Feasibility Study on the Etherification of Fermentative-Produced Isobutylene to Fully Renewable Ethyl Tert-Butyl Ether (ETBE)
Abstract
:1. Introduction
2. Results and Discussion
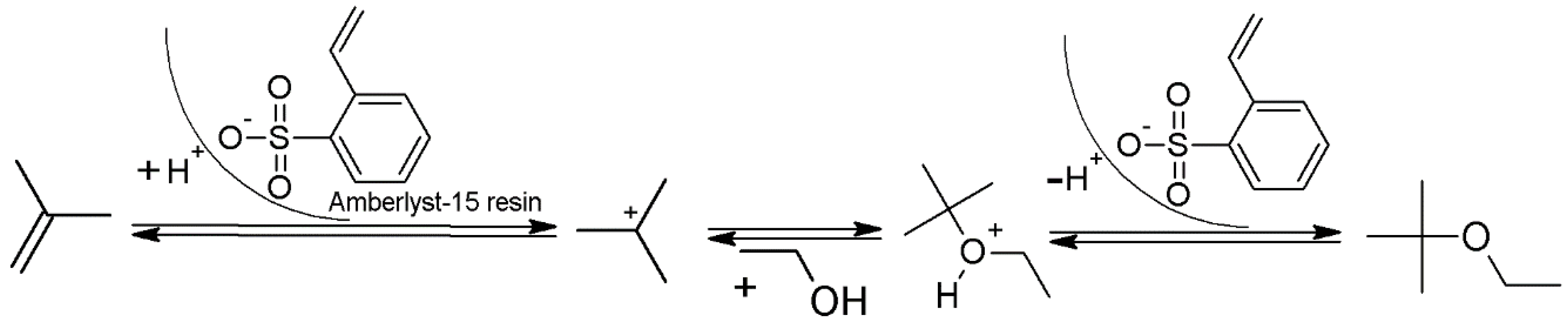
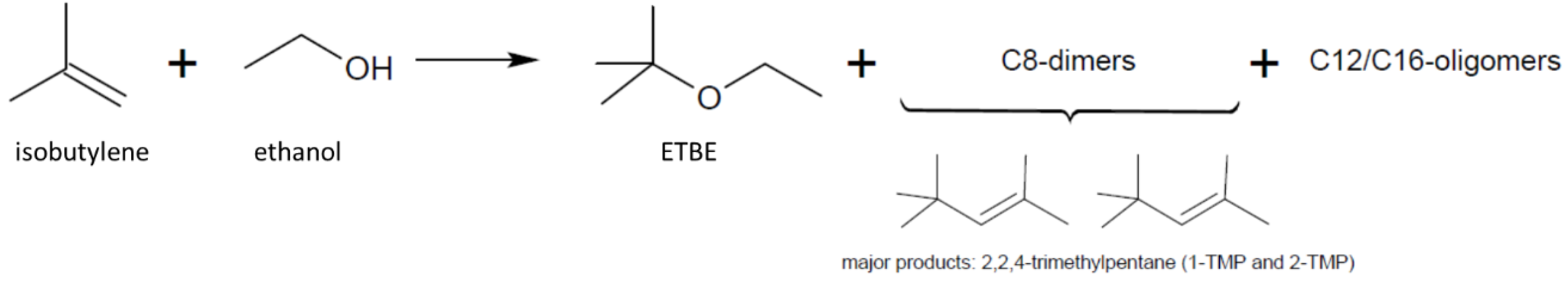
2.1. Initial Screening Using Excess of Ethanol
2.2. Optimization of the Continuous Synthesis of ETBE Using Fossil Isobutylene
2.3. Technology Transfer of the Continuous Synthesis of ETBE Using Bio-Sourced Isobutylene and Production of Sample Amounts
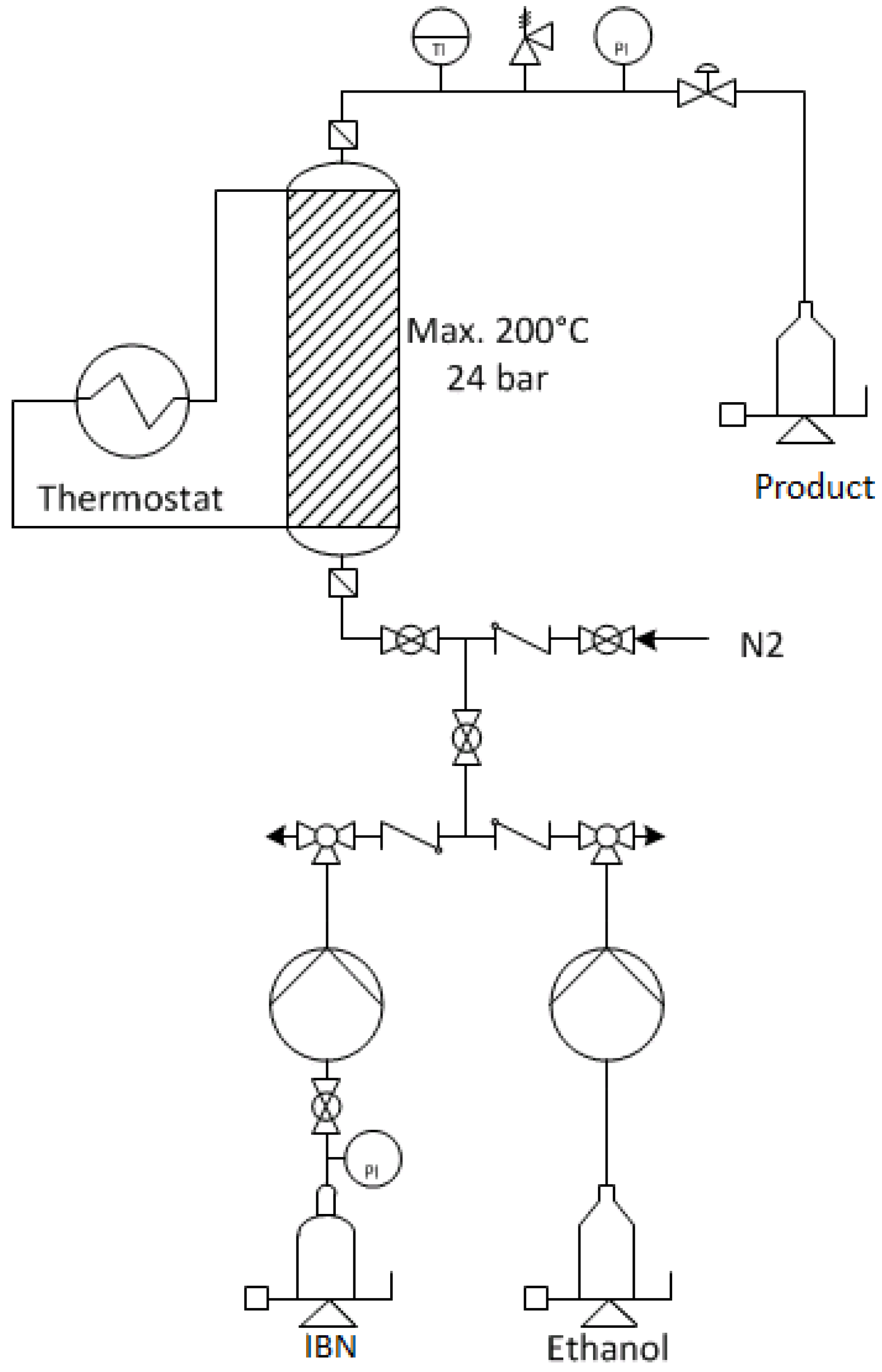
3. Materials and Methods
3.1. Chemicals and Equipment
3.2. Methods for Catalysis and Purification
3.3. Analytical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- EU-Regulation Number 510/2011. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32011R0510 (accessed on 2 November 2018).
- US EPA, Office of Water (OW). Chapter 13 (MTBE) of Regulatory Determinations Support Document for Selected Contaminants from the Second Drinking Water Contaminant Candidate List (CCL 2). July 2008. Available online: https://www.epa.gov/ccl/regulatory-determination-2-contaminants-second-drinking-water-contaminant-candidate-list (accessed on 2 November 2018).
- US EPA. Federal Register/Rules and Regulations, 71, 106, 2006. Available online: https://www.federalregister.gov/documents/2006/04/03/06-3132/regulation-of-fuel-and-fuel-additives-gasoline-and-diesel-fuel-test-methods (accessed on 2 November 2018).
- Bundesgesetzblatt Teil 1, Nr. 41. Gesetz zur Änderung der Förderung von Biokraftstoffen, 19 July 2009. Available online: https://www.bgbl.de/xaver/bgbl/start.xav?start=%2F%2F*[%40attr_id%3D%27bgbl109s1804.pdf%27]#__bgbl__%2F%2F*%5B%40attr_id%3D%27bgbl109s1804.pdf%27%5D__1541147863998 (accessed on 2 November 2018).
- Hart Energy Publishing and its Global offices, Bio-ETBE: The Right Road to High Quality 21st Century Motor Fuels. 2008. Available online: https://de.scribd.com/document/148045860/ 2008-Hart-Publication-on-Bio-ETBE. (accessed on 7 February 2017).
- Global Bioenergies. First Production in History of Fully Renewable ETBE, 7t February 2017. Available online: http://www.global-bioenergies.com/first-production-in-history-of-fully-renewable-etbe/?lang=en (accessed on 2 November 2018).
- Sun, J.; Liu, C.; Wang, Y.; Martin, K.; Venkitasubramanian, P. Process for Making Biobased Fuel Additives. Patent US 020150239812 A1, 27 August 2015. [Google Scholar]
- Marliere, P. Method for the Enzymatic Production of 3-hydroxy-3-methylbutyric Acid from Acetone and Acetyl-CoA. Patent EP 2295593 A1, 16 March 2011. [Google Scholar]
- Marliere, P. Production of Alkenes by Enzymatic Decarboxylation of 3-hydroxyalkanoic Acids. Patent WO 2010001078 A3, 1 July 2010. [Google Scholar]
- Marliere, P.; Allard, M. Method for Producing Isobutylene from 3-methylcrotonyl-CoA. Patent WO 2016042011 A1, 24 March 2016. [Google Scholar]
- Allard, M.; Anissimova, M.; Marliere, P. Methods for Producing Isobutylene from 3-methylcrotonic Acid. Patent WO 2017085167 A2, 26 May 2017. [Google Scholar]
- Weber de Menezes, E.; Cataluna, R. Optimization of the ETBE (ethyl tert-butyl ether) production process. Fuel Process. Technol. 2008, 89, 1148–1152. [Google Scholar] [CrossRef]
- Yee, K.F.; Mohamed, A.R.; Tan, S.H. A review on the evolution of ethyl tert-butyl ether(ETBE) and its future prospects. Renew. Sustain. Energy Rev. 2013, 22, 604–620. [Google Scholar] [CrossRef]
- Shpantseva, L.V.; Aksenow, V.I.; Komarov, S.V.; Yu, A.; Tyulentseva, L.E. Method for Manufacturing High-octane Additive to Gasoline Containing Ethyl tert-butyl Ether. Patent RU 2391329 C2, 10 June 2010. [Google Scholar]
- Goto, S. Method for Synthesizing ETBE. JP 2005162669A, 23 June 2003. [Google Scholar]
- Assabumrungrat, S.; Kiatkittipong, W.; Sevitoon, N.; Praserthdam, P.; Goto, S. Kinetics of liquid phase synthesis of ethyl tert-butyl ether from tert-butyl alcohol and ethanol catalyzed by β-zeolite supported on monolith. Int. J. Chem. Kinet. 2002, 34, 292–299. [Google Scholar] [CrossRef]
- Jensen, K.L.; Datta, R. Ethers from Ethanol. 1. Equilibrium Thermodynamic Analysis of the Liquid-Phase Ethyl tert-Butyl Ether Reaction (ETBE). Ind. Eng. Chem. Res. 1995, 34, 392–399. [Google Scholar] [CrossRef]
- Girolamo, M.D.; Lami, M.; Marchionna, M.; Pescaroll, E.; Tagliabue, L.; Ancillotti, F. Liquid-phase etherification/dimerization of isobutylene over sulfonic acid resins. Ind. Eng. Chem. Res. 1997, 36, 4452–4458. [Google Scholar] [CrossRef]
- Krivan, E.; Valkai, I.; Hancsok, J. Investigation of production of motor fuel components on heterogeneous catalysts with oligomerization. Top. Catal. 2013, 56, 831–838. [Google Scholar] [CrossRef]
- Robert, K.; Meitzner, E.A.; Oline, J.A.; Fisher, S.A.; Norman, F. Characterization of Amberlyst-15. macroreticular sulfonic acid cation exchange resin, Ind. Eng. Chem. Prod. Res. Dev. 1962, 1, 140–144. [Google Scholar]
- Rammohan, P.; Taradas, S.; Shampa, K. Amberlyst-15 in organic synthesis. Top. Catal. 2013, 56, 831–838. [Google Scholar]
- Alcantara, R.; Alcantara, E.; Canoira, L.; Franco, M.J.; Herrera, M.; Navarro, M. Trimerzation of isobutene over Amberlyst-15 catalyst. Reac. Funct. Polym. 2000, 45, 19–27. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Sacia, E.R.; Bell, A. T Etherfication and reductive etherfication of 5-(hydroxymethyl)fufural: 5-(alkoxymethyl)fufurals and 2,5-bis(alkoxymethyl)furans as potential bio-diesel candidates. Green Chem. 2012, 14, 1626–1634. [Google Scholar] [CrossRef]
- Giwa, A. Sensitivity Analysis of ETBE Production Process Using Aspen PLUS, Sensitivity Analysis of ETBE. Production Process. Using Aspen PLUS 2015, 3, 293–303. [Google Scholar]
- Tau, L.-M.; Davis, B.H. Acid catalyzed formation of ethyl tertiary butyl ether (ETBE). Appl. Catal. 1989, 53, 263–271. [Google Scholar] [CrossRef]
- Bakshi, A.; Jones, E.M.; Strain, B.A. Process for the Preparation of ETBE. Patent US 5248836A, 28 September 1993. [Google Scholar]
- Global Bioenergies. First Car Driving with Global Bioenergies’ Renewable Gasoline. 5 April 2018. Available online: http://www.global-bioenergies.com/first-car-driving-with-global-bioenergies-renewable-gasoline/?lang=en (accessed on 2 November 2018).
| Entry | IBN/EtOH Molar Ratio | Process Conditions (a) | Flow (mL/min) | Yield C8 (%) | Formation Rate ETBE (mol/s/gcat.) | Yield ETBE (%) |
|---|---|---|---|---|---|---|
| 1 | (1:4.0) | 22 °C/2.4 MPa | 5.3:13.1 | - | 6.07 × 10−7 | 2.6 |
| 2 | (1:2.3) | 22 °C/2.4 MPa | 7.5:10.9 | - | 9.92 × 10−7 | 3.0 |
| 3 | (1:1.5) | 22 °C/2.4 MPa | 9.5:8.9 | - | 1.25 × 10−6 | 3.0 |
| 4 | (1:4.0) | 40 °C/2.4 MPa | 5.3:13.1 | - | 5.86 × 10−6 | 25.1 |
| 5 | (1:2.3) | 40 °C/2.4 MPa | 9.5:8.9 | - | 8.42 × 10−6 | 20.1 |
| 6 | (1:4.0) | 60 °C/2.4 MPa | 5.3:13.1 | - | 1.36 × 10−5 | 58.2 |
| 7 | (1:1.5) | 60 °C/2.4 MPa | 7.5:10.9 | - | 2.42 × 10−5 | 73.2 |
| 8 | (1:2.3) | 60 °C/2.4 MPa | 9.5:8.9 | - | 3.07 × 10−5 | 73.4 |
| 9 | (1:1.0) | 60 °C/2.4 MPa | 11.4:7.0 | - | 3.77 × 10−5 | 75.0 |
| 10 | (1:1.0) | 60 °C/1.5 MPa | 11.4:7.0 | 9.8 (b) | 3.97 × 10−5 | 79.0 |
| Entry | IBN/EtOH Molar Ratio | T (°C) (a) | Flow (mL/min) | Residence Time (min) | Formation Rate ETBE (mol/s/gcat.) | Yield ETBE (%) | Purity ETBE (wt.%) |
|---|---|---|---|---|---|---|---|
| 1 | (1:1.2) | 53 | 9.71:6.88 | 2.0 | 3.63 × 10−5 | 85 | 80 |
| 2 | (1:1.0) | 55 | 12.91:8.16 | 1.6 | 4.83 × 10−5 | 85 | 85 |
| 3 | (1:0.9) | 54 | 13.61:7.60 | 1.6 | 4.96 × 10−5 | 92 | 88 |
| 4 | (1:0.8) | 52 | 16.05:6.95 | 1.4 | 5.54 × 10−5 | 98 | 86 |
| Entry | IBN/EtOH Molar Ratio | T (°C) (a) | Flow (mL/min) | Residence Time (min) | Formation Rate ETBE (mol/s/gcat.) | Yield ETBE (%) | Purity ETBE (wt.%) |
|---|---|---|---|---|---|---|---|
| 1 | (1:0.9) | 54 | 13.61:7.60 | 1.6 | 4.96 × 10−5 | 92 | 88 |
| 2 | (1:1.0) | 55 | 13.22:7.52 | 1.6 | 5.42 × 10−5 | 93 | 88 |
| 3 | (1:1.1) | 53 | 9.69:7.50 | 1.9 | 3.54 × 10−5 | 83 | 79 |
| 4 | (1:1.5) | 52 | 7.96:7.65 | 2.1 | 2.80 × 10−5 | 80 | 65 |
| 5 | (1:1.8) | 52 | 7.28:7.65 | 2.2 | 2.76 × 10−5 | 86 | 66 |
| 6 | (1:3.0) | 49 | 4.15:7.56 | 2.8 | 1.68 × 10−5 | 92 | 49 |
| Entry | IBN/EtOH Molar Ratio | T (°C) (a) | Flow (mL/min) | Residence Time (min) | Formation Rate ETBE (mol/s/gcat.) | Yield ETBE (%) | Purity ETBE (wt.%) (b) | Water Content (wt.%) |
|---|---|---|---|---|---|---|---|---|
| 1 | (1:0.9) | 53 | 13.09:7.56 | 1.6 | 4.77 × 10−5 | 97 | 89 | - |
| 2 | (1:0.4) | 54 | 25.97:7.10 | 1.0 | 4.26 × 10−5 | 34 | 84/99 (b),(c) | 0.02/0.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tretbar, M.; Witzel, T.; Hauffe, A.; Junghans, U.; Bulc, A.; Pufky-Heinrich, D. Feasibility Study on the Etherification of Fermentative-Produced Isobutylene to Fully Renewable Ethyl Tert-Butyl Ether (ETBE). Catalysts 2018, 8, 514. https://doi.org/10.3390/catal8110514
Tretbar M, Witzel T, Hauffe A, Junghans U, Bulc A, Pufky-Heinrich D. Feasibility Study on the Etherification of Fermentative-Produced Isobutylene to Fully Renewable Ethyl Tert-Butyl Ether (ETBE). Catalysts. 2018; 8(11):514. https://doi.org/10.3390/catal8110514
Chicago/Turabian StyleTretbar, Maik, Thomsen Witzel, Anika Hauffe, Ulrike Junghans, Aleš Bulc, and Daniela Pufky-Heinrich. 2018. "Feasibility Study on the Etherification of Fermentative-Produced Isobutylene to Fully Renewable Ethyl Tert-Butyl Ether (ETBE)" Catalysts 8, no. 11: 514. https://doi.org/10.3390/catal8110514
APA StyleTretbar, M., Witzel, T., Hauffe, A., Junghans, U., Bulc, A., & Pufky-Heinrich, D. (2018). Feasibility Study on the Etherification of Fermentative-Produced Isobutylene to Fully Renewable Ethyl Tert-Butyl Ether (ETBE). Catalysts, 8(11), 514. https://doi.org/10.3390/catal8110514





