2. Results and Discussion
We chose several probes with different chemical characteristics, such as rhodamine B, rhodamine 6G, phenol red, methylene blue, and phenol (p). All the probes, which exhibit aromatic characteristics, as well as the structure of nylon 6,6 are depicted in
Figure 1; UV detection was used on their filtrates at maximum absorbance.
During the screening experiment using 5 mL of all probe samples at a concentration of 10 ppm, we observed that phenol, methylene blue, and rhodamine 6G were not removed, while 96% and 97% of phenol red and rhodamine B were removed completely (see
Figure S1: filtration setup, Supplemeantary Materials). This can be seen in
Figure 2 and
Figure 3.
Figure 2 compares the color of the solution before and after filtration. Both phenol red and rhodamine B were almost totally removed by passing through the nylon filter membrane; however, the color of the methylene blue and rhodamine 6G samples did not undergo any change after passing through the nylon filter membrane. Since a phenol solution is colorless, the concentration of phenol before and after passing through the nylon filter membrane was measured using a spectrophotometer, as can be seen in
Figure 3.
Figure 3 shows no change at all in the absorption spectra of phenol after passing its solution through the membrane filter, compared to its prior absorption. It is good to note that even at at low concentration (5 ppm), a negligible removal (less than 3%) was observed for phenol, methylene blue, and rhodamine 6G. These results were confirmed by total organic carbon (TOC) analysis that showed 96% and 97% removal of phenol red and rhodamine B and no removal of phenol, methylene blue, and rhodamine 6G after the filtration process. Moreover, a 5 mL mixture of phenol red and phenol, each 10 ppm, for a total concentration of 20 ppm, was tested using a filter paper. Complete removal of phenol red and zero removal of phenol were observed. Therefore, it can be concluded that the adsorption of phenol red is not affected by phenol that is not adsorbed on the filter (see
Figure S7 in Supplementary Materials).
It should be pointed out that three different batches of filters were tested, and the results were reproducible.
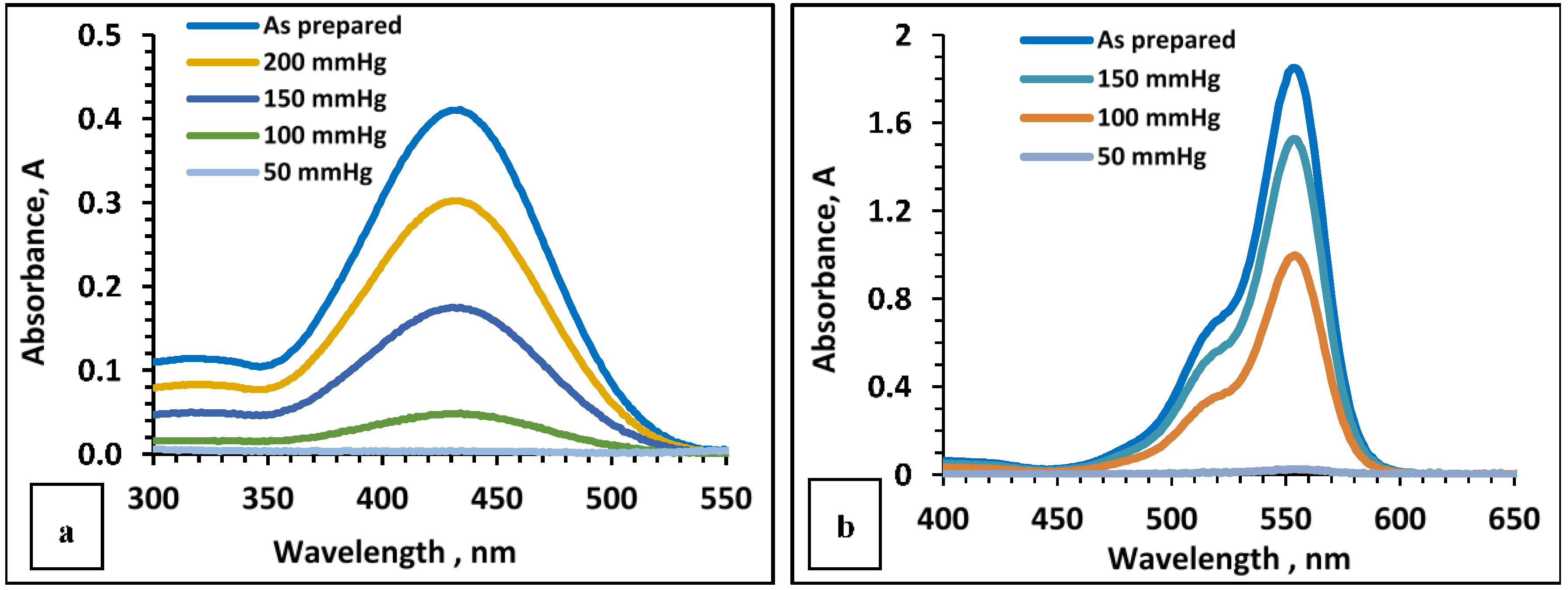
Further experiments focused on changing the applied pressure during the filtration process of 5 mL of different solutions of rhodamine B and phenol red at different concentrations of 5, 10, and 20 ppm, using a membrane filter (
Figure 4a,b). It is worth mentioning that the choice of the concentrations (20 and 10 ppm) was based on the concentrations reported in the literature [
7,
17,
19,
20,
21]. A high vacuum (i.e., low pressure and high suction power) enhanced the removal of the dyes in all cases. Regarding 5 ppm phenol red, only a slight increase in the removal was observed at a pressure increase from 50 to 100 mmHg with no further unchange until 150 mmHg, at which point the removal increased from 96% to 98.2%. Then, the removal decreased to 91.6% at 200 mmHg. For solutions with increased concentration of phenol red to 10 ppm, the effect of the applied pressure was more pronounced. The previously observed 95.9% removal of phenol red at 50 mmHg was decreased to 91.3% at 100 mmHg and then decreased drastically to 57.2% and 39.8% at 150 and 200 mmHg, respectively. A similar trend was observed with 20 ppm phenol red. The effect of pressure was more noticeable in the case of rhodamine B than in the case of phenol red. During experiments with a 5 ppm solution of rhodamine B at 50, 100, and 150 mmHg, we observed a progressive decrease in dye removal corresponding to 97.6%, 47.1%, and 17.8%, respectively. A similar trend was observed at a higher concentration of rhodamine B. It is important to note, as clearly shown in
Figure 4, that the dye removals from 5, 10, 20 ppm solutions of phenol red at 50 mmHg, without adjustment of pH, were quantitatively very close to each other, and the same situation was observed for rhodamine B. This led to the conclusion that 50 mmHg is the optimal vacuum pressure for studying the removal of dyes at different concentrations and, especially, their mixtures.
The UV–vis spectra of filtrates from 5 mL solutions at 10 ppm concentration of phenol red and rhodamine B using different vacuum pressures are depicted in
Figure 5a,b.
The efficient removal of the dyes under study at higher vacuum pressures was attributed to the membrane filter structure that is porous, as can be seen in the SEM picture (
Figure 6). A higher vacuum (lower pressure, i.e., higher suction power), therefore, allows a more efficient dye penetration through the pores of the membrane filter and increases the selective permeation region of the filter porous membrane, increasing the contact area between the dyes and the structure of the membrane. The influence of higher vacuum on dye removal was more pronounced in the case of phenol red; thus, we assume a stronger interaction of phenol red with the filtration setup. Increasing the concentration of the dyes under study led to a decreased removal capacity of the membrane filter. An enhancement of dye removal can be achieved by using a multiple-filter membrane assembly, as it was investigated in a comparison of dye removal from different volumes of a 20 ppm mixture of phenol red and rhodamine B (each 10 ppm) using one and three nylon filter membranes (FP) at 50 mmHg (
Table 1). Regarding phenol red, the removal percentage changed upon increasing the volume from 5, to 10, and to 50 mL, reaching values of 99.1%, 98.4%, and 65.5%, respectively, for one membrane, and 99.6%, 99.6%, and 97.9%, respectively, for the three-membrane assembly. Similarly, for rhodamine B, using a one-membrane filter, the removal percentage from 5, 10, and 50 mL solutions were 98.2%, 82.5%, and 36.1%, respectively, while, when applying the three-filter membrane, the removal increased to 99.7%, 99.7%. and 75.9%, respectively. With dye concentrations three-times higher, the use of three filters enhances the removal linearly, as investigated with 30 ppm solutions of phenol red and rhodamine B at 50 mm Hg, for which complete removal of phenol red (99.8%) and rhodamine B (99.2%) was observed. This is due to the increase in the membrane surface area (See
Figures S2 and S3 in the Supplementary Materials).
To study the removal capacity, the dye distribution and profile in cross sections of the membrane filters was investigated using confocal microscopy (
Figure 7). It should be pointed out that attempts for confocal microscopy investigation directly from the top of the samples were carried out; however, the optical properties of the dense mesh of the membrane did not allow for this type of measurement. The arrows in
Figure 7 indicate the direction of filtration. Two areas were chosen for investigation that differed for their contact with the filtrate. The first area corresponded to clear membrane filter where filtration took place, whereas the second area corresponded to a membrane region in contact with the ceramic support.
Figure 7a,b shows that no precipitation formed, and both rhodamine B and phenol red were distributed gradually from the upper to the bottom part of the membrane. Intensity was much higher in the area were filtration took place (7a) compared to the areas in contact with the ceramic support (7c), suggesting the presence of an interaction between the dye and the membrane filter nylon material. Concerning the part of the filter in contact with ceramic, although the concentration of the dye was much lower, after normalization of the intensity, a similar gradual intensity profile of dye through the filter was observed (
Figure 7, bottom row). For phenol, rhodamine 6G, and methylene blue it was observed that their fluorescence was negligible.
Since we assumed an interaction of the nylon filter with some probe, we conducted an X-ray Photoelectron Spectroscopy (XPS) investigation. XPS is a technique providing simple analyses of elements and their surface chemical state.
Figure 8 shows the XPS spectra region of N1s for rhodamine B, a nylon membrane filter, and a nylon membrane filter + rhodamine B. The binding energies for N1s can be observed at 339.37, 399.53, and 399.56 eV for rhodamine B, nylon, and rhodamine B + nylon, respectively. On the basis of full-width at half-maximum (FWHM), N1s’ peak of rhodamine B + nylon is broader (FWHM of 1.59) than that of nylon and rhodamine B (FWHW of 1.46 and 1.12, respectively). The increase in the broadening of N1s in rhodamine B + nylon could be attributed to the overlap between the N1s’ peaks of rhodamine B and nylon.
The XPS of phenol red (
Figure 9) showed the presence of two spectra lines for S2p, at 167.69 and 168.83 eV for 3/2 and 1/2 spin-orbit coupling. These peaks represent the SO
3 group in the phenol red structure. Following filtration with the nylon membrane, the same S2p peak was observed, but broader and less intense, which might be due to the low intensity of phenol red on a nylon membrane filter.
After these observations, we were curious about the mechanism of membrane adsorption of particular dyes. We assumed that the adsorption of some dyes, such as phenol red and rhodamine B, can be ascribed to the chemical characters of these dyes and of the filter. Since all investigated molecules contain large aromatic conjugated systems, π–π interactions are unlikely. Phenol red and rhodamine B at pH 7 are in the form of a carboxylate which, as a negatively charged molecule, can interact through electrostatic interactions with the Whatman nylon membrane filter, while phenol, rhodamine 6G, and methylene blue do not have such negatively charged moieties in their molecules, so they do not show any interaction with the nylon membrane. Phenol is neutral, rhodamine 6G has a stable ester moiety with only a positive charge on nitrogen in its structure, and methylene blue contains positively charged moieties. Phenol red and rhodamine B contain, at pH 7, a sulfo- and carboxylic group in their molecules, respectively, as in
Figure 10. Since ion-pairs are more stable than free ions, interactions occur most probably with the charged terminal groups in the nylon molecules, which could form during the fabrication process of the membrane. Nylon 6,6 is a polyamide prepared by a polycondensation reaction from hexamethylenediamine and adipic acid, and it is known that, during the fabrication process, each polymer chain is terminated with functional groups such as the amino group. The terminal group is more exposed to the environment and, in the case of the amino group, it prefers a hydrophilic environment. FT–IR of the membrane filter was performed to obtain information about the functional groups present, as shown in
Figure 11. The main absorption peaks at 3330, 3080, 1650, and 1535 cm
−1 are related to N–H stretching, N–H angular deformation, C = O stretching, and N–H in-plane deformation, respectively, as regards the amide group. Moreover, the absorption peaks at 3020, 2950, and 2840 cm
−1 correspond to C–H symmetric, CH
2 asymmetric, and CH
2 symmetric vibrations, respectively, in the main polymer structure. Additionally, there is indication of the amino-like group in FT–IR, attributed to aliphatic C–N stretching at 1140 cm
−1. This supports the hypothesis of an interaction between the terminal groups of the polymeric chain with the charged dyes.
The higher removal percentage for rhodamine B at lower pH can be attributed to the formation of an ammonium salt on the membrane filter, involving the amino group on the membrane surface that electrostatically interacts with rhodamine B in the carboxylic acid form, whereas, at high pH, the amino group can be in the amine form, which reduces the electrostatic interactions with rhodamine B. This can form a lactone neutral form. Similarly, the lower removal of phenol red at a lower pH, at which phenol red is transformed to the neutral lactone form, suggests the same principle as previously explained for rhodamine B.
The measurements of the removal percentage at different pH values support fully this assumption, as is seen in
Figure 12. Decrease of the removal percentage occurred at higher pH, as rhodamine B transformed into the neutral lactone form. Phenol red transformed at a lower pH to the lactone form, as depicted in
Figure 12b, and, similarly, its removal percentage from the sample decreased.
Chemical differences also exist between rhodamine B and phenol red, as the organic sulfate group of phenol red has a much lower pKa compared to the pKa of the carboxylate group in phenol red. This difference in functionality influences ion pairing between negative and positive charges. Stronger ion pairing is formed between the more acidic sulfate group in phenol red and ammonium from the filter mesh, which is in agreement with our observations at high concentrations and vacuum pressures.
It is good to mention that during the experiments and data collection, we considered the alternative idea that CONH in the nylon could act as a hydrogen bond donor; however, such type of hydrogen bond is rather weak (about 2 kcal/mol) to strongly interact with a simple molecule, as in the case we examined [
22]. Moreover, if the key and responsible interaction was a hydrogen bonding between N–H from nylon acting as H- acceptor and the COO or SO
3 group acting as a donor, then also rhodamine 6G would react with the H- acceptor as well, which did not happen in our experiments. Additionally, FTIR data clearly indicated the presence of C–NH as an amine-like molecule, in addition to the peak related to the amide CONH group present in the main polymer backbone of the nylon membrane. On the other hand, the size of the molecule is not a factor for the adsorption of phenol red and rhodamine B on the filters. Key factors for the adsorption of the dyes are the electrostatic interactions and pKa. Phenol has a much higher pKa than a carboxylic acid, thus, it cannot form a strong electrostatic interaction as in the case of carboxylic acids or sulfonic acid derivatives.
To evaluate how many times a filter can be used, consecutive runs using one filter membrane with 10 ppm phenol red were tested. In each run, 5 mL of phenol red was filtered, and the percentage of removal of phenol red was calculated. These repetitive runs were done 10 times, then the filter was inverted, washed with distilled water under high vacuum, and subjected to two more runs. It was observed that, after four runs, the removal percentage was nearly the same as after the first run, corresponding to 98% removal, then it decreased gradually in the subsequent runs, to reach 64.3% after the 10th run. After washing the filter, its efficiency increased to 88% (see
Table S1 in the Supplementary Materials).
To evaluate the possible error that can be associated with the use of a nylon filter for heterogenous catalysis, a photodegradation of 10 ppm phenol red with TiO
2 as a photocatalyst using a 150 W Xe lamp as the irradiation source was done. Before photocatalysis, a 100 mL of phenol red with TiO
2 was stirred in a dark room, then dye separation was performed, using either a centrifuge or a nylon filter paper, to determine the initial concentration of phenol red. After complete adsorption was accomplished, the suspended solution (phenol red + TiO
2) was subjected to Xe illumination for 5 min. A 93% removal of phenol red was observed before illumination when using a nylon filter, while no removal of phenol red was observed when using a centrifuge. After 5 min illumination, a 98.9% removal of phenol red was measured when a nylon filter was used, while only 73.9% degradation was obtained when a centrifuge was used. Therefore, it can be concluded that an error corresponding to 25% can be associated to the use of a nylon filter paper in the photodegradation of 10 ppm phenol red after 5 min, using Xe illumination and TiO
2 as a photocatalyst (see
Figures S4–S6 in Supplementary Materials)