2007, 126, 164705. [Google Scholar] [CrossRef] [PubMed]
- Phatak, A.A.; Delgass, W.N.; Ribeiro, F.H.; Schneider, W.F. Density Functional Theory Comparison of Water Dissociation Steps on Cu, Au, Ni, Pd, and Pt. J. Phys. Chem. C 2009, 113, 7269–7276. [Google Scholar] [CrossRef]
- González-Castaño, M.; Ivanova, S.; Laguna, O.H.; Martínez Tejada, L.M.; Centeno, M.A.; Odriozola, J.A. Structuring Pt/CeO2/Al2O3 WGS catalyst: Introduction of buffer layer. Appl. Catal. B Environ. 2017, 200, 420–427. [Google Scholar] [CrossRef]
- Olympiou, G.G.; Kalamaras, C.M.; Zeinalipour-Yazdi, C.D.; Efstathiou, A.M. Mechanistic aspects of the water-gas shift reaction on alumina-supported noble metal catalysts: In situ DRIFTS and SSITKA-mass spectrometry studies. Catal. Today 2007, 127, 304–318. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Petallidou, K.C.; Efstathiou, A.M. The effect of La3+-doping of CeO2 support on the water-gas shift reaction mechanism and kinetics over Pt/Ce1-xLaxO2-δ. Appl. Catal. B Environ. 2013, 136–137, 225–238. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I. Effect of the nature of the support on the catalytic performance of noble metal catalysts for the water-gas shift reaction. Catal. Today 2006, 112, 49–52. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T.; Kondarides, D.I. Water-gas shift activity of doped Pt/CeO2 catalysts. Chem. Eng. J. 2007, 134, 16–22. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Liu, P.; Hrbek, J.; Evans, J.; Pérez, M. Water Gas Shift Reaction on Cu and Au Nanoparticles Supported on CeO2(111) and ZnO(0001): Intrinsic Activity and Importance of Support Interactions. Angew. Chem. Int. Ed. 2007, 46, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Thinon, O.; Diehl, F.; Avenier, P.; Schuurman, Y. Screening of bifunctional water-gas shift catalysts. Catal. Today 2008, 137, 29–35. [Google Scholar] [CrossRef]
- García-Moncada, N.; Bobadilla, L.F.; Poyato, R.; López-Cartes, C.; Romero-Sarria, F.; Centeno, M.Á.; Odriozola, J.A. A direct in situ observation of water-enhanced proton conductivity of Eu-doped ZrO2: Effect on WGS reaction. Appl. Catal. B Environ. 2018, 231, 343–356. [Google Scholar] [CrossRef]
- García-Moncada, N.; González-Castaño, M.; Ivanova, S.; Centeno, M.Á.; Romero-Sarria, F.; Odriozola, J.A. New concept for old reaction: Novel WGS catalyst design. Appl. Catal. B Environ. 2018, 238, 1–5. [Google Scholar] [CrossRef]
- García Moncada, N. On the Rate-Limiting Step of the WGS Reaction: Design of the Model Catalyst. Ph.D. Thesis, University of Sevilla, Seville, Spain, 2017. [Google Scholar]
- Tonkovich, A.L.Y.; Zilka, J.L.; LaMont, M.J.; Wang, Y.; Wegeng, R.S. Microchannel reactors for fuel processing applications. I. Water gas shift reactor. Chem. Eng. Sci. 1999, 54, 2947–2951. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Liu, Y.; Ruettinger, W.; Ilinich, O.; Shore, L.; Giroux, T. Precious Metal Catalysts Supported on Ceramic and Metal Monolithic Structures for the Hydrogen Economy. Catal. Rev. Sci. Eng. 2007, 49, 141–196. [Google Scholar] [CrossRef]
- González-Castaño, M.; Reina, T.R.; Ivanova, S.; Martínez Tejada, L.M.; Centeno, M.A.; Odriozola, J.A. O2-assisted Water Gas Shift reaction over structured Au and Pt catalysts. Appl. Catal. B Environ. 2016, 185, 337–343. [Google Scholar] [CrossRef]
- Milt, V.G.; Ivanova, S.; Sanz, O.; Domínguez, M.I.; Corrales, A.; Odriozola, J.A.; Centeno, M.A. Au/TiO2 supported on ferritic stainless steel monoliths as CO oxidation catalysts. Appl. Surf. Sci. 2013, 270, 169–177. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H.B. Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- Potemkin, D.I.; Snytnikov, P.V.; Belyaev, V.D.; Sobyanin, V.A. Preferential CO oxidation over Cu/CeO2-x catalyst: Internal mass transport limitation. Chem. Eng. J. 2011, 176–177, 165–171. [Google Scholar] [CrossRef]
- Laguna, O.H.; González Castaño, M.; Centeno, M.A.; Odriozola, J.A. Microreactors technology for hydrogen purification: Effect of the catalytic layer thickness on CuOx/CeO2-coated microchannel reactors for the PROX reaction. Chem. Eng. J. 2015, 275, 45–52. [Google Scholar] [CrossRef]
- Ayastuy, J.L.; Gutiérrez-Ortiz, M.A.; González-Marcos, J.A.; Aranzabal, A.; González-Velasco, J.R. Kinetics of the Low-Temperature WGS Reaction over a CuO/ZnO/Al2O3 catalyst. Ind. Eng. Chem. Res. 2005, 44, 41–50. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1998; ISBN 9686165037. [Google Scholar]
- Vannice, M.A. Kinetics of Catalytic Reactions; Springer: New York, NY, USA, 2005; ISBN 0387246495. [Google Scholar]
- Hougen, O.A.; Watson, K.M. Solid catalysts and reaction rates. General Principles. Ind. Eng. Chem. 1943, 35, 529–541. [Google Scholar] [CrossRef]
- Yang, K.H.; Hougen, O.A. Determination of mechanism of catalyzed gaseous reactions. Chem. Eng. Prog. 1950, 46, 146–157. [Google Scholar]
- Ratnasamy, C.; Wagner, J.P. Water Gas Shift Catalysis. Catal. Rev. Sci. Eng. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Thinon, O.; Rachedi, K.; Diehl, F.; Avenier, P.; Schuurman, Y. Kinetics and Mechanism of the Water-Gas Shift Reaction over Platinum Supported Catalysts. Top. Catal. 2009, 52, 1940–1945. [Google Scholar] [CrossRef]
- Sun, J.; DesJardins, J.; Buglass, J.; Liu, K. Noble metal water gas shift catalysis: Kinetics study and reactor design. Int. J. Hydrogen Energy 2005, 30, 1259–1264. [Google Scholar] [CrossRef]
- Byron, S.R.J.; Loganathan, M.; Shekhar Shantha, M. A Review of the Water Gas Shift Reaction Kinetics. Int. J. Chem. React. Eng. 2010, 8, 1–32. [Google Scholar] [CrossRef]
- Maestri, M.; Vlachos, D.G.; Beretta, A.; Groppi, G.; Tronconi, E. Steam and dry reforming of methane on Rh: Microkinetic analysis and hierarchy of kinetic models. J. Catal. 2008, 259, 211–222. [Google Scholar] [CrossRef]
- Maestri, M.; Livio, D.; Beretta, A.; Groppi, G. Hierarchical Refinement of Microkinetic Models: Assessment of the Role of the WGS and r-WGS Pathways in CH4 Partial Oxidation on Rh. Ind. Eng. Chem. Res. 2014, 53, 10914–10928. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir-Hinshelwood kinetics—A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Vannice, M.A.; Hyun, S.H.; Kalpakci, B.; Liauh, W.C. Entropies of Adsorption in Heterogeneous Catalytic Reactions. J. Catal. 1979, 56, 358–362. [Google Scholar] [CrossRef]
- Moe, J.M. Design of water-gas shift reactors. Chem. Eng. Prog. 1962, 58, 33–36. [Google Scholar]
- Germani, G.; Alphonse, P.; Courty, M.; Schuurman, Y.; Mirodatos, C. Platinum/ceria/alumina catalysts on microstructures for carbon monoxide conversion. Catal. Today 2005, 110, 114–120. [Google Scholar] [CrossRef]
- Wang, X.; Gorte, R.J.; Wagner, J.P. Deactivation Mechanisms for Pd/Ceria during the Water–Gas-Shift Reaction. J. Catal. 2002, 212, 225–230. [Google Scholar] [CrossRef]
- Ruettinger, W.; Liu, X.; Farrauto, R.J. Mechanism of aging for a Pt/CeO2-ZrO2 water gas shift catalyst. Appl. Catal. B Environ. 2006, 65, 135–141. [Google Scholar] [CrossRef]
- Hilaire, S.; Wang, X.; Luo, T.; Gorte, R.J.; Wagner, J. A comparative study of water-gas-shift reaction over ceria supported metallic catalysts. Appl. Catal. A Gen. 2001, 215, 271–278. [Google Scholar] [CrossRef]
- Liu, X.; Ruettinger, W.; Xu, X.; Farrauto, R. Deactivation of Pt/CeO2 water-gas shift catalysts due to shutdown/startup modes for fuel cell applications. Appl. Catal. B Environ. 2005, 56, 69–75. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 3393–3403. [Google Scholar] [CrossRef]
- Zhang, F.X.; Tracy, C.L.; Lang, M.; Ewing, R.C. Stability of fluorite-type La2Ce2O7 under extreme conditions. J. Alloys Compd. 2016, 674, 168–173. [Google Scholar] [CrossRef]
- Lv, J.; Wang, L.; Lei, D.; Guo, H.; Kumar, R.V. Sintering, chemical stability and electrical conductivity of the perovskite proton conductors BaCe0.45Zr0.45M0.1O3-δ (M = In, Y, Gd, Sm). J. Alloys Compd. 2009, 467, 376–382. [Google Scholar] [CrossRef]
- Phair, J.W.; Badwal, S.P.S. Review of proton conductors for hydrogen separation. Ionics 2006, 12, 103–115. [Google Scholar] [CrossRef]
- Joulia, A.; Vardelle, M.; Rossignol, S. Synthesis and thermal stability of Re2Zr2O7, (Re=La, Gd) and La2(Zr1−xCex)2O7−δ compounds under reducing and oxidant atmospheres for thermal barrier coatings. J. Eur. Ceram. Soc. 2013, 33, 2633–2644. [Google Scholar] [CrossRef]


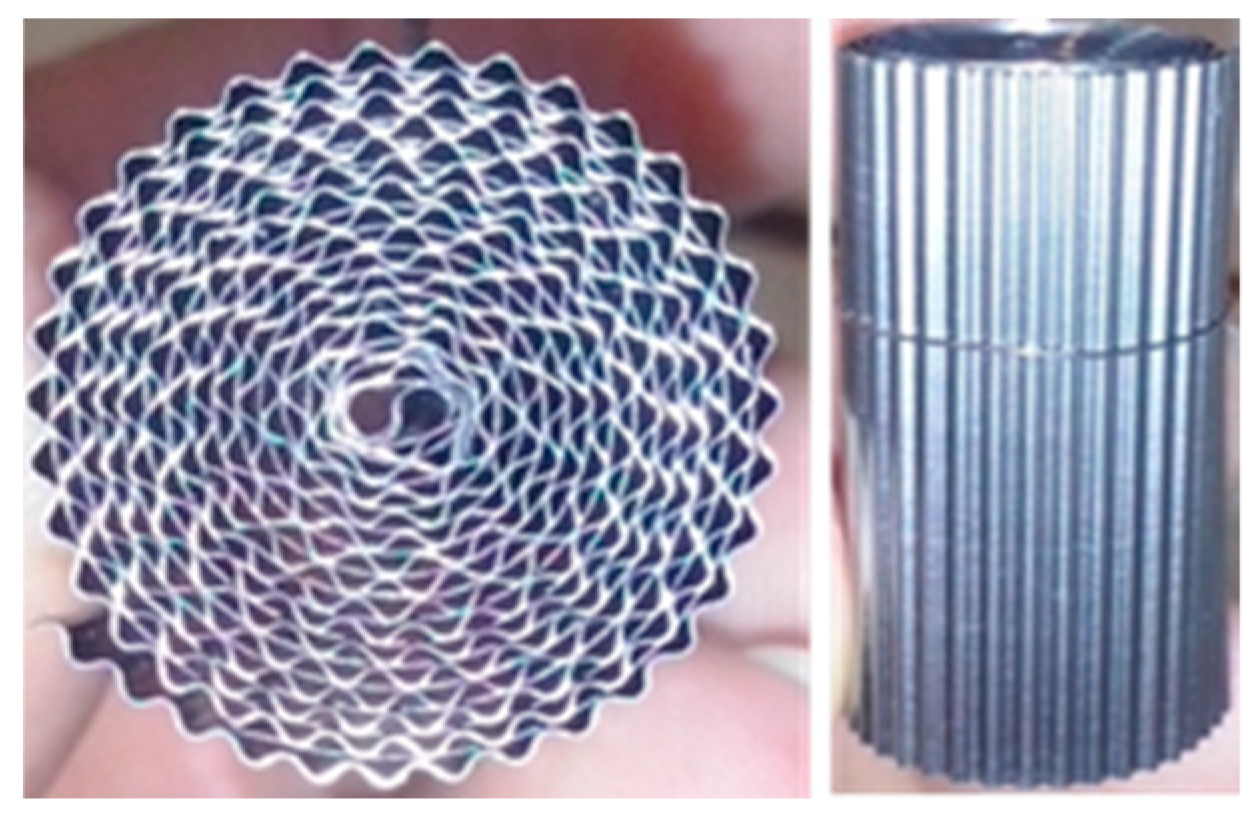
| Test | CO | H2O | CO2 | H2 | N2 |
|---|---|---|---|---|---|
| Reference A | 1.28 | 2.87 | 0 | 0 | 95.85 |
| Reference B | 1.30 | 2.82 | 0 | 0 | 95.88 |
| CO_1 | 1.65 | 2.81 | 0 | 0 | 95.54 |
| CO_2 | 2.19 | 2.80 | 0 | 0 | 95.01 |
| CO_3 | 4.20 | 2.83 | 0 | 0 | 92.97 |
| Reference C | 1.30 | 2.76 | 0 | 0 | 95.94 |
| H2_1 | 1.28 | 3.03 | 0 | 1.93 | 93.76 |
| H2_2 | 1.28 | 2.80 | 0 | 5.30 | 90.62 |
| H2_3 | 1.26 | 2.87 | 0 | 10.69 | 85.18 |
| H2_4 | 1.25 | 2.87 | 0 | 21.29 | 74.59 |
| Reference D | 1.29 | 2.87 | 0 | 0 | 95.84 |
| H2O_1 | 1.31 | 3.58 | 0 | 0 | 95.11 |
| H2O_2 | 1.40 | 5.1 | 0 | 0 | 93.50 |
| Reference E | 1.27 | 2.78 | 0 | 0 | 95.95 |
| k0 | 8.38 × 10−8 |
| A | 0.052 |
| Ea (kJ/mol) | 67.40 |
| β (H2O) | 0.359 |
| T0 (K) | 473.15 |
| α (H2) | 0.428 |
| Test | CO | H2O | CO2 | H2 | N2 |
|---|---|---|---|---|---|
| Reference A | 4.39 | 20.35 | 7.11 | 19.82 | 48.33 |
| CO_1 | 11.12 | 20.96 | 7.13 | 20.13 | 40.66 |
| H2_1 | 4.67 | 20.14 | 7.28 | 40.76 | 27.15 |
| Reference B | 4.39 | 20.32 | 7.15 | 19.97 | 48.17 |
| CO_2 | 16.72 | 20.75 | 7.13 | 20.24 | 35.16 |
| CO2_1 | 4.38 | 20.16 | 0.00 | 20.26 | 55.20 |
| Reference C | 4.41 | 20.60 | 7.17 | 20.03 | 47.79 |
| H2O_1 | 4.33 | 8.63 | 6.97 | 19.33 | 60.74 |
| H2O_2 | 4.62 | 31.23 | 7.50 | 21.20 | 35.45 |
| Reference D | 4.51 | 20.48 | 7.24 | 20.29 | 47.48 |
| H2O_3 | 4.25 | 14.81 | 6.89 | 19.22 | 54.83 |
| Reference E | 4.49 | 20.26 | 7.22 | 20.24 | 47.79 |
| k0 | 2.08 × 10−7 |
| A | 0.052 |
| Ea (kJ/mol) | 67.40 |
| β (H2O) | 0.359 |
| T0 (K) | 473.15 |
| α (H2) | 0.428 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Moncada, N.; Groppi, G.; Beretta, A.; Romero-Sarria, F.; Odriozola, J.A. Metal Micro-Monoliths for the Kinetic Study and the Intensification of the Water Gas Shift Reaction. Catalysts 2018, 8, 594. https://doi.org/10.3390/catal8120594
García-Moncada N, Groppi G, Beretta A, Romero-Sarria F, Odriozola JA. Metal Micro-Monoliths for the Kinetic Study and the Intensification of the Water Gas Shift Reaction. Catalysts. 2018; 8(12):594. https://doi.org/10.3390/catal8120594
Chicago/Turabian StyleGarcía-Moncada, Nuria, Gianpiero Groppi, Alessandra Beretta, Francisca Romero-Sarria, and José Antonio Odriozola. 2018. "Metal Micro-Monoliths for the Kinetic Study and the Intensification of the Water Gas Shift Reaction" Catalysts 8, no. 12: 594. https://doi.org/10.3390/catal8120594
APA StyleGarcía-Moncada, N., Groppi, G., Beretta, A., Romero-Sarria, F., & Odriozola, J. A. (2018). Metal Micro-Monoliths for the Kinetic Study and the Intensification of the Water Gas Shift Reaction. Catalysts, 8(12), 594. https://doi.org/10.3390/catal8120594