Catalyst-Doped Anodic TiO2 Nanotubes: Binder-Free Electrodes for (Photo)Electrochemical Reactions
Abstract
1. Introduction
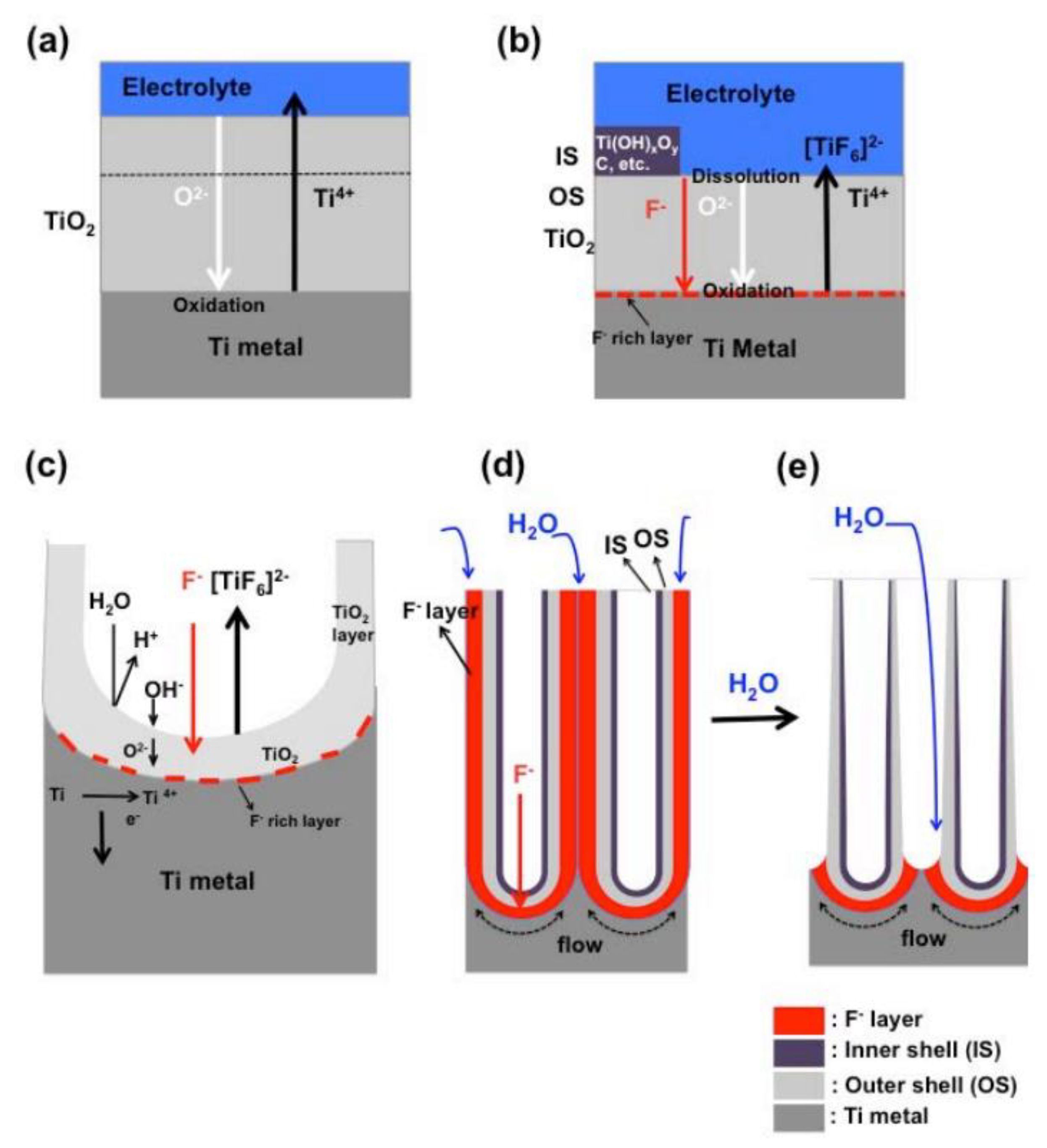
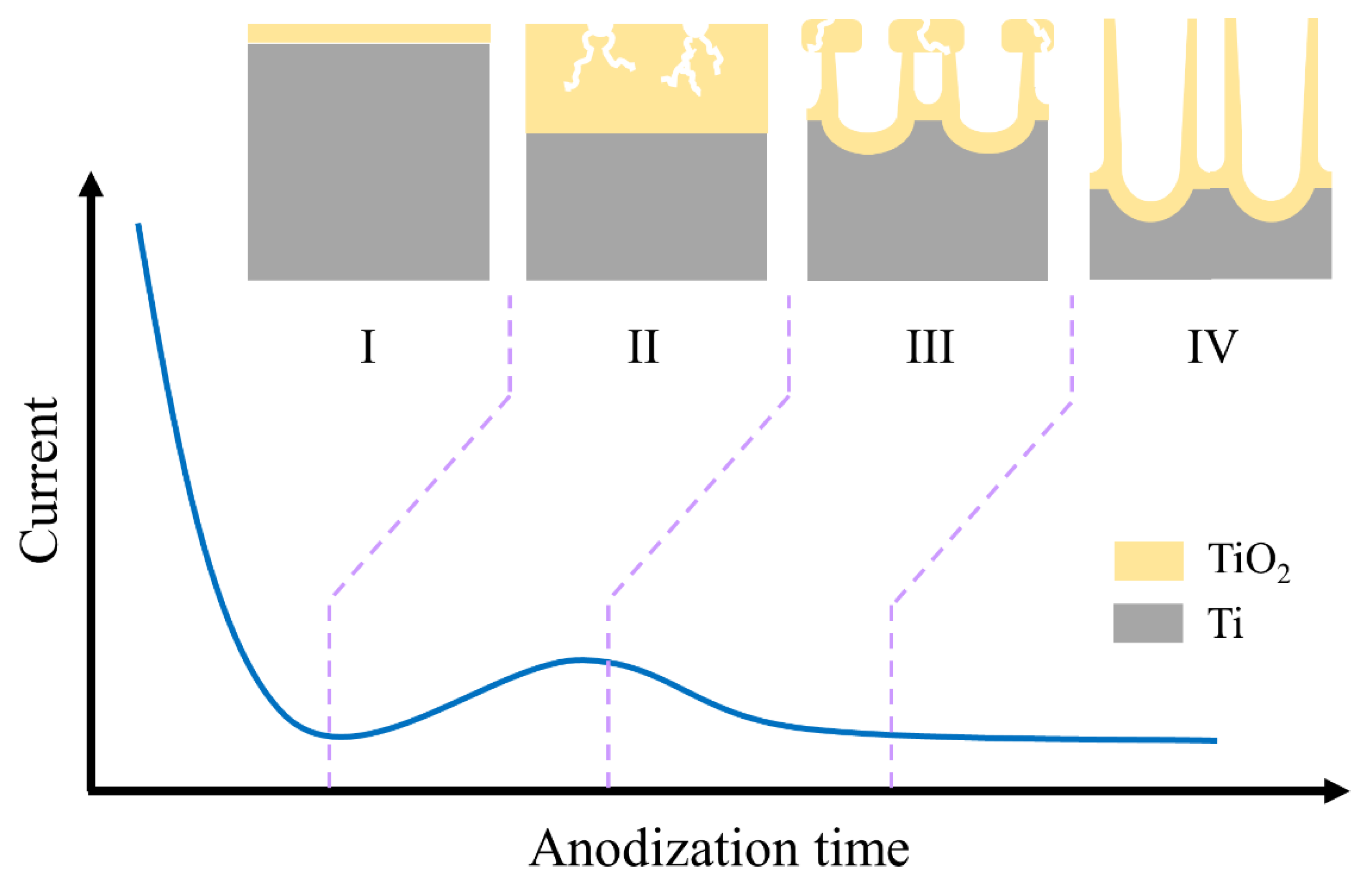
2. Formation Mechanisms of TiO2 Nanotubes
2.1. Reaction Mechanism
2.2. Reaction Parameters
3. Doping of TiO2 Nanotubes
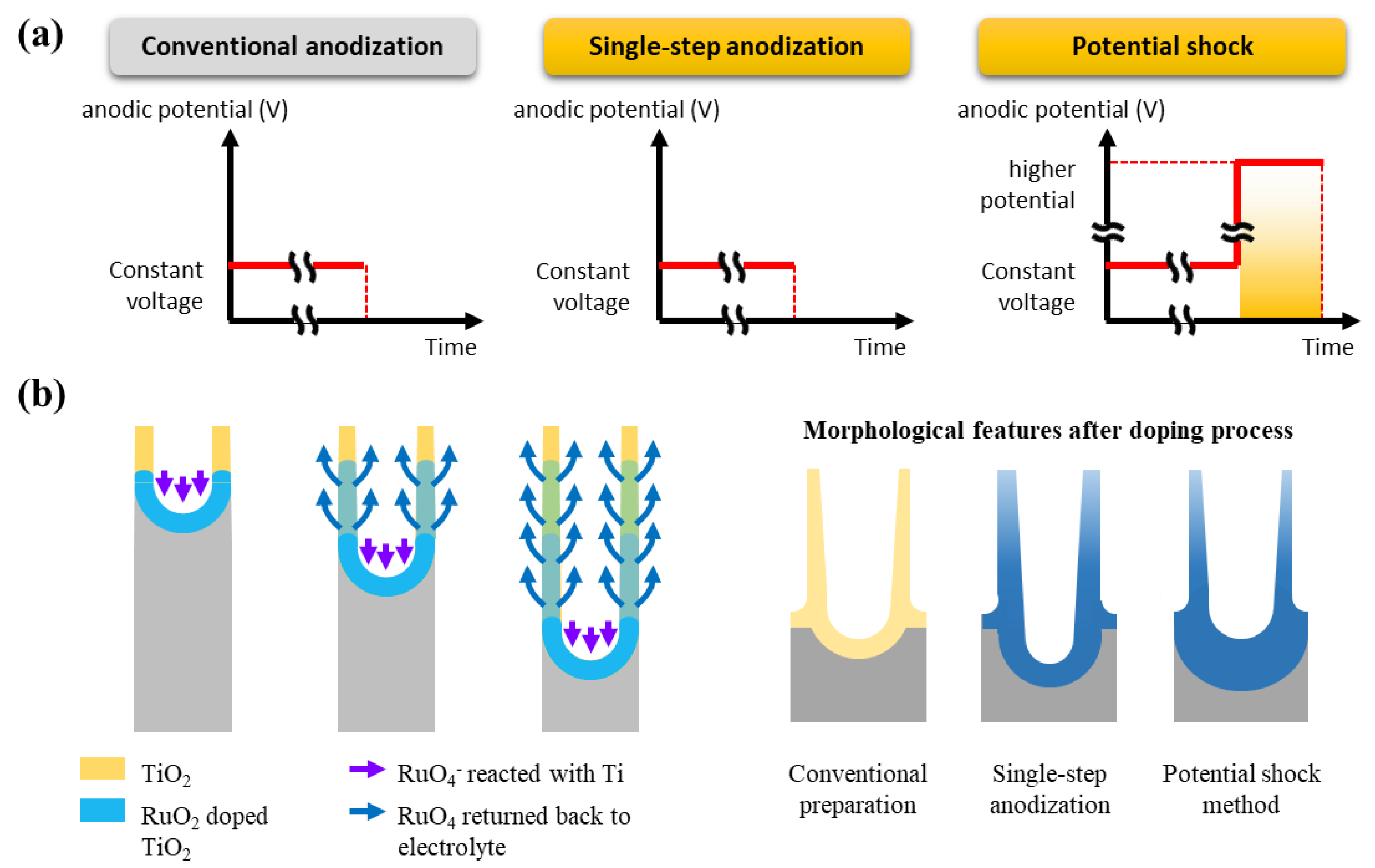
3.1. Electrochemical Doping Methods
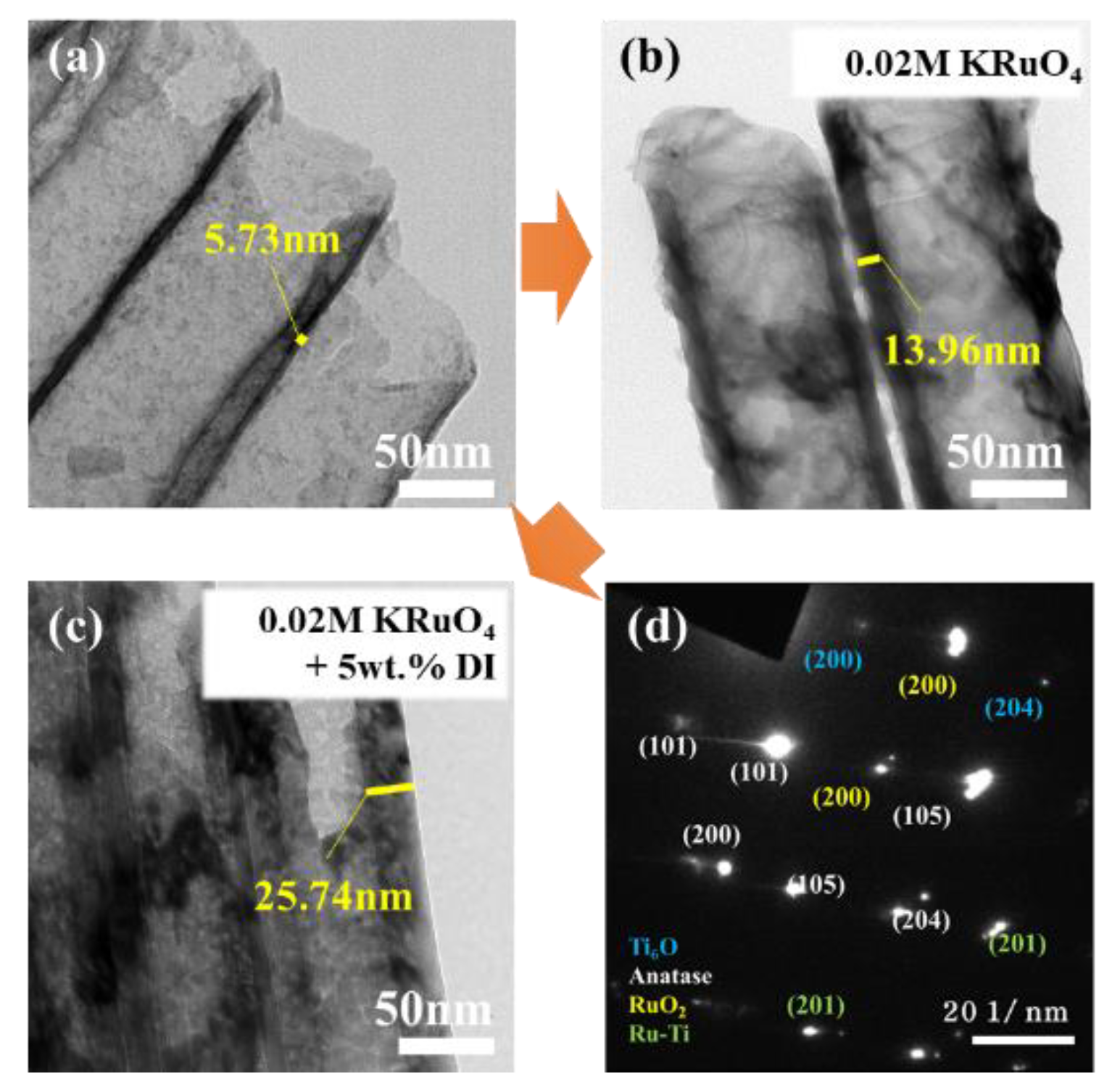
3.1.1. Single-Step Anodization
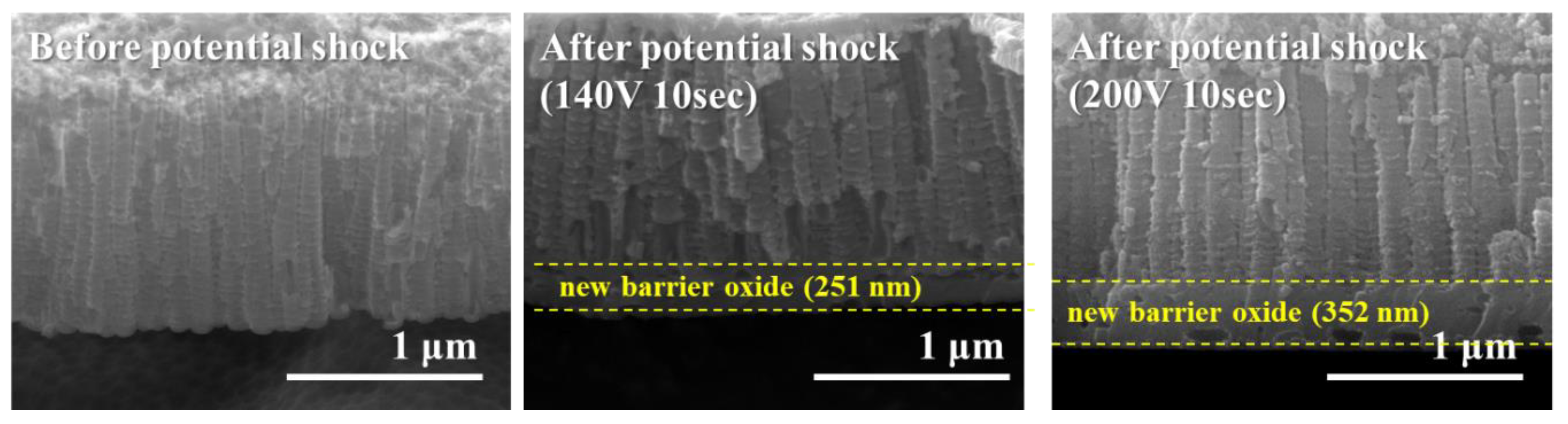
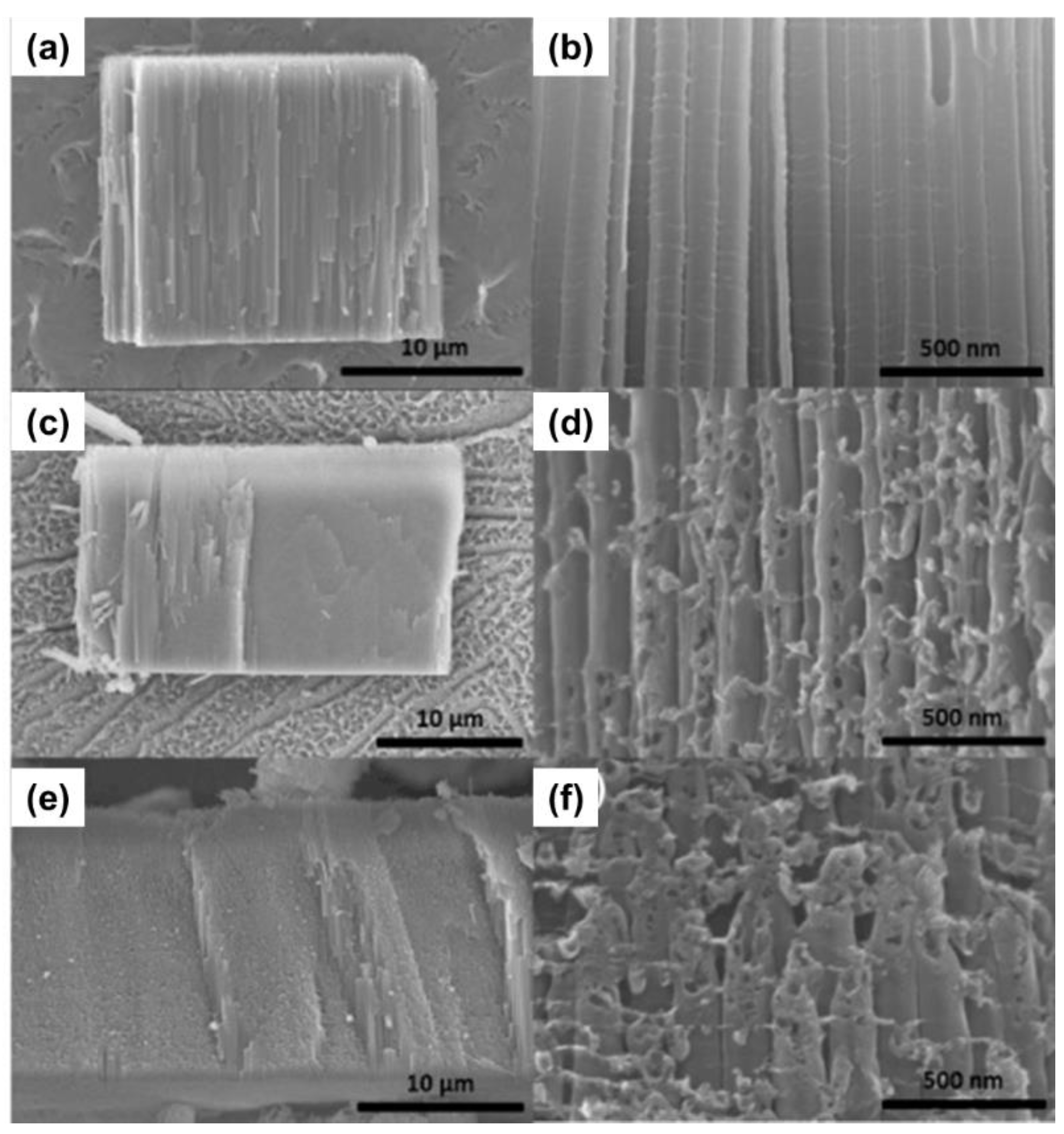
3.1.2. Anodic Potential Shock
3.2. Doping via Thermal Treatment
3.3. Alloy-Based Anodization
4. Applications
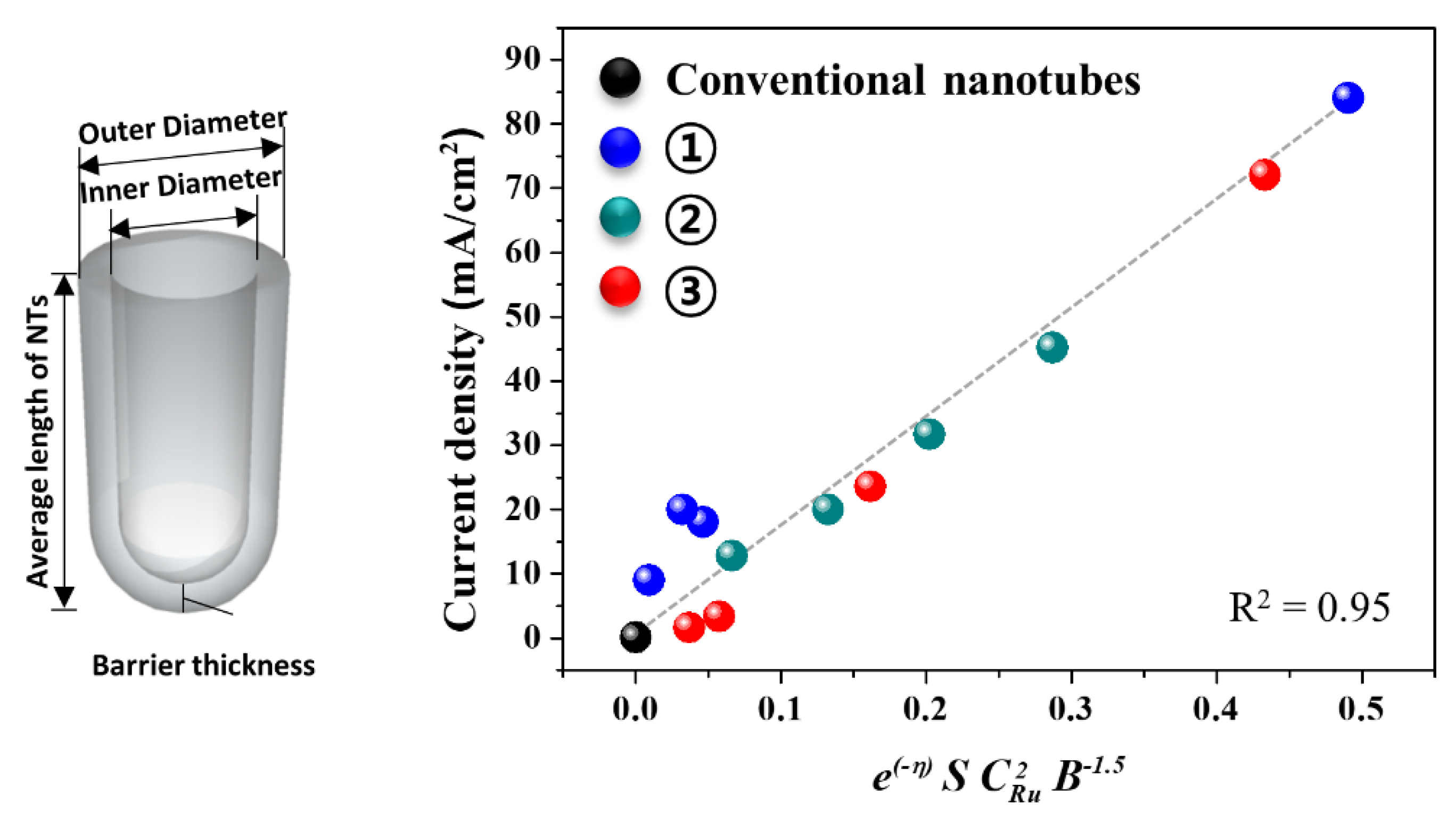
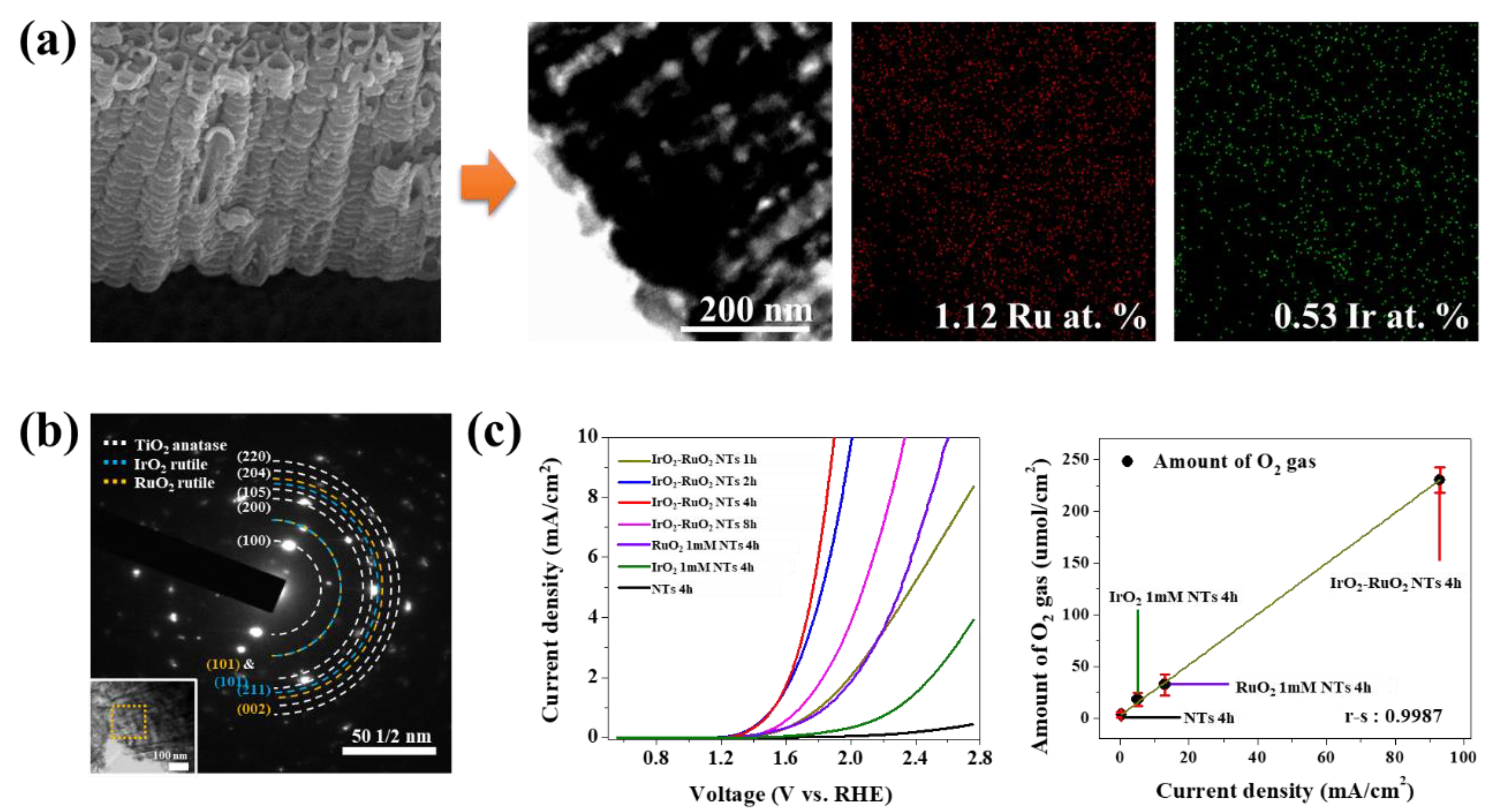
4.1. Electrochemical Water Electrolysis
- i = end current density of LSV for OER
- η = overpotential (0.1 M KOH vs. RHE)
- CRu = average concentration of Ru determined by TEM EDS
- S = calculated reaction surface area of electrode
- B = average thickness of barrier oxide
4.2. Photoanodes
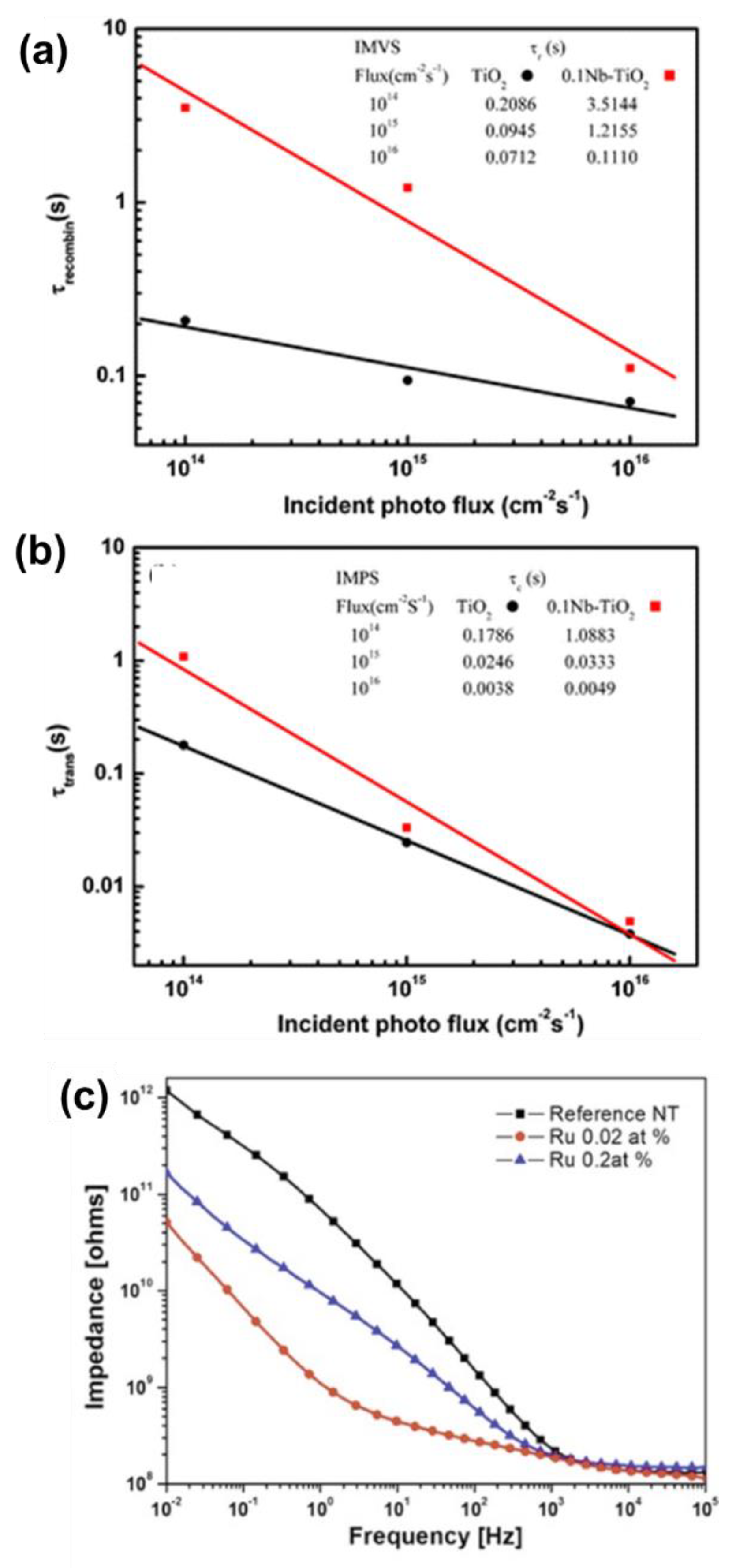
4.2.1. Photocatalysis and Photoelectrochemical Water Splitting
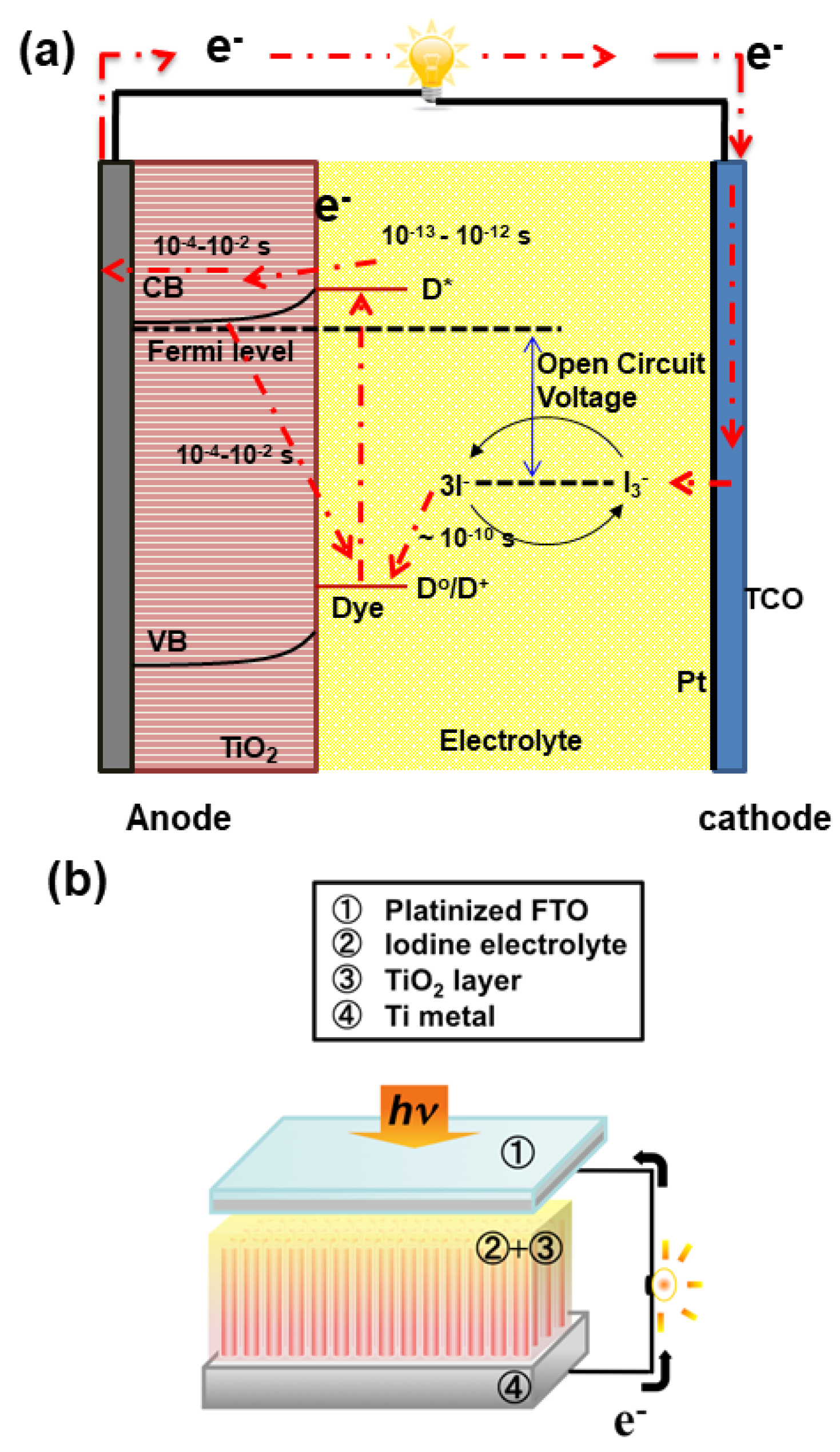
4.2.2. Solar Cells
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Keller, F.; Hunter, M.; Robinson, D.L. Structural features of oxide coatings on aluminum. J. Electrochem. Soc. 1953, 100, 411–419. [Google Scholar] [CrossRef]
- Choi, J. Fabrication of monodomain porous alumina using nano-imprint lithography and its applications. Ph. D. Thesis, Martin-Luther-Universität, Halle-Wittenberg, Germany, 2004. [Google Scholar]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Yamada, H.; Satoh, M.; Asoh, H.; Nakao, M.; Tamamura, T. Highly ordered nanochannel-array architecture in anodic alumina. Appl. Phys. Lett. 1997, 71, 2770–2772. [Google Scholar] [CrossRef]
- Jessensky, O.; Müller, F.; Gösele, U. Self-organized formation of hexagonal pore arrays in anodic alumina. Appl. Phys. Lett. 1998, 72, 1173–1175. [Google Scholar] [CrossRef]
- Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Highly ordered anodic alumina nanofibers fabricated via two distinct anodizing processes. ECS Electrochem. Lett. 2015, 4, H14–H17. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmiki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.-Z.; Cao, C.-Y.; Huang, J.-Y.; Li, S.-H.; Zhang, S.-N.; Deng, S.; Li, Q.-S.; Zhang, K.-Q.; Lai, Y.-K. Synthesis, modification, and photo/photoelectrocatalytic degradation applications of TiO2 nanotube arrays: A review. Nanotechnol. Rev. 2013, 2, 27–45. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Lee, K. Anodic Growth of Porous Metal Oxides and Their Applications. Ph.D. Thesis, Friedrich-Alexander-Universität, Erangen-Nürnberg, Germany, 2013. [Google Scholar]
- Park, J.; Lee, G.; Choi, J. Key anodization factors for determining the formation of TiO2 microcones vs nanotubes. J. Electrochem. Soc. 2017, 164, D640–D644. [Google Scholar] [CrossRef]
- Lim, J.H.; Choi, J. Titanium oxide nanowires originated from anodically-grown nanotubes: The bamboo-splitting-model. Small 2007, 3, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Riboni, F.; Nguyen, N.T.; So, S.; Schmuki, P. Aligned metal oxide nanotube arrays: Key-aspects of anodic TiO2 nanotube formation and properties. Nanoscale Horiz. 2016, 1, 445–466. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matuoka, M.; Takeuchi, M.; Zhang, J.; Horiuci, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Choi, Y.-W.; Choi, J. Ruthenium oxide-doped TiO2 nanotubes by single-step anodization for water-oxidation applications. ChemCatChem 2015, 7, 643–647. [Google Scholar] [CrossRef]
- Gong, J.; Lai, Y.; Lin, C. Electrochemically multi-anodized TiO2 nanotube arrays for enhancing hydrogen generation by photoelectrocatalytic water splitting. Electrochim. Acta 2010, 55, 4776–4782. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis: Understanding the success of DSA®. Electrochim. Acta 2000, 45, 2377–2385. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karkhick, K.; Mishra, S.; Kundu, S. Recent trends and perspectives in electrochemical water splitting with an emphasis on sulfide, selenide, and phosphide catalysts of Fe, Co, and Ni: A review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sato, E. Electrocatalytic properties of transition metal oxides for oxygen evolution reaction. Mater. Chem. Phys. 1986, 15, 397–426. [Google Scholar] [CrossRef]
- Bockris, J.O.; Otagawa, T. Mechanism of oxygen evolution on perovskites. J. Phys. Chem. 1983, 87, 2960–2971. [Google Scholar] [CrossRef]
- Rungtaweevoranit, B.; Zhao, Y.; Choi, K.M.; Yaghi, O.M. Cooperative effects at the interface of nanocrystalline metal-organic frameworks. Nano Res. 2016, 9, 47–58. [Google Scholar] [CrossRef]
- Yoo, H.; Oh, K.; Lee, Y.R.; Row, K.H.; Lee, G.; Choi, J. Simultaneous co-doping of RuO2 and IrO2 into anodic TiO2 nanotubes: A binary catalysts for electrochemical water splitting. Int. J. Hydrogen Energy 2017, 42, 6657–6664. [Google Scholar] [CrossRef]
- Murphy, A.B. Does carbon doping of TiO2 allow water splitting in visible light? Comments on “Nanotube enhanced photoresponse of carbon modified (CM)-n-TiO2 for efficient water splitting”. Sol. Energy Mater. Solar Cells 2008, 92, 363–367. [Google Scholar] [CrossRef]
- Ghicov, A.; Macak, J.M.; Tsuchiya, H.; Kunze, J.; Haeublein, V.; Frey, L.; Schmuki, P. Ion implantation and annealing for an efficient N-doping of TiO2 nanotubes. Nano Lett. 2006, 6, 1080–1082. [Google Scholar] [CrossRef]
- Ghicov, A.; Macak, J.M.; Tsuchiya, H.; Kunze, J.; Haeublein, V.; Kleber, S.; Schmuki, P. TiO2 nanotube layers: Dose effects during nitrogen doping by ion implantation. Chem. Phys. Lett. 2006, 419, 426–429. [Google Scholar] [CrossRef]
- Macak, J.M.; Ghicov, A.; Hahn, R.; Tsuchiya, H.; Schmuki, P. Photoelectrochemical properties of N-doped self-organized titania nanotube layers with different thicknesses. J. Mater. Res. 2006, 21, 2824–2828. [Google Scholar] [CrossRef]
- Vitiello, R.P.; Macak, J.M.; Ghicov, A.; Tsuchiya, H.; Dick, L.F.P.; Schmuki, P. N-Doping of anodic TiO2 nanotubes using heat treatment in ammonia. Electrochem. Commun. 2006, 8, 544–548. [Google Scholar] [CrossRef]
- Jha, H.; Hahn, R.; Schmuki, P. Ultrafast oxide nanotube formation on TiNb, TiZr and TiTa alloys by rapid breakdown anodization. Electrochim. Acta 2010, 55, 8883–8887. [Google Scholar] [CrossRef]
- Yoo, H. TiO2-based electrodes for electrochemical energy conversion and storage. Ph.D. Thesis, Inha University, Incheon, South Korea, August 2018. [Google Scholar]
- Gim, Y.; Seong, M.; Choi, Y.-W.; Choi, J. RuO2-doping into high-aspect-ratio anodic TiO2 nanotubes by electrochemical potential shock for water oxidation. Electrochem. Commun. 2015, 52, 37–40. [Google Scholar] [CrossRef]
- Kim, S.; Yoo, H.; Rhee, O.; Choi, J. Doping of Pt into anodic TiO2 nanotubes for water oxidation: Underpotential shock method in Cl− solution. J. Phys. Chem. C 2015, 119, 21497–21503. [Google Scholar] [CrossRef]
- Seong, M.; Kim, S.; Yoo, H.; Choi, J. Doping of anodic nanotubular TiO2 electrodes with MnO2 for use as catalysts in water oxidation. Catal. Today 2016, 260, 135–139. [Google Scholar] [CrossRef]
- Albu, S.P.; Tsuchiya, H.; Fujimoto, S.; Schmuki, P. TiO2 nanotubes—Annealing effects on detailed morphology and structure. Eur. J. Inorg. Chem. 2010, 2010, 4351–4356. [Google Scholar] [CrossRef]
- Choi, J.; Wehrspohn, R.B.; Lee, J.; Gösele, U. Anodization of nanoimprinted titanium: A comparison with formation of porous alumina. Electrochim. Acta 2004, 49, 2645–2652. [Google Scholar] [CrossRef]
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A review of photocatalysis using self-organized TiO2 nanotubes and other ordered oxide nanostructures. Small 2012, 8, 3073–3103. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.R.; Ray, R.S.; Carlson, K.; Sarma, B.; Misra, M. Self-ordered titanium dioxide nanotube arrays: Anodic synthesis and their photo/electro-catalytic applications. Materials 2013, 6, 2892–2957. [Google Scholar] [CrossRef] [PubMed]
- Altomare, M.; Nguyen, N.T.; Schmuki, P. Templated dewetting: Designing entirely self-organized platforms for photocatalysis. Chem. Sci. 2016, 7, 6865–6886. [Google Scholar] [CrossRef] [PubMed]
- Po, C.C.; Chien, C.C.; Shih, H.C. A review on production, characterization, and photocatalytic applications of TiO2 nanoparticles and nanotubes. Curr. Nanosci. 2017, 13, 373–393. [Google Scholar] [CrossRef]
- Fu, Y.; Mo, A. A review on the electrochemically self-organized titania nanotube arrays: Synthesis, modifications, and biomedical applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Awad, N.K.; Edwards, S.L.; Morsi, Y.S. A review of TiO2 NTs on Ti metal: Electrochemical synthesis, functionalization and potential use as bone implants. Mater. Sci. Eng. C 2017, 76, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Kim, D.; Lee, K.; Spiecker, E.; Schmuki, P. TiO2 nanotubes and their application in dye-sensitized solar cells. Nanoscale 2010, 2, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Seong, M.; Choi, J. Rapid breakdown anodization for the preparation of titania nanotubes in halogen-free acid. J. Electrochem. Soc. 2015, 162, C205–C208. [Google Scholar] [CrossRef]
- Hahn, R.; Macák, J.M.; Schmuki, P. Rapid anodic growth of TiO2 and WO3 nanotubes in fluoride free electrolytes. Electrochem. Commun. 2007, 9, 947–957. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Bhargava, Y.V.; Devine, T.M. Titania nanotube formation in chloride and bromide containing electrolytes. Electrochem. Commun. 2008, 10, 471–475. [Google Scholar] [CrossRef]
- Yoo, H.; Oh, K.; Nah, Y.-C.; Choi, J.; Lee, K. Single-step anodization for formation of WO3-doped TiO2 nanotubes toward enhanced electrochromic performance. ChemElectroChem 2018, 5, 3379–3382. [Google Scholar] [CrossRef]
- Macák, J.M.; Tsuchiyam, H.; Schmuki, P. High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew. Chem. Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.G.; Nam, S.-C.; Choi, J. Anodic TiO2 nanotubes as anode electrode in Li-air and Li-ion batteries. Curr. Appl. Phys. 2012, 12, 1580–1585. [Google Scholar] [CrossRef]
- Paulose, M.; Shankar, K.; Yoriya, S.; Prakasam, H.E.; Varghese, O.K.; Mor, G.K.; Latempa, T.A.; Fitzgerald, A.; Grimes, C.A. Anodic growth of highly ordered TiO2 nanotube arrays to 134 µm in length. J. Phys. Chem. B 2006, 110, 16179–16184. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Mor, G.; Fitzgerald, K.A.; Grimes, C.A. Cation effect on the electrochemical formation of very high aspect ratio TiO2 nanotube arrays in formamide−water mixtures. J. Phys. Chem. C 2007, 111, 21–26. [Google Scholar] [CrossRef]
- Yin, H.; Liu, H.; Shen, W.Z. The large diameter and fast growth of self-organized TiO2 nanotube arrays achieved via electrochemical anodization. Nanotechnol. 2010, 21, 035601. [Google Scholar] [CrossRef] [PubMed]
- Elzarka, A.; Liu, N.; Hwang, I.; Kamal, M.; Schmuki, P. Large-diameter TiO2 nanotubes enable wall engineering with conformal hierarchical decoration and blocking layers for enhanced efficiency in dye-sensitized solar cells (DSSC). Chem. Eur. J. 2017, 23, 12995–12999. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Schmuki, P. Bottom sealing and photoelectrochemical properties of different types of anodic TiO2 nanotubes. Electrhcim. Acta 2013, 100, 229–235. [Google Scholar] [CrossRef]
- Chen, X.; Schriver, M.; Suen, T.; Mao, S.S. Fabrication of 10 nm diameter TiO2 nanotube arrays by titanium anodization. Thin Solid Films 2007, 515, 8511–8514. [Google Scholar] [CrossRef]
- Hoseinzadeh, T.; Ghorannevis, Z.; Ghoranneviss, M.; Sari, A.H.; Salem, M.K. Effects of various applied voltages on physical properties of TiO2 nanotubes by anodization method. J. Theor. Appl. Phys. 2017, 11, 243–248. [Google Scholar] [CrossRef]
- Atyaoui, A.; Cachet, H.; Sutter, E.M.M.; Bousselmi, L. Effect of the anodization voltage on the dimensions and photoactivity of titania nanotubes arrays. Surf. Interface Anal. 2013, 45, 1751–1759. [Google Scholar] [CrossRef]
- Cha, G.; Lee, H.J.; Choi, J. Preparation of binder-free thin film Li4Ti5O12 anode with an adjustable thickness through anodic TiO2 nanotubes. Curr. Appl. Phys. 2013, 13, 1788–1795. [Google Scholar] [CrossRef]
- Kim, D.; Ghicov, A.; Schmuki, P. TiO2 Nanotube arrays: Elimination of disordered top layers (“nanograss”) for improved photoconversion efficiency in dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1835–1838. [Google Scholar] [CrossRef]
- Ma, X.; Sun, Z.; Hu, X. Synthesis of tin and molybdenum co-doped TiO2 nanotube arrays for the photoelectrocatalytic oxidation of phenol in aqueous solution. Mater. Sci. Semicond. Proc. 2018, 85, 150–159. [Google Scholar] [CrossRef]
- Jo, Y.; Jung, I.; Lee, I.; Choi, J.; Tak, Y. Fabrication of through-hole TiO2 nanotubes by potential shock. Electrochem. Comm. 2010, 12, 616–619. [Google Scholar] [CrossRef]
- Shin, S.; Kim, K.; Choi, J. Fabrication of ruthenium-doped TiO2 electrodes by one-step anodization for electrolysis applications. Electrochem. Commun. 2013, 36, 88–91. [Google Scholar] [CrossRef]
- Shin, S.; Choi, Y.-W.; Choi, J. Water splitting by dimensionally stable anode prepared through micro-arc oxidation. Mater. Lett. 2013, 105, 117–119. [Google Scholar] [CrossRef]
- Wei, W.; Oltean, G.; Tai, C.-W.; Edström, K.; Björefors, F.; Nyholm, L. Hogh energy and power density TiO2 nanotube electrodes for 3D Li-ion microbatteries. J. Mater. Chem. A 2013, 1, 8160–8170. [Google Scholar] [CrossRef]
- Sitler, S.J.; Raja, K.S.; Karmiol, Z.; Chidambaram, D. Photoelectrochemical characterization of dual-layered anodic TiO2 nanotubes with honeycomb morphology. J. Phys. D Appl. Phys. 2016, 50, 035502. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Sun, L. A Two-step anodization to grow high-aspect-ratio TiO2 nanotubes. Thin Solid Films 2011, 519, 4694–4698. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Aldabergenova, S.; Drechsel, P.; LeClere, D.; Thompson, G.E.; Macak, J.M.; Schmuki, P. Formation of double-walled TiO2 nanotubes and robust anatase membranes. Adv. Mater. 2008, 20, 4135–4139. [Google Scholar] [CrossRef]
- Beranek, R.; Tsuchiya, H.; Sugishima, T.; Macak, J.M.; Taveira, L.; Fujimoto, S.; Kisch, H.; Schmuki, P. Enhancement and limits of the photoelectrochemical response from anodic TiO2 nanotubes. Appl. Phys. Lett. 2005, 87, 243114. [Google Scholar] [CrossRef]
- Beranek, R.; Hildebrand, H.; Schmuki, P. Self-organized porous titanium oxide prepared in H2SO4/HF electrolytes. Electrochem. Sol. State Lett. 2003, 6, B12–B14. [Google Scholar] [CrossRef]
- Wang, H.; Lewis, J.P. Second-generation photocatalytic materials: Anion-doped TiO2. J. Phys. Condens. Matter. 2006, 18, 421–434. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2−xNx powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Q. Mechanism of higher photocatalytic activity of anatase TiO2 doped with nitrogen under visible-light irradiation from density functional theory calculation. J. Phys. D Appl. Phys. 2008, 41, 025105. [Google Scholar] [CrossRef]
- Qiu, X.; Burda, C. Chemically synthesized nitrogen-doped metal oxide nanoparticles. Chem. Phys. Lett. 2007, 339, 1–10. [Google Scholar] [CrossRef]
- Prokes, S.M.; Gole, J.L.; Chen, X.; Burda, C.; Carlos, W.E. Defect-related optical behavior in surface modified TiO2 nanostructures. Adv. Funct. Mater. 2005, 15, 161–167. [Google Scholar] [CrossRef]
- Liu, S.; Yang, L.; Xu, S.; Luo, S.; Cai, Q. Photocatalytic activities of C–N-doped TiO2 nanotube array/carbon nanorod composite. Electrochem. Commun. 2009, 11, 1748–1751. [Google Scholar] [CrossRef]
- Beranek, R.; Macak, J.M.; Gartner, M.; Meyer, K.; Schmuki, P. Enhanced visible light photocurrent generation at surface-modified TiO2 nanotubes. Electrochim. Acta 2009, 54, 2640–2646. [Google Scholar] [CrossRef]
- Mazare, A.; Paramasivam, I.; Schmidt-Stein, F.; Lee, K.; Demetrescu, I.; Schmuki, P. Flame annealing effects on self-organized TiO2 nanotubes. Electrochim. Acta 2012, 66, 12–21. [Google Scholar] [CrossRef]
- Yang, K.; Dai, Y.; Huang, B.; Whangbo, M.-H. Density functional characterization of the visible-light absorption in substitutional C-anion- and C-cation-doped TiO2. J. Phys. Chem. C 2009, 1, 2624–2629. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.; Bard, A.J. Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano Lett. 2006, 6, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.; Ghicov, A.; Salonen, J.; Lehto, V.-P.; Schmuki, P. Carbon doping of self-organized TiO2 nanotube layers by thermal acetylene treatment. Nanotechnology 2007, 18, 105604. [Google Scholar] [CrossRef]
- Barborini, E.; Conti, A.M.; Kholmanov, I.; Piseri, P.; Podesta, A.; Milani, P.; Cepek, C.; Sakho, O.; Macovez, R.; Sancrotti, M. Nanostructured TiO2 films with 2 eV optical gap. Adv. Mater. 2005, 17, 1842–1846. [Google Scholar] [CrossRef]
- Yang, L.; Luo, S.; Liu, S.; Cai, Q. Graphitized carbon nanotubes formed in TiO2 nanotube arrays: A novel functional material with tube-in-tube nanostructure. J. Phys. Chem. C 2008, 112, 8939–8943. [Google Scholar] [CrossRef]
- Hahn, R.; Schmidt-Stein, F.; Salonen, J.; Thiemann, S.; Song, Y.Y.; Kunze, J.; Lehto, V.-P.; Schmuki, P. Semimetallic TiO2 nanotubes. Angew. Chem. Int. Ed. 2009, 48, 7236–7239. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Y.; Roy, P.; Paramasivam, I.; Schmuki, P. Voltage-induced payload release and wettability control on TiO2 and TiO2 nanotubes. Angew. Chem. Int. Ed. 2010, 49, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Aruna, S.T.; Tirosh, S.; Zaban, A. Nanosize rutile titania particle synthesis via a hydrothermal method without mineralizers. J. Mater. Chem. 2000, 10, 2388–2391. [Google Scholar] [CrossRef]
- Kim, D.; Tsuchiya, H.; Fujimoto, S.; Schmidt-Stein, F.; Schmuki, P. Nitrogen-doped TiO2 mesosponge layers formed by anodization of nitrogen-containing Ti alloys. J. Solid State Electrochem. 2012, 16, 89–92. [Google Scholar] [CrossRef]
- Nah, Y.-C.; Ghicov, A.; Kim, D.; Berger, S.; Schmuki, P. TiO2−WO3 Composite nanotubes by alloy anodization: Growth and enhanced electrochromic properties. J. Am. Chem. Soc. 2008, 130, 16154–16155. [Google Scholar] [CrossRef] [PubMed]
- Ghicov, A.; Yamamoto, M.; Schmuki, P. Lattice Widening in niobium-doped TiO2 nanotubes: Efficient ion intercalation and swift electrochromic contrast. Angew. Chem. Int. Ed. 2008, 47, 7934–7937. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Paramasivam, I.; Shrestha, N.K.; Schmuki, P. MoO3 in Self-organized TiO2 nanotubes for enhanced photocatalytic activity. Chem. Asian J. 2010, 5, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Czoska, A.; Livraghi, S.; Chiesa, M.; Giamello, E.; Agnoli, S.; Granozzi, S.; Finazzi, E.; Valentin, C.D.; Pacchioni, G. The nature of defects in fluorine-doped TiO2. J. Phys. Chem. C 2008, 112, 8951–8956. [Google Scholar] [CrossRef]
- Osorio-Guillen, J.; Lany, S.; Zunger, A. Atomic control of conductivity versus ferromagnetism in wide-gap oxides via selective doping: V, Nb, Ta in anatase TiO2. Phys. Rev. Lett. 2008, 100, 036601. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Doren, D. Electronic structures of V-doped anatase TiO2. Solid State Commun. 2005, 136, 142–146. [Google Scholar] [CrossRef]
- Valentin, C.D.; Pacchioni, G.; Onishi, H.; Kudo, A. Cr/Sb co-doped TiO2 from first principles calculations. Chem. Phys. Lett. 2009, 469, 166–171. [Google Scholar] [CrossRef]
- Shao, G.J. Red shift in manganese- and iron-doped TiO2: A DFT+U analysis. Phys. Chem. C 2009, 113, 6800–6808. [Google Scholar] [CrossRef]
- Hotsenpiller, P.A.M.; Bolt, J.D.; Farneth, W.E.; Lowekamp, J.B.; Rohrer, G.S. Orientation dependence of photochemical reactions on TiO2 surfaces. J. Phys. Chem. B 1998, 102, 3216–3226. [Google Scholar] [CrossRef]
- Casarin, M.; Maccato, C.; Vittadini, A. Electronic structure of Nb impurities in and on TiO2. Phys. Chem. Chem. Phys. 1999, 1, 3793–3799. [Google Scholar] [CrossRef]
- Atashbar, M.Z.; Sun, H.T.; Gong, B.; Wlodarski, W.; Lamb, R. XPS study of Nb-doped oxygen sensing TiO2 thin films prepared by sol-gel method. Thin Solid Films 1998, 326, 238–244. [Google Scholar] [CrossRef]
- Stodolny, M.; Laniecki, M. Synthesis and characterization of mesoporous Ta2O5–TiO2 photocatalysts for water splitting. Catal. Today 2009, 142, 314–319. [Google Scholar] [CrossRef]
- Fernandez-Garcia, M.; Martinez-Arias, A.; Fuerte, A.; Conesa, J.C. Nanostructured Ti−W mixed-metal oxides: Structural and electronic properties. J. Phys. Chem. B 2005, 109, 6075–6083. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Macak, J.M.; Ghlcov, A.; Schmuki, P. Self-organization of anodic nanotubes on two size scales. Small 2006, 2, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Akaki, T.; Terada, D.; Tsuji, N.; Minamino, Y.; Schmuki, P.; Fujimoto, S. Metallurgical aspects on the formation of self-organized anodic oxide nanotube layers. Electrochim. Acta 2009, 54, 5155–5162. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Akaki, T.; Nakata, J.; Terada, D.; Tsuji, N.; Koizumi, Y.; Minamino, Y.; Schmuki, P.; Fujimoto, S. Anodic oxide nanotube layers on Ti–Ta alloys: Substrate composition, microstructure and self-organization on two-size scales. Corros. Sci. 2009, 51, 1528–1533. [Google Scholar] [CrossRef]
- Feng, X.J.; Macak, J.M.; Albu, S.P.; Schmuki, P. Electrochemical formation of self-organized anodic nanotube coating on Ti–28Zr–8Nb biomedical alloy surface. Acta Biomater. 2008, 4, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Schmuki, P. Control of morphology and composition of self-organized zirconium titanate nanotubes formed in (NH4)2SO4/NH4F electrolytes. Electrochim. Acta 2007, 52, 4053–4061. [Google Scholar] [CrossRef]
- Yasuda, K.; Macak, J.M.; Berger, S.; Ghicov, A.; Schmuki, P. Mechanistic aspects of the self-organization process for oxide nanotube formation on valve metals. J. Electrochem. Soc. 2007, 154, C472–C478. [Google Scholar] [CrossRef]
- Yasuda, K.; Schmuki, P. Electrochemical formation of self-organized zirconium titanate nanotube multilayers. Electrochem. Commun. 2007, 9, 615–619. [Google Scholar] [CrossRef]
- Kamkin, A.N.; Fishgoit, L.A.; Davydov, A.D. Composition and structure of anodic oxide films on titanium–aluminum alloys by fast electron reflection diffraction, rutherford backscattering, and secondary neutral particle mass spectrometry. Russ. J. Electrochem. 2003, 39, 665–670. [Google Scholar] [CrossRef]
- Yasuda, K.; Schmuki, P. Formation of self-organized zirconium titanate nanotube layers by alloy anodization. Adv. Mater. 2007, 19, 1757–1760. [Google Scholar] [CrossRef]
- Nah, Y.C.; Ghicov, A.; Kim, D.; Schmuki, P. Enhanced electrochromic properties of self-organized nanoporous WO3. Electrochem. Commun. 2008, 10, 1777–1780. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Nah, Y.C.; Tsuchiya, H.; Schmuki, P. Self-organized nano-tubes of TiO2-MoO3 with enhanced electrochromic properties. Chem. Commun. 2009, 15, 2008–2010. [Google Scholar] [CrossRef] [PubMed]
- Benoit, A.; Paramasivam, I.; Nah, Y.C.; Roy, P.; Schmuki, P. Decoration of TiO2 nanotube layers with WO3 nanocrystals for high-electrochromic activity. Electrochem. Commun. 2009, 11, 728–732. [Google Scholar] [CrossRef]
- Yang, M.; Shrestha, N.K.; Schmuki, P. Thick porous tungsten trioxide films by anodization of tungsten in fluoride containing phosphoric acid electrolyte. Electrochem. Commun. 2009, 11, 1908–1911. [Google Scholar] [CrossRef]
- So, S.; Lee, K.; Schmuki, P. Ru-doped TiO2 nanotubes: Improved performance in dye-sensitized solar cells. Phys. Status Solidi RRL 2012, 6, 169–171. [Google Scholar] [CrossRef]
- Doyle, R.L.; Lyons, M.E.G. The Oxygen Evolution Reaction: Mechanistic Concepts and Catalyst Design; Giménez, S., Bisquert, J., Eds.; Photoelectrochemical Solar Fuel Production; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Reier, T.; Nong, H.N.; Teschner, D.; Schlögl, R.; Strasser, P. Electrocatalytic oxygen evolution reaction in acidic environments—Reaction mechanisms and catalysts. Adv. Energy Mater. 2017, 7, 1601275–1601293. [Google Scholar] [CrossRef]
- Matyushcov, V. Standard electrode potential, Tafel equation, and the solvation thermodynamics. J. Chem. Phys. 2009, 130, 234704–234804. [Google Scholar] [CrossRef] [PubMed]
- Trasatti, S. Electrocatalysis in the anodic evolution of oxygen and chlorine. Electrochim. Acta 1984, 29, 1503–1512. [Google Scholar] [CrossRef]
- Castelli, P.; Trasatti, S.; Pollak, F.H.; Ogrady, W.E. Single crystals as model electrocatalysts: Oxygen evolution on RuO2 (110). J. Electroanal. Chem. 1986, 210, 189–194. [Google Scholar] [CrossRef]
- Yoo, H.; Oh, K.; Lee, G.; Choi, J. RuO2-doped Anodic TiO2 nanotubes for water oxidation: Single-step anodization vs potential shock method. J. Electrochem. Soc. 2017, 164, H104–H111. [Google Scholar] [CrossRef]
- Bockris, J.O.; Nagy, Z. Symmetry factor and Transfer coefficient; A source of confusion in electrode kinetics. J. Chem. Edu. 1973, 50, 839–843. [Google Scholar] [CrossRef]
- Conway, B.E.; Salomon, M. Electrochemical reaction orders: Applications to the hydrogen- and oxygen-evolution reactions. Electrochim. Acta 1964, 9, 1599–1615. [Google Scholar] [CrossRef]
- Conway, B.E.; Bai, L.; Sattar, M.A. Role of the transfer coefficient in electrocatalysis: Applications to the H2 and O2 evolution reactions and the characterization of participating adsorbed intermediates. Int. J. Hydrogen Energy 1987, 12, 607–621. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Logadottir, A.; Norskov, J.K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005, 319, 178–184. [Google Scholar] [CrossRef]
- Medford, A.J.; Voivodic, A.; Hummelshøj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Fabbri, E.; Havereder, A.; Waltar, K.; Kötz, R.; Schmidt, T.J. Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2014, 4, 3800–3821. [Google Scholar] [CrossRef]
- Kötz, R.; Stucki, S. Stabilization of RuO2 by IrO2 for anodic oxygen evolution in acid media. Electrochim. Acta 1986, 31, 1311–1316. [Google Scholar] [CrossRef]
- Yeo, R.S.; Orehotsky, J.; Visscher, W.; Srinivasan, S. Ruthenium-based mixed oxides as electrocatalysts for oxygen evolution in acid electrolytes. J. Electrochem. Soc. 1981, 128, 1900–1904. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Eugene, Y.-J.K.; Khiavi, N.D.; Katal, R.; Gong, X. Aluminum-incorporated p-CuO/n-ZnO photocathode coated with nanocrystal-engineered TiO2 protective layer for photoelectrochemical water splitting and hydrogen generation. J. Mater. Chem. A 2018, 6, 11951–11965. [Google Scholar] [CrossRef]
- Katal, R.; Panah, S.M.; Zarinejad, M.; Salehi, M.; Jiangyong, H. Synthesis of self-gravity settling faceted-anatase TiO2 with dominant {010} facets for the photocatalytic degradation of acetaminophen and study of the type of generated oxygen vacancy in faceted-TiO2. Water 2018, 10, 1462. [Google Scholar] [CrossRef]
- Katal, R.; Salehi, M.; Farahani, M.H.D.A.; Masudy-Panah, S.; Ong, S.L.; Hu, J. Preparation of a new type of black TiO2 under a vacuum atmosphere for sunlight photocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 35316–35326. [Google Scholar] [CrossRef] [PubMed]
- Moakhar, R.S.; Masudy-Panah, S.; Jalali, M.; Goh, G.K.L.; Dolati, A.; Ghorbani, M.; Riahi-Noori, N. Sunlight driven photoelectrochemical light-to-electricity conversion of screen-printed surface nanostructured TiO2 decorated with plasmonic Au nanoparticles. Electrochim. Acta 2016, 219, 386–393. [Google Scholar] [CrossRef]
- Ghosh, M.; Liu, J.; Chuang, S.S.C.; Jana, S.C. Fabrication of hierarchical V2O5 nanorods on TiO2 nanofibers and their enhanced photocatalytic activity under visible light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Paramasivam, I.; Macak, J.M.; Schmuki, P. Photocatalytic activity of TiO2 nanotube layers loaded with Ag and Au nanoparticles. Electrochem. Commun. 2008, 10, 71–75. [Google Scholar] [CrossRef]
- Macak, J.M.; Zlamal, M.; Krysa, J.; Schmuki, P. Self-organized TiO2 nanotube layers as highly efficient photocatalysts. Small 2007, 3, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.S.; Smith, Y.R.; Misra, M.; Subramanian, V. Electrochemically assisted photocatalytic degradation of methyl orange using anodized titanium dioxide nanotubes. Appl. Catal. B Environ. 2008, 84, 372–378. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Zhang, F.; Tao, K.; Pippel, E.; Domen, K. Spontaneous phase and morphology transformations of anodized titania nanotubes induced by water at room temperature. Nano Lett. 2011, 11, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, I.; Nah, Y.-C.; Das, C.; Shrestha, N.K.; Schmuki, P. WO3/TiO2 nanotubes with strongly enhanced photocatalytic activity. Chem. Eur. J. 2010, 16, 8993–8997. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.K.; Misra, M.; Mahajan, V.K.; Raja, K.S. A novel method for the synthesis of titania nanotubes using sonoelectrochemical method and its application for photoelectrochemical splitting of water. J. Catal. 2007, 246, 362–369. [Google Scholar] [CrossRef]
- Allam, N.K.; Shankar, K.; Grimes, C.A. Photoelectrochemical and water photoelectrolysis properties of ordered TiO2 nanotubes fabricated by Ti anodization in fluoride-free HCl electrolytes. J. Mater. Chem. 2008, 18, 2341–2348. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Misra, M.; Mahajan, V.K.; Raja, K.S. Design of a highly efficient photoelectrolytic cell for hydrogen generation by water splitting: Application of TiO2−xCx nanotubes as a photoanode and Pt/TiO2 nanotubes as a cathode. J. Phys. Chem. C 2007, 111, 8677–8685. [Google Scholar] [CrossRef]
- Mahajan, V.K.; Misra, M.; Raja, K.S.; Mohapatra, S.K. Self-organized TiO2 nanotubular arrays for photoelectrochemical hydrogen generation: Effect of crystallization and defect structure. J. Phys. D Appl. Phys. 2008, 41, 125307. [Google Scholar] [CrossRef]
- Nam, W.; Han, G.Y. Preparation and characterization of anodized Pt–TiO2 nanotube arrays for water splitting. J. Chem. Eng. Jpn. 2007, 40, 266–269. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmidt, B.; Kunze, J.; Schmuki, P. Photoresponse in the visible range from Cr doped TiO2 nanotubes. Chem. Phys. Lett. 2007, 433, 323–326. [Google Scholar] [CrossRef]
- Das, C.; Roy, P.; Yang, M.; Jha, H.; Schmuki, P. Nb doped TiO2 nanotubes for enhanced photoelectrochemical water-splitting. Nanoscale 2011, 3, 3094–3096. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Das, C.; Lee, K.; Hahn, R.; Ruff, T.; Moll, M.; Schmuki, P. Oxide nanotubes on Ti−Ru alloys: Strongly enhanced and stable photoelectrochemical activity for water splitting. J. Am. Chem. Soc. 2011, 133, 5629–5631. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jha, H.; Liu, N.; Schmuki, P. Increased photocurrent response in Nb-doped TiO2 nanotubes. J. Mater. Chem. 2011, 21, 15205–15208. [Google Scholar] [CrossRef]
- Altomare, M.; Lee, K.; Killian, M.S.; Selli, E.; Schmuki, P. Ta-Doped TiO2 nanotubes for enhanced solar-light photoelectrochemical water splitting. Chem. Eur. J. 2013, 19, 5841–5844. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Sakata, T. Conversion of carbohydrate into hydrogen fuel by a photocatalytic process. Nature 1980, 286, 474–476. [Google Scholar] [CrossRef]
- Sakata, T.; Hashimoto, K.; Kawai, T. Catalytic properties of ruthenium oxide on n-type semiconductors under illumination. J. Phys. Chem. 1984, 88, 5214–5221. [Google Scholar] [CrossRef]
- Blondeel, G.; Harriman, A.; Porter, G.; Urwin, D.; Kiwi, J. Design, preparation and characterization of ruthenium dioxide/titanium dioxide catalytic surfaces active in photooxidation of water. J. Phys. Chem. 1983, 87, 2629–2636. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Nazeeruddin, M.K.; Angelis, F.D.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, B.; Grätzel, M. Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, Y.; Moser, J.E.; Grätzel, M.; Klug, D.R.; Durrant, J.R. Subpicosecond interfacial charge separation in dye-sensitized nanocrystalline titanium dioxide films. J. Phys. Chem. 1996, 100, 20056–20062. [Google Scholar] [CrossRef]
- Peter, L.M. Characterization and modeling of dye-sensitized solar cells. J. Phys. Chem. C 2007, 111, 6601–6612. [Google Scholar] [CrossRef]
- Bailes, M.; Cameron, P.J.; Lobato, K.; Peter, L.M. Determination of the density and energetic distribution of electron traps in dye-sensitized nanocrystalline solar cells. J. Phys. Chem. B 2005, 109, 15429–15435. [Google Scholar] [CrossRef] [PubMed]
- Cameron, P.J.; Peter, L.M. How does back-reaction at the conducting glass substrate influence the dynamic photovoltage response of nanocrystalline dye-sensitized solar cells? J. Phys. Chem. B 2005, 109, 7392–7398. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.R.; Peter, L.M. A Reappraisal of the electron diffusion length in solid-state dye-sensitized solar cells. J. Phys. Chem. C 2007, 111, 16100–16104. [Google Scholar] [CrossRef]
- Fujihara, K.; Kumar, A.; Jose, R.; Ramakrishna, S.; Uchida, S. Spray deposition of electrospun TiO2 nanorods for dye-sensitized solar cell. Nanotechnology 2007, 18, 365709. [Google Scholar] [CrossRef]
- Zhu, K.; Vinzant, T.B.; Neale, N.R.; Frank, A.J. Removing structural disorder from oriented tio2 nanotube arrays: Reducing the dimensionality of transport and recombination in dye-sensitized solar cells. Nano Lett. 2007, 7, 3739–3746. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.R.; Ghicov, A.; Peter, L.M.; Schmuki, P.; Walker, A.B. Dye-sensitized solar cells based on oriented TiO2 nanotube arrays: Transport, trapping, and transfer of electrons. J. Am. Chem. Soc. 2008, 130, 13364–13372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xie, Y.; Xiong, D.; Zeng, X.; Li, Z.; Wang, M.; Cheng, Y.-B.; Chen, W.; Yang, K.; Yang, S. TiO2 nanorods: A facile size- and shape-tunable synthesis and effective improvement of charge collection kinetics for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 9698–9704. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kim, D.; Jha, H.; Lee, K.; Paul, J.; Schmuki, P. Nb doping of TiO2 nanotubes for an enhanced efficiency of dye-sensitized solar cells. Chem. Commun. 2011, 47, 2032–2034. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Schmuki, P. Ta doping for an enhanced efficiency of TiO2 nanotube based dye-sensitized solar cells. Electrochem. Commun. 2012, 25, 11–14. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.; Kim, M.; Kim, Y.-T.; Lee, K.; Choi, J. Catalyst-Doped Anodic TiO2 Nanotubes: Binder-Free Electrodes for (Photo)Electrochemical Reactions. Catalysts 2018, 8, 555. https://doi.org/10.3390/catal8110555
Yoo H, Kim M, Kim Y-T, Lee K, Choi J. Catalyst-Doped Anodic TiO2 Nanotubes: Binder-Free Electrodes for (Photo)Electrochemical Reactions. Catalysts. 2018; 8(11):555. https://doi.org/10.3390/catal8110555
Chicago/Turabian StyleYoo, Hyeonseok, Moonsu Kim, Yong-Tae Kim, Kiyoung Lee, and Jinsub Choi. 2018. "Catalyst-Doped Anodic TiO2 Nanotubes: Binder-Free Electrodes for (Photo)Electrochemical Reactions" Catalysts 8, no. 11: 555. https://doi.org/10.3390/catal8110555
APA StyleYoo, H., Kim, M., Kim, Y.-T., Lee, K., & Choi, J. (2018). Catalyst-Doped Anodic TiO2 Nanotubes: Binder-Free Electrodes for (Photo)Electrochemical Reactions. Catalysts, 8(11), 555. https://doi.org/10.3390/catal8110555




