Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review
Abstract
:1. Introduction
2. Biodiesel
3. Biochar
3.1. Activation of Biochars through Chemical Treatment
3.2. Biodiesel Formation through Transesterification Reaction Using Different Biochars as Catalysts
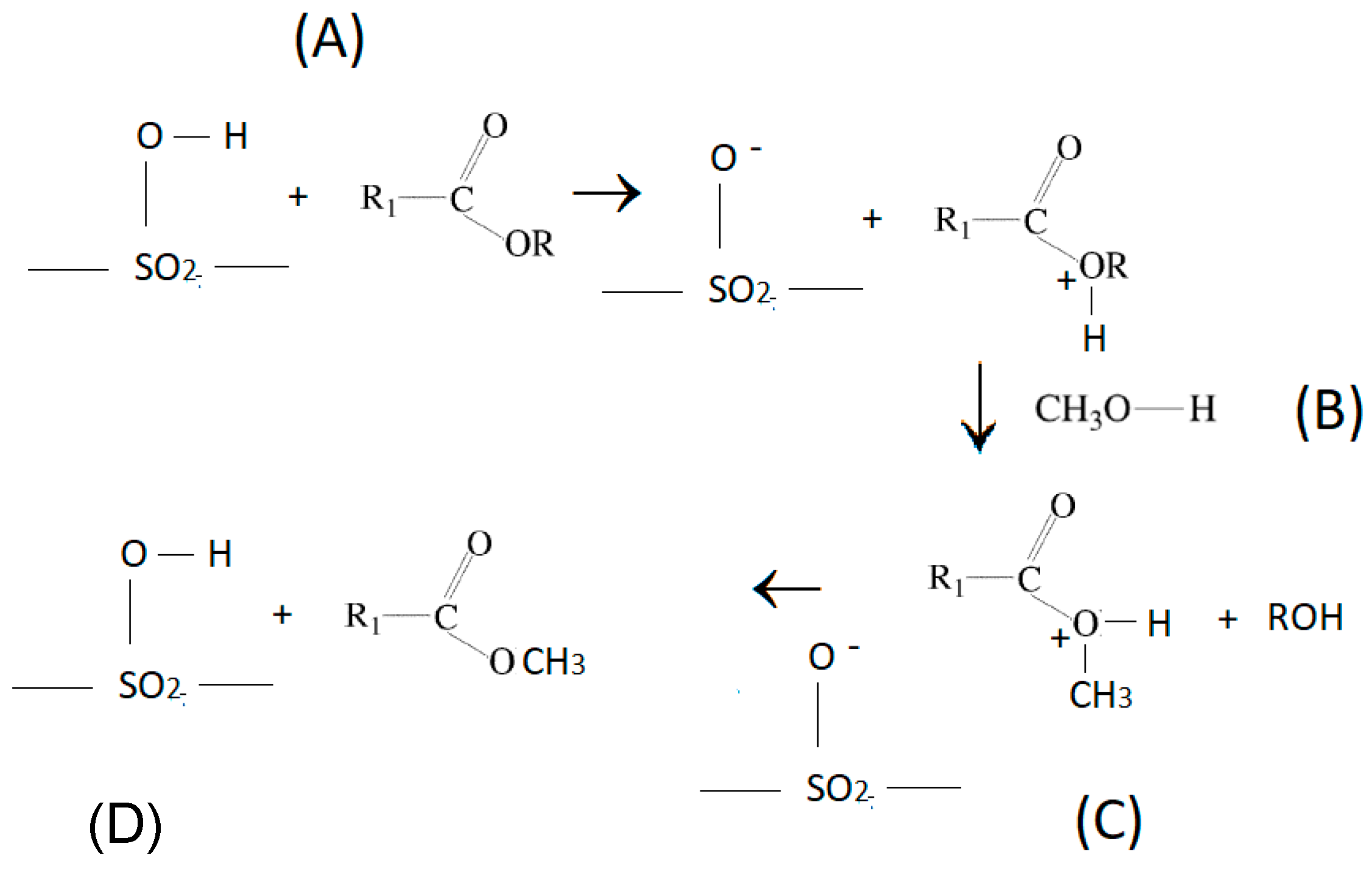
3.2.1. Acid Biochars Catalysts
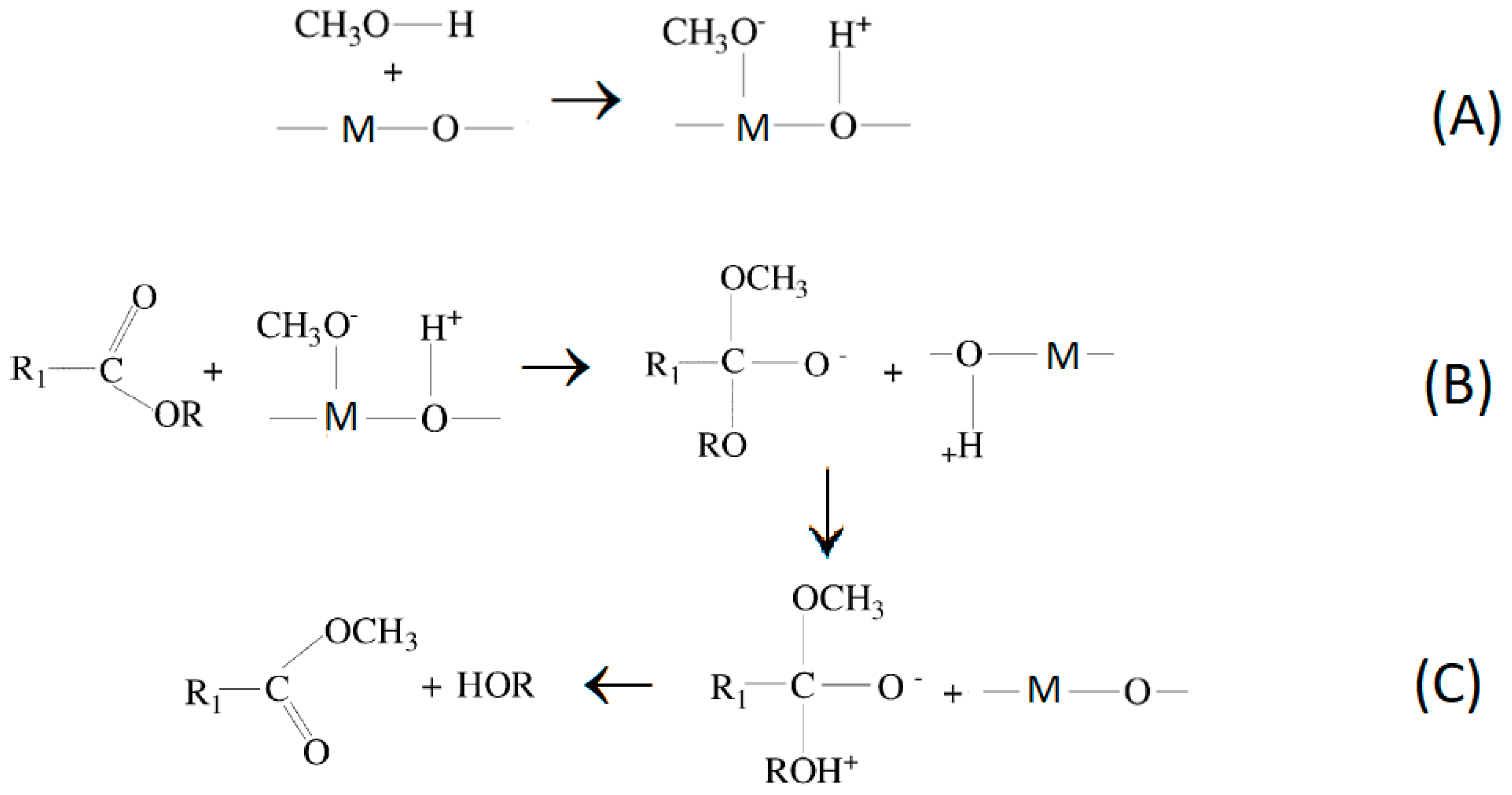
3.2.2. Basic Biochars Catalysts
3.2.3. Pseudo Catalytic Transesterification
4. Conclusions
Funding
Conflicts of Interest
References
- Liew, W.H.; Hassim, M.H.; Ng, D.K.S. Review of evolution, technology and sustainability assessments of biofuel production. J. Clean. Prod. 2014, 71, 11–29. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Kordulis, C.; Bourikas, K.; Gousi, M.; Kordouli, E.; Lycourghiotis, A. Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel: A critical review. Appl. Catal. B Environ. 2016, 181, 156–196. [Google Scholar] [CrossRef]
- Kordouli, E.; Pawelec, B.; Bourikas, K.; Kordulis, C.; Fierro, J.L.G.; Lycourghiotis, A. Mo promoted Ni-Al2O3 co-precipitated catalysts for green diesel production. Appl. Catal. B Environ. 2018, 229, 139–154. [Google Scholar] [CrossRef]
- Kordouli, E.; Sygellou, L.; Kordulis, C.; Bourikas, K.; Lycourghiotis, A. Probing the synergistic ratio of the NiMo/γ-Al2O3 reduced catalysts for the transformation of natural triglycerides into green diesel. Appl. Catal. B Environ. 2017, 209, 12–22. [Google Scholar] [CrossRef]
- Kordouli, E.; Kordulis, C.; Lycourghiotis, A.; Cole, R.; Vasudevan, P.T.; Pawelec, B.; Fierro, J.L.G. HDO activity of carbon-supported Rh, Ni and MoNi catalysts. Mol. Catal. 2017, 441, 209–220. [Google Scholar] [CrossRef]
- Kordouli, E.; Pawelec, B.; Kordulis, C.; Lycourghiotis, A.; Fierro, J.L.G. Hydrodeoxygenation of phenol on bifunctional Ni-based catalysts: Effects of Mo promotion and support. Appl. Catal. B Environ. 2018, 238, 147–160. [Google Scholar] [CrossRef]
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azida, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Tang, Z.E.; Lim, S.; Pang, Y.L.; Ong, H.C.; Lee, K.T. Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Katsoulidis, A.K.; Pomonis, P.G.; Manos, G.; Kontominas, M.G. Transesterification of rapeseed oil for the production of biodiesel using homogeneous and heterogeneous catalysis. Fuel Process. Technol. 2009, 90, 1016–1022. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Al-Resayes, S.I.; Nehdi, I.A. Recent progress in synthesis and surface functionalization of mesoporous acidic heterogeneous catalysts for esterification of free fatty acid feedstocks: A review. Energy Convers. Manag. 2017, 141, 183–205. [Google Scholar] [CrossRef]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T.L. Selection of Direct Transesterification as the Preferred Method for Assay of Fatty Acid Content of Microalgae. Lipids 2010, 45, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Semwal, S.; Arora, A.K.; Badoni, R.P.; Tuli, D.K. Biodiesel production using heterogeneous catalysts. Bioresour. Technol. 2011, 102, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Suppes, G.J.; Dasari, M.A.; Doskocil, E.J.; Mankidy, P.J.; Goff, M.J. Transesterification of soybean oil with zeolite and metal catalysts. Appl. Catal. A Gen. 2004, 257, 213–223. [Google Scholar] [CrossRef]
- Mbaraka, I.K.; Shanks, B.H. Conversion of oils and fats using advanced mesoporous heterogeneous catalysts. J. Am. Oil Chem. Soc. 2006, 83, 79–91. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, M.R.; Pengmei, L.; Santacesaria, E. Heterogeneous catalysts for biodiesel production. Energy Fuels 2008, 22, 207–217. [Google Scholar] [CrossRef]
- Boutsika, L.G.; Karapanagioti, H.K.; Manariotis, I.D. Effect of chloride and nitrate salts on Hg(II) sorption by raw and pyrolyzed malt spent rootlets. J. Chem. Technol. Biotechnol. 2017, 92, 1912–1918. [Google Scholar] [CrossRef]
- Manariotis, I.D.; Fotopoulou, K.N.; Karapanagioti, H.K. Preparation and Characterization of Biochar Sorbents Produced from Malt Spent Rootlets. Ind. Eng. Chem. Res. 2015, 54, 9577–9584. [Google Scholar] [CrossRef]
- Kemmou, L.; Frontistis, Z.; John Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Waqas, M.; Aburiazaiza, A.S.; Miandad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of biochar as fuel and catalyst in energy recovery technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Härmas, M.; Thomberg, T.; Kurig, H.; Romann, T.; Jänes, A.; Lust, E. Microporous-mesoporous carbons for energy storage synthesized by activation of carbonaceous material by zinc chloride, potassium hydroxide or mixture of them. J. Power Sources 2016, 326, 624–634. [Google Scholar] [CrossRef]
- Iqbaldin, M.N.M.; Khudzir, I.; Azlan, M.I.M.; Zaidi, A.G.; Surani, B.; Zubri, Z. Properties of coconut shell activated carbon. J. Trop. Sci. 2013, 25, 497–503. [Google Scholar]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Production of high surface area mesoporous activated carbons from waste biomass using hydrogen peroxide-mediated hydrothermal treatment for adsorption applications. Chem. Eng. J. 2015, 273, 622–629. [Google Scholar] [CrossRef]
- Bourikas, K.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Potentiometric mass titrations: Experimental and theoretical establishment of a new technique for determining the point of zero charge (PZC) of metal (hydr)oxides. J. Phys. Chem. 2003, 107, 9441–9451. [Google Scholar] [CrossRef]
- Lloyd, T.A.; Wyman, C.E. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour. Technol. 2005, 96, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Gratuito, M.K.B.; Panyathanmaporn, T.; Chumnanklang, R.A.; Sirinuntawittaya, N.; Dutta, A. Production of activated carbon from coconut shell: Optimization using response surface methodology. Bioresour. Technol. 2008, 99, 4887–4895. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, C.; Ca, H.; Zhao, H.; Zhang, Y.; Fan, Z. KOH self-templating synthesis of three-dimensional hierarchical porous carbon materials for high performance supercapacitors. J. Mater. Chem. A 2014, 2, 14844–14851. [Google Scholar] [CrossRef]
- Bazargan, A.; Kostić, M.D.; Stamenković, O.S.; Veljković, V.B.; McKay, G. A calcium oxide-based catalyst derived from palm kernel shell gasification residues for biodiesel production. Fuel 2015, 150, 519–525. [Google Scholar] [CrossRef]
- Shan, R.; Zhao, C.; Lv, P.; Yuan, H.; Yao, J. Catalytic applications of calcium rich waste materials for biodiesel: Current state and perspectives. Energy Conver. Manag. 2016, 127, 273–283. [Google Scholar] [CrossRef]
- Jung, J.M.; Cho, J.; Kim, K.H.; Kwon, E.E. Pseudo catalytic transformation of volatile fatty acids into fatty acid methyl esters. Bioresour. Technol. 2016, 203, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Khosla, K.; Rathour, R.; Maurya, R.; Maheshwari, N.; Gnansounou, E.; Larroche, C.; Thakur, I.S. Biodiesel production from lipid of carbon dioxide sequestrating bacterium and lipase of psychrotolerant Pseudomonas sp. ISTPL3 immobilized on biochar. Bioresour. Technol. 2017, 245, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, J.M.; Oh, J.I.; Ok, Y.S.; Lee, S.R.; Kwon, E.E. Evaluating the effectiveness of various biochars as porous media for biodiesel synthesis via pseudo-catalytic transesterification. Bioresour. Technol. 2017, 231, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Niu, S.; Li, J. Activity of the carbon-based heterogeneous acid catalyst derived from bamboo in esterification of oleic acid with ethanol. Energy Convers. Manang. 2016, 114, 188–196. [Google Scholar] [CrossRef]
- Chellappan, S.; Nair, V.; Sajith, V.; Aparna, K. Experimental validation of biochar based green Bronsted acid catalysts for simultaneous esterification and transesterification in biodiesel production. Bioresour. Technol. Rep. 2018, 2, 38–44. [Google Scholar] [CrossRef]
- Ahmad, J.; Rashid, U.; Patuzzi, F.; Baratieri, M.; Taufiq-Yap, Y.H. Synthesis of char-based acidic catalyst for methanolysis of waste cooking oil: An insight into a possible valorization pathway for the solid by-product of gasification. Energy Conver. Manag. 2018, 158, 186–192. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S. Sustainable utilization of waste palm oil and sulfonated carbon catalyst derived from coconut meal residue for biodiesel production. Bioresour. Technol. 2018, 248, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Endut, A.; Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Hamid, S.H.A.; Lananan, F.; Kamarudin, M.K.A.; Umar, R.; Juahir, H.; Khatoon, H. Optimization of biodiesel production by solid acid catalyst derived from coconut shell via response surface methodology. Int. Biodeterior. Biodegrad. 2017, 124, 250–257. [Google Scholar] [CrossRef]
- Hidayat, A.; Rochmadi, W.K.; Nurdiawati, A.; Kurniawan, W.; Hinode, H.; Yoshikawa, K.; Budiman, A. Esterification of Palm Fatty Acid Distillate with High Amount of Free Fatty Acids Using Coconut Shell Char Based Catalyst. Energy Procedia 2015, 75, 969–974. [Google Scholar] [CrossRef]
- Ngaosuwan, K.; Goodwin, J.G., Jr.; Prasertdham, P. A green sulfonated carbon-based catalyst derived from coffee residue for esterification. Renew. Energy 2016, 86, 262–269. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.; Li, W.; Shi, C.; Wang, Y. Preparation and characterization of biomass carbon-based solid acid catalyst for the esterification of oleic acid with methanol. Bioresour. Technol. 2013, 133, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Zong, M.-H.; Duan, Z.-Q.; Lou, W.-Y.; Smith, T.J.; Wu, H. Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem. 2007, 9, 434–437. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R. Methyl ester production from palm fatty acid distillate using sulfonated glucose-derived acid catalyst. Renew. Energy 2015, 81, 347–354. [Google Scholar] [CrossRef]
- Lien, Y.-S.; Hsieh, L.-S.; Wu, J.C.S. Biodiesel Synthesis by Simultaneous Esterification and Transesterification Using Oleophilic Acid Catalyst. Ind. Eng. Chem. Res. 2010, 49, 2118–2121. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Ayodele, O.; Xin, J.; Zhang, S.; Yan, D. Effective conversion of non-edible oil with high free fatty acid into biodiesel by sulphonated carbon catalyst. Appl. Energy 2014, 114, 819–826. [Google Scholar] [CrossRef]
- Dong, T.; Gao, D.; Miao, C.; Yu, X.; Degan, C.; Garcia-Pérez, M.; Rasco, B.; Salbani, S.S.; Chen, S. Two-step microalgal biodiesel production using acidic catalyst generated from pyrolysis-derived bio-char. Energy Convers. Manag. 2015, 105, 1389–1396. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Pang, Y.L. Investigation of carbon-based solid acid catalyst from Jatropha curcas biomass in biodiesel production. Energy Convers. Manag. 2017, 144, 10–17. [Google Scholar] [CrossRef]
- Rafi, J.M.; Rajashekar, A.; Srinivas, M.; Rao, B.V.S.K.; Prasad, R.B.N.; Lingaiah, N. Esterification of glycerol over a solid acid biochar catalyst derived from waste biomass. RSC Adv. 2015, 5, 44550–44556. [Google Scholar] [CrossRef]
- Fu, X.; Li, D.; Chen, J.; Zhang, Y.; Huang, W.; Zhu, Y.; Yang, J.; Zhang, C. A microalgae residue based carbon solid acid catalyst for biodiesel production. Bioresour. Technol. 2013, 146, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.T.; Dehkhoda, A.M.; Ellis, N. Development of biochar-based catalyst for transesterification of canola oil. Energy Fuels 2011, 25, 337–344. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Reyes, D.; Romero-Hermoso, L.; Hidalgo, P.; Meier, S.; Benito, N.; Navia, R. Functionalization of biochar derived from lignocellulosic biomass using microwave technology for catalytic application in biodiesel production. Energy Conver. Manag. 2017, 137, 165–173. [Google Scholar]
- Konwar, L.J.; Das, R.; Thakur, A.J.; Mäki-Arvelac, E.S.P.; Narendra, K.; Mikkola, J.P.; Deka, D. Biodiesel production from acid oils using sulfonated carbon catalyst derived from oil-cake waste. J. Mol. Catal. A Chem. 2014, 388–389, 167–176. [Google Scholar] [CrossRef]
- Santos, E.M.; Teixeira, A.P.D.C.; da Silva, F.G.; Cibaka, T.E.; Araújo, M.H.; Oliveira, W.X.C.; Medeiros, F.; Brasil, A.N.; Oliveira, L.S.; Lago, R.M. New heterogeneous catalyst for the esterification of fatty acid produced by surface aromatization/sulfonation of oilseed cake. Fuel 2015, 150, 408–414. [Google Scholar] [CrossRef]
- Akinfalabi, S.I.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Synthesis of biodiesel from palm fatty acid distillate using sulfonated palm seed cake catalyst. Renew. Energy 2017, 111, 611–619. [Google Scholar] [CrossRef]
- Ezebor, F.; Khairuddean, M.; Abdullah, A.Z.; Boey, P.L. Esterification of oily-FFA and transesterification of high FFA waste oils using novel palm trunk and bagasse derived catalysts. Energy Convers. Manag. 2014, 88, 1143–1150. [Google Scholar] [CrossRef]
- Rao, B.V.S.K.; Chandra Mouli, K.; Rambabu, N.; Dalai, A.K.; Prasad, R.B.N. Carbon-based solid acid catalyst from de-oiled canola meal for biodiesel production. Catal. Commun. 2011, 14, 20–26. [Google Scholar] [CrossRef]
- Kastner, J.R.; Miller, J.; Geller, D.P.; Locklin, J.; Keith, L.H.; Johnson, T. Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catal. Today 2012, 190, 122–132. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, S.; Gong, W.; Wang, G.; Qiu, J.; Chen, H. Synthesis, characterization and acid catalysis of solid acid from peanut shell. Appl. Catal. A Gen. 2014, 469, 284–289. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Bioresour. Technol. 2014, 154, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, A.; Meng, Y.; Wang, L.; Jiang, H.; Li, G. Catalytic Performance of Biomass Carbon-Based Solid Acid Catalyst for Esterification of Free Fatty Acids in Waste Cooking Oil. Catal. Surv. Asia 2015, 19, 61–67. [Google Scholar] [CrossRef]
- Li, M.; Chen, D.; Zhu, X. Preparation of solid acid catalyst from rice husk char and its catalytic performance in esterification. Chin. J. Catal. 2013, 34, 1674–1682. [Google Scholar] [CrossRef]
- Chellappan, S.; Nair, V.; Sajith, V.; Aparna, K. Synthesis, optimization and characterization of biochar based catalyst from sawdust for simultaneous esterification and transesterification. Chin. J. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Haile, M. Integrated volarization of spent coffee grounds to biofuels. Biofuel Res. J. 2014, 1, 65–69. [Google Scholar] [CrossRef]
- Haile, M.; Asfaw, A.; Asfaw, N. Investigation of waste coffee ground as a potential raw material for biodiesel production. Int. J. Renew. Energy Res. 2013, 3, 854–860. [Google Scholar]
- Shu, Q.; Zhang, Q.; Xu, G.; Nawaz, Z.; Wang, D.; Wang, J. Synthesis of biodiesel from cottonseed oil and methanol using a carbon-based solid acid catalyst. Fuel Process. Technol. 2009, 90, 1002–1008. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Ellis, N. Biochar-based catalyst for simultaneous reactions of esterification and transesterification. Catal. Today 2013, 207, 86–92. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Konwar, L.J.; Boro, J.; Deka, D. Review on latest developments in biodiesel production using carbon-based catalysts. Renew. Sustain. Energy Rev. 2014, 29, 546–564. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Peng, Y.-P.; Amesho, K.T.T.; Chen, C.-E.; Jhang, S.-R.; Chou, F.-C.; Lin, Y.-C. Optimization of Biodiesel Production from Waste Cooking Oil Using Waste Eggshell as a Base Catalyst under a Microwave Heating System. Catalysts 2018, 8, 81. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, H.; Wang, Y.; Shana, R. Transesterification of vegetable oil on low cost and efficient meat and bone meal biochar catalysts. Energy Convers. Manag. 2017, 150, 214–221. [Google Scholar] [CrossRef]
- Kostić, M.D.; Bazargan, A.; Stamenković, O.S.; Veljković, V.B.; McKay, G. Optimization and kinetics of sunflower oil methanolysis catalyzed by calcium oxide-based catalyst derived from palm kernel shell biochar. Fuel 2016, 163, 304–313. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Shan, R.; Wang, Y.; Yuan, H. A novel peat biochar supported catalyst for the transesterification reaction. Energy Conver. Manag. 2017, 139, 89–96. [Google Scholar] [CrossRef]
- Wang, S.; Shan, R.; Wang, Y.; Lu, L.; Yuan, H. Synthesis of calcium materials in biochar matrix as a highly stable catalyst for biodiesel production. Renew. Energy 2019, 130, 41–49. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, L.; Xing, S.; Luo, W.; Wang, Z.; Lv, P. Biodiesel production by a highly effective renewable catalyst from pyrolytic rice husk. J. Clean. Prod. 2018, 199, 772–780. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, P.; Yang, L.; Xing, S.; Luo, W.; Wang, Z. Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Conver. Manag. 2018, 160, 477–485. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2019, 50, 14–34. [Google Scholar] [CrossRef]
- Glisic, S.B.; Orlović, A.M. Review of biodiesel synthesis from waste oil under elevated pressure and temperature: Phase equilibrium, reaction kinetics, process design and techno-economic study. Renew. Sustain. Energy Rev. 2014, 31, 708–725. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.-M.; Oh, J.-I.; Ok, Y.S.; Kwon, E.E. Establishing a green platform for biodiesel synthesis via strategic utilization of biochar and dimethyl carbonate. Bioresour. Technol. 2017, 241, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Kusudiana, D.; Saka, S. Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 2001, 80, 693–698. [Google Scholar] [CrossRef]
- Kusudiana, D.; Saka, S. Effect of water on biodiesel fuel production by supercritical methanol treatment. Bioresour. Technol. 2004, 91, 289–295. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Convser. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.-M.; Lee, J.; Kim, K.-H.; Choi, T.O.; Kim, J.-K.; Jeon, Y.J.; Kwon, E.E. Pyrogenic transformation of Nannochloropsis oceanica into fatty acid methyl esters without oil extraction for estimating total lipid content. Bioresour. Technol. 2016, 212, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Posarac, D.; Ellis, N. Process simulation and economic analysis of biodiesel production processes using fresh and waste vegetable oil and supercritical methanol. Chem. Eng. Res. Des. 2011, 89, 2626–2642. [Google Scholar] [CrossRef]
- Jung, J.-M.; Lee, J.; Choi, D.; Oh, J.-I.; Lee, S.-R.; Kim, J.-K.; Kwon, E.E. Biochar as porous media for thermally-induced non-catalytic transesterification to synthesize fatty acid ethyl esters from coconut oil. Energy Convers. Manag. 2017, 145, 308–313. [Google Scholar] [CrossRef]
- Lee, J.; Jung, M.Y.; Ok, Y.S.; Kwon, E.E. Sustainable approach to biodiesel synthesis via thermally induced transesterification using biochar as surrogate porous media. Energy Convers. Manag. 2017, 151, 601–606. [Google Scholar] [CrossRef]
- Jung, J.M.; Lee, J.; Kim, K.H.; Kwon, E.E. Pyrogenic transformation of oil-bearing biomass into biodiesel without lipid extraction. Energy Convers. Manag. 2016, 123, 317–323. [Google Scholar] [CrossRef]
- Jung, J.M.; Lee, J.; Kim, K.H.; Lee, S.R.; Song, H.; Kwon, E.E. Biodiesel conversion via thermal assisted in-situ transesterification of bovine fat using dimethyl carbonate as an acyl acceptor. ACS Sustain. Chem. Eng. 2016, 4, 5600–5605. [Google Scholar] [CrossRef]



| Raw Material | Characteristics | Reaction Temp. °C | % Conversion or % Yield | Reference | ||
|---|---|---|---|---|---|---|
| SSA (m2/g) | %S | –SO3H mmol/g | ||||
| Bamboo | 2.7 | 6 | 65 | 98.4 | [35] | |
| Cassava peel, irul sawdust and coconut shell | 2 | 85 | 96.8–85.2 | [36] | ||
| Char from a commercial small-scale gasification plant | 337–354 | 2.37 | 96 | [37] | ||
| Coconut meal residue | 1.3–4 | 6–14 | BP | 92.7 | [38] | |
| Coconut shell | 24.2 | 0.5 | 60 | 88 | [39] | |
| Coconut shell char | 244 | 4.55 | 60 | 80% | [40] | |
| Coffee | 992–1218 | 0.45–0.72 | 60 | 71.5 | [41] | |
| Corn | 7.8 | 60 | 92 | [42] | ||
| d-glucose | 4.13 | 11.9 | 80 | >95 | [43] | |
| d-glucose | 4−10.67 | 0.12–4.23 | 93 | [44] | ||
| d-glucose | 0.31 | 2.7 | 60 | 90 | [45] | |
| d-glucose or C. inophyllum seed cake | 0.2–3.4 | 2.5 | 130–200 | 99 | [46] | |
| Douglas fir | 3.51 | 2 | 65 | 99 | [47] | |
| Hardwood | 2.74–14.38 | 1.7–2.1 | 65 | 77–89 | [48] | |
| Jatropha curcas seed cake | 1.62−2.35 | 23 | 45–70 | 99.1 | [49] | |
| karanja seed shells | 13–16 | 7.5–16 | 120 | 95.6 | [50] | |
| Microalgae residue | 1 | 80 | 98 | [51] | ||
| Mixture of wood waste, white wood bark, and shavings | 2−1411 | 1.2–2.69 | 65 | 7.6–18.9 | [52] | |
| Oat hull. | 5−49.3 | 3–7.5 | 100–140 | 27–72 | [53] | |
| Oil cake waste | 8−777 | 1.2–2.4 | 0.16–2.21 | 65 | 7 | [54] |
| Oilseed cake | ≅0 | 3–3.3 | 60 | 94 | [55] | |
| Palm seed cake | 483 | 3.16 | 55–75 | 98 | [56] | |
| Palm trunk and bagasse | 3−4.5 | 2.7–2.98 | 0.94–1.48 | 65 | 83.2 | [57] |
| Partially carbonized de-oiled canola meal | <10 | 0.2–4.4 | 65 | 93.8 | [58] | |
| Peanut hulls, pine logging residues, and wood chips | 0–242 | 0.1–2.82 | 0–0.86 | 55–60 | 90–100 | [59] |
| Peanut shell | 4–12 | 0–3.25 | 120 | 90.2 | [60] | |
| Rice husk | 4 | 5.7 | 70–110 | 98.2 | [61] | |
| Rice husk | 0.43–2.43 | 66 | 87 | [62] | ||
| Rice husk | 4 | 2.25 | 70 | 96 | [63] | |
| Sawdust | 2.35–33 | 0–6.62 | 85 | 95.6 | [64] | |
| Spent coffee grounds | 60 | 15.6–21.5 | [65] | |||
| Spent coffee grounds | 60 | 73.4 | [66] | |||
| Vegetable oil asphalt | 7.48–41.27 | 2.21 | 180–260 | 89.93 | [67] | |
| Woody biomass | 857–990 | 0.36 | 150 | 48.1 | [68] | |
| Raw Material | Active Phase | SSA (m2/g) | Reaction Tempe. (°C) | % Conversion or % Yield | Reference |
|---|---|---|---|---|---|
| Meat and bone meal | K2CO3 | 45–430 | 65 | 98 | [73] |
| Palm kernel shells | CaO | - | 45–65 | 99 | [74] |
| Peat | K2CO3 | 14–25 | 65 | 98.6 | [75] |
| Peat | 20–40% wt K2CO3 | 80–230 | 65 | 98 | [76] |
| Rice husk | CaO | 20–342 | 65 | 98 | [77] |
| Waste pomelo peel | 15–35% wt K2CO3 | 3–31 | 65 | 98 | [78] |
| Raw Material | % Conversion or % Yield | Comment | Reference |
|---|---|---|---|
| Maize residue | 95.4 | 380 °C and molar ratio of DMC to olive oil (36:1) | [81] |
| Maize residue | 87 | 380 °C | [87] |
| Maize residue | 90.9 | 300 °C | [88] |
| Charcoal-activate aloumina silica | 95 | thermally induced transesterification | [32] |
| Silica | 95 | 380 °C | [89] |
| Silica | 96.1 | 380 °C | [90] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562. https://doi.org/10.3390/catal8110562
Vakros J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts. 2018; 8(11):562. https://doi.org/10.3390/catal8110562
Chicago/Turabian StyleVakros, John. 2018. "Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review" Catalysts 8, no. 11: 562. https://doi.org/10.3390/catal8110562
APA StyleVakros, J. (2018). Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts, 8(11), 562. https://doi.org/10.3390/catal8110562




