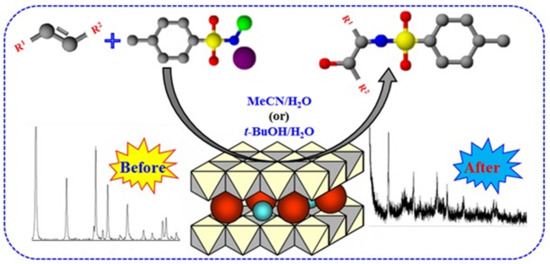
The Heterogeneous Aminohydroxylation Reaction Using Hydrotalcite-Like Catalysts Containing Osmium
Abstract
:1. Introduction
2. Results
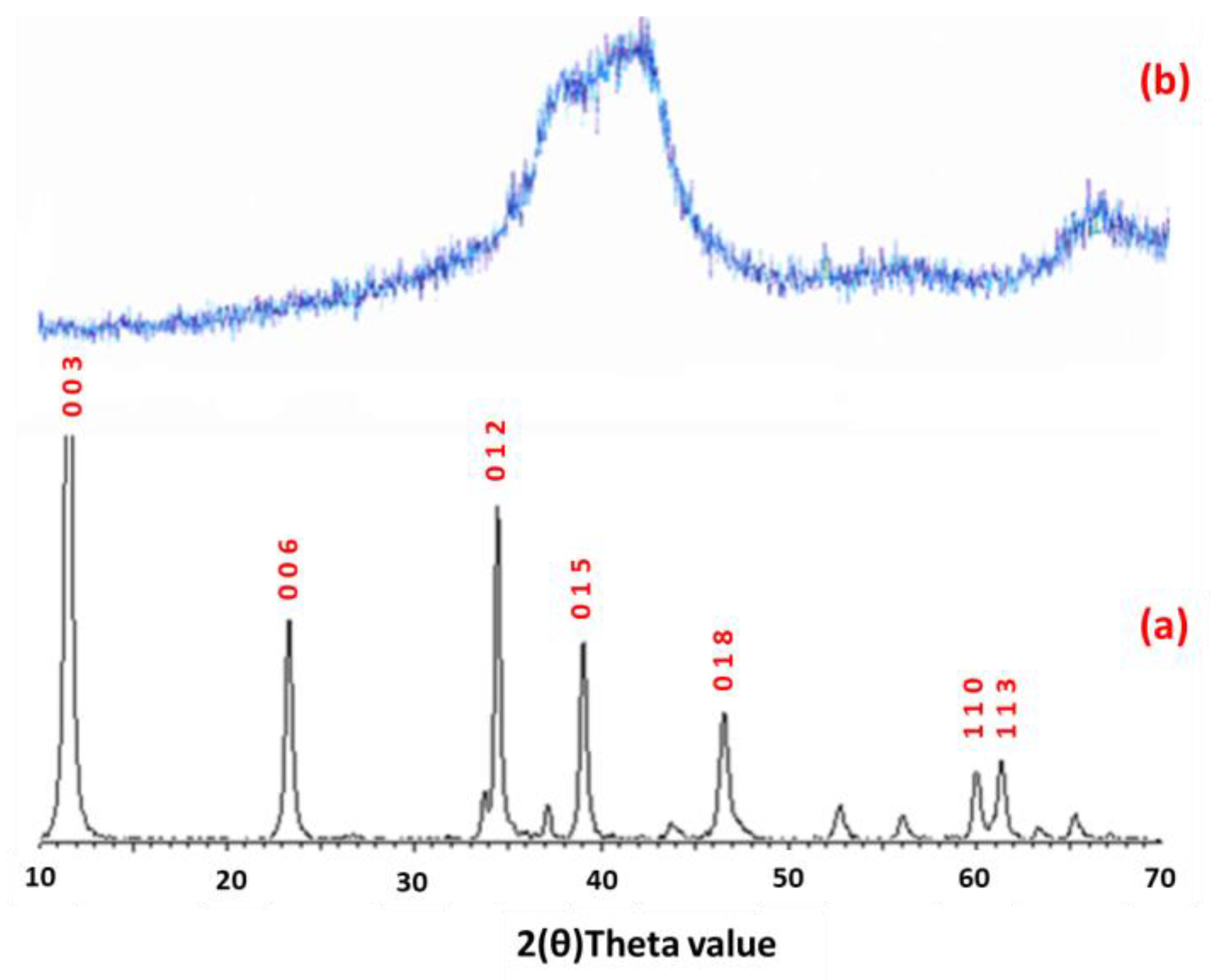
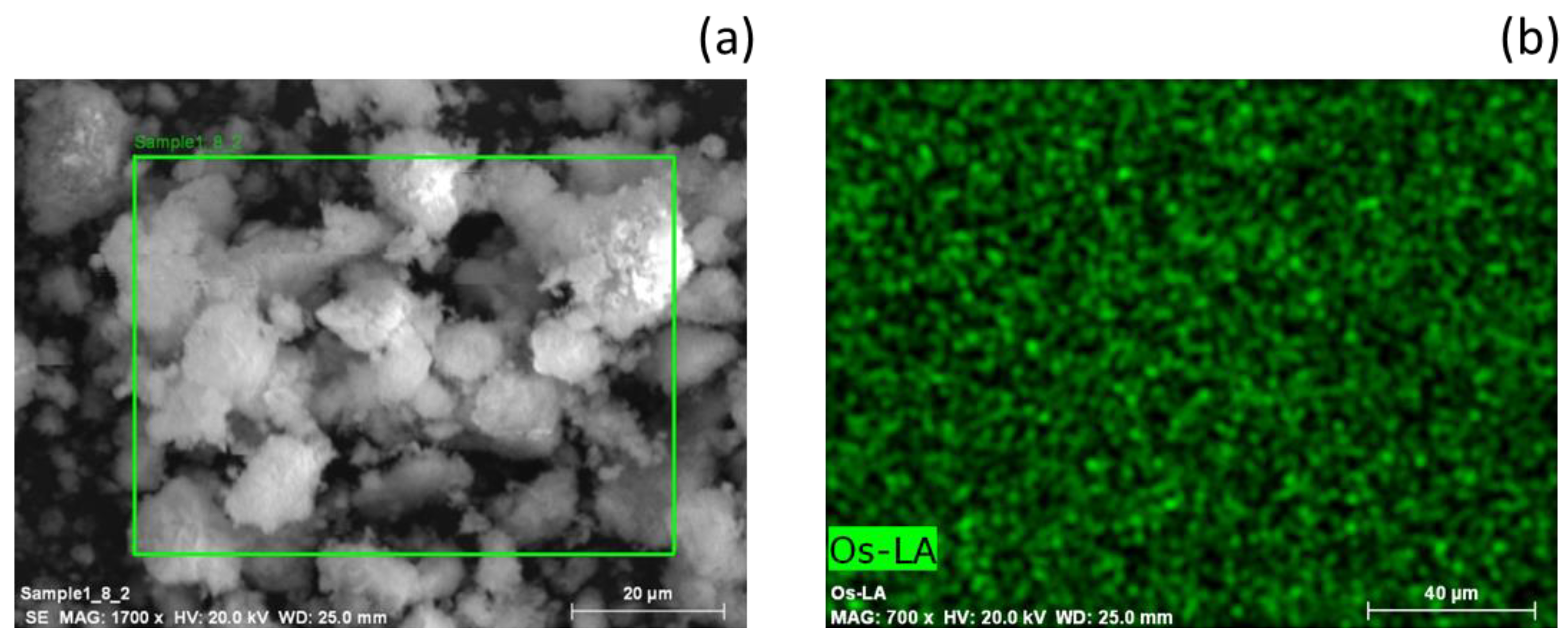
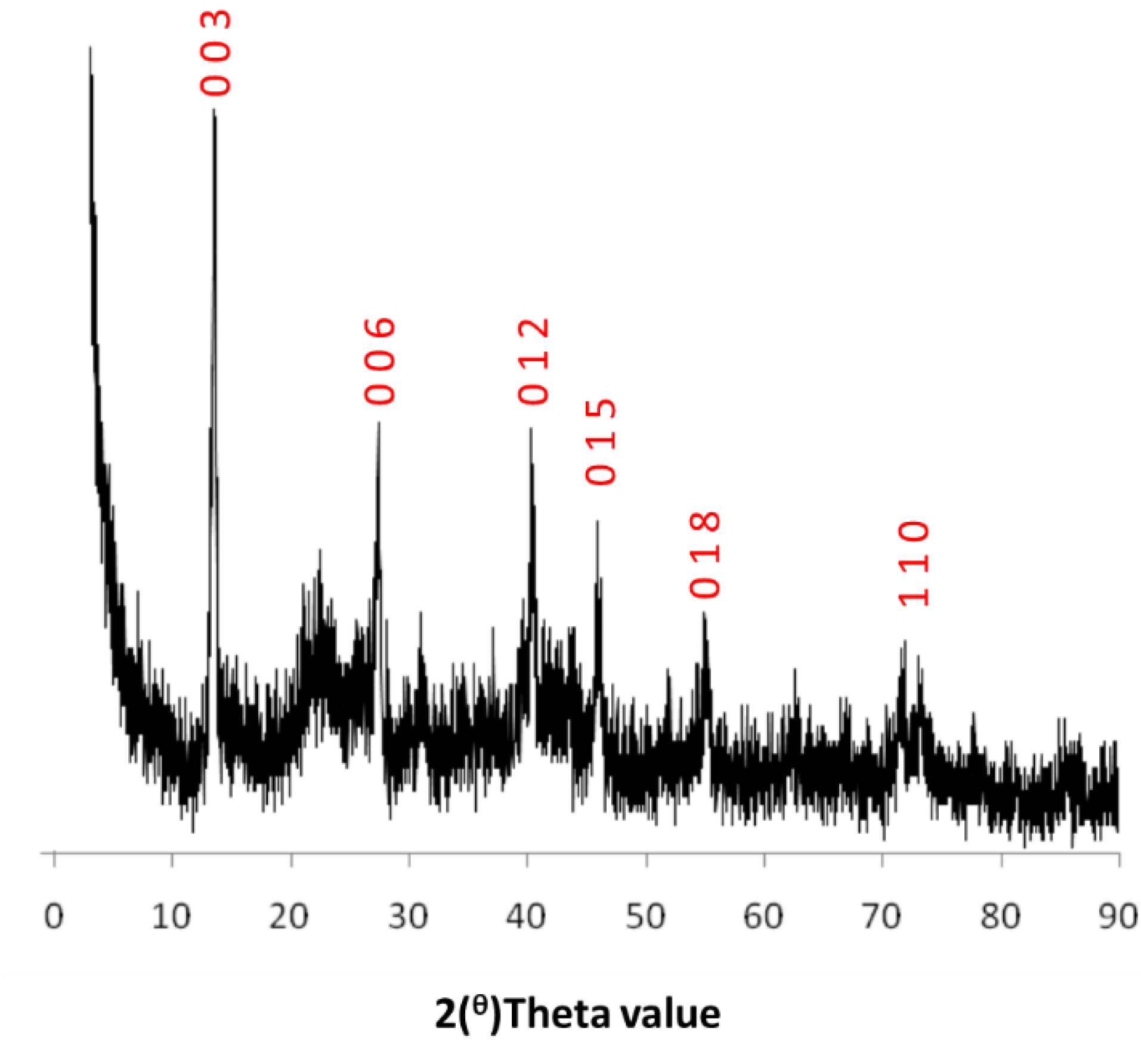
2.1. Catalyst Characterization
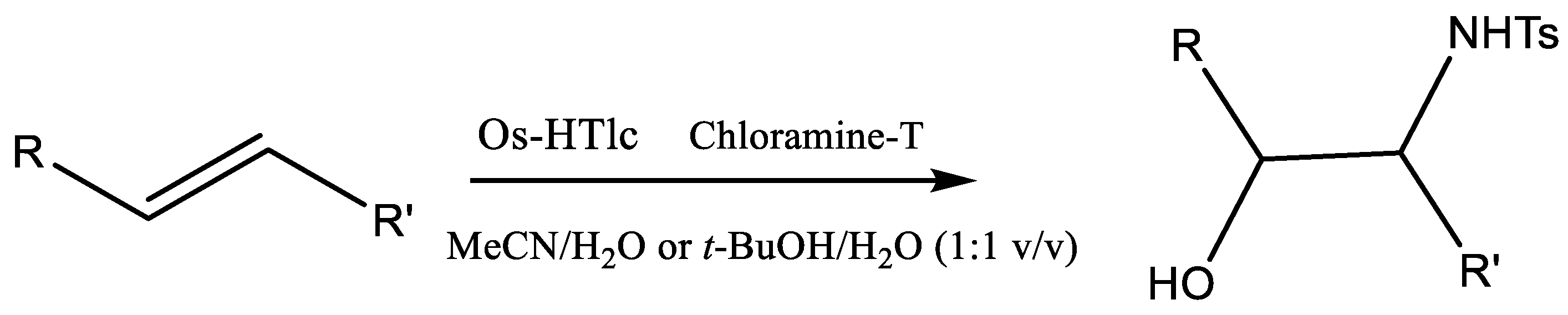
2.2. Catalytic Testing
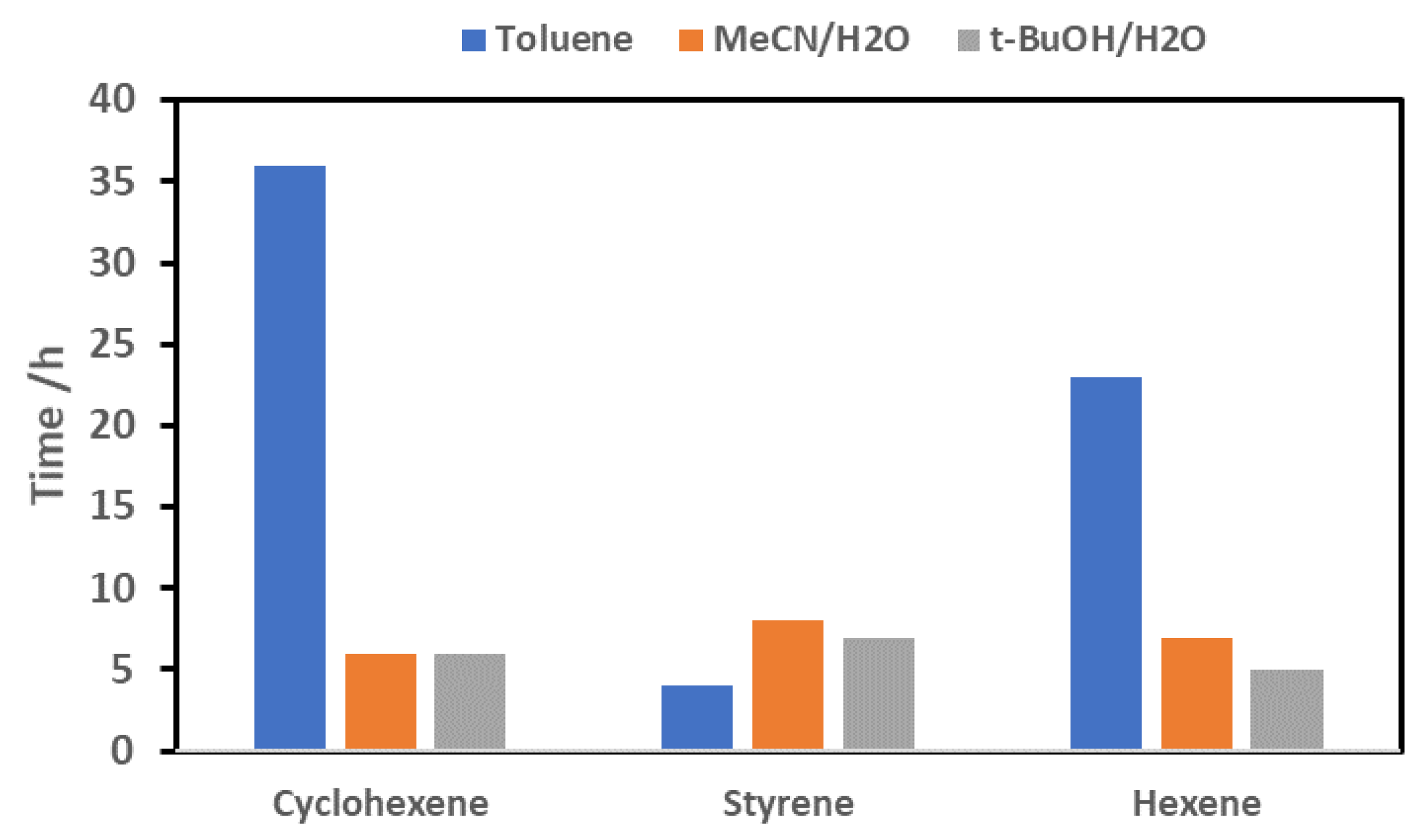
2.2.1. Effect of Solvent on Reaction Time
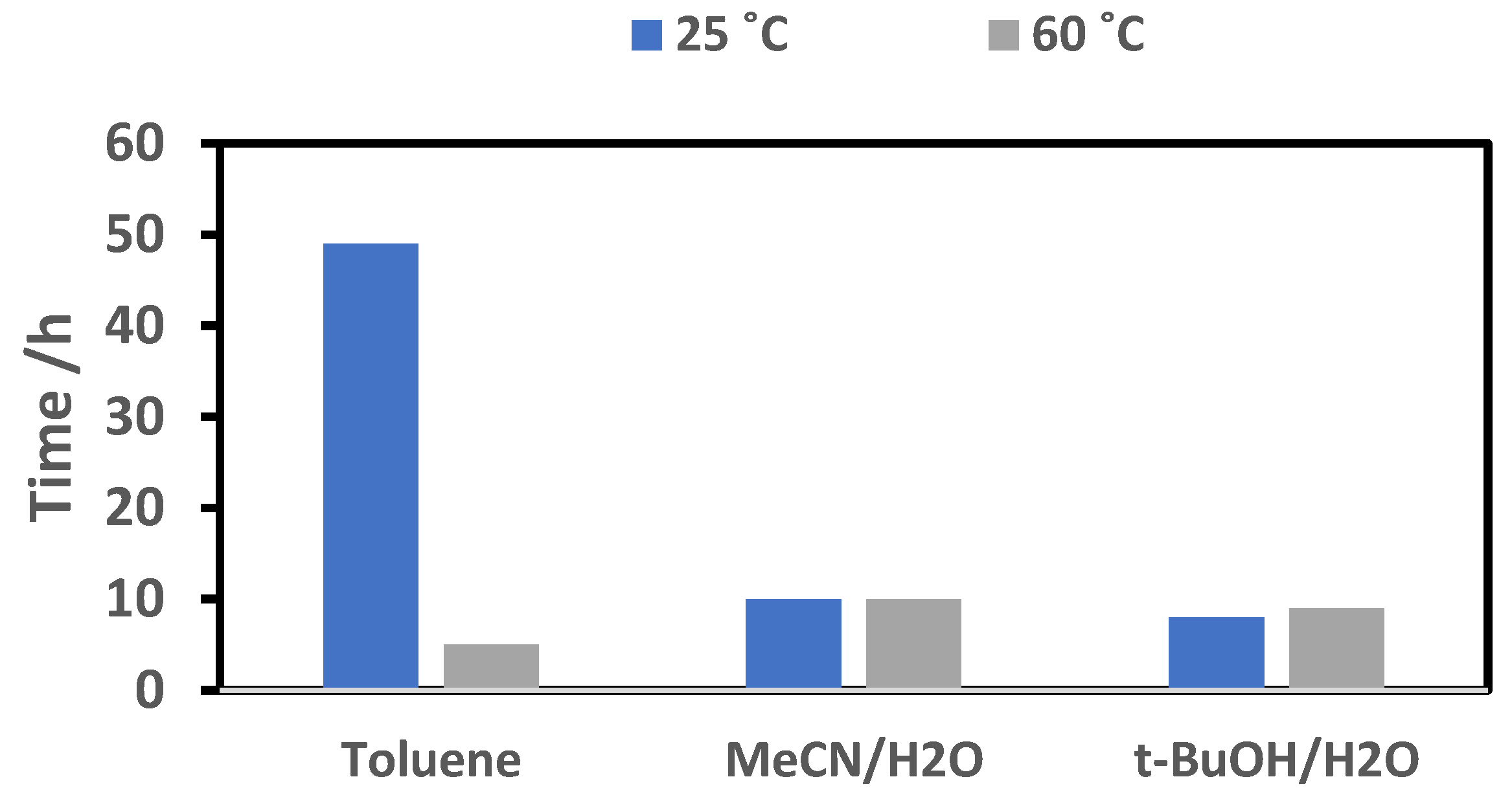
2.2.2. Effect of Temperature on Reaction Time
2.2.3. Effect of Catalyst Structure on Reaction Time
2.2.4. Screening of Different Classes of Olefins
2.3. Leaching Test
2.4. Recycling Test
2.5. Spent Catalyst Characterisation
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Activity
4.3.1. Standard Catalytic Testing Method
4.3.2. Investigation into the Effect of the Solvent on the Reaction
4.3.3. Investigation into the Effect of the Temperature on the Reaction
4.3.4. Investigation into the Effect of the Catalyst Structure on the Reaction
4.3.5. β-Amino Alcohols Product Characterization
4.4. Leaching Test
4.5. Recycling Test
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolb, H.C.; Sharpless, K.B. Asymmetric aminohydroxylation. In Transition Metals for Organic Synthesis; Matthias Beller, C.B., Ed.; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Kolb, H.C.; VanNieuwenhze, M.S.; Sharpless, K.B. Catalytic asymmetric dihydroxylation. Chem. Rev. 1994, 94, 2483–2547. [Google Scholar] [CrossRef]
- Dupau, P.; Epple, R.; Thomas, A.A.; Fokin, V.V.; Sharpless, K.B. Osmium-catalyzed dihydroxylation of olefins in acidic media: Old process, new tricks. Adv. Synth. Catal. 2002, 344, 421–433. [Google Scholar] [CrossRef]
- Li, G.; Chang, H.-T.; Sharpless, K.B. Catalytic asymmetric aminohydroxylation (aa) of olefins. Angew. Chem. Int. Ed. Engl. 1996, 35, 451–454. [Google Scholar] [CrossRef]
- Li, G.; Angert, H.H.; Sharpless, K.B. N-halocarbamate salts lead to more efficient catalytic asymmetric aminohydroxylation. Angew. Chem. Int. Ed. Engl. 1996, 35, 2813–2817. [Google Scholar] [CrossRef]
- Nilov, D.; Reiser, O. The sharpless asymmetric aminohydroxylation—Scope and limitation. Adv. Synth. Catal. 2002, 344, 1169–1173. [Google Scholar] [CrossRef]
- Bergmeier, S.C. The synthesis of vicinal amino alcohols. Tetrahedron 2000, 56, 2561–2576. [Google Scholar] [CrossRef]
- Lohray, B.B.; Thombare, P.S.; Bhushan, V. Advances in asymmetric aminhydroxylation. PINSA 2002, 68A, 391. [Google Scholar]
- Kattamuri, P.V.; Salmonsen, R.; McQuain, C.; Burstein, S.; Sun, H.; Li, G. Asymmetric synthesis of novel n-(1-phenyl-2,3-dihydroxypropyl)arachidonylamides and evaluation of their anti-inflammatory activity. Life Sci. 2013, 92, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Williamson, K.S.; Yoon, T.P. Iron-catalyzed aminohydroxylation of olefins. J. Am. Chem. Soc. 2010, 132, 4570–4571. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, D.J.; Shaffer, C.J.; Yoon, T.P. Copper(ii)-catalyzed aminohydroxylation of olefins. J. Am. Chem. Soc. 2007, 129, 1866–1867. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Oh, C.; Lee, S.; Sherrington, D. Heterogeneous asymmetric aminohydroxylation of alkenes using a silica gel-supported bis-cinchona alkaloid. Chem. Commun. 1998, 22, 2435–2436. [Google Scholar] [CrossRef]
- Mandoli, A.; Pini, D.; Agostini, A.; Salvadori, P. Heterogeneous asymmetric catalytic aminohydroxylation promoted by a bis-cinchona alkaloid derivative supported onto an insoluble organic polymeric matrix. Tetrahedron Asymmetry 2000, 11, 4039–4042. [Google Scholar] [CrossRef]
- Choudary, B.M.; Chowdari, N.S.; Jyothi, K.; Kantam, M.L. Heterogeneous catalytic asymmetric aminohydroxylation of olefins using ldh-supported oso4. J. Mol. Catal. A Chem. 2003, 196, 151–156. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Othman, M.R.; Helwani, Z.; Fernando, W.J.N. Synthetic hydrotalcites from different routes and their application as catalysts and gas adsorbents: A review. Appl. Organomet. Chem. 2009, 23, 335–346. [Google Scholar] [CrossRef]
- Oswald, H.R.; Asper, R. Physics and Chemistry of Materials with Layered Structures; Reidel: Dordrecht, The Netherlands, 1977; Volume 73. [Google Scholar]
- Friedrich, H.B.; Govender, M.; Makhoba, X.; Ngcobo, T.D.; Onani, M.O. The os/cu-al-hydrotalcite catalysed hydroxylation of alkenes. Chem. Commun. 2003, 23, 2922–2923. [Google Scholar] [CrossRef]
- Naicker, T.; Datye, A.K.; Friedrich, H.B. A comparative study of os-hydrotalcites for the cis-dihydroxylation of cyclohexene. Appl. Catal. A Gen. 2008, 350, 96–102. [Google Scholar] [CrossRef]
- Miyata, S. Hydrotalcites in relation to composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Kosower, E.M. The effect of solvent on spectra. I. A new empirical measure of solvent polarity: Z-values. J. Am. Chem. Soc. 1958, 80, 3253–3260. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Wallau, M.; Arends, I.W.C.E.; Schuchardt, U. Heterogeneous catalysts for liquid-phase oxidations: Philosophers’ stones or trojan horses? Acc. Chem. Res. 1998, 31, 485–493. [Google Scholar] [CrossRef]
- Yang, X.-W.; Liu, H.-Q.; Xu, M.-H.; Lin, G.-Q. A highly efficient and practical new peg-bound bi-cinchona alkaloid ligand for the catalytic asymmetric aminohydroxylation of alkenes. Tetrahedron Asymmetry 2004, 15, 1915–1918. [Google Scholar] [CrossRef]
- Hagen, J. Industrial Catalysis: A Practical Approach; Wiley-VCH: Weinheim-Hohensachsen, Germany, 2006. [Google Scholar]
- Gagliardi, L.G.; Castells, C.B.; Ràfols, C.; Rosés, M.; Bosch, E. Static dielectric constants of acetonitrile/water mixtures at different temperatures and debye−hückel a and a0b parameters for activity coefficients. J. Chem. Eng. Data 2007, 52, 1103–1107. [Google Scholar] [CrossRef]
- Akerlof, G. Dielectric constants of some organic solvent-water mixtures at various temperatures. J. Am. Chem. Soc. 1932, 54, 4125–4139. [Google Scholar] [CrossRef]
| Catalyst | Crystallite Size/Å | a Parameter/Å | c Parameter/Å | BET-SURFACE Area/m2 g−1 | ICP-OES of Os/Zn/Al |
|---|---|---|---|---|---|
| Catalyst A | 196 | 3.06 | 23.09 | 87 | 0.29/3.5/1 |
| Catalyst B | - | - | - | 150 | 0.17/3.56/1 |
| Entry | Olefin | Reaction Time/h in MeCN/H2O (t-BuOH/H2O) | % Yield of β-Amino Alcohol, in MeCN/H2O (t-BuOH/H2O) |
|---|---|---|---|
| 1 | 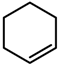 | 7 (8) | 99 (99) |
| 2 | 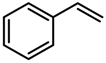 | 9.5 (10) | 99 (99) |
| 3 |  | 7 (6) | 99 (99) |
| 4 | 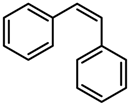 | 24 (24) | 99 (99) |
| 5 | 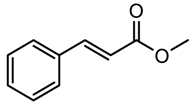 | 23 (24) | 99 (99) |
| 6 | 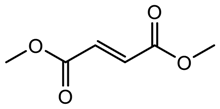 | 24 (24) | 99 (99) |
| 7 | 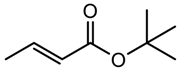 | 22 (23) | 99 (99) |
| Recycle Number | Reaction Time/h | Conversion/Yield % * |
|---|---|---|
| 1 | 7 | >99 |
| 2 | 7.5 | >99 |
| 3 | 8.5 | >99 |
| Catalyst | Os | Zn | Al |
|---|---|---|---|
| Before reaction | 0.31 | 3.70 | 1.00 |
| After reaction | 0.22 | 3.50 | 1.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadlalla, M.I.; Maguire, G.E.M.; Friedrich, H.B. The Heterogeneous Aminohydroxylation Reaction Using Hydrotalcite-Like Catalysts Containing Osmium. Catalysts 2018, 8, 547. https://doi.org/10.3390/catal8110547
Fadlalla MI, Maguire GEM, Friedrich HB. The Heterogeneous Aminohydroxylation Reaction Using Hydrotalcite-Like Catalysts Containing Osmium. Catalysts. 2018; 8(11):547. https://doi.org/10.3390/catal8110547
Chicago/Turabian StyleFadlalla, Mohamed I., Glenn E. M. Maguire, and Holger B. Friedrich. 2018. "The Heterogeneous Aminohydroxylation Reaction Using Hydrotalcite-Like Catalysts Containing Osmium" Catalysts 8, no. 11: 547. https://doi.org/10.3390/catal8110547
APA StyleFadlalla, M. I., Maguire, G. E. M., & Friedrich, H. B. (2018). The Heterogeneous Aminohydroxylation Reaction Using Hydrotalcite-Like Catalysts Containing Osmium. Catalysts, 8(11), 547. https://doi.org/10.3390/catal8110547