Selective Oxidation of Glycerol Using 3% H2O2 Catalyzed by Supported Nano-Au Catalysts
Abstract
1. Introduction
2. Results and Discussion
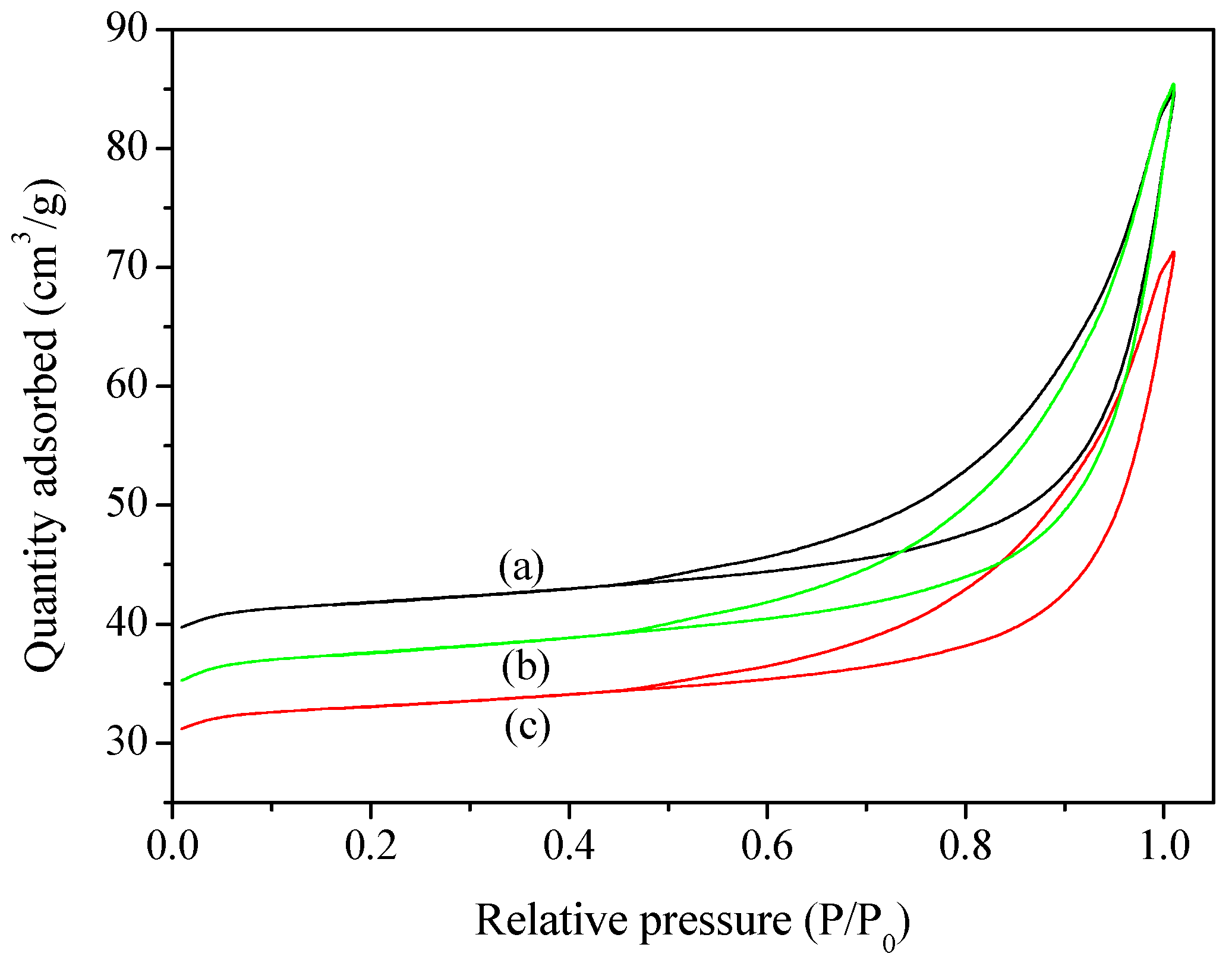
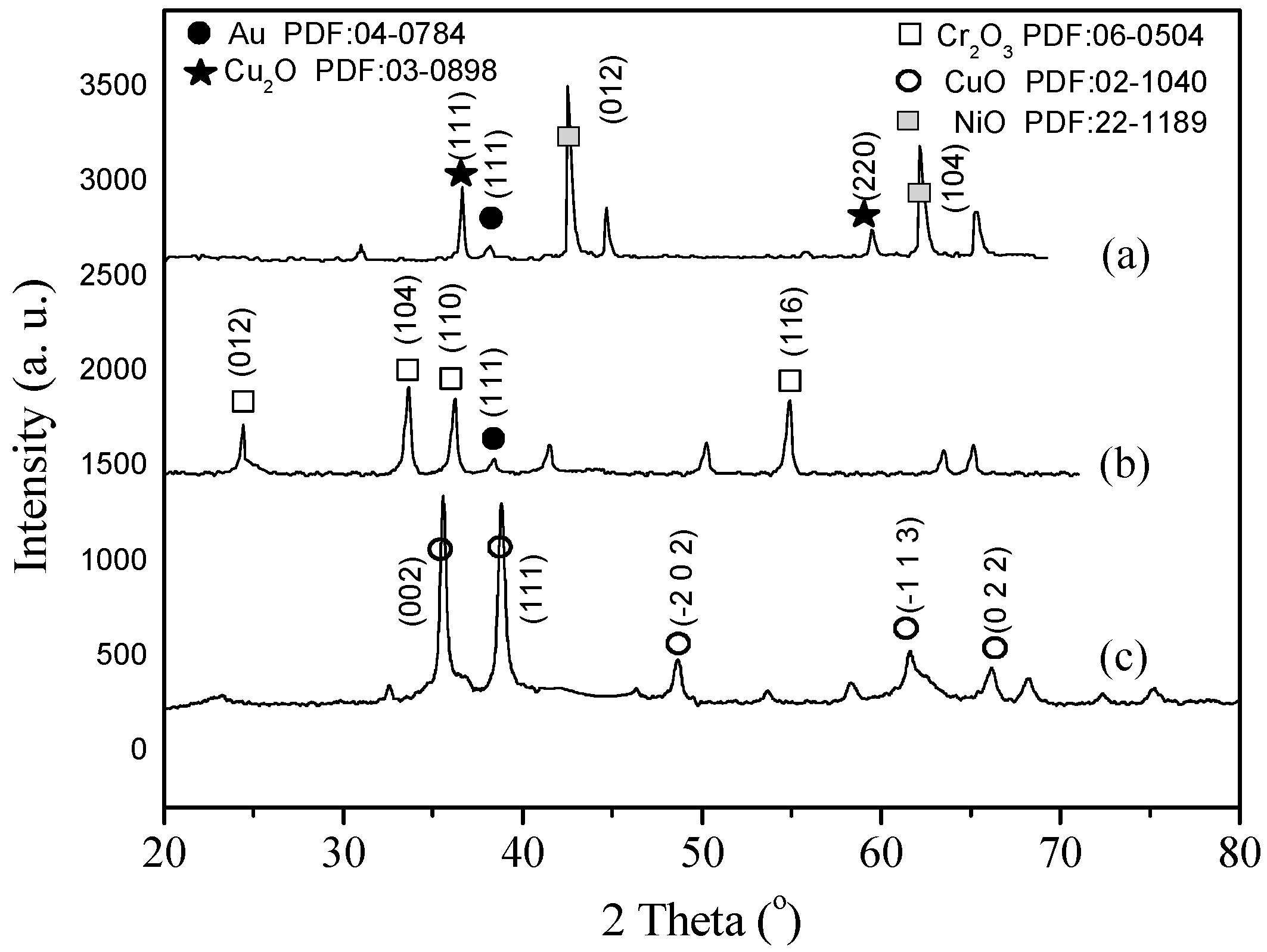
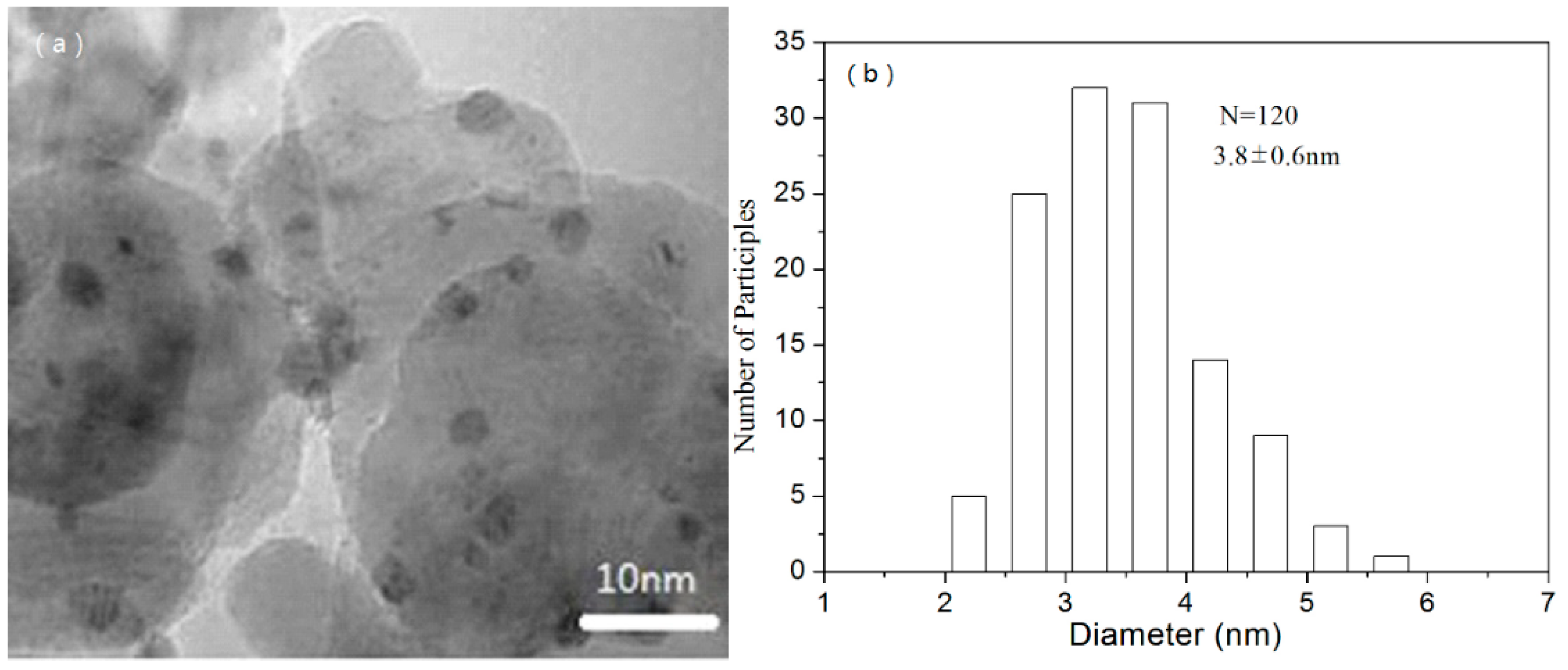
2.1. Characterization of Catalysts
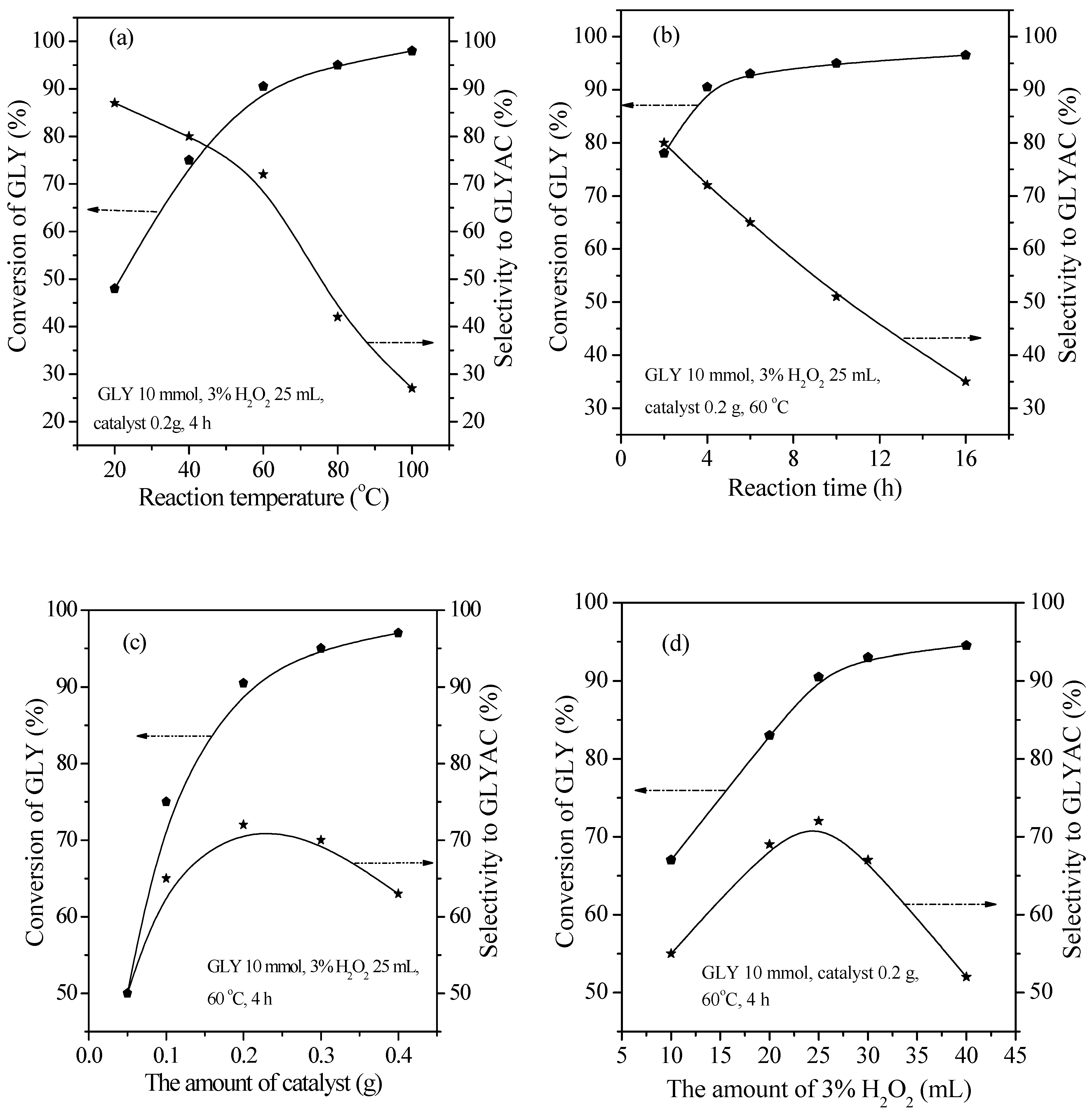
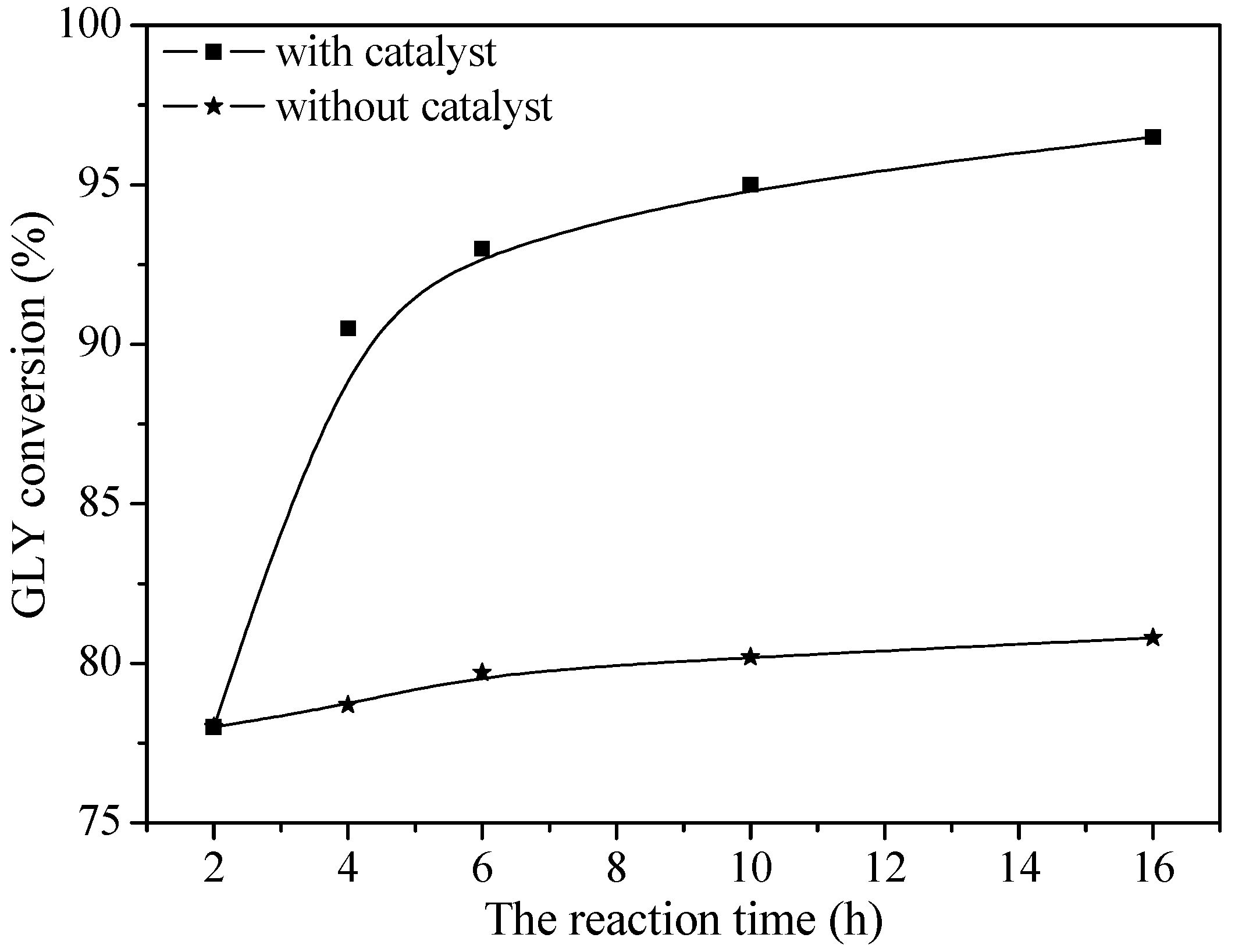
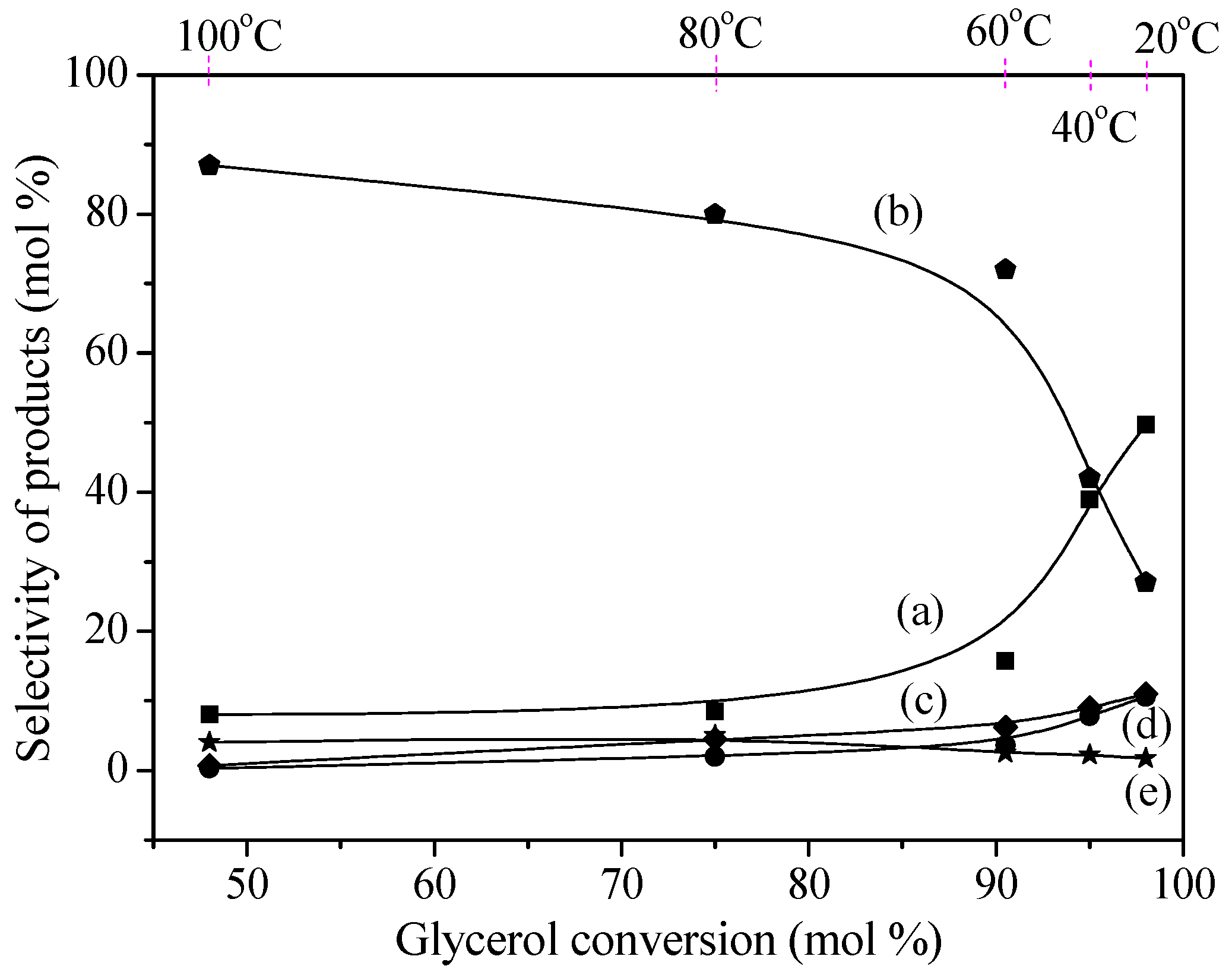
2.2. Catalytic Performance
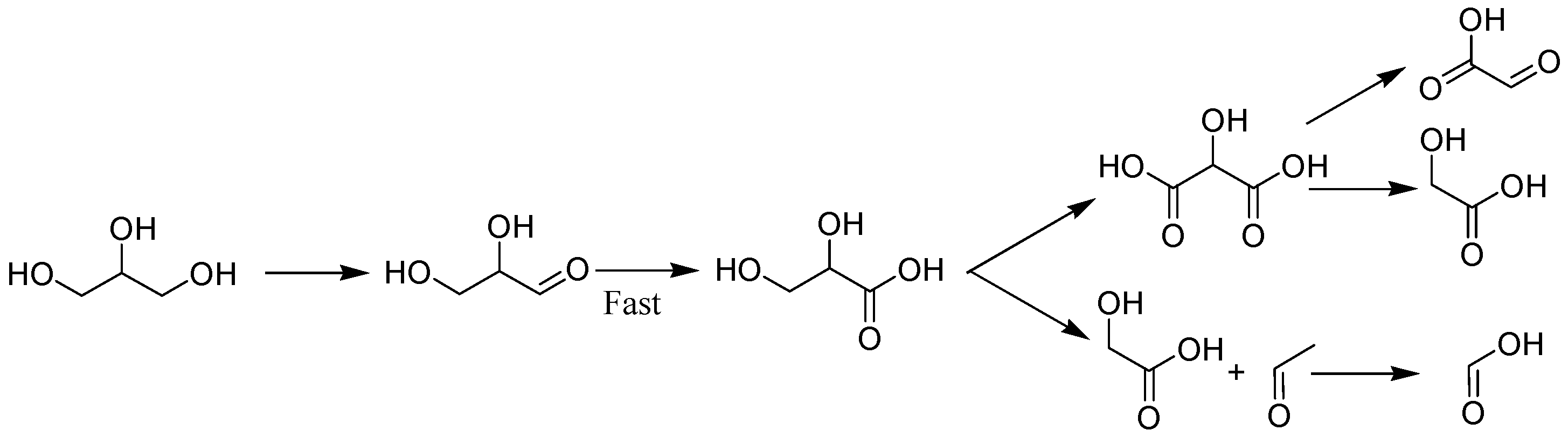
2.3. Reaction Process
3. Experimental Section
3.1. Catalyst Preparation
3.1.1. Preparation of Single Metal Oxides
3.1.2. Preparation of Polymetallic Mixed Oxides
3.1.3. Preparation of Au Loaded Oxides
3.2. Characterization
3.3. Catalytic Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cespi, X.D.; Passarini, F.; Mastragostino, G.; Vassura, I.; Larocca, S.; Iaconi, A.; Chieregato, A.; Duboise, J.L.; Cavani, F. Glycerol as feedstock in the synthesis of chemicals: A life cycle analysis for acrolein production. Green Chem. 2015, 17, 343–355. [Google Scholar] [CrossRef]
- Xiang, Y.Z.; Davis, R.J. Glycerol-intercalated Mg-Al hydrotalcite as a potential solid base catalyst for transesterification. Clay Clay Miner. 2010, 58, 475–485. [Google Scholar]
- Zhou, C.H.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Ch.; Wang, T.; Ding, Y.J. One-step synthesis of pyruvic acid from glycerol oxidation over Pb promoted Pt/activated carbon catalysts. Chin. J. Catal. 2017, 38, 928–938. [Google Scholar] [CrossRef]
- Zhang, Ch.; Wang, T.; Liu, X.; Ding, Y.J. Cu-promoted Pt/activated carbon catalyst for glycerol oxidation to lactic acid. J. Mol. Catal. A Chem. 2016, 44, 91–97. [Google Scholar] [CrossRef]
- Yu, K.O.; Kim, S.W.; Han, S.O. Reduction of glycerol production to improve ethanol yield in an engineered saccharomyces cerevisiae using glycerol as substrate. J. Biotechnol. 2010, 150, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Zope, B.N.; Hibbitts, D.D.; Neurock, M.; Davis, R.J. Reactivity of the gold/water interface during selective oxidation catalysis. Science 2010, 330, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Kaskow, I.; Decyk, P.; Sobczak, I. The effect of copper and silver on the properties of Au-ZnO catalyst and its activity in glycerol oxidation. Appl. Surf. Sci. 2018, 444, 197–207. [Google Scholar] [CrossRef]
- Rodrigues, E.G.; Carabineiro, S.A.C.; Delgado, J.J.; Chen, X.; Pereira, M.F.R.; Órfäo, J.J.M. Gold supported on carbon nanotubes for the selective oxidation of glycerol. J. Catal. 2012, 285, 83–91. [Google Scholar] [CrossRef]
- Wang, X.L.; Wu, G.D.; Liu, X.F.; Zhang, F.; Xue, Y.B.; Tang, X.; Jin, T.F. Selective Oxidation of Glycerol to Glyceric Acid Catalyzed by Supported Nanosized Au/Cr2O3. J. Mol. Catal. (China) 2017, 31, 334–340. [Google Scholar]
- Feng, J.J.; Chem, S.S.; Chen, X.L.; Zhang, X.F.; Wang, A.J. On-pot fabrication of reduced grapheme oxide supported dendritic core-shell gold @ gold-palladium nanoflowers for glycerol oxidation. J. Colloid Interface Sci. 2018, 509, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.T.; Chen, L.X.; Li, D.N.; Wang, A.J.; Zhang, Q.L.; Feng, J.J. On-pot controlled synthesis of AuPd@Pd core-shell nanocrystals with enhanced electrocatalytic performances for formic acid oxidation and glycerol oxidation. J. Colloid Interface Sci. 2017, 508, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Kapkowski, M.; Bartczak, P.; Korzec, M.; Sitko, R.; Szade, J.; Balin, K.; Lelątko, J.; Polanski, J. SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation in the liquid phase. J. Catal. 2014, 319, 110–118. [Google Scholar] [CrossRef]
- Sharma, A.S.; Kaur, H.; Shah, D. Selective oxidation of alcohols by supported gold nanoparticles: Recent advances. RSC Adv. 2016, 6, 28688–28727. [Google Scholar] [CrossRef]
- Prati, L.; Villa, A.; Chan-Thaw, C.E.; Arrigo, R.; Wang, D.; Su, D.S. Gold catalyzed liquid phase oxidation of alcohol: The issue of selectivity. Faraday Discuss. 2011, 152, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.G.; Pereira, F.R.; Chen, X.W.; Delgado, J.J.; Orfao, J.J.M. Selective oxidation of glycerol over platinm-based catalysts supported on carbon nanotubes. Ind. Eng. Chem. Res. 2013, 52, 17390–17398. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Portilho, M.F.; Silva, A.C.; Taroco, H.A.; Souza, P.P. Modified niobia as a bifunctional catalyst for simultaneous dehydration and oxidation of glycerol. Appl. Catal. B Envioron. 2012, 117, 29–35. [Google Scholar] [CrossRef]
- Wang, F.F.; Shao, S.; Liu, C.L.; Xu, C.L.; Yang, R.Z.; Dong, W.S. Selective oxidation of glycerol over Pt supported on mesoporous carbon nitride in base-free aqueous solution. Chem. Eng. J. 2015, 264, 336–343. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Nie, R.F.; Wang, L.; Shi, J.J.; Du, W.C.; Hou, Z.Y. Selective oxidation of glycerol over carbon nanofibers supported Pt catalysts in a base-free aqueous solution. Catal. Commun. 2015, 59, 5–9. [Google Scholar] [CrossRef]
- Zhao, G.F.; Yang, F.; Chen, Z.J.; Liu, Q.F.; Ji, Y.G.; Zhang, Y.; Niu, Z.Q.; Mao, J.J.; Bao, X.H.; Hu, P.J.; Li, Y.D. Metal/oxide interfacial effects on the selective oxidation of primary alcohols. Nat. Commun. 2017, 8, 14039. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Nishimura, S.; Takagaki, A.; Ebitani, K. Selective oxidation of glycerol by using a hydrotalcite-supported platinum catalyst under atmospheric oxygen pressure in water. Chem. Sus. Chem. 2011, 4, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Horikawa, M.; Takei, T.; Murayama, T.; Haruta, M. Correlation between catalytic activity of supported gold catalysts for carbon monoxide oxidation and metal-oxygen binding energy of the support metal oxides. Chin. J. Catal. 2016, 37, 1651–1655. [Google Scholar] [CrossRef]
- Wu, G.D.; Wang, X.L.; Huang, Y.; Liu, X.F.; Zhang, F.; Ding, K.Q.; Yang, X.L. Selective oxidation of glycerol with O2 catalyzed by low-cost CuNiAl hydrotalcites. J. Mol. Catal. A Chem. 2013, 379, 185–191. [Google Scholar] [CrossRef]
- Wang, X.L.; Wu, G.D.; Wang, F.; Ding, K.Q.; Zhang, F.; Liu, X.F.; Xue, Y.B. Base-free selective oxidation of glycerol with 3% H2O2 catalyzed by sulphonato-salen-chromium(III) intercalated LDH. Catal. Commun. 2012, 28, 73–76. [Google Scholar] [CrossRef]


| Entry | Catalysts | Au Loading (wt %) | Molar Ratio (M1/M2/M3) | SBET (m2 g−1) | Au/SBET a (mol m−2 × 10−7) | GLY Con. (%) | GLYAC Sel. (%) | H2O2 Efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Cr2O3 | …… | …… | 45 | …… | 13.8 | 0 | 15.5 |
| 2 | CuO | …… | …… | 42 | …… | 12.5 | 0 | 13.8 |
| 3 | NiO | …… | …… | 40 | …… | 11.9 | 0 | 12.0 |
| 4 | CoO | …… | …… | 47 | …… | 7.5 | 0 | 8.7 |
| 5 | ZnO | …… | …… | 45 | …… | 4.0 | 0 | 5.1 |
| 6 | Fe2O3 | …… | …… | 39 | …… | 6.3 | 0 | 7.2 |
| 7 | MCM-41 | …… | …… | 1020 | …… | 0 | 0 | 0 |
| 8 | SBA-15 | …… | …… | 785 | …… | 0 | 0 | 0 |
| 9 | CuNiAlO | …… | Cu/Ni/Al = 0.85/0.85/1 | 220 | …… | 38.5 | 27.3 | 41.5 |
| 10 | FeNiAlO | …… | Fe/Ni/Al = 0.86/0.87/1 | 223 | …… | 22.1 | 12.2 | 24.6 |
| 11 | CoNiAlO | …… | Co/Ni/Al = 0.85/0.84/1 | 219 | …… | 25.0 | 19.5 | 27.3 |
| 12 | CrNiAlO | …… | Cr/Ni/Al = 0.85/0.83/1 | 218 | …… | 33.6 | 30.1 | 37.0 |
| 13 | ZnNiAlO | …… | Zn/Ni/Al = 0.85/0.86/1 | 221 | …… | 23.0 | 16.9 | 25.9 |
| 14 | Au/Cr2O3 | 0.95 | …… | 37 | 13.0 | 81.5 | 67.0 | 83.0 |
| 15 | Au/CuO | 0.96 | …… | 35 | 13.9 | 83.0 | 68.2 | 85.5 |
| 16 | Au/NiO | 0.92 | …… | 35 | 13.3 | 65.7 | 58.5 | 67.2 |
| 17 | Au/CoO | 0.93 | …… | 39 | 12.1 | 51.3 | 57.0 | 57.5 |
| 18 | Au/ZnO | 0.91 | …… | 38 | 12.2 | 50.5 | 53.7 | 52.3 |
| 19 | Au/Fe2O3 | 0.95 | …… | 32 | 15.0 | 65.0 | 54.8 | 69.2 |
| 20 | Au/MCM-41 | 0.98 | …… | 976 | 0.5 | 33.2 | 77.6 | 37.5 |
| 21 | Au/SBA-15 | 0.95 | …… | 702 | 0.7 | 35.8 | 76.3 | 38.1 |
| 22 | Au/CuNiAlO | 0.93 | Cu/Ni/Al = 0.85/0.86/1 | 206 | 2.3 | 90.5 | 72.0 | 89.5 |
| 23 | Au/FeNiAlO | 0.95 | Fe/Ni/Al = 0.86/0.86/1 | 203 | 2.4 | 79.7 | 65.4 | 80.2 |
| 24 | Au/CoNiAlO | 0.92 | Co/Ni/Al = 0.85/0.85/1 | 205 | 2.3 | 73.0 | 63.1 | 76.4 |
| 25 | Au/CrNiAlO | 0.90 | Cr/Ni/Al = 0.85/0.84/1 | 203 | 2.2 | 89.5 | 70.8 | 87.3 |
| 26 | Au/ZnNiAlO | 0.94 | Zn/Ni/Al = 0.86/0.85/1 | 202 | 2.4 | 60.5 | 60.0 | 67.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wu, G.; Jin, T.; Xu, J.; Song, S. Selective Oxidation of Glycerol Using 3% H2O2 Catalyzed by Supported Nano-Au Catalysts. Catalysts 2018, 8, 505. https://doi.org/10.3390/catal8110505
Wang X, Wu G, Jin T, Xu J, Song S. Selective Oxidation of Glycerol Using 3% H2O2 Catalyzed by Supported Nano-Au Catalysts. Catalysts. 2018; 8(11):505. https://doi.org/10.3390/catal8110505
Chicago/Turabian StyleWang, Xiaoli, Gongde Wu, Tongfa Jin, Jie Xu, and Shihao Song. 2018. "Selective Oxidation of Glycerol Using 3% H2O2 Catalyzed by Supported Nano-Au Catalysts" Catalysts 8, no. 11: 505. https://doi.org/10.3390/catal8110505
APA StyleWang, X., Wu, G., Jin, T., Xu, J., & Song, S. (2018). Selective Oxidation of Glycerol Using 3% H2O2 Catalyzed by Supported Nano-Au Catalysts. Catalysts, 8(11), 505. https://doi.org/10.3390/catal8110505






